Abstract
The application of chemical pesticides to protect agricultural crops from pests and diseases is discouraged due to their harmful effects on humans and the environment. Therefore, alternative approaches for crop protection through microbial or microbe-originated pesticides have been gaining momentum. Wheat blast is a destructive fungal disease caused by the Magnaporthe oryzae Triticum (MoT) pathotype, which poses a serious threat to global food security. Screening of secondary metabolites against MoT revealed that antimycin A isolated from a marine Streptomyces sp. had a significant inhibitory effect on mycelial growth in vitro. This study aimed to investigate the inhibitory effects of antimycin A on some critical life stages of MoT and evaluate the efficacy of wheat blast disease control using this natural product. A bioassay indicated that antimycin A suppressed mycelial growth (62.90%), conidiogenesis (100%), germination of conidia (42%), and the formation of appressoria in the germinated conidia (100%) of MoT at a 10 µg/mL concentration. Antimycin A suppressed MoT in a dose-dependent manner with a minimum inhibitory concentration of 0.005 μg/disk. If germinated, antimycin A induced abnormal germ tubes (4.8%) and suppressed the formation of appressoria. Interestingly, the application of antimycin A significantly suppressed wheat blast disease in both the seedling (100%) and heading stages (76.33%) of wheat at a 10 µg/mL concentration, supporting the results from in vitro study. This is the first report on the inhibition of mycelial growth, conidiogenesis, conidia germination, and detrimental morphological alterations in germinated conidia, and the suppression of wheat blast disease caused by a Triticum pathotype of M. Oryzae by antimycin A. Further study is required to unravel the precise mode of action of this promising natural compound for considering it as a biopesticide to combat wheat blast.
Keywords: natural compound, biological control, inhibition, biopesticide
1. Introduction
The wheat blast fungus Magnaporthe oryzae Triticum (MoT) pathotype is considered one of the utmost destructive pathogens of the major food crop, wheat [1,2,3,4]. This fungal pathogen has been a threat to three million hectares of wheat crop in some South American countries including Brazil, Argentina, Bolivia, and Paraguay since its first outbreak in Brazil in 1985. Recently, this fearsome wheat killer was introduced into Bangladesh and Zambia, posing a serious threat to global food and nutritional security [3,5,6,7]. The MoT fungus can infect wheat plants at all growth stages, but infection at the heading stage is considered the most destructive [4,8,9]. Infection on the spike at the heading stage blocks the vascular system, resulting in whitehead symptoms with shriveled or no grains in the spike [1,3,4]. Under a favorable environment, yield loss due to wheat blast can be up to 100% [3]. Infected seeds are thought to be the source of inoculum for the long-distance dispersion of this pathogen, while both seeds and airborne asexual spores serve as inoculum for the short-distance dispersion of the pathogen [10,11]. The asexual spore of the fungus is called conidium, a three-celled, hyaline, and pyriform structure, which attaches to the surface of the host by secreting adhesive biochemicals [3,8,12]. After attachment to the host surface, the conidium germinates to form a germ tube. Later on, an appressorium and infection peg is formed to rupture the host epidermis to proceed with the infection process [12,13]. The process of plant tissue invasion is accomplished by the penetrating fungal hypha through the host epidermis and invading the plasma membrane of the host [8,12,13]. Therefore, disruption of any of these asexual life stages eliminates the possibility of pathogenesis by this fungus [14].
The use of synthetic fungicides is one of the approaches to control plant disease. Strobilurin (QoI) fungicides, either alone or in combination with other fungicides, have been used to manage wheat blast disease. However, indiscriminate and frequent use of strobilurin (QoI) fungicides has led to the emergence of pathogenic strains resistant to this group of fungicides [15,16,17]. In addition, extensive use of these fungicides can cause serious damage to human health and animals, and can disturb the natural ecosystem [18,19]. Moreover, traditional breeding strategies take a longer time to develop resistant varieties, which often break down under field conditions after a few years due to the development of new pathogen races [15,20]. Due to hexaploidy, the genetic modification of wheat is considered extremely complicated [21]. Therefore, an eco-friendly alternative approach is needed to manage this fearsome disease of wheat.
Biological control may offer a better alternative to the management of plant diseases. Several bacterial genera including Bacillus, Pseudomonas, and Streptomyces are well known for their biocontrol activity [22]. They produce a wide array of antifungal compounds such as lytic enzymes, and antibiotics to suppress growth of pathogens or induce systemic resistance in plants. Among the bacterial genera, the genus Streptomyces has received special attention from researchers due to its potential to produce a diverse class of antibiotics [23]. Approximately two-thirds of economically important antibiotics have been isolated from Streptomyces spp. that were used for disease management in agriculture [24,25]. The biocontrol activity of these isolated compounds against phytopathogens has been reported by various researchers [25,26,27,28,29,30]. Several studies were conducted using biopesticides as biocontrol agents with specific formulations against plant diseases [31,32]. Moreover, they also produce lytic enzymes such as chitinases and glucanases, which are also used to control phytopathogens [33,34,35]. Either whole cells or metabolites have been used to formulate Streptomyces-based fungicides. For example, the mycelia and spores of Streptomyces have been used for formulation of Mycostop (containing S. griseoviridis K61), Actinovate and Actino-Iron (containing Streptomyces lydicus WYEC 108), and RhizovitR (S. rimosus) for the control of the foliar and root diseases of various crops [36,37,38,39]. In addition, three secondary metabolites, polyoxin D, streptomycin, and kasugamycin, produced by Streptomyces spp. have been marked as foliar fungicides and bactericides [40]. Secondary metabolite-based products provide advantages over live organism-based products due to their higher shelf life and being not amenable to compromising the efficacy due to changes in the environmental conditions.
Antimycin A, a member of antimycin antibiotics isolated from marine Streptomyces sp., is composed of acyl and alkyl side chains and a nine-member dilactone ring [41]. Antimycins are known as specific inhibitors of the mitochondrial respiratory chain at the level of complex III [42]. Antimycin A has drawn considerable attention due to its toxicity toward both human and phytopathogenic fungi [42,43,44]. They inhibit the growth and development of the fungi Rhizoctonia solani and Magnaporhe grisea [45,46]. In a screening of secondary metabolites against a newly introduced destructive wheat blast disease caused by MoT, we found that antimycin A isolated from a marine Streptomyces sp. significantly inhibited growth of MoT in vitro. This study aimed to investigate, in detail, the effects of antimycin A on asexual development of wheat blast fungus in vitro and evaluate the control of disease in vivo. Therefore, the specific objectives of this study were to (i) investigate the effect of antimycin A on the suppression of the mycelial growth of MoT; (ii) test its effect on conidiogenesis, conidial germination, and subsequent steps of the asexual development of MoT; and (iii) evaluate the suppression of blast disease at the seedling and heading stages of wheat.
2. Materials and Methods
2.1. Chemicals
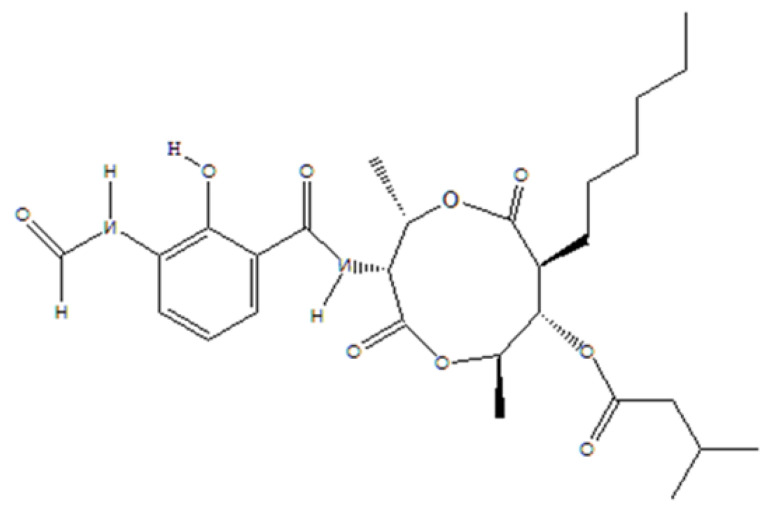
Antimycin A (AMA) (Figure 1) is a chemical compound produced by a marine Streptomyces sp. [41]. This pure compound was generously provided by Dr. Hartmut Laatsch of Georg-August University Goettingen, Germany. The fungicide Nativo® 75 WG (a combination of 50% tebuconazole and 25% trifloxystrobin) was purchased from Bayer Crop Science Ltd. Dhaka, Bangladesh.
Figure 1.
Structure of antimycin A.
2.2. Culture of Wheat Blast Isolate
A fungal isolate BTJP 4–5 was obtained in pure culture from the field-infected wheat blast samples by picking up a single conidium and then preserving it at 4 °C on dry filter paper, following the method described earlier [47]. For this study, this virulent isolate BTJP 4–5 of MoT was retrieved from storage in potato dextrose agar (PDA) medium and incubated at 25 °C for conidia production [47].
2.3. Preparation of Chemical Solution and Conidial Suspension
A stock solution of antimycin A was prepared using a small quantity of dimethyl sulfoxide (DMSO). The preparation of 1, 5, and 10 μg/disk concentrations of this compound was then carried out in distilled water, where the final concentration of DMSO never exceeded 1% (v/v) in the final solution, which is proven not to affect the hyphal growth or sporulation of MoT. The Nativo® 75 WG concentrations of 1, 5, and 10 μg/disk were prepared in distilled water. Sterilized water with 1% DMSO served as a negative control. The conidial suspension was prepared from 10-day-old culture plates and the spore concentration was adjusted to ca. 5 × 104 conidia mL−1, as described by [47].
