Abstract
Background:COVID-19 sequelae among veterans need evaluation. Design: Propensity-score-matched retrospective cohort study. Participants: A total 778,738 veterans, who were tested for COVID-19 at VA facilities between 20 February 2020–27 March 2021. Main Outcomes: Development of new physical and mental health conditions (incidence) during the follow-up period of 7 days to 3 months after the diagnosis of COVID-19. Results: Out of 778,738 veterans, 149,205 (19.2%) were inpatients and 629,533 (80.8%) were outpatients. 123,757 (15.9%) diagnosed with COVID-19. Mean age was 61 ± 15.4, mostly men (89%) who were White (68%) and non-Hispanic (88%). In hospitalized patients, COVID-19 is associated with significantly higher incidences of physical conditions (venous thromboembolism (5.8% vs. 2.9%, p < 0.001), pulmonary circulation disorder (5.1% vs. 2.9%, p < 0.001), chronic lung disease (8.4% vs. 4.3%, p < 0.001), acute kidney injury (16.4% vs. 9.3%, p < 0.001), chronic kidney disease (6.5% vs. 4.8%, p < 0.001), cardiac arrhythmia (15.2% vs. 10.9%, p < 0.001), complicated hypertension (12% vs. 8.5%, p < 0.001), coagulopathy (6.1% vs. 2.6%, p < 0.001), fluid/electrolyte disorders (24.4% vs. 12.6%, p < 0.001) and neurological disorders (7.1% vs. 3.8%, p < 0.001)) and mental health conditions (depressive episode (6.6% vs. 4.3%, p < 0.001), adjustment disorder (2.5% vs. 1.7%, p < 0.001), insomnia (4.9% vs. 3.2%, p < 0.001) and dementia (3.0% vs. 1.9%, p < 0.001)) compared to propensity-matched hospitalized COVID-19 negative patients. In outpatient settings, COVID-19 diagnosis is associated with smaller increase in the incidences of the physical sequelae. Conclusions: In this propensity-score-matched analysis of US veterans, COVID-19 survivors, especially those who were hospitalized, developed new physical and mental health sequelae at a significantly higher rate than those without COVID-19.
Keywords: COVID-19, mental sequelae, physical sequelae, veterans
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection presents with a wide clinical spectrum ranging from asymptomatic cases to life-threatening illness. During the early part of the Coronavirus disease 2019 (COVID-19) pandemic, the emphasis was on life-threatening health consequences like severe respiratory failure, cytokine storm, thromboembolism, and death. However, as the experience with the COVID-19 has grown, a greater recognition of post-acute sequelae emerged [1,2] This could be due to the persistence of the virus in several organs and the vascular endothelium [3,4,5,6] The predictors and clinical burden of this syndrome that spans mental and physical health are being recognized across many populations but need to be described among the US Veterans.
Veterans have been vaccinated from the early part of 2021, and from mid-2021 less virulent COVID-19 variants (Alpha, Beta, Gamma, Delta, Omicron, etc.) have emerged. Vaccination, less virulent strains, and better therapeutic options have positively affected the severity and mortality of COVID-19. In general, as the years passed, milder forms of COVID-19 emerged. However, the question remains as to how the infection by the initial strain of SARS-CoV-2 affected the veterans. This vulnerable group of patients tends to be older, have higher comorbidities and disabilities, and be from lower socioeconomically disadvantaged groups than the general US population [7,8,9]. Given the larger disease burden in veterans at baseline, it is important to investigate the potential consequences of COVID-19 beyond the acute illness phase. In this study, we have investigated the development of new physical and mental conditions among veterans after the initial phase of COVID-19 infection.
2. Method
2.1. Data Source
Veteran Affairs (VA) has the largest integrated health care system in the US. All healthcare data were extracted to the VA’s Corporate Data Warehouse (CDW), which is a national electronic health data repository. To facilitate COVID-19 research, VA Informatics and Computing Infrastructure (VINCI) analysts created COVID-19 Shared Data Resource, which includes analytic tables extracted from the VA’s CDW for all patients tested for SARS-CoV-2.
2.2. Definition of Positive or Negative COVID-19 and Index Date
Patients were defined as COVID-19-positive if they had at least one positive polymerase chain reaction test during the study period. Patients were defined as COVID-19-negative if all polymerase chain reaction tests were negative. Final adjudication of COVID-19 status was performed by the VA National Surveillance Tool: the single, authoritative data source for the determination of positive and negative cases within the Veterans Health Administration.
