Abstract
In Brazil, sporotrichosis has transitioned from a rural to urban disease, driven by a shift in the initiation of infection from the accidental inoculation of organic matter to the traumatic implantation of the fungus by cats. Since the emergence of zoonotic sporotrichosis caused by Sporothrix brasiliensis, investigations have largely ignored the environmental habitat of the pathogen due to its association with domestic cats. Therefore, we investigated 18 environmental samples collected from rural areas of two cities where zoonotic sporotrichosis is endemic, but where domestic cats are scarce. We utilized traditional culture methods, and samples were also examined with two molecular methods used for the clinical diagnosis of sporotrichosis: a nested-PCR targeting the ITS region and a species-specific PCR targeting the calmodulin gene. No Sporothrix colonies were identified by traditional culture methods. However, the nested-PCR and the species-specific PCR for S. brasiliensis were positive for 18 and 5 samples, respectively. Sequencing revealed that positive results with the nested-PCR were due to non-specific amplification of other Ophiostomatales DNA, rather than Sporothrix spp. Three of the five amplicons from the species-specific PCR were suitable for sequencing and confirmed the presence of S. brasiliensis DNA. Hence, we confirmed that S. brasiliensis, as with other Sporothrix species, has an environmental habitat. Our findings underscore the challenges of nested-PCR for Sporothrix environmental studies and highlight that sequencing must follow PCR protocols to definitively identify Sporothrix spp. in environmental samples.
Keywords: soil, molecular biology, PCR, Sporothrix
1. Introduction
Sporotrichosis is a subcutaneous mycosis with a worldwide distribution and broad spectrum of disease manifestations [1]. In immunocompetent people, sporotrichosis is typically a benign disease, and the most severe forms are usually observed in people living with HIV/AIDS (PLWHA) [2] and in individuals with other underlying immunosuppressive conditions [3,4].
Initially, sporotrichosis was known as rose gardener’s disease [1,5]. Its classical transmission is characterized by traumatic inoculation of contaminated material in the subcutaneous tissue, and the injury is frequently associated with vegetation, wood and soil manipulation [5,6]. In this scenario, the major at-risk individuals are those involved with horticulture, farming, mining and wood exploration [7]. This transmission form is becoming relatively less frequent in Brazil, where zoonotic transmission prevails [8,9,10,11].
Over the past 20 years, cats have played an important role in the transmission of sporotrichosis in Rio de Janeiro state, Brazil [1]. More recently, zoonotic sporotrichosis has spread throughout the Brazilian territory [11,12]. In addition, other countries are also reporting cases of zoonotic sporotrichosis transmitted by naturally infected cats [13,14,15]. The immune system of cats is not able to adequately combat Sporothrix, contributing substantially to the increase in the number of sporotrichosis cases among the animals [16], and consequently, increasing cat-to-human transmission, which mostly occurs in housewives [11].
Independently of the transmission form, sporotrichosis is caused by a thermodimorphic species of the Sporothrix genus, which under laboratorial culture at 25–30 °C or in environmental niches, grows in the filamentous form, whereas it grows in a yeast-like form in parasitism or when cultivated on enriched culture media at 35–37 °C [1,5,7]. At least 50 species have been described in the Sporothrix genus, but the vast majority are saprobiotic [17]. Some species have been described as pathogenic to humans and other mammals, mainly Sporothrix brasiliensis, Sporothrix schenckii and Sporothrix globosa [18,19].
Sporothrix spp. has been isolated from environmental samples in several countries worldwide [13,20,21,22]. Recently, a systematic review described ecological determinants of the sporotrichosis agents, mainly S. schenckii, such as growth in soils with temperatures ranging from 6.6 °C to 28.84 °C and humidity ranging from 37.5% to 99.06%. Moreover, Sporothrix spp. is highly associated with decomposition of organic material, which potentially increases its ability to proliferate [20]. In Brazil, a study aiming to isolate and/or identify sporotrichosis agents in soil samples from a geographic area with reports of sporotrichosis cases was unsuccessful [23].
To improve our knowledge about the relationship of Sporothrix spp. with the environment and the suitability of different molecular methods of Sporothrix DNA detection, we applied classical and molecular methods to detect Sporothrix spp. in soil samples collected in two cities within the sporotrichosis hyperendemic area in the Rio de Janeiro state, Brazil, where Sporothrix spp. has not yet been associated with environmental samples.
2. Materials and Methods
2.1. Sample Collection and Processing
Soil samples were obtained in Seropédica (22°46′52.3′′ S, 043°45′27.1′′ W) and Nova Iguaçu (22°40′37.3′′ S, 043°24′17.7′′ W), both cities located in Rio de Janeiro state, Brazil. The samples were taken in rural areas where Paracoccidioides brasiliensis was recently described [24]. Approximately 100 g of soil were collected using sterile shovels, and stored in sealed sample collection bags (Nasco Sampling/Whirl-Pak®, Madson, WI, USA). Samples were processed for culture within 24 h after the collection. One gram of each soil sample was diluted in 9 mL sterile saline, vortexed for 10 min and particles were allowed to settle for 5 min. Serial ten-fold dilutions of the upper homogenous suspensions were plated on Potato Dextrose Agar (PDA) Mycosel Agar (Becton, Dickinson and Company, Sparks, MD, USA) and incubated at 25 °C for 60 days. Putative Sporothrix colonies were submitted to micro- and macromorphological tests to confirm genus identification [1].