2.4. Fungal Growth Inhibition and Morphological Changes of Hyphae
The hyphal growth inhibition of MoT isolate BTJP 4–5 by antimycin A and Nativo® 75 WG was calculated using the modified disk diffusion method [14,48]. A series of concentrations ranging from 0.005 to 2 μg/disk of antimycin A and Nativo® 75 WG were prepared by dissolving the required amounts in DMSO and water. Nine-millimeter diameter filter-paper disks (Sigma-Aldrich Co., St. Louis, MO, USA) were soaked with the test compounds. The treated disks were placed at a 2 cm distance from one side of 9 cm diameter Petri dishes containing 20 mL of fungal growth media. Five-millimeter diameter mycelial blocks were placed on the opposite side of the filter paper disk. Filter paper disks treated with DMSO followed by evaporation at room temperate functioned as a negative control. The Petri dishes were incubated at 25 °C until the fungal colony fully covered the media surface of the control plates and the experiment was repeated five times. The radial growth of the fungal colony was measured in centimeters with a ruler along with two perpendicular lines drawn on the lower side of each plate. After 10 days of incubation, the data were recorded by measuring the inhibition zone formed by the test compounds and corresponding mycelial growth. The radial growth inhibition percentage (RGIP) (± standard error) [49] was determined from mean values as: RGIP = (Radial growth in control plate − Radial growth in treated plate) ÷ (Radial growth in control plate) × 100.
A Zeiss Primo Star microscope at 40× and 100× (100× was an oil immersion lens) was used to observe the hyphal morphology at the leading edge of the colonies facing the treated and control disks. A Canon DOS 700D digital camera was used to capture images of the disk diffusion experiment. Photographs of the hyphae were captured with a Zeiss Axiocam ERc 5 s microscope.
2.5. Inhibition of Conidiogenesis
To induce conidiogenesis, the mycelia of a 10-day-old MoT culture plate were washed to reduce nutrients [3,14,50]. MoT mycelial agar blocks measuring 10 mm were treated with 50 μL of antimycin A and Nativo® 75 WG at 1, 5, and 10 μg/mL and put into Nunc multi-well plates. The same amount of sterilized water was used on the MoT mycelial block with 1% DMSO serving as a negative control. Then, the treated MoT mycelial agar blocks were incubated at 28 °C with >90% RH. Additionally, light and dark periods were adjusted at 14 h and 10 h, respectively. After 24 h, conidiogenesis was examined using a Zeiss Primo Star microscope at 40× magnification. All of the images were captured with a Zeiss Axiocam ERc 5 s microscope and the experiment was repeated five times.
2.6. Germination Inhibition and Morphological Modifications of Germinated Conidia
The conidial germination assay was conducted according to the previously described protocol [14,51]. For each treatment, a 100 μL solution of respective concentration was added directly to 100 μL of 5 × 104 conidia mL−1 of MoT to make a final volume of 200 μL into a well of a 96-well plate. A glass rod was used to mix the solution immediately, and the solution was incubated at 25 °C. In this experiment, 1% DMSO with sterile water served as a control. A moisture chamber was used to incubate the multi-well plate at 25 °C for 6 h, 12 h, and 24 h in the dark. For each replication, a total of 100 conidia were observed under a Zeiss Primo Star at 100× magnification. Calculations were made of the of germination percentage of the conidia and its developmental process was examined, and photographs were captured with a Zeiss Axiocam ERc 5 s. The time course was repeated five times. The conidial germination percentage (±standard error) was calculated from mean values as: CG% = (C − T)/C × 100, where, CG = conidial germination, C = percentage of germinated conidia in the control samples, and T = percentage of germinated conidia in the treated samples.
2.7. Growing of Seedlings
Wheat seeds of Bangladeshi cultivar BARI Gom-26 were surface-sterilized with 70% ethanol for 10 min, soaked in 1.5% active chlorine for 1 hour, and rinsed five times in sterile distilled water (SDW) [52]. Twenty-five seeds were planted in each of the 20 cm diameter plastic pots filled with NPK fertilizer-amended soil. Finally, 20 healthy seedlings per pot were allowed to grow under natural conditions until the seedling bioassay, following the previously described protocol by Gupta et al. [47]. Watering was done as a regular management practice.
2.8. Field Evaluation of Antimycin A against Wheat Blast
2.8.1. Preparation of Land and Fertilization
The experiment was set at confined land in the research field of Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU), Gazipur, Bangladesh. The experimental site was located at 24.09° north latitude and 90.26° east longitude with an elevation of 8.4 m from the mean sea level. The land was well ploughed and cleaned properly by uprooting weeds and stubbles. Well-decomposed cow dung was applied in adequate amounts during land preparation. Chemical fertilizers like nitrogen, triple super phosphate, muriate of potash, and gypsum were applied at the rate of 70-28-50-11 kg ha−1 N-P-K-S, respectively [53]. Two-thirds of urea and all other fertilizers were applied at the final land preparation as a basal dose 3–4 days before seed sowing. Immediately after sowing, the plots were lightly irrigated to ensure uniform germination. Irrigation and other intercultural operations were done whenever necessary. The rest one-third of urea was top dressed at first irrigation at 20 days after sowing (DAS). A randomized complete block design (RCBD) was followed for conducting the experiment.
2.8.2. Seed Sowing and Management of the Plot
The seeds of wheat variety BARI Gom-26 were sown in the first week of December. Seed treatment was carried out with fungicide Vitavex 200 (3 g/kg seed) before sowing. All plots were properly labeled. Irrigation and other intercultural operations were conducted as necessary.
2.9. Plant Infection Assay at Seedling Stage
After 14 days of emergence, the pots were covered with sterilized transparent polyethylene bags to maintain humidity and laid in a completely randomized design. Two sets of experiments were conducted for seedling assay. One was preventive and another was curative. In the case of the preventive assay, the seedlings were sprayed with freshly prepared test compounds at the respective concentrations mentioned above and left overnight to dry. The pots were then inoculated by spraying a conidial suspension containing 5 × 104 MoT conidia mL−1. Inoculated seedlings were incubated inside sterilized transparent polyethylene bags (>95% relative humidity) at 25 °C and kept in the dark for 24 h after inoculation. The other seedlings were sprayed with a conidial suspension containing 5 × 104 MoT conidia mL−1 in the case of the curative assay. The pots were then incubated overnight inside sterilized transparent polyethylene bags (>95% relative humidity) at 25 °C and kept in the dark for 24 h after inoculation for disease development. Then, the seedlings were sprayed with freshly prepared test compounds at the respective concentrations mentioned above. For both preventive and curative assays, the seedlings were then transferred into a growth room operating at 28 ± 1 °C and a minimum of 90% relative humidity with 12 h light per day [54]. In addition, sterilized water was sprayed on the seedlings five to seven times a day to provide a conducive environment for disease development in the growth room conditions. The disease development data were recorded after five days of inoculation. Each treatment was replicated five times.
2.10. Infection Assay in Wheat Field at Reproductive Phase
Freshly prepared 1, 5, and 10 μg/disk concentrations of the test compound were sprayed in the respective plots and left overnight to dry; sterilized water with 1% DMSO served as a negative control. Spore suspension was applied in wheat fields just after the flowering stage of the wheat plant. The fungicide Nativo® 75 WG was applied as a positive control and deionized distilled water was applied as a negative control. Before inoculation, the plots were covered with transparent polyethylene sheets to ensure humid condition congenial for spore germination.
2.11. Recording of Data, Measurement of Disease Intensity and Severity
At the reproductive phase, the data were collected on total tiller, effective tiller, and infected tiller hill−1, full length and infected part of spike, seeds spike−1, 1000-grain weight, and grain yield hill−1. At the vegetative phase, the data were collected on total seedlings, infected seedlings pot−1, full length, and infected part of the leaves. The disease intensity (DI) was calculated using the formula:
| DI = (Total number of infected plants) ÷ (Total number of plant observed) × 100 |
Likewise, the blast disease severity assessment was done using a five-scale basis, where % infection means the length of the spike infected by blast. The scales were 0 = no lesions; 1 = 1–25% infection; 2 = 26–50% infection; 3 = 51–75% infection, and 4 = 76–100% length of the spikes infected by blast. The severity of blast was calculated using the formula:
DS = disease severity,
n = number of spikes infected by blast,
v = value score of each category attack,
N = number of spikes observed,
V = value of highest score.
2.12. Design of Experiment and Statistical Analysis
The experiments in the laboratory and field conditions were performed using a completely randomized design (CRD) and a randomized complete block design (RCBD), respectively, to determine the fungicidal activities of the pure antimycin A compound compared to a standard fungicide. All statistical analyses were conducted using the statistical software package IBM SPSS Statistics 25, and the Microsoft Office Excel 2015 program package. The analysis of means comparison of the treatments was accomplished by Tukey’s honest significance difference (HSD) test (p ≤ 0.05). Each treatment was replicated five times and the mean value ± standard error was used in the tables and figures.