The index date for all analyses was defined as the date of the earliest positive test (for COVID-19-positive patients) or the date of the earliest negative test (for COVID-19-negative patients), unless the patient had been admitted to a VA hospital during the preceding 15 days, in which case the date of admission served as the index date.
2.3. Study Population
We identified patients who were tested for COVID-19 at VA facilities between 20 February 2020 and 27 March 2021, for any indication, and who had at least one primary care follow-up in the previous 18 months. We excluded patients who are defined as employees and others, keeping only veterans with proven established care at the VA healthcare system. We excluded patients who died within 3 months of the index date or who did not have a minimum of 3 months of follow-up after the index date.
COVID-19-positive patients who were initially admitted and then discharged were categorized as the COVID-19-positive hospitalized cohort, whereas COVID-19 positive patients who were managed as outpatients at the time of diagnosis were categorized as the COVID-19-positive outpatient cohort.
Patients who were admitted and then discharged for other health conditions during the study period with consistent COVID-19 negative tests were categorized as COVID-19-negative hospitalized cohort, whereas patients who had COVID-19 negative tests and were managed as outpatients were categorized as the COVID-19-negative outpatient cohort.
2.4. Data Extraction
Available data included demographics variables like age, sex, ethnicity, race, body-mass index, and comorbid conditions. We extracted baseline comorbid conditions from CDW based on ICD-10 diagnosis codes occurring in the 2 years prior to the index date from outpatient or inpatient setting. We used the Charlson Comorbidity Index (CCI) to estimate the overall burden of baseline comorbidity.
Prevalent conditions are collected for all patients. To be considered, a prevalent health condition should have been previously recorded as ICD-10 codes in either inpatient or outpatient settings any time before the index date. For an example, to define that a patient has ischemic heart disease as a prevalent health condition, he/she should have one of the listed ICD-10 codes (ischemic heart disease—I20, I21, I22, I23 or I25) on any previous inpatient or outpatient visit. Detailed list of all ICD-10 codes for all physical and mental health conditions are listed in the supplement.
Incident conditions are defined as the development of new physical and mental health conditions (ICD-10 codes) during the follow-up, in the patients who did not have those physical and mental health conditions (ICD-10 codes) as prevalent conditions before the index date. The follow-up period is defined as 7 days to 3 months after the index date. For example, if the patient did not have a depressive episode diagnosis (ICD-10 code F32) before the index date and was subsequently, during the follow-up period, diagnosed with a depressive episode based on the ICD-10 code, then it will be considered an incident condition.
2.5. Outcomes
The primary outcome was the development of new physical and mental health conditions (incidence) during follow-up period in the COVID-19 positive versus the COVID-19 negative patients.
We have analyzed the following four groups: (A) COVID-19-positive hospitalized patient. (B) COVID-19-negative hospitalized patients. (C) COVID-19-positive patients managed as an outpatient. (D) COVID-19-negative patients managed as an outpatient.
2.6. Statistical Analysis
Patient characteristics were summarized using means and standard deviations (std) for continuous variables and percentages for categorical variables, and differences were tested with t-tests and χ2 tests, respectively.
Propensity score matching: To minimize the effects of potential confounders, and to adjust for the potential bias due to the nonrandom balance of the baseline characteristics between the COVID-19-positive and -negative patients, a propensity-matched analysis was applied [10]. Propensity scores were calculated for each subject as the predicted probability of testing positive for COVID-19 using a logistic regression model, adjusting for: age, sex, race, ethnicity, BMI, smoking status, CCI at 2 years, and state of residence. Since the patient is required to have a minimum of 3 months of the follow-up period, patients with an index date later than 27 December 2020 are not included in the analysis. Predominantly all patients were unvaccinated and infected with the initial strain of SARS-CoV-2. So, we did not match for vaccination status and SARS-CoV-2 variants. Patients were matched 1:1 without replacement using a nearest-neighbor approach with caliper restrictions. The covariate balance between the full and matched samples were evaluated using the standardized mean difference (see Supplement Section SX). Outcomes were analyzed in the propensity-matched samples using χ2 tests and unadjusted logistic regressions.
Stratified analysis of the incidence of the different physical and mental conditions in the full sample and in the propensity-matched samples was performed to evaluate the differences between the COVID-19 positive and negative cohorts; stratified by being in the hospitalized or the outpatient cohorts. Then a comparison between the hospitalized patients and the outpatients was performed among the COVID-19-positive patients only. All analyses were limited to patients who did not have the outcome condition prior to the index date.