2.2. Soil DNA Extraction
DNA extraction was performed as described [24] using the DNeasy® PowerSoil® Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions.
2.3. Nested-PCR
Nested-PCR was performed in order to detect low amounts of Sporothrix DNA [25,26]. The reaction mixture consisted of a total volume of 25 µL, with final concentrations of 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 0.4 µM concentrations of primers (Table S1), 1.5 U of Taq DNA polymerase (Invitrogen, Waltham, MA, USA), and 200 µM concentration of each dNTP (Invitrogen). For the first reaction, 4 µL of total extracted soil DNA was used and for the second, 4 µL of the first amplicon. Reactions were as follows: 95 °C for 5 min, 35 cycles of 95 °C for 30 s, 60 °C for 45 s, 72 °C for 30 s and 72 °C for 10 min [26]. The positive control was performed including the DNA of a reference strain for S. brasiliensis (CBS 120339), and ultrapure water (Gibco, Walthan, MA, USA) as negative control. As a positive result, an amplified fragment with 152 bp was expected, compared to a 1 kb plus DNA ladder (Invitrogen).
2.4. Species-Specific PCR
Species-specific PCR was performed on the environmental DNA samples for the three main relevant species. The reactions (final volume of 25 µL) were obtained using 3 mM MgCl2, 400 mM each deoxynucleotide triphosphate, and 50 U/mL Taq Polymerase (Invitrogen); 9.5 μL water, 1 μL (10 pmol/μL) of forward and reverse primers (Invitrogen) described in Table S1 and 4 μL of target DNA (25 ng/μL). The reactions were performed as described [27].
2.5. DNA Sequencing and Phylogenetic Analyses
The calmodulin amplicons obtained from the species-specific PCR products as well as the nested-PCR products were excised from the gel, purified with the IllustraTM GFXTM PCR DNA and Gel Band Purification Kit (GE Healthcare, Buckinghamshire, UK) and sequenced. Amplified fragments were purified with the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany), and sequenced at the Fiocruz Technological Platforms Network, Rio de Janeiro, Brazil [28]. The sequences were edited with the Sequencher Software Package version 4.9 (GeneCodes Corporation, Ann Arbor, MI, USA) and aligned with MEGA software version 10 [29]. The sequences were used for the phylogenetic analysis with sequences deposited in the GenBank database. A maximum likelihood (ML) tree was created using 1000 bootstrap replicates. The same dataset was used within DNAsp 5.10 (Universitat de Barcelona, Barcelona, Spain) [30] to calculate the distribution and diversity of haplotypes and the median-joining network was built and visualized using Network Software (Version 5.0.1.0, Fluxus Technology Ltd., Stanway, UK) [31].
3. Results
Eighteen soil samples were included in this study, nine from Seropédica and nine from Nova Iguaçú. After 60 days of incubation at 25 °C, fungal growth compatible with Sporothrix spp. did not occur in any of the soil samples from either city.
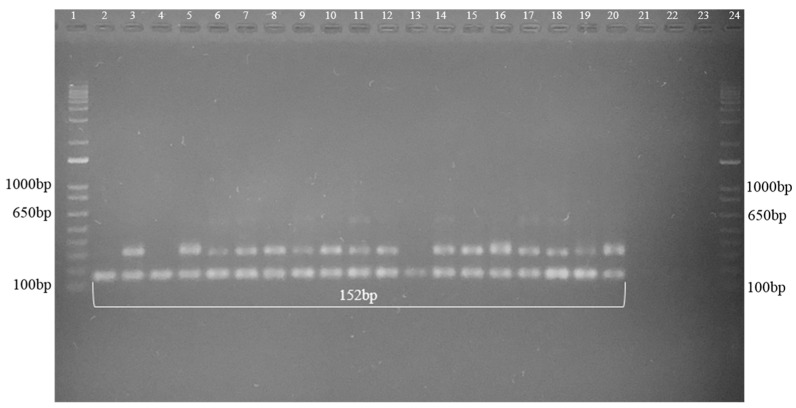
The nested-PCR demonstrated the presence of DNA amplicons of 152 bp in all 18 studied samples (Figure 1).
Figure 1.
Representative nested-PCR agarose gel of the DNA extracted from soil samples, demonstrating 16 amplified fragments with the amplification of 152 bp fragments. 1 and 24 = Molecular Weight (1 kb Plus–Invitrogen), 2 to 10 = samples from Seropédica, and 11 to 19 = samples from Nova Iguaçu, 20 = Positive control S. brasiliensis (IPEC 16490) and 21, 22 and 23 = Negative control.