3. Results
3.1. In Vitro Assays of Fungal Growth Inhibition
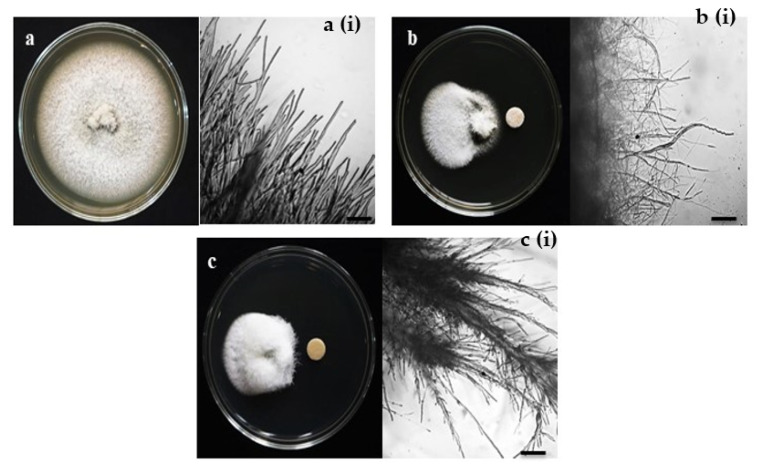
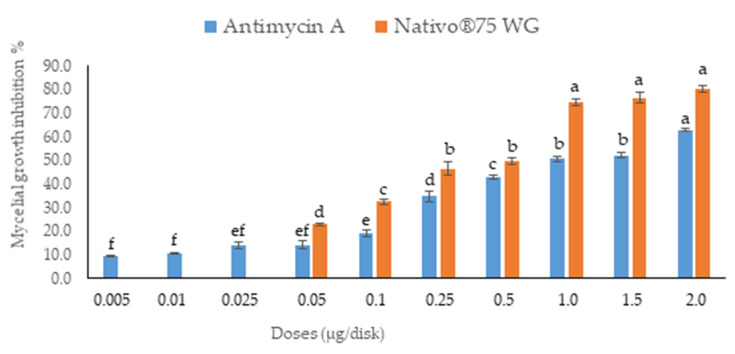
The antifungal activity was tested by measuring the inhibition of fungal growth by antimycin A using a plate assay against wheat blast fungus MoT (Figure 2). The compound antimycin A showed a strong inhibition of hyphal growth of the fungus MoT. The mycelial growth inhibition by antimycin A was 62.9 ± 0.42% at 2 µg/disk (Figure 3). Comparative pictures of the suppression of fungal growth by test compounds are shown in Figure 2.
Figure 2.
Macroscopic and microscopic view of in vitro antifungal activity of antimycin A and the commercial fungicide Nativo® 75 WG against M. oryzae Triticum (MoT) at 20 µg/disk. The macroscopic images are (a) control, (b) antimycin A, and (c) Nativo® 75 WG, whereas, (a(i)), (b(i)), and (c(i)) are microscope images of control, antimycin A, and Nativo® 75 WG, respectively. Bar = 50 μm.
Figure 3.
Inhibitory effects of antimycin A and the commercial fungicide Nativo® 75 WG on mycelial growth of MoT in PDA media. The data are the mean ± standard errors of five replicates for each concentration of the compound tested at a 5% level based on the Tukey HSD (Honest Significance Difference) post-hoc statistic. Bars having a common letter are not significantly different at the 5% level of significance.
The bioassay revealed that antimycin A inhibited the growth of mycelia in a concentration-dependent manner. The inhibitory effects of antimycin A increased with the increasing doses, ranging from 0.005 to 2 µg/disk. Based on the lower and higher concentrations, the inhibition percentages of mycelial growth by antimycin A were 9.6 ± 0.38% and 62.9 ± 0.42%, respectively (Figure 3). Antimycin A showed a slightly lower inhibition rate of fungal mycelia than Nativo® 75 WG (Figure 3).
Antimycin A showed extensive inhibition of hyphal growth at 2 μg/disk (62.9 ± 0.42%), followed by 1.5 μg/disk (52.3 ± 1.29%) and 1 μg/disk (50.6 ± 1.19%), which was indicative of a positive correlation of suppression with an increase in concentration. However, this natural product did not show any activity against MoT lower than the dose of 0.005 µg/disk.
The minimum inhibitory concentration (MIC) required for growth inhibition for each inhibitor were obtained. The MIC of Nativo® 75 WG was 0.05 μg/disk, which was 10 times higher than the MIC of antimycin A (0.005 μg/disk). The percentages of fungal growth inhibition at MICs of antimycin A and Nativo® 75 WG were 9.6 ± 0.38 and 22.9 ± 0.64, respectively.
The observation of hyphal growth under a microscope revealed that the hyphae of untreated MoT had polar and tubular growth with smooth, hyaline, regularly branched, septate, plump, and intact structures [Figure 2a(i)]. The MoT-containing Petri dish treated with antimycin A showed irregular growth and frequently increased branch-per-unit length of fungal hyphae. The antimycin A-treated Petri dish also showed rough hyphal cell walls, but displayed ridges with a corrugated existence and irregular swelling of cells [Figure 2b(i)]. Likewise, morphological abnormality also occurred in the case of MoT where the hyphae were close to the disk containing the fungicide Nativo® 75 WG [Figure 2c(i)]. Nevertheless, the altered morphological appearance of MoT by antimycin A was slightly different than those observed with the Nativo®75 WG, signifying a possible dissimilar mode of action. Overall, antimycin A is a stronger inhibitor than the commercial fungicide, Nativo® 75 WG.
3.2. Antimycin A Block Conidiogenesis in MoT
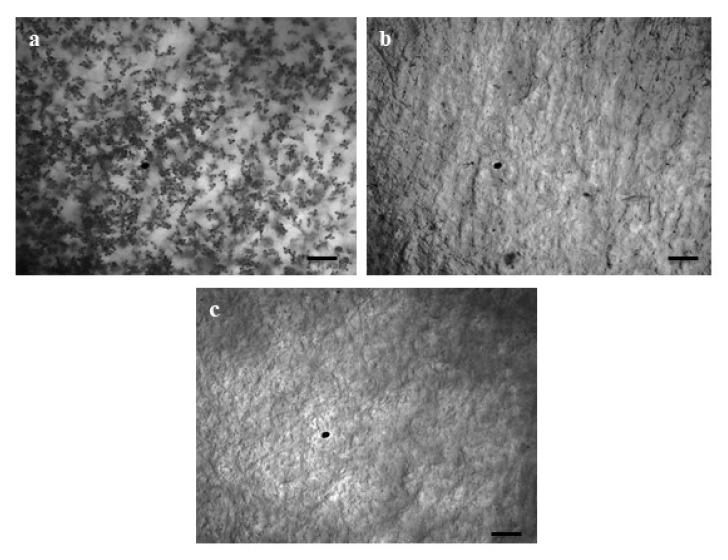
The generation of conidia asexually from conidiophores is critical for infecting wheat plants with MoT. Both antimycin A and the fungicide Nativo® 75 WG significantly decreased the conidia formation in MoT at 5 and 10 μg/mL when compared to the control. Inhibition increased with an increase in concentration from 1, 5, and 10 μg/mL (Figure 4). MoT failed to develop any conidia at the concentration of 10 μg/mL of both natural antimycin A and the synthetic fungicide Nativo® 75 WG. Microscopic investigation showed broken mycelial tips and a complete lack of conidiophores when the mycelia were treated with 10 μg/mL of both tested compounds. On the other hand, the control dish treated with sterilized water containing 1% DMSO produced 5–6 × 105 conidia/mL.
Figure 4.
Effects of antimycin A and the commercial fungicide Nativo® 75 WG on the suppression of conidiogenesis of MoT in Nunc multidisc at 10 µg/mL. (a) Control, (b) antimycin A, (c) Nativo® 75 WG. Bar = 50 μm.
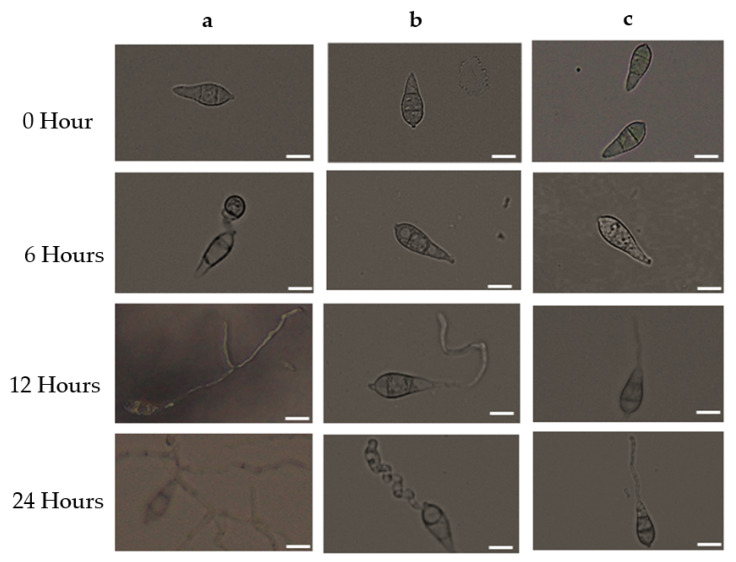
3.3. Antimycin A Alters Conidia Germination and Developmental Transitions of Germinated Conidia
To determine the germination of conidia and the formation of appressoria of MoT, we used antimycin A and Nativo® 75 WG at 10 μg/mL in multi-well plates. The percentages of germinated conidia and their altered morphology were recorded after 6, 12, and 24 h of incubation (Table 1). Both treatments remarkably reduced conidial germination after six hours of incubation compared to the control. At the same time, 100% conidial germination was observed in water, whereas 44.3 ± 2.33% was observed in plates treated with Nativo® 75 WG. In the antimycin A solution, fungal spore germination was 42.1 ± 0.35%, whereas the commercial fungicide exhibited 44.3 ± 2.33% after 6 h of incubation. At all incubation times (6 h, 12 h, and 24 h), the control treatment supported 100% germination of conidia, developed normal germ tubes, and showed standard mycelial growth at 25 °C in the dark (Table 1 and Figure 5a). At the 10 μg/mL concentration, antimycin A displayed significant adverse effects on both germinations of conidia and impaired the post-germination developmental process of MoT. Overall, antimycin A showed abnormal transitional advancement from one step to the next during the developmental processes of germinated conidia.