Incidence rates were compared in the propensity-matched samples using χ2 tests. The odds ratios and 95% confidence intervals of acquiring the physical or mental condition between the comparison groups were estimated using unadjusted logistic regressions, regressing the mental or physical condition on the COVID-19 diagnosis status to compare between positive and negative cases, or on the hospitalization status to compare between the outpatient and hospitalized cohorts.
As a sensitivity analysis, the follow-up period was redefined as 15 days post index date to 3 months, and the outcomes were extracted from that period. All analyses were repeated using this definition. Another sensitivity analysis was performed by matching the hospitalized to outpatient patients among the COVID-19-positive sample. The comparison in the outcomes between hospitalized and outpatient cohorts was repeated using this matched sample.
All statistical analyses were performed using SAS Enterprise Guide version 8.2 (SAS Inc., Cary, NC, USA). Two-sided p< 0.001 was considered statistically significant.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This study was approved by the institutional review board of the Central Virginia VA healthcare system. Being a retrospective cohort analysis, a waiver of the informed consent was granted.
3. Results
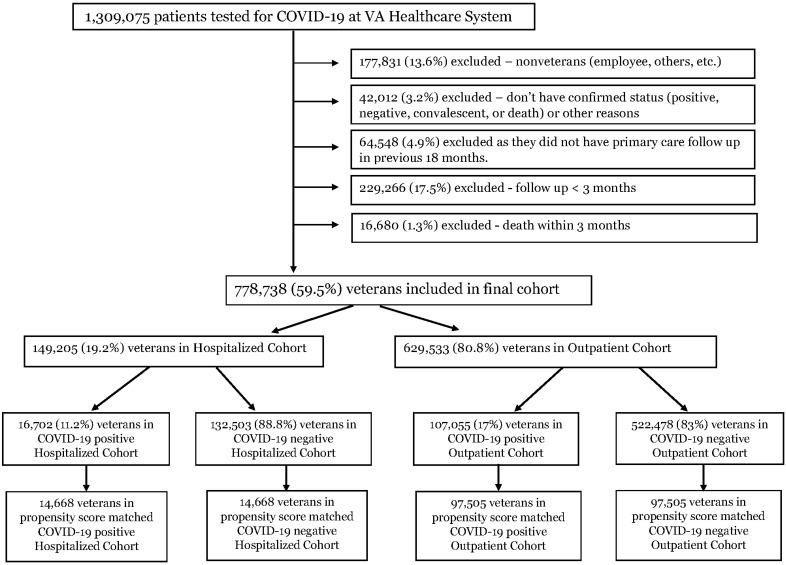
Patients: We analyzed 1,309,075 patients from the VA healthcare system who were tested for COVID-19 during the study period of 20 February 2020 to 27 March 2021. After excluding various conditions as showed in Figure 1, we included 778,738 veterans for the final cohort analysis. Out of these, 149,205 (19.2%) were in the hospitalized cohort and 629,533 (80.8%) in the outpatient cohort, and 16,702 (11.2%) veterans were diagnosed with COVID-19 in the hospitalized cohort, while 107,055 (17.0%) veterans were diagnosed with COVID-19 in the outpatient cohort (Figure 1).
Figure 1.
Flowchart.
Mean age was 61 (±15.4). Most of the patients were male (89%), non-Hispanic ethnicity (88%), and White (68%). (Table S1). In bivariate analysis of demographic variables at baseline, Hispanic ethnicity, Black race, and higher BMI are associated with COVID-19 positivity. However, slightly higher comorbidity burden (CCI) and older age were found in COVID-19 negative patients (Table 1).
Table 1.
Baseline Characteristics of patients in different cohorts.