The BLAST analysis of the amplified DNA obtained in the nested-PCR (GenBank sequences ON306930 to ON306947) demonstrated a nonspecific alignment with Sporothrix spp. Individual sequences showed a high similarity (100%) and query cover (100%) with several fungi from the Ophiostomataceae family, such as Grosmannia crassivaginata, Raffaelea canadensis, Ophiostoma nigrocarpum, Ophiostoma ambrosium, Leptographium huntii and Grosmannia serpens, most of them phytopathogenic and geophilic (Figure S1).
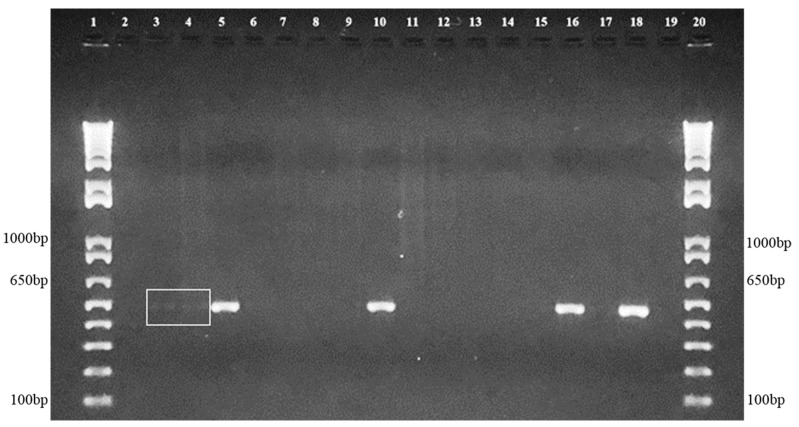
The PCR using species-specific primers demonstrated the absence of S. schenckii and S. globosa DNA. However, amplification with S. brasiliensis species-specific primers identified this pathogen’s DNA in soil samples from Seropédica (n = 4) and Nova Iguaçu (n = 1), as depicted in Figure 2.
Figure 2.
Representative agarose gel of the positive soil samples, demonstrating species-specific amplification of 469 bp fragments compatible with S. brasiliensis. 1 and 20 = Molecular Weight (1 kb Plus–Invitrogen), 2 to 10 samples from Seropédica and 11 to 17 samples from Nova Iguaçu, 18 = Positive control S. brasiliensis (IPEC 16490) and 19 = Negative control. The white square highlights two positive samples with low-intensity bands.
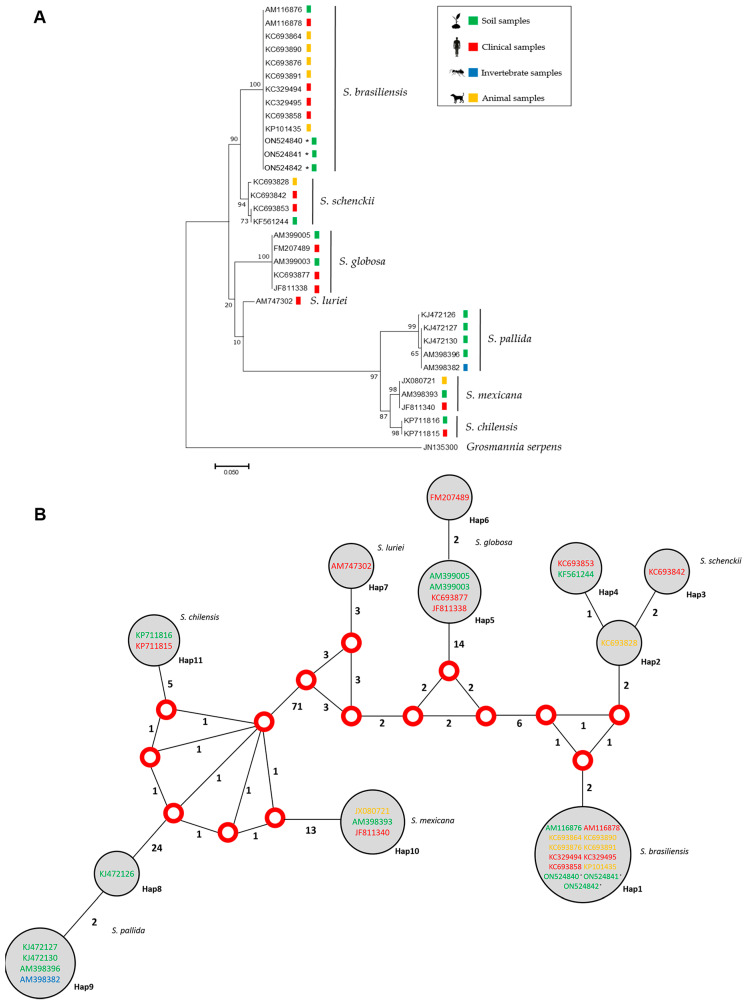
Phylogenetic analysis and haplotype network performed with the sequences obtained from the amplification of the species-specific primer (GenBank sequences ON524840 to ON524842, respectively, for samples 5, 10 and 16 in Figure 2) and sequences deposited in GenBank demonstrated that the three sequences from this study grouped with other sequences of S. brasiliensis, including isolates from clinical, animal and environmental sources (Figure 3).