Table 1.
Effects of antimycin A and the fungicide Nativo® 75 WG on germination of conidia and morphology of germ tubes and appressoria of Magnaporthe oryzae Triticum at 10 μg/mL in vitro.
| Treatment | Time (h) | Germination of Conidia, Morphology of Germ Tubes, and Appressorial Formation | |
|---|---|---|---|
| Germinated Conidia (% ± SE a) | Morphological Change/Developmental Transitions in the Treated Conidia | ||
| Water | 0 | 0.0 ± 0.00 b | No germination |
| 6 | 100.0 ± 0.00 a | Germination with normal germ tube and normal appressoria | |
| 12 | 100.0 ± 0.00 a | Normal mycelial growth | |
| 24 | 100.0 ± 0.00 a | Normal mycelial growth | |
| Antimycin A | 0 | 0.0 ± 0.00 c | No germination |
| 6 | 42.1 ± 0.35 a | 26.7 ± 0.41% short germ tube and 15.4 ± 0.44% conidia lysed | |
| 12 | 26.7 ± 0.41 b | 12.6 ± 0.40% normal germ tube, 9.3 ± 0.68% short and 4.8 ± 0.29% Abnormally elongated germ tube | |
| 24 | 0.0 ± 0.00 c | No appressoria, no mycelial growth | |
| Nativo® 75 WG | 0 | 0.0 ± 0.00 b | No germination |
| 6 | 44.3 ± 2.33 a | Germinated with a short germ tube | |
| 12 | 44.3 ± 2.33 a | Normal germ tube | |
| 24 | 0.0 ± 0.00 b | No appressoria; no mycelial growth | |
a The data presented here are the mean value ± SE of three replicates in each compound. Means within the column followed by the same letter(s) are not significantly different from those assessed by Tukey’s honest significance difference (HSD) post hoc (p ≤ 0.05). Conidia germination percent at different incubation times is not cumulative, but rather at different time intervals in separate experimental units.
Figure 5.
The time-dependent alterations in MoT germination of conidia and subsequent morphological changes in the presence of antimycin A and the commercial fungicide Nativo® 75 WG. Dose of antimycin A was 10 μg/mL. (a) Control, (b) antimycin A, and (c) Nativo® 75 WG. Germinated conidia; short germ tube; elongated germ tube. Bar = 10 μm.
After 6 h, in the presence of antimycin A, 26.7 ± 0.41% of conidia germinated with shorter germ tubes compared to the control and 15.4 ± 0.44% conidia lysed. Similar developmental abnormalities were also observed among the germinated spores after 12 h of incubation, which showed 12.6 ± 0.40% normal and 4.8 ± 0.29% with the formation of abnormally elongated germ tubes, while no additional germination was found after 24 h of incubation (Table 1, Figure 5b).
In the presence of Nativo® 75 WG, the germination of conidia was the same at 6 and 12 h of incubation (44.3 ± 2.33), although after 6 h, the conidia showed shorter germ tubes and after 12 h, all of the conidia exhibited normal germ tubes. However, they did not form any appressoria. Similar to antimycin A, the commercial fungicide Nativo® 75 WG also completely suppressed the germination of spores after 24 h (Table 1, Figure 5c). Interestingly, antimycin A yielded abnormally short and long germ tubes and lysed conidia, while the fungicide did not exhibit such changes. Both the natural compound antimycin A and the synthetic fungicide blocked the formation of appressoria that are essential for pathogenesis, suggesting their potential for the control of wheat blast.
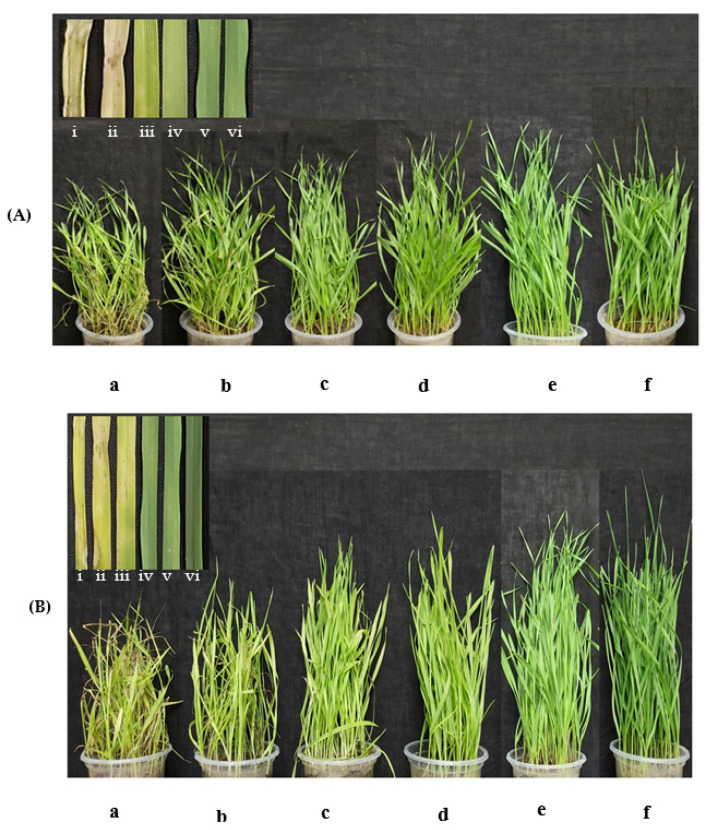
3.4. Antimycin A Suppresses Wheat Blast Disease at Seedling Stage
The application of antimycin A significantly inhibited the development of blast symptoms in the leaves of artificially inoculated wheat seedlings. In this study, blast lesions on wheat seedlings were treated with antimycin A and Nativo® 75 WG fungicide and were compared with a water treatment control. In the case of antimycin A, the percentages of disease incidence and severity were 16.33 ± 2.19% and 10.67 ± 2.96%, respectively, at the 1 μg/mL preventive dose, whereas 5 μg/mL produced 6.67 ± 0.88 and 3.33 ± 0.88% disease incidence and severity, respectively (Figure 6A(i) and Table 2). Wheat blast caused the highest level (100 ± 0%) of disease intensity and severity (82 ± 4.73%) in the case of the untreated control, whereas the healthy control did not show any blast symptoms (Figure 6B(i) and Table 2).
Figure 6.
Suppression of wheat blast disease by antimycin A and Nativo® 75 WG. Herein, blast lesions suppressed by different doses of antimycin A, a commercial dose of Nativo® 75 WG, as well as untreated and healthy control wheat seedlings were presented as (A) preventive, and (B) curative. Both preventive and curative assays included (a) water control + MoT, (b–d) antimycin A + MoT inoculation, (e) commercial dose of Nativo®75 WG + MoT inoculation, and (f) non-inoculated, non-treated seedlings. The 1 μg/mL, 5 μg/mL, and 10 μg/mL dose of antimycin A are represented as (b), (c), and (d), respectively. In inset, the representative leaf sample is presented as (i) water control + MoT, (ii–iv) antimycin A at 1 μg/mL, 5 μg/mL, and 10 μg/mL, respectively + MoT inoculation, (v) commercial dose of Nativo® 75 WG + MoT inoculation, and (vi) non-inoculated, non-treated seedlings as a healthy control. Photos were taken 21 days after inoculation.
Table 2.
Effect of antimycin A in suppression of wheat blast disease development in artificially inoculated wheat seedlings. Means ± standard errors having a common letter are not significantly different at the 5% level of significance.
| Parameter | Untreated Control | Healthy Control | Commercial Fungicide Nativo® 75 WG | Preventive (μg/mL) | Curative (μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 1 | 5 | 10 | ||||
| % disease incidence | 100 ± 0.00 a | 0 ± 0.00 d | 0 ± 0.00 d | 16.33 ± 2.19 b | 6.67 ± 0.88 c | 0.00 ± 0.00 d | 19 ± 1.15 b | 8.33 ± 0.67 c | 0.00 ± 0.00 d |
| % disease severity | 82 ± 4.73 a | 0 ± 0.00 c | 0 ± 0.00 c | 10.67 ± 2.96 b | 3.33 ± 0.88 b | 0 ± 0.00 c | 12.33 ± 2.40 b | 5.33 ± 1.20 bc | 0.00 ± 0.00 c |
Additionally, in both of the curative doses, 1 and 5 μg/mL developed 19 ± 1.15 and 8.33 ± 0.67% of plant infection as well as 12.33 ± 2.40 and 5.33 ± 1.20% of leaf infection, respectively. However, the 10 μg/mL preventive and curative doses of antimycin A did not show any blast symptoms on the wheat seedlings (Table 2). However, in both the preventive and curative control measures, 100% suppression of wheat blast was achieved at a 10 μg/mL concentration of antimycin A as well as the commercial fungicide (Figure 6A(d,e) and Table 2).