| Hospitalized Cohort | Outpatient Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total, n (%) | COVID-19-Negative, n (%) | COVID-19-Positive, n (%) | p Value | Total, n (%) | COVID-19-Negative, n (%) | COVID-19-Positive, n (%) | p Value | ||
| Age, mean (±SD) | 68.0 ± 12.8 | 68.1 ± 12.8 | 67.8 ± 13.3 | 0.02 | 59.4 ± 15.5 | 59.5 ± 15.4 | 58.4 ± 16.2 | <0.001 | |
| Gender | |||||||||
| Male | 140,125 (93.9%) | 124,371 (93.9%) | 15,754 (94.3%) | 0.02 | 550,403 (87.4%) | 455,736 (87.2%) | 94,667 (88.4%) | <0.001 | |
| Female | 9080 (6.1%) | 8132 (6.2%) | 948 (5.7%) | 79,130 (12.6%) | 66,742 (12.8%) | 12,388 (11.6%) | |||
| Ethnicity | |||||||||
| Non-Hispanic | 135,545 (90.8%) | 120,639 (91.0%) | 14,906 (89.2%) | <0.001 | 552,005 (87.7%) | 460,315 (88.1%) | 91,690 (85.6%) | <0.001 | |
| Hispanic | 9314 (6.2%) | 7963 (6.0%) | 1351 (8.09%) | 56,244 (9.0%) | 44,698 (8.6%) | 11,546 (10.8%) | |||
| Unknown | 4346 (2.9%) | 3901 (2.9%) | 445 (2.7%) | 21,284 (3.3%) | 17,465 (3.3%) | 3819 (3.6%) | |||
| Race | |||||||||
| White | 103,974 (69.7%) | 93,517 (70.6%) | 10,457 (62.6%) | <0.001 | 426,534 (67.8%) | 354,595 (67.9%) | 71,939 (67.2%) | <0.001 | |
| Black or African American | 34,237 (22.9%) | 29,344 (22.1%) | 4893 (29.3%) | 140,655 (22.3%) | 116,821 (22.4%) | 23,834 (22.3%) | |||
| American Indian or Alaska Native | 1121 (0.8%) | 973 (0.7%) | 148 (0.9%) | 5178 (0.8%) | 4159 (0.8%) | 1019 (1.0%) | |||
| Asian | 807 (0.5%) | 687 (0.5%) | 120 (0.7%) | 8045 (1.3%) | 6990 (1.3%) | 1055 (1.0%) | |||
| Native Hawaiian or Other Pacific Islander | 1009 (0.7%) | 862 (0.7%) | 147 (0.9%) | 5921 (0.9%) | 4906 (0.9%) | 1015 (1.0%) | |||
| BMI, mean (±SD) | 29.2 ± 6.96 | 29.1 ± 6.94 | 30.1 ± 7.04 | <0.001 | 30.3 ± 6.23 | 30.1 ± 6.21 | 31.2 ± 6.23 | <0.001 | |
| CCI, mean (±SD) | 3.18 ± 2.73 | 3.18 ± 2.73 | 3.14 ± 2.73 | 0.09 | 1.66 ± 2.08 | 1.70 ± 2.11 | 1.48 ± 1.94 | <0.001 | |
Incidence of physical and mental health conditions between COVID-19-negative and COVID-19-positive patients’ unmatched samples, stratified by the hospitalized and outpatient cohort, is reported in Table S2.
After propensity-score-matched analysis, the hospitalized COVID-19-positive and -negative groups had 14,668 patients each, and each of the outpatient COVID-19-positive and -negative groups had 97,505 patients (Figure 1).
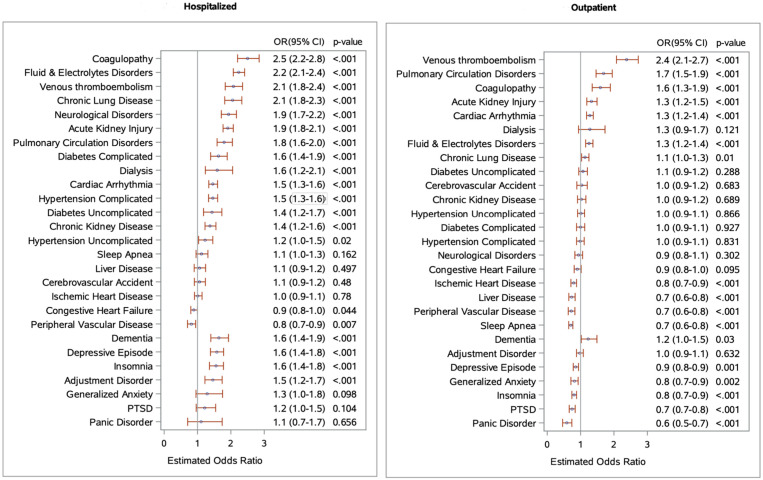
Incidence of the physical and mental health conditions is reported in Table 2. Likelihoods of developing the physical and mental health conditions among COVID-19-positive patients compared to COVID-19-negative patients are reported in Figure 2.
Table 2.