Figure 3.
(A) Maximum likelihood phylogenetic relationships based on calmodulin partial sequencing between members of clinical, animal and environmental isolates from the Sporothrix species with clinical interest. The colored squares represent the origin source of each isolate/sequence. (B) Median vector haplotype network demonstrating the phylogenetic relationships among the 33 isolates/sequences of the present study, demonstrating that, in both cases, the three sequences obtained grouped with S. brasiliensis. Red dots represent median vectors. Circle size is proportional to the frequency of isolates (h). The numbers around each vertex represent the amount of mutations separating each haplotype and the different colors in the Genbank code represent the isolate/sequence origins. * Indicate sequences generated in this work.
Figure 4 shows the sites where the positive samples for S. brasiliensis DNA were collected. They comprise the soil around an acerola fruit tree (Malpighia emarginata), around a coconut tree (Cocos nucifera) and enriched with cow dung, around an artificial lake, and from the bottom of a stream. An additional positive sample was collected from a soil sample with a spider web.
Figure 4.
Soil samples with Sporothrix brasiliensis DNA detection. (A) Soil around an acerola fruit tree (Malpighia emarginata). (B) Soil around a coconut tree (Cocos nucifera) enriched with cow dung. (C) Soil around an artificial lake. (D) Soil from the bottom of a stream. Sites A to C are located in Seropédica. Site D is located in Nova Iguaçú.
4. Discussion
Over the past two decades, despite a significant increase in research on sporotrichosis and its agents [32], there have been few studies focusing on the isolation and/or molecular identification of Sporothrix spp. from environmental sources. Initially, sporotrichosis was known as gardener’s disease due to its association with horticulture-associated injuries [1]. The zoonotic epidemic in Brazil, primarily driven by S. brasiliensis, has led to a major shift in the mechanisms for disease acquisition, as traumatic inoculation is now associated with scratches and/or bites from cats [11]. However, the interaction of cats—and other animals—with soil and the transmission of Sporothrix to and/or from the environment has been poorly explored, which has resulted in an important knowledge gap in the pathogenesis of cat-associated sporotrichosis.
Soil is the ecological niche of several human pathogens, including thermodimorphic fungi, such as P. brasiliensis, Coccidioides posadasii and Histoplasma capsulatum [33,34]. The relationship of these fungi with environmental sources is better described when compared to Sporothrix spp. [35]. For example, H. capsulatum is primarily present in soils with high concentrations of nitrogen compounds, especially those with bird excreta and bat guano [36]. Here, we present some soil characteristics that may improve the knowledge about S. brasiliensis environmental habitat. The soil samples where S. brasiliensis DNA was detected did not receive direct solar light and/or present high humidity. This last characteristic was previously described for other Sporothrix species [20]. Moreover, urban sporotrichosis due to S. brasiliensis usually occurs in areas with low infrastructure and precarious sanitation, usually near polluted water sources [37,38].
Some factors may influence the isolation of pathogens from the environment. There are many other fungal species that have faster growth rates in culture that limit the growth of the fungus of interest, which could influence the lack of success in the isolation of Sporothrix spp. Recently, our group reported the isolation of a S. brasiliensis fungal colony from a wood sample present in a house were several cats with sporotrichosis lived [39]. The presence of sick cats, with high fungal burden in their lesions around this material probably contributed to increase the number of fungal cells present in the wood, which may have facilitated the culture isolation. Both cities from which we collected soil samples report zoonotic sporotrichosis cases [37]. As we were looking for the habitat of S. brasiliensis, we chose to collect the materials from an area with some rural characteristics, such as the presence of trees, small rivers and moist soil, among others. We did not observe cats in the area where samples were collected during the harvest of soil samples. In the present study, we consider the possibility of contamination of the environmental material with S. brasiliensis by infected cats to be very low.
To overcome the difficulties with isolating fastidious thermodimorphic fungi from the environment, molecular methods are increasingly being used [24,33,40]. An important factor of success is the method used for fungal detection, and in other models, such as P. brasiliensis, the most effective technique is nested-PCR [24,41]. Nested-PCR drastically increases the possibility of a successful detection as the number of DNA amplification cycles is high, making it possible to amplify small quantities of DNA that would not be detected by conventional PCR. Nested-PCR yielded positive results in 18 samples; however, sequencing results demonstrated unspecific amplifications with other Ophiostomataceae for the nested-PCR targeting the ITS region. The members of this family are traditional saprobes that are frequently found in soil samples [42]. Nested-PCR has been successfully used in clinical samples, where Ophiostomataceae members besides Sporothrix spp. are unlikely to occur [25,26]. The present study demonstrates that this methodology is not appropriate for environmental studies, due to the frequent amplification of DNA from saprobe fungi.