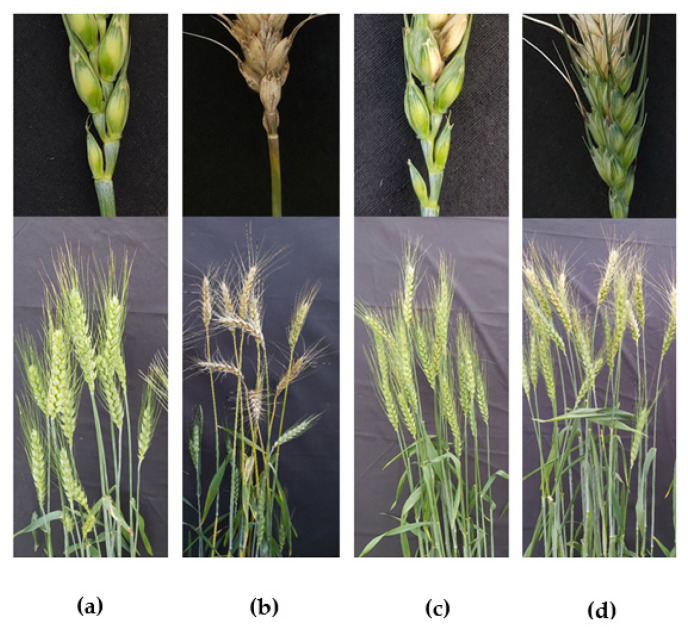
3.5. Suppression of Wheat Blast Disease by Antimycin A at Heading Stage of Wheat under Field Conditions
Wheat blast is predominantly a head disease. To assess the efficacy of the natural product antimycin A in suppressing wheat blast disease in artificially inoculated wheat spikes, we conducted an experiment under field conditions together with a commercial fungicide, Nativo®75 WG, at 10 μg/mL. In the field conditions, the application of antimycin A remarkably reduced the incidence of wheat blast (33%) (Figure 7c, Table 3), whereas the disease incidence in the untreated control plot was 87% (Figure 7b, Table 3).
Figure 7.
Suppression of wheat blast symptoms with antimycin A and Nativo® 75 WG. Herein, (a) non-inoculated, non-treated seedlings as a healthy control, (b) water control + MoT, (c) antimycin A at 10 μg/mL + MoT inoculation, and (d) blast lesions suppressed by commercial dose of Nativo® 75 WG. In inset, clear images of representative spike samples are presented.
Table 3.
Effect of antimycin A on yield or yield components of the wheat variety BARI Gom-26 under field conditions after artificial inoculation with wheat blast fungus.
| Treatment | Grain Yield Per Spike (gm) * | 1000-Grain Weight (gm) * | Disease Incidence (%) * | Disease Severity (%) * |
|---|---|---|---|---|
| Healthy control | 2.05 ± 0.05 a | 53.57 ± 1.37 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| Untreated control | 0.86 ± 0.04 b | 34.43 ± 0.27 c | 87.00 ± 2.91 a | 73.67 ± 2.65 a |
| Antimycin A | 1.95 ± 0.06 a | 42.73 ± 0.71 b | 33.00 ± 1.45 b | 23.67 ± 2.31 b |
| Nativo® 75 WG | 2.05 ± 0.13 a | 44.94 ± 1.55 b | 31.71 ± 2.96 b | 23.33 ± 2.95 b |
* Any two means ± standard errors with a common letter are not significantly different at the 5% level of significance.
Antimycin A-treated wheat plants had a blast severity of 23.67% compared to 73.67% in the untreated control. The application of both antimycin A (1.95 ± 0.06 gm) and Nativo® 75 WG (2.05 ± 0.13 gm) had a statistically similar, but significantly increased grain yield compared to the untreated control (0.86 ± 0.04 gm). The grain yield in both antimycin A and Nativo treatments were comparable to the negative (non-inoculated, non-treated) check (2.05 ± 0.05 gm) (no artificial inoculation) (Table 3).
We also recorded 1000-grain weight data for the treatments and found 44.94, 42.73, and 53.57 gm weights for the Nativo® 75 WG, antimycin A, and negative control plot, respectively. These grain yields were significantly higher than those of the untreated control plot (34.43 gm) (Table 3).
4. Discussion
Microorganisms are a vital source of novel antimicrobials, as they typically yield toxins to combat different microbes. The biological activity of microbe-derived secondary metabolites can successfully prevent the growth and developmental morphological features of wheat blast spores. In the current study, a natural product, antimycin A, isolated from a marine Stretomyces sp., significantly inhibited the mycelial growth and pre-infectional development of wheat blast fungus MoT in vitro. Interestingly, the bioactivity of antimycin A was stronger than that of the widely used commercial fungicide Nativo® 75 WG. Furthermore, this natural compound also suppressed wheat blast disease in artificially inoculated wheat seedlings and spikes, which is comparable to the commercial fungicide Nativo® 75 WG. The bioassay revealed that the inhibition of conidial germination, suppression of appressoria formation, and induction of abnormal mycelial growth by antimycin A are likely to be correlated with blast disease suppression in wheat. To the best of our knowledge, this is the first report of the suppression of the devastating wheat blast fungus by antimycin A extracted from the Streptomyces sp., which has the potential to become a fungicidal product or used as a lead compound for controlling the MoT, a killer of wheat.
The biological activities of antimycins are well documented [55,56,57,58,59,60,61]. In an earlier study, a crystalline antibiotic isolated from Streptomyces kitazawaensis nov. sp. significantly inhibited the growth of rice blast fungus, the Pyricularia oryzae Oryzae pathotype [62]. The antibiotic was identified as antimycin A based on its physical, chemical, and biological properties. Due to its high cost, antimycin A was initially viewed as a promising agent to control rice blast in the greenhouse rather than in the field [62]. Later, a series of experiments in mammalian cells revealed the mode of action of this bioactive secondary metabolite. Antimycin A inhibits mitochondrial electron transport, which disrupts the mitochondrial membrane potentials of mitochondria through the proton gradient across the mitochondrial inner membrane [63,64]. Additionally, antimycin A significantly increases the production of reactive oxygen species (ROS) and causes ATP inhibition as well as glutathione depletion [55,56,57,58,59,60,61]. ROS production and membrane depolarization by antimycin A lead to apoptosis by opening the mitochondrial permeability transition pore. Thus, it releases pro-apoptotic molecules such as cytochrome c into the cytoplasm [64,65,66]. In some instances, antimycin A-induced cell death can be associated with the increased activity of caspase and DNA damage [59,66,67]. Although several researchers reported the activity of antimycin A in mammalian cells, only a few reports are available on the antifungal activity of antimycin A to control plant pathogenic fungi [42,44]. As antimycin A inhibits the electron transport chain in the mitochondria to prevent spore germination, a fungicide can be designed against wheat blast using it as a lead compound.
In this study, we found that hyphal the growth of MoT was inhibited at a lower concentration of antimycin A compared to the commercial fungicide Nativo®75 WG. The microscopic observation suggested that antimycin A leads to irregular hyphal growth with frequent branching per unit length of fungal hyphae (Figure 2b(i)). These observations are consistent with a recent observation made by Chakraborty et al. [14], where the authors demonstrated that two Streptomyces sp. produced secondary metabolites (oligomycin B and F) and altered the morphological features of MoT hyphae [14]. Some other Streptomyces-derived secondary metabolites were also found to cause hyphal growth deformity and inhibition [44].
One of the notable findings of this study is the antimycin A-induced swelling of the MoT hyphae [Figure 2b(i),c(i)], which is generally considered as a mode of inhibitory action of a compound against the normal growth and development of pathogenic fungi [68,69]. We examined a series of concentrations of antimycin A ranging from 0.005 to 2 μg/disk. We observed increased hyphal swelling with an increase in concentrations of antimycin A (data not shown). This type of hyphal swelling has been reported in various fungal hyphae by the treatment of polyoxin B [70], fengycin [71], tensin [72], linear lipopeptides, and oligomycins B and F [14,22]. Morphological changes such as profuse branching and swelling of the hyphae of an oomycete pathogen viz. Aphanomyces cochlioides by phloroglucinols isolated from Pseudomonas fluorescence and xanthobaccin A extracted from Lysobacter sp. SB-K88 have been documented [73,74,75,76]. A few studies also evaluated the antifungal properties of antimycin A. Nakayama et al. [62] showed that antimycin A exhibits remarkable inhibitory effects against various fungi, including Pyricularia oryzae, Alternaria kikuchiana, Gloeosporium laeticolor, Torula utilis, Candida alicans, C. krusei, C. parakrusei, and Penicillium chrysogenum [62]. Rice blast fungus is now considered a Magnaporthe oryzae pathotype Oryzae (MoO), which is different from the wheat-infecting pathotype MoT. Thus, our work with antimycin A and MoT is the first report of the development of swelling-like structures in the hyphae of this devastating new wheat pathotype.