Incidence of physical and mental conditions between COVID-19-negative vs. -positive patients (Matched sample).
| Hospitalized Cohort | Outpatient Cohort | ||||||
|---|---|---|---|---|---|---|---|
| COVID-19 Negative, n (%) |
COVID-19 Positive, n (%) |
p Value | COVID-19 Negative, n (%) |
COVID-19 Positive, n (%) |
p Value | ||
| Pulmonary | |||||||
| Venous Thromboembolism | 384 (2.9%) | 769 (5.8%) | <0.001 | 293 (0.3%) | 696 (0.7%) | <0.001 | |
| Pulmonary Circulation Disorders | 386 (2.9%) | 673 (5.1%) | <0.001 | 299 (0.3%) | 507 (0.5%) | <0.001 | |
| Sleep Apnea | 306 (3.4%) | 330 (3.8%) | 0.16 | 1238 (2.1%) | 906 (1.5%) | <0.001 | |
| Chronic Lung Disease | 404 (4.3%) | 768 (8.4%) | <0.001 | 777 (1.1%) | 907 (1.2%) | 0.01 | |
| Renal | |||||||
| Acute Kidney Injury | 1070 (9.3%) | 1834 (16.4%) | <0.001 | 506 (0.6%) | 677 (0.7%) | <0.001 | |
| Chronic Kidney Disease | 510 (4.8%) | 665 (6.5%) | <0.001 | 514 (0.6%) | 523 (0.6%) | 0.69 | |
| Dialysis | 98 (0.7%) | 154 (1.1%) | <0.001 | 73 (0.1%) | 93 (0.1%) | 0.12 | |
| Cardiovascular | |||||||
| Ischemic Heart Disease | 664 (7.5%) | 679 (7.6%) | 0.78 | 751 (1.0%) | 601 (0.8%) | <0.001 | |
| Cerebrovascular Accident | 321 (2.5%) | 332 (2.7%) | 0.48 | 333 (0.4%) | 342 (0.4%) | 0.68 | |
| Congestive Heart Failure | 717 (6.6%) | 644 (6.0%) | 0.04 | 610 (0.7%) | 557 (0.6%) | 0.10 | |
| Peripheral Vascular Disease | 386 (3.5%) | 319 (2.8%) | 0.007 | 486 (0.6%) | 355 (0.4%) | <0.001 | |
| Cardiac Arrhythmia | 929 (10.9%) | 1317 (15.2%) | <0.001 | 1106 (1.5%) | 1442 (1.9%) | <0.001 | |
| Hypertension Uncomplicated | 270 (10.6%) | 312 (12.7%) | 0.02 | 717 (2.0%) | 720 (2.0%) | 0.87 | |
| Hypertension Complicated | 845 (8.5%) | 1168 (12.0%) | <0.0001 | 590 (0.7%) | 585 (0.7%) | 0.83 | |
| Endocrine | |||||||
| Diabetes Uncomplicated | 192 (2.4%) | 249 (3.4%) | <0.001 | 529 (0.8%) | 539 (0.8%) | 0.29 | |
| Diabetes Complicated | 289 (3.3%) | 428 (5.2%) | <0.001 | 525 (0.7%) | 506 (0.7%) | 0.93 | |
| Others | |||||||
| Liver Disease | 294 (2.4%) | 311 (2.5%) | 0.50 | 546 (0.6%) | 407 (0.5%) | <0.001 | |
| Coagulopathy | 341 (2.6%) | 817 (6.1%) | <0.001 | 219 (0.2%) | 351 (0.4%) | <0.001 | |
| Fluid and Electrolytes Disorders | 1229 (12.6%) | 2316 (24.4%) | <0.001 | 912 (1.1%) | 1167 (1.4%) | <0.001 | |
| Neurological Disorders | 458 (3.8%) | 817 (7.1%) | <0.001 | 512 (0.6%) | 484 (0.6%) | 0.30 | |
| Mental Disorders | |||||||
| Depressive Episode | 428 (4.3%) | 655 (6.6%) | <0.001 | 979 (1.5%) | 875 (1.3%) | <0.001 | |
| Panic Disorder | 36 (0.3%) | 40 (0.3%) | 0.66 | 189 (0.2%) | 111 (0.1%) | <0.001 | |
| Generalized Anxiety | 74 (0.5%) | 96 (0.7%) | 0.10 | 481 (0.6%) | 394 (0.4%) | 0.002 | |
| PTSD | 126 (1.1%) | 151 (1.4%) | 0.10 | 625 (1.0%) | 486 (0.7%) | <0.001 | |
| Adjustment Disorders | 214 (1.7%) | 308 (2.5%) | <0.001 | 730 (0.9%) | 720 (0.9%) | 0.63 | |
| Insomnia | 367 (3.2%) | 553 (4.9%) | <0.001 | 1118 (1.5%) | 932 (1.2%) | <0.001 | |
| Dementia | 251 (1.9%) | 393 (3.0%) | <0.001 | 197 (0.2%) | 240 (0.3%) | 0.03 | |
Figure 2.