Real time-PCR has been reported as superior to nested-PCR to detect H. capsulatum in environmental samples [43]. The species-specific PCR used in our study was developed for clinical diagnosis purposes [27]. When we applied this method to environmental samples, we identified five positive samples and two patterns of amplifications were observed. The soil samples with high DNA amplification harbored S. brasiliensis by sequencing and subsequent phylogenetic analyses. The two samples that presented low amplification could not be sequenced, due to the small quantity of DNA. Nevertheless, there is a high possibility that these faint bands represent small amounts of S. brasiliensis DNA. However, as we observed with the nested-PCR, we cannot rule out the possibility of non-specific amplification of other close related fungi, since only members of the genus Ophiostoma, among all Ophiostomataceae family, were tested for cross-reaction in the standardization of the method for Sporothrix DNA amplification using species-specific primers.
The paucity of studies associating S. brasiliensis with the classic environmental transmission of sporotrichosis tends to suggest that this species is exclusively transmitted through the zoonotic pathway. However, S. brasiliensis has been isolated from patients with sporotrichosis who did not have contact with infected cats [44,45]. Moreover, S. brasiliensis has been isolated from soil in Argentina [46]. In Brazil, the largest environmental study attempting to detect S. brasiliensis was unsuccessful in both the isolation and detection of its DNA in 101 samples studied [23]. However, the isolation of S. schenckii was demonstrated in soil samples from armadillo burrows. Although S. schenckii was not isolated after direct plating of the soil in culture media, it was grown form organs of mice and hamsters inoculated with sand from the armadillo burrow [47]. An environmental relationship of S. brasiliensis was observed through fungal isolation from a sample of cat feces buried in sand in São Paulo state, Brazil [48].
Undoubtedly, cats play a major role in zoonotic sporotrichosis transmission. In contrast with the soil, which appears to have a low number of fungal cells, these animals have increased numbers of S. brasiliensis parasitic cells in their lesions and they may carry the fungus on their claws, which can traumatically inoculate the fungus into humans and other cats [49]. However, it is noteworthy that the close relationship of these animals with natural sources likely contributed to the emergence and persistence of S. brasiliensis as a feline and human pathogen. The strong epidemiological relationship reported between cats and sporotrichosis has incorrectly led to the belief that S. brasiliensis-caused sporotrichosis is only zoonotically transmitted, ignoring the original association of sporotrichosis with environmental sources [8]. From the authors’ point of view, it is principally the human–cat interaction that increases the odds of traumatic inoculation of the fungus, as these animals frequently seek human contact and harbor a high fungal burden. On the other hand, the lower amounts of S. brasiliensis in soil compared to naturally infected cats makes the classic transmission of S. brasiliensis via traumatic injury with vegetation or soil more difficult, but not inconceivable. Furthermore, the sporotrichosis expansion in Rio de Janeiro is caused by a clonal species [50] and, in this study, we did not observe phylogenetic differences among clinical or environmental S. brasiliensis strains.
5. Conclusions
This study demonstrates the environmental detection of S. brasiliensis DNA in a hyperendemic area of sporotrichosis, primarily due to S. brasiliensis. This reinforces the initial historical association of sporotrichosis as a disease related to soil and organic material. The detection of Sporothrix spp. in environmental samples can be facilitated by implementing existing molecular methods with high sensitivity and specificity. Importantly, sequencing of PCR amplicons must be used to confirm Sporothrix spp. identification. A deeper understanding of the ecological niches of Sporothrix spp. is a significant step forward in the efforts to resolve this important public health problem in endemic regions.
Acknowledgments
The authors thank Joshua Nosanchuk for his editorial assistance and helpful comments on the text. The authors also thank Bodo Wanke (in memorian), Luciana Trilles and Marcia dos Santos Lazéra who helped to collect the environmental samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8060604/s1, Figure S1: BLAST Maximum likelihood phylogenetic relationships of amplified sequences obtained in the nested-PCR, presenting similarity with sequences from 16 different fungal species deposited in the GanBank. Table S1: PCR primers used in this study.