Most of the pathogenic fungi enter host plants via infecting propagule-like spores or conidia, and the process by which conidia are produced is known as conidiogenesis [77,78]. The suppression of conidiogenesis and germination of conidia reduces the chance of infection by the pathogen. Antimycin A inhibited both conidiogenesis and the conidial germination of MoT in a dose-dependent manner in this study. In addition, other unique antagonistic modes of effect discovered in this study include conidia lysis and abnormally extended hypha-like germ tubes (Figure 5b,c). Consistent with these findings, Chakraborty et al. [14] reported that secondary metabolites from Streptomyces sp. suppressed conidiogenesis and the germination of conidia of MoT. Similar work by other investigators revealed that reveromycins A and B from Streptomyces sp. inhibited the spore germination of B. cinerea and R. stolonifer [79]. It has also been reported that during the conidiogenesis and conidial germination process, fungal cells need high energy production in the form of ATP [80]. Antimycin A has been reported to interfere with mitochondrial electron transport by targeting ubiquinol–cytochrome c oxidoreductase, which breaks down membrane potentials and inhibits ATP synthesis. The inhibition of conidiogenesis and conidial germination by antimycin A is likely to be linked with the inhibition of ATP synthesis in MoT cells. A recent experiment with Fusarium oxysporum cells provides evidence that an elevated level of ATP is positively associated with conidial germination. Higher ATP production supports the breaking of the dormancy and formation of a germ tube [80]. Therefore, a reasonable justification for the inhibition of the spore germination and hyphal growth of MoT described in this study might be associated with the inhibition of ATP synthesis in mitochondria by antimycin A, indicating that the mode of action of antimycin A for MoT suppression may parallel strobilurin fungicides. More research is needed to unravel the mechanisms involving the interactions of the microbe-derived antibiotic compounds that suppress MoT growth and development. It is also possible that, apart from ubiquinol–cytochrome c oxidoreductase, antimycin A targets an additional protein, Bcl-2 [81]. The Bcl-2 protein is found in the mitochondria of Colletotricum gloeosporioides, as well as in other cellular compartments including the ER, and is involved in many stages of the fungal life cycle, including growth, morphogenesis, morpho-pathogenesis, and reproduction. In comparison to the wildtype, Bcl-2 isolates produced 8–10 times more conidia. Furthermore, Bcl-2 isolates are more virulent than wildtype and cause infection faster [82]. The Bcl-2 family has been reported to regulate apoptosis in mammals by controlling the mitochondria efflux of cytochrome c and other apoptosis-related proteins [82]. Whether antimycin A inhibits conidiogenesis in MoT fungus by interfering with the function of Bcl-2 needs to be confirmed by further investigations.
The hallmark of the findings of this study is that antimycin A remarkably suppressed wheat blast disease in both greenhouse and field conditions, which is comparable to the commercial fungicide Nativo® 75 WG (Figure 6). Herein, the wheat seedlings treated with antimycin A had a lower infection rate than the untreated control (Figure 6), supporting the in vitro results. In contrast, the water-treated healthy control seedlings had a very high percentage of infection, indicating that the efficacy trial was conducted under a conducive environment for higher infection and disease. Products or active compounds that are capable of reducing plant disease under high disease pressure in an experiment should be considered as viable options for future commercial use. In our study, blast lesions did not form on the seedlings treated with antimycin A and Nativo® 75 WG at the highest concentration (Figure 6), indicating the high potential of this antibiotic compound for commercialization in the future to control the wheat blast pathogen MoT. At the heading stage of wheat, we found similar results, where antimycin A significantly inhibited the blast disease development on the artificially inoculated wheat spikes (Figure 7). Excitingly, most of the antifungal effect of antimycin A on the inhibition of wheat blast fungus in vitro was found to be equivalent to or stronger than that of the commercial fungicide at the same concentration. The commercial fungicide Nativo® 75 WG has two main active ingredients, namely, tebuconazole and trifloxystrobin. Tebuconazole acts as a demethylase inhibitor (DMI), which is known as a systemic triazole fungicide. Demethylase inhibitors suppress the biosynthesis of ergosterol, which is a key component of the plasma membrane of certain fungi crucial for fungal growth and development [83]. On the other hand, trifloxystrobin is a strobilurin fungicide that interferes with the respiration of plant pathogenic fungi by inhibiting energy production in the mitochondria, thereby halting the germination of fungal conidia [84]. The adverse effects on conidial germination caused by antimycin A (10 μg/mL) reduced the infection of wheat seedlings by MoT conidia, suggesting a potential role of antimycin A in blast disease suppression. Taken together, both the in vitro and in vivo field tests revealed that antimycin A inhibited mycelial growth, conidiogenesis, and conidial germination, thereby reducing the disease incidence in wheat plants. Our results suggest that antimycin A has a high potential for formulating an effective biopesticide against wheat blast, either using it directly or as a lead compound. Further study is required to elucidate the underlying mechanism of wheat blast disease control by the marine natural product, antimycin A.
5. Conclusions
Our experimental findings revealed that a marine natural product, antimycin A, significantly inhibited mycelial growth, asexual sporulation, and the developmental transitions of the conidia of wheat blast fungus MoT in vitro. In vivo seedling assays and field evaluation confirmed that antimycin A was effective in suppressing wheat blast disease in artificially inoculated wheat at both the seedling and heading stages. These assessments proved that this natural compound could be considered as a biofungicide or a lead compound to design a new fungicide to control the destructive wheat blast disease. Further extensive research is also needed to understand the precise mode of action of antimycin A against the devastating fungus, M. oryzae Triticum.
Author Contributions
Conceptualization, writing-review, editing and supervision, T.I.; investigation, visualization, writing-original draft preparation and editing, S.K.P.; methodology and software, N.U.M.; investigation and formal analysis, M.C., M.M.R., A.M.A., J.U.A. and D.R.G.; writing—review and editing, A.A.M.R., A.S., M.R. and M.A.H.; project administration, T.I.; funding acquisition, M.R. and T.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
No human participants and/or animals were involved in this research content.
Informed Consent Statement
This manuscript has not been published or presented elsewhere in part or entirety and is not under consideration by another journal. We have read and understood your journal policies, and we believe that neither the manuscript nor the study violates any of these. All the authors have been personally and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content. All co-authors agreed to this submission.
Data Availability Statement
All data are included in this manuscript as Figures and Tables.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Krishi Gobeshona Foundation (KGF) of Bangladesh under project Nos. KGF TF50-C/17 and TF 92-FNS/21 to Tofazzal Islam of the Institute of Biotechnology, and Genetic Engineering of BSMRAU, Bangladesh.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Igarashi S., Utiamada C.M., Igarashi L.C., Kazuma A.H., Lopes R.S. Pyricularia em trigo. 1. Ocorrencia de Pyricularia sp noestado do Parana. Phytopathol. Bras. 1986;11:351–352. [Google Scholar]
- 2.Kohli M.M., Mehta Y.R., Guzman E., Viedma L., Cubilla L.E. Pyricularia blast-a threat to wheat cultivation. Czech J. Genet. Plant Breed. 2011;47:130–134. doi: 10.17221/3267-CJGPB. [DOI] [Google Scholar]
- 3.Islam M.T., Croll D., Gladieux P., Soanes D.M., Persoons A., Bhattacharjee P., Hossain M.S., Gupta D.R., Rahman M.M., Mahboob M.G., et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016;14:84. doi: 10.1186/s12915-016-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam M.T., Kim K.H., Choi J. Wheat blast in Bangladesh: The current situation and future impacts. Plant Pathol. J. 2019;35:1. doi: 10.5423/PPJ.RW.08.2018.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam M.T., Gupta D.R., Hossain A., Roy K.K., He X., Kabir M.R., Singh P.K., Khan M., Rahman A., Rahman M., et al. Wheat blast: A new threat to food security. Phytopathol. Res. 2020;2:28. doi: 10.1186/s42483-020-00067-6. [DOI] [Google Scholar]