Likelihood of development of new physical and mental health conditions in COVID-19-positive patients compared to COVID-19-negative patients. The figure (a) represents the odds ratio for the development of new physical and mental health conditions among propensity-score-matched hospitalized COVID-19 positive patients versus hospitalized COVID-19-negative patients during the follow-up period of 7 days to 3 months of diagnosis. For example—COVID-19-positive patients are 1.4 times (OR: 1.4, 95% CI 1.2–1.6) more likely to develop chronic kidney disease than COVID-19-negative patients during the follow-up period of 7 days to 3 months of diagnosis. The figure (b) represents the odds ratio for the development of new physical and mental health conditions among matched outpatient COVID-19-positive patients versus outpatient COVID-19-negative patients.
3.1. COVID-19 Positive versus Negative Comparisons
Pulmonary: In the hospitalized cohort, COVID-19 diagnosis is associated with a 5.8% incidence of venous thromboembolism (VTE) compared to 2.9% in the COVID-19-negative group (p < 0.001). Similarly, the incidence of pulmonary circulation disorder and chronic lung disease are 5.1% and 8.4% in the COVID-19-positive group, compared to 2.9% and 4.3% in the COVID-19-negative group, respectively (p < 0.001). When we compared the outpatient cohort, we found that COVID-19 diagnosis is associated with a rise in the incidence of the above conditions, but the incidence rate is much smaller. (Table 2).
Renal: In the hospitalized cohort, we noted a 16.4% incidence of acute kidney injury (AKI) in the COVID-19-positive group compared to 9.3% in the COVID-19-negative group (p < 0.001). Interestingly, AKI incidence was also high in the COVID-19-positive outpatient cohort. Incidence of dialysis (1.1% vs. 0.69%, p < 0.001) and chronic kidney disease (CKD) (6.5% vs. 4.8%, p < 0.001) were high in the hospitalized COVID-19-positive group compared to the hospitalized COVID-19-negative group. However, they were not much different in the outpatient cohort. (Table 2).
Cardiovascular: We found a higher incidence of cardiac arrhythmias (15.2% vs. 10.9%, p < 0.001) and complicated hypertension (12% vs. 8.5%, p < 0.001) in hospitalized COVID-19-positive patients versus hospitalized COVID-19-negative patients. A higher incidence of cardiac arrhythmias (1.9% vs. 1.5%, p < 0.001) was also noted in the outpatient setting in the COVID-19-positive patients. Interestingly, the incidence of CHF and PVD were higher in hospitalized COVID-19-negative cohort, while the incidence of ischemic heart disease and cerebrovascular accidents (CVA) were not much different between groups. (Table 2).
Other physical conditions: COVID-19 diagnosis was also associated with higher incidence of fluid and electrolyte disorders (24.4% vs. 12.6%, p < 0.001), coagulopathy (6.1% vs 2.6%, p < 0.001), and neurological disorders (7.1% vs. 3.8%, p < 0.001) in the hospitalized cohort. Even with the outpatient cohort, a higher incidence of coagulopathy and fluid–electrolyte disorder were noted, but absolute values were low. (Table 2).
Mental health disorders: In the hospitalized cohort, COVID-19 was associated with a significantly higher incidence of depressive disorder (6.6% vs. 4.3%, p < 0.001), adjustment disorder (2.5% vs. 1.7%, p < 0.001), insomnia (4.9% vs. 3.2%, p < 0.001), and dementia (3.0% vs. 1.9%, p < 0.001) compared to the matched COVID-19-negative patients. There was not much difference in the outpatient cohort. (Table 2).
3.2. COVID-19 Positive Patients Hospitalized versus Outpatient Comparison
This comparison showed that hospitalized patients with COVID-19 had a significantly higher incidence and odds of development of physical and mental health conditions compared to those who were managed as an outpatient. (Table 3, Figure S1).
Table 3.