Author Contributions
Conceptualization, P.M.d.M. and R.A.-P.; methodology, F.A.-S. and R.A.-P.; validation, F.A.-S.; formal analysis, F.A.-S.; investigation, F.A.-S., V.B.d.S.R. and B.d.S.S.-C.; resources, R.M.Z.-O. and P.M.d.M.; data curation, F.A.-S. and V.B.d.S.R.; writing—original draft preparation, F.A.-S. and R.A.-P.; writing—review and editing, V.B.d.S.R., B.d.S.S.-C., R.M.Z.-O. and P.M.d.M.; visualization, F.A.-S., P.M.d.M. and R.A.-P.; supervision, R.M.Z.-O. and R.A.-P.; project administration, R.M.Z.-O., P.M.d.M. and R.A.-P.; funding acquisition, R.M.Z.-O. and P.M.d.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 28 April 2022) [51].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The “Programa Jovens Pesquisadores (INI/Fiocruz)” supported this study. This work was supported, in part, by Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq 302796/2017-7] and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro [FAPERJ E-26/202.527/2019].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Orofino-Costa R., de Macedo P.M., Rodrigues A.M., Bernardes-Engemann A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira J.A.S., Freitas D.F.S., Lamas C.C. The impact of sporotrichosis in HIV-infected patients: A systematic review. Infection. 2015;43:267–276. doi: 10.1007/s15010-015-0746-1. [DOI] [PubMed] [Google Scholar]
- 3.Ramos V., Astacio G.S.-M., do Valle A.C.F., de Macedo P.M., Lyra M.R., Almeida-Paes R., Oliveira M.M.E., Zancopé-Oliveira R.M., Brandão L.G.P., Quintana M.S.B., et al. Bone sporotrichosis: 41 cases from a reference hospital in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2021;15:e0009250. doi: 10.1371/journal.pntd.0009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichman V., Marques de Macedo P., Francis Saraiva Freitas D., Carlos Francesconi do Valle A., Almeida-Silva F., Reis Bernardes-Engemann A., Zancopé-Oliveira R.M., Almeida-Paes R., Clara Gutierrez-Galhardo M. Zoonotic sporotrichosis in renal transplant recipients from Rio de Janeiro, Brazil. Transpl. Infect. Dis. 2021;23:e13485. doi: 10.1111/tid.13485. [DOI] [PubMed] [Google Scholar]
- 5.De Barros M.B.L., de Almeida Paes R., Schubach A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Araujo M.L., Rodrigues A.M., Fernandes G.F., de Camargo Z.P., de Hoog G.S. Human sporotrichosis beyond the epidemic front reveals classical transmission types in Espírito Santo, Brazil. Mycoses. 2015;58:485–490. doi: 10.1111/myc.12346. [DOI] [PubMed] [Google Scholar]
- 7.Lopes-Bezerra L.M., Schubach A., Costa R.O. Sporothrix schenckii and sporotrichosis. An. Acad. Bras. Cienc. 2006;78:293–308. doi: 10.1590/S0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 8.Rossow J.A., Queiroz-Telles F., Caceres D.H., Beer K.D., Jackson B.R., Pereira J.G., Ferreira Gremião I.D., Pereira S.A. A One Health approach to combatting Sporothrix brasiliensis: Narrative review of an emerging zoonotic fungal pathogen in South America. J. Fungi. 2020;6:247. doi: 10.3390/jof6040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macêdo-Sales P.A., Souto S.R.L.S., Destefani C.A., Lucena R.P., Machado R.L.D., Pinto M.R., Rodrigues A.M., Lopes-Bezerra L.M., Rocha E.M.S., Baptista A.R.S. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro state, Brazil: A comparison between infected and non-infected populations. BMC Vet. Res. 2018;14:19. doi: 10.1186/s12917-018-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchotene K.O., Madrid I.M., Klafke G.B., Bergamashi M., Della Terra P.P., Rodrigues A.M., de Camargo Z.P., Xavier M.O. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–658. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- 11.Rabello V.B.S., Almeida M.A., Bernardes-Engemann A.R., Almeida-Paes R., de Macedo P.M., Zancopé-Oliveira R.M. The historical burden of sporotrichosis in Brazil: A systematic review of cases reported from 1907 to 2020. Braz. J. Microbiol. 2022;53:231–244. doi: 10.1007/s42770-021-00658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gremião I.D.F., Oliveira M.M.E., Monteiro de Miranda L.H., Saraiva Freitas D.F., Pereira S.A. Geographic expansion of sporotrichosis, Brazil. Emerg. Infect. Dis. 2020;26:621–624. doi: 10.3201/eid2603.190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etchecopaz A., Toscanini M.A., Gisbert A., Mas J., Scarpa M., Iovannitti C.A., Bendezú K., Nusblat A.D., Iachini R., Cuestas M.L. Sporothrix brasiliensis: A review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J. Fungi. 2021;7:170. doi: 10.3390/jof7030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etchecopaz A.N., Lanza N., Toscanini M.A., Devoto T.B., Pola S.J., Daneri G.L., Iovannitti C.A., Cuestas M.L. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: Case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J. Mycol. Med. 2020;30:100908. doi: 10.1016/j.mycmed.2019.100908. [DOI] [PubMed] [Google Scholar]