- 6.Kamoun S., Talbot N.J., Islam M.T. Plant health emergencies demand open science: Tackling a cereal killer on the run. PLoS Biol. 2019;17:e3000302. doi: 10.1371/journal.pbio.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tembo B., Mulenga R.M., Sichilima S., M’siska K.K., Mwale M., Chikoti P.C., Singh P.K., He X., Pedley K.F., Peterson G.L., et al. Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE. 2020;15:e0238724. doi: 10.1371/journal.pone.0238724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceresini P.C., Castroagudı’n V.L., Rodriguez F.A., Rios J.A., Aucique-Pe´rez C.E., Moreira S.I. Wheat blast: From its origin in South America to its emergence as a global threat. Mol. Plant Pathol. 2019;20:155–172. doi: 10.1111/mpp.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue K., Suzuki T., Ikeda K., Jiang S., Hosogi N., Hyon G.S. Extracellular matrix of Magnaporthe oryzae may have a role in host adhesion during fungal penetration and is digested by matrix metalloproteinases. J. Gen. Plant Pathol. 2007;73:388–398. doi: 10.1007/s10327-007-0048-2. [DOI] [Google Scholar]
- 10.Urashima A.S., Hashimoto Y., Le Don D., Kusaba M., Tosa Y., Nakayashiki H., Mayama S. Molecular analysis of the wheat blast population in Brazil with a homolog of retrotransposon MGR583. Jpn. J. Phytopathol. 1999;65:429–436. doi: 10.3186/jjphytopath.65.429. [DOI] [Google Scholar]
- 11.Singh P.K., Gahtyari N.C., Roy C., Roy K.K., He X., Tembo B., Xu K., Juliana P., Sonder K., Kabir M.R., et al. Wheat blast: A disease spreading by intercontinental jumps and its management strategies. Front. Plant Sci. 2021;12:710707. doi: 10.3389/fpls.2021.710707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale A.T., Graham R.D.M., Andreas M., Jean-Benoit M., Lucie M., Lesley A.B. Wheat blast: Histopathology and transcriptome reprogramming in response to adapted and non-adapted Magnaporthe isolates. New Phytol. 2009;184:473–484. doi: 10.1111/j.1469-8137.2009.02970.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R.A., Talbot N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009;7:185–195. doi: 10.1038/nrmicro2032. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty M., Mahmud N.U., Muzahid A.N.M., Rabby S.M.F., Islam T. Oligomycins inhibit Magnaporthe oryzae Triticum and suppress wheat blast disease. PLoS ONE. 2020;15:e0233665. doi: 10.1371/journal.pone.0233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castroagudín V.L., Ceresini P.C., de Oliveira S.C., Reges J.T., Maciel J.L., Bonato A.L., Dorigan A.F., McDonald B.A. Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology. 2015;105:284–294. doi: 10.1094/PHYTO-06-14-0184-R. [DOI] [PubMed] [Google Scholar]
- 16.Cruz C.D., Valent B. Wheat blast disease: Danger on the move. Trop. Plant Pathol. 2017;42:210–222. doi: 10.1007/s40858-017-0159-z. [DOI] [Google Scholar]
- 17.Poloni N.M., Carvalho G., Nunes Campos Vicentini S., Francis Dorigan A., Nunes Maciel J.L., McDonald B.A., Intra Moreira S., Hawkins N., Fraaije B.A., Kelly D.E., et al. Widespread distribution of resistance to triazole fungicides in Brazilian populations of the wheat blast pathogen. Plant Pathol. 2021;70:436–448. doi: 10.1111/ppa.13288. [DOI] [Google Scholar]
- 18.Petriccione M., Mastrobuoni F., Zampella L., Nobis E., Capriolo G., Scortichini M. Effect of chitosan treatment on strawberry allergen-related gene expression during ripening stages. J. Food Sci. Technol. 2017;54:1340–1345. doi: 10.1007/s13197-017-2554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez M., Núñez F. Novel approaches to minimizing mycotoxin contamination. Toxins. 2020;12:216. doi: 10.3390/toxins12040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueroa E., Lee-Estévez M., Valdebenito I., Farías J.G., Romero J. Potential biomarkers of DNA quality in cryopreserved fish sperm: Impact on gene expression and embryonic development. Rev. Aquac. 2020;12:382–391. doi: 10.1111/raq.12323. [DOI] [Google Scholar]
- 21.Wulff B.B., Dhugga K.S. Wheat-the cereal abandoned by GM. Science. 2018;361:451–452. doi: 10.1126/science.aat5119. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty M., Mahmud N.U., Gupta D.R., Tareq F.S., Shin H.J., Islam T. Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae Triticum. Front. Microbiol. 2020;11:665. doi: 10.3389/fmicb.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ōmura S., Ikeda H., Ishikawa J., Hanamoto A., Takahashi C., Shinose M., Takahashi Y., Horikawa H., Nakazawa H., Osonoe T., et al. Genome sequence of an industrial microorganism Streptomyces avermitilis: Deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA. 2001;98:12215–12220. doi: 10.1073/pnas.211433198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehdi R.B.A., Sioud S., Fguira L.F.B., Bejar S., Mellouli L. Purification and structure determination of four bioactive molecules from a newly isolated Streptomyces sp. TN97 strain. Process Biochem. 2006;41:1506–1513. doi: 10.1016/j.procbio.2006.02.010. [DOI] [Google Scholar]
- 25.Kaur T., Kaur A., Sharma V., Manhas R.K. Purification and Characterization of a New Antifungal Compound 10-(2, 2-dimethyl-cyclohexyl)-6, 9-dihydroxy-4, 9-dimethyl-dec-2-enoic Acid Methyl Ester from Streptomyces hydrogenans Strain DH16. Front. Microbiol. 2016;7:1004. doi: 10.3389/fmicb.2016.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doumbou C.L., Salove M.K.H., Crawford D.L., Beaulieu C. Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection. 2001;82:85–102. doi: 10.7202/706219ar. [DOI] [Google Scholar]
- 27.Xiong Z.Q., Zhang Z.P., Li J.H., Wei S.J., Tua G.Q. Characterization of Streptomyces padanus JAU4234, a producer of actinomycin X2, fungichromin, and a new polyene macrolide antibiotic. Appl. Environ. Microbiol. 2012;78:589–592. doi: 10.1128/AEM.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palaniyandi S.A., Yang S.H., Zhang L., Suh J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013;97:9621–9636. doi: 10.1007/s00253-013-5206-1. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen X.H., Naing K.W., Lee Y.S., Kim Y.H., Moon J.H., Kim K.Y. Antagonism of antifungal metabolites from Streptomyces griseus H7602 against Phytophthora capsici. J. Basic Microbiol. 2015;55:45–53. doi: 10.1002/jobm.201300820. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.T., Monkhung S., Lee Y.S., Kim K.Y. Effects of Lysobacter antibioticus HS124, an effective biocontrol agent against Fusarium graminearum, on crown rot disease and growth promotion of wheat. Can. J. Microbiol. 2019;65:904–912. doi: 10.1139/cjm-2019-0285. [DOI] [PubMed] [Google Scholar]
- 31.Fathi F., Saberi-Riseh R., Khodaygan P. Survivability and controlled release of alginate-microencapsulated pseudomonas fluorescens vupf506 and their effects on biocontrol of Rhizoctonia solani on potato. Int. J. Biol. Macromol. 2021;183:627–634. doi: 10.1016/j.ijbiomac.2021.04.159. [DOI] [PubMed] [Google Scholar]
- 32.Moradi-Pour M., Saberi-Riseh R., Mohammadinejad R., Hosseini A. Investigating the formulation of alginate-gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int. J. Biol. Macromol. 2019;133:603–613. doi: 10.1016/j.ijbiomac.2019.04.071. [DOI] [PubMed] [Google Scholar]
- 33.Taechowisan T., Peberdy J.F., Lumyong S. Chitinase production by endophytic Streptomyces aureofaciens CMUAc130 and its antagonism against phytopathogenic fungi. Ann. Microbiol. 2003;53:447–461. [Google Scholar]
- 34.Taechowisan T., Lu C., Shen Y., Lumyong S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology. 2005;151:1691–1695. doi: 10.1099/mic.0.27758-0. [DOI] [PubMed] [Google Scholar]
- 35.Quecine M.C., Araujo W.L., Marcon J., Gai C.S., Azevedo J.L., Pizzirani-Kleiner A.A. Chitinolytic activity of endophytic Streptomyces and potential for biocontrol. Lett. Appl. Microbiol. 2008;47:486–491. doi: 10.1111/j.1472-765X.2008.02428.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuan W.M., Crawford D.L. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl. Environ. Microbiol. 1995;61:3119–3128. doi: 10.1128/aem.61.8.3119-3128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahvonen R., Avikainen H. The biological control of seed-borne Alternaria brassicicola of cruciferous plants with a powdery preparation of Streptomyces sp. Agric. Food Sci. 1987;59:199–208. doi: 10.23986/afsci.72264. [DOI] [Google Scholar]
- 38.Law J.W.F., Ser H.L., Khan T.M., Chuah L.H., Pusparajah P., Chan K.G. The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae) Front. Microbiol. 2017;8:3. doi: 10.3389/fmicb.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubici G. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Rev. Perspective. Agric. Vet. Sci. Nutr. Nat. Resour. 2018;13:1–15. doi: 10.1079/PAVSNNR201813050. [DOI] [Google Scholar]
- 40.Santra H.K., Maity S., Banerjee D. Production of bioactive compounds with broad spectrum bactericidal action, bio-film inhibition and antilarval potential by the secondary metabolites of the endophytic fungus Cochliobolus sp. APS1 Isolated from the Indian Medicinal Herb Andrographis paniculata. Molecules. 2022;27:1459. doi: 10.3390/molecules27051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukku V.J., Speitling M., Laatsch H., Helmke E. New butenolides from two marine streptomycetes. J. Nat. Prod. 2000;63:1570–1572. doi: 10.1021/np0001676. [DOI] [PubMed] [Google Scholar]
- 42.Hosotani N., Kumagai K., Nakagawa H., Shimatani T., Saji I. Antimycins A10 approximately A16, seven new antimycin antibiotics produced by Streptomyces spp. SPA-10191 and SPA-8893. J. Antibiot. 2005;58:460–467. doi: 10.1038/ja.2005.61. [DOI] [PubMed] [Google Scholar]
- 43.Rieske J.S. Antibiotics. Springer; Berlin/Heidelberg, Germany: 1967. Antimycin A; pp. 542–584. [Google Scholar]
- 44.Nafis A., Elhidar N., Oubaha B., Samri S.E., Niedermeyer T., Ouhdouch Y., Hassani L., Barakate M. Screening for Non-polyenic Antifungal Produced by Actinobacteria from Moroccan Habitats: Assessment of Antimycin A19 Production by Streptomyces albidoflavus AS25. Int. J. Mol. Cell. Med. 2018;7:133–145. doi: 10.22088/IJMCM.BUMS.7.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan L.L., Han N.N., Zhang Y.Q., Yu L.Y., Chen J., Wei Y.Z., Li Q.P., Tao L., Zheng G.H., Yang S.E., et al. Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. J. Antibiot. 2010;63:259–261. doi: 10.1038/ja.2010.21. [DOI] [PubMed] [Google Scholar]