Incidence of physical and mental conditions between COVID-19-positive hospitalized vs. COVID-19-positive outpatient cohort (matched sample).
| All COVID-19-Positive Patients | TOTAL, n (%) | Hospitalized, n (%) |
Outpatients, n (%) | p Value |
|---|---|---|---|---|
| Pulmonary | ||||
| Venous Thromboembolism | 912 (3.37%) | 763 (5.74%) | 149 (1.08%) | <0.001 |
| Pulmonary Circulation Disorders | 790 (2.92%) | 667 (5.03%) | 123 (0.892%) | <0.001 |
| Sleep Apnea | 457 (2.59%) | 333 (3.83%) | 124 (1.39%) | <0.001 |
| Chronic Lung Disease | 950 (5.09%) | 769 (8.44%) | 181 (1.89%) | <0.001 |
| Renal | ||||
| Acute Kidney Injury | 2013 (8.45%) | 1826 (16.3%) | 187 (1.48%) | <0.001 |
| Chronic Kidney Disease | 777 (3.75%) | 664 (6.48%) | 113 (1.08%) | <0.001 |
| Dialysis | 186 (0.661%) | 154 (1.10%) | 32 (0.226%) | <0.001 |
| Cardiovascular | ||||
| Ischemic Heart Disease | 799 (4.32%) | 675 (7.56%) | 124 (1.30%) | <0.001 |
| Cerebrovascular Accident | 409 (1.63%) | 334 (2.69%) | 75 (0.588%) | <0.001 |
| Congestive Heart Failure | 799 (3.55%) | 642 (5.94%) | 157 (1.34%) | <0.001 |
| Peripheral Vascular Disease | 407 (1.77%) | 318 (2.82%) | 89 (0.758%) | <0.001 |
| Cardiac Arrhythmia | 1560 (8.46%) | 1312 (15.1%) | 248 (2.53%) | <0.001 |
| Hypertension Uncomplicated | 396 (7.28%) | 312 (12.6%) | 84 (2.82%) | <0.001 |
| Hypertension Complicated | 1326 (6.46%) | 1165 (11.9%) | 161 (1.50%) | <0.001 |
| Endocrine | ||||
| Diabetes Uncomplicated | 333 (2.27%) | 250 (3.42%) | 83 (1.12%) | <0.001 |
| Diabetes Complicated | 514 (3.07%) | 431 (5.21%) | 83 (0.979%) | <0.001 |
| Others | ||||
| Liver Disease | 371 (1.50%) | 310 (2.53%) | 61 (0.486%) | <0.001 |
| Coagulopathy | 911 (3.37%) | 811 (6.11%) | 100 (.728%) | <0.001 |
| Fluid and Electrolytes Disorders | 2603 (12.5%) | 2318 (24.4%) | 285 (2.51%) | <0.001 |
| Neurological Disorders | 949 (3.98%) | 815 (7.10%) | 134 (1.09%) | <0.001 |
| Mental Disorders | ||||
| Depressive Episode | 783 (3.83%) | 654 (6.56%) | 129 (1.23%) | <0.001 |
| Panic Disorder | 56 (0.196%) | 40 (0.280%) | 16 (0.112%) | <0.001 |
| Generalized Anxiety | 133 (0.489%) | 95 (0.698%) | 38 (0.280%) | <0.001 |
| PTSD | 209 (0.953%) | 151 (1.37%) | 58 (0.532%) | <0.001 |
| Adjustment Disorder | 412 (1.64%) | 304 (2.44%) | 108 (0.855%) | <0.001 |
| Insomnia | 682 (2.97%) | 550 (4.86%) | 132 (1.13%) | <0.001 |
| Dementia | 465 (1.78%) | 392 (3.04%) | 73 (0.553%) | <0.001 |
The sensitivity analysis by redefining the follow-up period as 15 days post index date to 3 months showed consistent results for all outcomes. (Tables S3 and S4).
4. Discussion
In a large propensity-score-matched analysis of the Veteran population we found that COVID-19 survivors have a significantly higher rate of the development of new physical and mental health sequelae. The risk of developing these sequelae increase in those who required hospitalization for COVID-19.
Veterans are predisposed to several mental health disorders stemming from war-related and other exposures. This burden is higher among US veterans compared to the general population. After critical illnesses, a subgroup of patients develops adjustment disorders or even PTSD. We found that veterans who required hospitalization for COVID-19 have higher incidence of development of new mental health disorders, mainly depressive episodes, adjustment disorder, PTSD, insomnia, and dementia, compared to matched COVID-19-negative hospitalized patients and the matched COVID-19-positive outpatient group. This increase was not trivial since it was 1.3 to 10 folds compared to the matched groups. The increase was highest in dementia, insomnia, and depressive disorder, and relatively lower for PTSD and panic episodes. These data extend other studies into the US veteran realm [1,11,12]. In a recently published French study, at 4-month follow-up telephone interviews 17.5% reported memory problems, and 20.7% reported cognitive symptoms [13] The neuroinvasive properties of SARS-CoV-2 and neuroinflammation are some of the speculative mechanisms that could explain higher neuropsychiatric manifestation in COVID-19 patients [3,4,14]. These are important consequences that need a priori goal-setting for the patient and the VHA system as a whole. Even under the usual circumstances, mental health disorders among veterans are independently associated with greater health care utilization, rates of disability, and mortality [15,16,17]. Social isolation, anxiety, fear of contagion, uncertainty, chronic stress, and the economic difficulties of the pandemic may lead to the development or exacerbation of depression, anxiety, substance use, and other psychiatric disorders among vulnerable populations [18]. Therefore, any additional increase in the incidence of mental health illnesses among veterans is a concerning finding and needs more attention to prevent long-term health consequences.