- 15.Siew H.H. The current status of feline sporotrichosis in Malaysia. Med. Mycol. J. 2017;58:E107–E113. doi: 10.3314/mmj.17.014. [DOI] [PubMed] [Google Scholar]
- 16.De Souza E.W., de Borba C.M., Pereira S.A., Gremião I.D.F., Langohr I.M., Oliveira M.M.E., de Oliveira R.d.V.C., da Cunha C.R., Zancopé-Oliveira R.M., de Miranda L.H.M., et al. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Sci. Rep. 2018;8:9074. doi: 10.1038/s41598-018-27447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Beer Z.W., Duong T.A., Wingfield M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016;83:165–191. doi: 10.1016/j.simyco.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti A., Bonifaz A., Gutierrez-Galhardo M.C., Mochizuki T., Li S. Global epidemiology of sporotrichosis. Med. Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Hagen F., Stielow B., Rodrigues A.M., Samerpitak K., Zhou X., Feng P., Yang L., Chen M., Deng S., et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia. 2015;35:1–20. doi: 10.3767/003158515X687416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramírez-Soto M.C., Aguilar-Ancori E.G., Tirado-Sánchez A., Bonifaz A. Ecological determinants of sporotrichosis etiological agents. J. Fungi. 2018;4:95. doi: 10.3390/jof4030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon D.M., Salkin I.F., Duncan R.A., Hurd N.J., Haines J.H., Kemna M.E., Coles F.B. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 1991;29:1106–1113. doi: 10.1128/jcm.29.6.1106-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feuerman E.J., Alteras I., Bashan D., Lehrer N.B. Isolation of Sporothrix schenckii in the soil in Israel in relation to a new case in Man. Sabouraudia. 1976;14:217–222. doi: 10.1080/00362177685190301. [DOI] [PubMed] [Google Scholar]
- 23.Poester V.R., Mendes J.F., Groll A.V., Klafke G.B., Brandolt T.M., Xavier M.O. Sporothrix spp. evaluation in soil of a hyperendenic area for sporotrichosis in southern Brazil. Ciênc. Anim. Bras. 2018;19:1–8. doi: 10.1590/1809-6891v19e-52571. [DOI] [Google Scholar]
- 24.De Macedo P.M., de Scramignon-Costa B.S., Almeida-Paes R., Trilles L., de Oliveira L.S.C., Zancopé-Oliveira R.M., do Valle A.C.F., Wanke B. Paracoccidioides brasiliensis habitat: Far beyond armadillo burrows? Mem. Inst. Oswaldo Cruz. 2020;115:e200208. doi: 10.1590/0074-02760200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira M.M.E., de Muniz M.M., Almeida-Paes R., Zancope-Oliveira R.M., Freitas A.D., Lima M.A., Gutierrez-Galhardo M.C., Freitas D.F.S. Cerebrospinal fluid PCR: A new approach for the diagnosis of CNS sporotrichosis. PLoS Negl. Trop. Dis. 2020;14:e0008196. doi: 10.1371/journal.pntd.0008196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu S., Chung W.-H., Hung S.-I., Ho H.-C., Wang Z.-W., Chen C.-H., Lu S.-C., Kuo T.-T., Hong H.-S. Detection of Sporothrix schenckii in clinical samples by a Nested PCR assay. J. Clin. Microbiol. 2003;41:1414–1418. doi: 10.1128/JCM.41.4.1414-1418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues A.M., de Hoog G.S., de Camargo Z.P. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2015;9:e0004190. doi: 10.1371/journal.pntd.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto T.D., Vasconcellos E.A., Gomes L.H.F., Moreira A.S., Degrave W.M., Mendonça-Lima L., Alves-Ferreira M. ChromaPipe: A pipeline for analysis, quality control and management for a DNA sequencing facility. Genet. Mol. Res. 2008;7:861–871. doi: 10.4238/vol7-3X-Meeting04. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 31.Bandelt H.J., Forster P., Röhl A. Median-Joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 32.Albuquerque P.C., Fonseca E., de Fonseca B.P., Zicker F., Zancopé-Oliveira R.M., Almeida-Paes R. Bibliometric assessment and key messages of sporotrichosis research (1945–2018) F1000Research. 2020;9:654. doi: 10.12688/f1000research.24250.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Macêdo R.C.L., Rosado A.S., da Mota F.F., Cavalcante M.A.S., Eulálio K.D., Filho A.D., Martins L.M.S., Lazéra M.S., Wanke B. Molecular identification of Coccidioides spp. in soil samples from Brazil. BMC Microbiol. 2011;11:108. doi: 10.1186/1471-2180-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taborda C.P., Buccheri R., Benard G., Duarte-Neto A.N., Nosanchuk J.D., Travassos L.R. Paracoccidioides spp. and Histoplasma capsulatum: Current and new perspectives for diagnosis and treatment. Curr. Top. Med. Chem. 2018;18:1333–1348. doi: 10.2174/1568026618666181002112231. [DOI] [PubMed] [Google Scholar]
- 35.Thompson G.R., Pasqualotto A.C. Endemic mycoses: Expansion of traditional geographic ranges and pitfalls in management. Mycoses. 2021;64:989–992. doi: 10.1111/myc.13326. [DOI] [PubMed] [Google Scholar]