- 46.Belakhov V.V., Boikova I.V., Novikova I.I., Kolodyaznaya V.A. Results of examination of the biological activity of nonmedical antibiotics with a view to finding environmentally friendly pesticides for plant protection. Russ. J. Gen. Chem. 2018;88:2982–2989. doi: 10.1134/S107036321813025X. [DOI] [Google Scholar]
- 47.Gupta D.R., Surovy M.Z., Mahmud N.U., Chakraborty M., Paul S.K., Hossain M., Bhattacharjee P., Mehebub M., Rani K., Yeasmin R., et al. Suitable methods for isolation, culture, storage and identification of wheat blast fungus Magnaporthe oryzae Triticum pathotype. Phytopathol. Res. 2020;2:30. doi: 10.1186/s42483-020-00070-x. [DOI] [Google Scholar]
- 48.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 49.Riungu G.M., Muthorni J.W., Narla R.D., Wagacha J.M., Gathumbi J.K. Management of Fusarium head blight of wheat and deoxynivalenol accumulation using antagonistic microorganisms. Plant Pathol. J. 2008;7:13–19. doi: 10.3923/ppj.2008.13.19. [DOI] [Google Scholar]
- 50.Urashima A.S., Igarashi S., Kato H. Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis. 1993;77:1211–1216. doi: 10.1094/PD-77-1211. [DOI] [Google Scholar]
- 51.Islam M.T., von Tiedemann A. 2,4-Diacetylphloroglucinol suppresses zoosporogenesis and impairs motility of Peronosporomycete zoospores. World J. Microb. Biotechnol. 2011;27:2071–2079. doi: 10.1007/s11274-011-0669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson R.J., Fraaije B.A., Clark I.M., Jackson R.W., Hirsch P.R., Mauchline T.H. Wheat seed embryo excision enables the creation of axenic seedlings and Koch’s postulates testing of putative bacterial endophytes. Sci. Rep. 2016;6:25581. doi: 10.1038/srep25581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bangladesh Agricultural Research Council (BARC) Fertilizer Recommendation Guide (FRG) Bangladesh Agricultural Research Council (BARC); Dhaka, Bangladesh: 2012. pp. 1–265. [Google Scholar]
- 54.Ha X., Koopmann B., von Tiedemann A. Wheat blast and Fusarium head blight display contrasting interaction patterns on ears of wheat genotypes differing in resistance. Phytopathology. 2016;106:270–281. doi: 10.1094/PHYTO-09-15-0202-R. [DOI] [PubMed] [Google Scholar]
- 55.Turrens J.F., Alexandre A., Lehninger A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 56.Turrens J.F. Mitochondrial formation of reactive oxygen species. Physiol. J. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Formigli L., Papucci L., Tani A., Schiavone N., Tempestini A., Orlandini G.E., Capaccioli S., Zecchi Orlandini S. Aponecrosis: Morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J. Cell. Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Park W.H., Han Y.W., Kim S.H., Kim S.Z. An ROS generator, antimycin A, inhibits the growth of HeLa cells via apoptosis. J. Cell. Biochem. 2007;102:98–109. doi: 10.1002/jcb.21280. [DOI] [PubMed] [Google Scholar]
- 59.Han Y.W., Kim S.Z., Kim S.H., Park W.H. The changes of intracellular H2O2 are an important factor maintaining mitochondria membrane potential of antimycin A-treated As4. 1 juxtaglomerular cells. Biochem. Pharmacol. 2007;73:863–872. doi: 10.1016/j.bcp.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Han Y.H., Moon H.J., You B.R., Kim S.Z., Kim S.H., Park W.H. p38 inhibitor intensified cell death in antimycin A-treated As4. 1 juxtaglomerular cells via the enhancement of GSH depletion. Anticancer Res. 2009;29:4423–4431. [PubMed] [Google Scholar]
- 61.Park W.H., You B.R. Antimycin A induces death of the human pulmonary fibroblast cells via ROS increase and GSH depletion. Int. J. Oncol. 2016;48:813–820. doi: 10.3892/ijo.2015.3276. [DOI] [PubMed] [Google Scholar]
- 62.Nakayama K., Okamoto F., Harada Y. Antimycin A: Isolation from a new Streptomyces and activity against rice plant blast fungi. J. Antibiot. Res. 1956;9:63–66. [PubMed] [Google Scholar]
- 63.Pham N.A., Robinson B.H., Hedley D.W. Simultaneous detection of mitochondrial respiratory chain activity and reactive oxygen in digitonin-permeabilized cells using flow cytometry. Cytom. J. Int. Soc. Anal. Cytol. 2000;41:245–251. doi: 10.1002/1097-0320(20001201)41:4<245::AID-CYTO2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 64.King M.A., Radicchi-Mastroianni M.A. Antimycin A-induced apoptosis of HL-60 cells. Cytom. J. Int. Soc. Anal. Cytol. 2002;49:106–112. doi: 10.1002/cyto.10156. [DOI] [PubMed] [Google Scholar]
- 65.Cai J., Jones D.P. Superoxide in apoptosis: Mitochondrial generation triggered by cytochromec loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]
- 66.King M.A. Antimycin A-induced killing of HL-60 cells: Apoptosis initiated from within mitochondria does not necessarily proceed via caspase 9. Cytom. J. Int. Soc. Anal. Cytol. 2005;63:69–76. doi: 10.1002/cyto.a.20107. [DOI] [PubMed] [Google Scholar]
- 67.Wolvetang E.J., Johnson K.L., Krauer K., Ralph S.J., Linnane A.W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339:40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- 68.Liao J.H., Chen P.Y., Yang Y.L., Kan S.C., Hsieh F.C., Liu Y.C. Clarification of the antagonistic effect of the lipopeptides produced by Bacillus amyloliquefaciens BPD1 against Pyricularia oryzae via in situ MALDI-TOF IMS analysis. Molecules. 2016;21:1670. doi: 10.3390/molecules21121670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L., Sun C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018;84:e00445-18. doi: 10.1128/AEM.00445-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Isono K., Nagatsu J., Kawashima Y., Suzuki S. Studies on polyoxins, antifungal antibiotics. Agric. Biol. Chem. 1965;29:848–854. [Google Scholar]
- 71.Tang Q., Bie X., Lu Z., Lv F., Tao Y., Qu X. Effects of fengycin from Bacillus subtilis fmbJ on apoptosis and necrosis in Rhizopus stolonifer. J. Microbiol. 2014;52:675–680. doi: 10.1007/s12275-014-3605-3. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen T.H., Thrane C., Christophersen C., Anthoni U., Sørensen J. Structure, production characteristics and fungal antagonism of tensin–a new antifungal cyclic lipopeptide from Pseudomonas fluorescens strain 96.578. J. Appl. Microbiol. 2000;89:992–1001. doi: 10.1046/j.1365-2672.2000.01201.x. [DOI] [PubMed] [Google Scholar]
- 73.Islam M.T., Hashidoko Y., Deora A., Ito T., Tahara S. Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes. Appl. Environ. Microbiol. 2005;71:3786–3796. doi: 10.1128/AEM.71.7.3786-3796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Islam M.T. Disruption of ultrastructure and cytoskeletal network is involved with biocontrol of damping off pathogen Aphanomyces cochlioides by Lysobacter sp. strain SB-K88. Biol. Control. 2008;46:312–321. doi: 10.1016/j.biocontrol.2008.02.006. [DOI] [Google Scholar]
- 75.Islam M.T. Mode of antagonism of a biocontrol bacterium Lysobacter sp. SB-K88 toward a damping-off pathogen Aphanomyces cochlioides. World J. Microb. Biotechnol. 2010;26:629–637. doi: 10.1007/s11274-009-0216-y. [DOI] [Google Scholar]
- 76.Islam M.T., Fukushi Y. Growth inhibition and excessive branching in Aphanomyces cochlioides induced by 2,4-diacetylphloroglucinol is linked to disruption of filamentous actin cytoskeleton in the hyphae. World J. Microb. Biotechnol. 2010;26:1163–1170. doi: 10.1007/s11274-009-0284-z. [DOI] [PubMed] [Google Scholar]
- 77.Kopecká M., Ilkovics L., Ramı´kova´ V., Yamaguchi M. Effect of cytoskeleton inhibitors on conidiogenesis and capsule in the long neck yeast Fellomyces examined by scanning electron microscopy. Chemotherapy. 2010;56:197–202. doi: 10.1159/000316330. [DOI] [PubMed] [Google Scholar]
- 78.Ohara T. REN1 is required for development of microconidia and macroconidia, but not of chlamydospores, in the plant pathogenic fungus Fusarium oxysporum. Genetics. 2004;166:113–124. doi: 10.1534/genetics.166.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyu A., Liu H., Che H., Yang L., Zhang J., Wu M., Chen W., Li G. Reveromycins A and B from Streptomyces sp. 3–10: Antifungal activity against plant pathogenic fungi in vitro and in a strawberry food model system. Front. Microbiol. 2017;8:550. doi: 10.3389/fmicb.2017.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang N., Song L., Xu Y., Pei X., Luisi B.F., Liang W. The decrotonylase FoSir5 facilitates mitochondrial metabolic state switching in conidial germination of Fusarium oxysporum. eLife. 2021;10:e75583. doi: 10.7554/eLife.75583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tzung S.P., Kim K.M., Basañez G., Giedt C.D., Simon J., Zimmerberg J., Zhang K.Y., Hockenbery D.M. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat. Cell Biol. 2001;3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 82.Barhoom S., Sharon A. Bcl-2 proteins link programmed cell death with growth and morphogenetic adaptations in the fungal plant pathogen Colletotrichum gloeosporioides. Fungal Genet. Biol. 2007;44:32–43. doi: 10.1016/j.fgb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Pring R.J. Effects of triadimefon on the ultrastructure of rust fungi infecting leaves of wheat and broad bean (Vicia faba) Pestic. Biochem. Phys. 1984;21:127–137. doi: 10.1016/0048-3575(84)90079-8. [DOI] [Google Scholar]
- 84.Sauter H., Steglich W., Anke T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. Engl. 1999;38:1328–1349. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this manuscript as Figures and Tables.