- 36.Deepe G.S. Outbreaks of histoplasmosis: The spores set sail. PLoS Pathog. 2018;14:e1007213. doi: 10.1371/journal.ppat.1007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Silva M.B.T., de Mattos Costa M.M., da Torres C.C.S., Galhardo M.C.G., do Valle A.C.F., de Magalhães M.A.F.M., Sabroza P.C., Oliveira R.M. Esporotricose urbana: Epidemia negligenciada no Rio de Janeiro, Brasil. Cad. Saúde Pública. 2012;28:1867–1880. doi: 10.1590/S0102-311X2012001000006. [DOI] [PubMed] [Google Scholar]
- 38.Alzuguir C.L.C., Pereira S.A., Magalhães M.A.F.M., Almeida-Paes R., Freitas D.F.S., Oliveira L.F.A., Pimentel M.I.F. Geo-epidemiology and socioeconomic aspects of human sporotrichosis in the municipality of Duque de Caxias, Rio de Janeiro, Brazil, between 2007 and 2016. Trans. R. Soc. Trop. Med. Hyg. 2020;114:99–106. doi: 10.1093/trstmh/trz081. [DOI] [PubMed] [Google Scholar]
- 39.Rabello V.B.S., Almeida-Silva F., de Scramignon-Costa B.S., Motta B.S., de Macedo P.M., de Teixeira M.M., de Almeida Paes R., Irinyi L., Meyer W., Zancope-Oliveira R.M. Environmental isolation of Sporothrix brasiliensis in an area with recurrent feline sporotrichosis cases. Front. Cell Infect. Microbiol. 2022;12:894297. doi: 10.3389/fcimb.2022.894297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez L.F., Torres I.P., del Pilar Jiménez-A M., McEwen J.G., de Bedout C., Peláez C.A., Acevedo J.M., Taylor M.L., Arango M. Detection of Histoplasma capsulatum in organic fertilizers by Hc100 nested polymerase chain reaction and its correlation with the physicochemical and microbiological characteristics of the samples. Am. J. Trop. Med. Hyg. 2018;98:1303–1312. doi: 10.4269/ajtmh.17-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theodoro R.C., Candeias J.M.G., Araújo J.P., Bosco S.D.M.G., Macoris S.A.D.G., Padula L.O.J., Franco M., Bagagli E. Molecular detection of Paracoccidioides brasiliensis in soil. Med. Mycol. 2005;43:725–729. doi: 10.1080/13693780500129418. [DOI] [PubMed] [Google Scholar]
- 42.Spor A., Roucou A., Mounier A., Bru D., Breuil M.-C., Fort F., Vile D., Roumet P., Philippot L., Violle C. Domestication-driven changes in plant traits associated with changes in the assembly of the rhizosphere microbiota in tetraploid wheat. Sci. Rep. 2020;10:12234. doi: 10.1038/s41598-020-69175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gómez L.F., Gade L., Litvintseva A.P., McEwen J.G., Peláez C.A., Arango M., del Jiménez M.P. Comparison between two molecular techniques: Nested and real-time polymerase chain reaction targeting 100-KDa Hc protein for detection of Histoplasma capsulatum in environmental samples. Am. J. Trop. Med. Hyg. 2022;106:1329–1332. doi: 10.4269/ajtmh.20-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fichman V., Freitas D.F.S., de Macedo P.M., do Valle A.C.F., Almeida-Silva F., Zancopé-Oliveira R.M., Almeida-Paes R., Gutierrez-Galhardo M.C. Sporotrichosis after tattooing caused by Sporothrix brasiliensis. Mycopathologia. 2022;187:137–139. doi: 10.1007/s11046-021-00611-8. [DOI] [PubMed] [Google Scholar]
- 45.Fichman V., Gremião I.D.F., Mendes-Júnior A.A.V., Sampaio F.M.S., Freitas D.F.S., Oliveira M.M.E., Almeida-Paes R., Valle A.C.F., Gutierrez-Galhardo M.C. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus) J. Eur. Acad. Dermatol. Venereol. 2018;32:e157–e158. doi: 10.1111/jdv.14661. [DOI] [PubMed] [Google Scholar]
- 46.Córdoba S., Isla G., Szusz W., Vivot W., Hevia A., Davel G., Canteros C.E. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses. 2018;61:441–448. doi: 10.1111/myc.12760. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues A.M., Bagagli E., de Camargo Z.P., de Bosco S.M.G. Sporothrix schenckii sensu stricto isolated from soil in an armadillo’s burrow. Mycopathologia. 2014;177:199–206. doi: 10.1007/s11046-014-9734-8. [DOI] [PubMed] [Google Scholar]
- 48.Montenegro H., Rodrigues A.M., Dias M.A.G., da Silva E.A., Bernardi F., de Camargo Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014;10:269. doi: 10.1186/s12917-014-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gremião I.D.F., Martins da Silva da Rocha E., Montenegro H., Carneiro A.J.B., Xavier M.O., de Farias M.R., Monti F., Mansho W., de Macedo Assunção Pereira R.H., Pereira S.A., et al. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz. J. Microbiol. 2021;52:107–124. doi: 10.1007/s42770-020-00365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues A.M., de Melo Teixeira M., de Hoog G.S., Schubach T.M.P., Pereira S.A., Fernandes G.F., Bezerra L.M.L., Felipe M.S., de Camargo Z.P. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GenBank Overview. [(accessed on 1 June 2022)]; Available online: https://www.ncbi.nlm.nih.gov/genbank/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 28 April 2022) [51].