These mental health changes were accompanied by major alterations in renal, lung, cardiovascular, and thrombotic complications. As expected, lung-related complications were greater in those recovering from COVID-19 with almost double the incidence of chronic lung disease and pulmonary circulatory disorder in COVID-19 survivors. Our result is consistent with previously reported data, where 63–71% COVID-19 survivors had radiological abnormalities consistent with pulmonary dysfunction, 19% had fibrotic lesions in the lung, and 25–53% had decreased diffusion capacity for carbon monoxide [13,19,20]. These results focused on the lung complications, and validated the dataset and potentially the methodology used to determine the incidence of complications after COVID-19 in this dataset. Prior analyses of renal complications in patients post-COVID have shown a worsening eGFR trajectory, proteinuria, hematuria, and new-onset kidney failure as an outpatient [13,21]. Our analyses point towards a greater rate of AKI in hospitalized veterans with COVID-19 compared to those hospitalized without it. Since we excluded patients who died within 3 months, our AKI incidence is lower than previously reported in the literature [22]. Also, in keeping with prior literature, we found almost a doubling of VTE incidence in hospitalized COVID-19 survivors. This is likely exacerbated by endothelial dysfunction, cytokine release, and various pro-inflammatory milieux in this condition [23]. Previously reported data showed the incidence of VTEs almost up to 50%; however, it also carries a very high mortality rate [24,25]. Since we have excluded veterans who died within 3 months of having COVID-19, our reported incidence is lower. We also found a higher incidence of cardiac arrhythmia in COVID-19 survivors. Some prior uncontrolled studies that did not distinguish prevalence from incidence describe a rate of cardiac arrhythmia in COVID-19 in the range of 6–21% [26,27,28]. Medications, direct COVID-19 cardiac injury, or electrolyte disorders could be contributory [29].
We also found that the incidences of several other conditions were higher in COVID-19-negative survivors compared to those with COVID-19. Specifically, these were related to CHF and PVD. Similar behavior seen in other conditions such as cirrhosis [30], it is likely that patients seeking admission for COVID-19-unrelated issues during the pandemic are more advanced in their disease process. At baseline, US veterans have a larger comorbid condition burden than the general US population. In our cohort, this was demonstrated by the higher baseline Charleston comorbidity index in the COVID-19-negative group. Therefore, these results as a whole demonstrate that, despite propensity-matching, there is a selective increase in incidence of mental health, thromboembolic, pulmonary, and renal complications in hospitalized survivors of COVID-19 but not in the usual conditions responsible for pre-COVID-19 care in VHA, such as PVD and CHF. This adds confidence that the data is specific for COVID-19 and not simply a function of hospitalization during the pandemic in the VA system.
Our study has several strengths. We have a large study population with propensity-score-matched analysis to focus on COVID-19-related impacts, have also analyzed hospitalized to non-hospitalized COVID-19 patients, and done sensitivity analysis. Because we have relied on administrative data, our study is not subject to biases inherent in self-report studies or questionnaire-based studies of long COVID-19 outcomes.
Our study has several limitations. First, our study population is predominantly male veterans, so the result cannot be generalized to other US populations. Second, although, we have only included veterans with established primary care in the VA healthcare system, some veterans might have obtained the care outside the VA system. Third, we have only captured illness based on ICD-10 codes. Fourth, despite the propensity score-matching, there could be residual confounding factors. Fifth, since there is no consistent definition of post-acute sequelae, we were not able to determine pre-discharge variables that could determine who develops this diagnosis. Instead, we treated all new diseases as potentially related to COVID-19 and analyzed the severity of COVID-19 (hospitalized or not) as a comparator. Lastly, clinicians might have paid more attention to the patient during the follow-up visit after the COVID-19 diagnosis and that could have led to improved detection of the health conditions (e.g., dementia), which had been present but remained undiagnosed before COVID-19 diagnosis.
We conclude that in a large propensity-score-matched analysis, veterans who survived COVID-19 developed new physical and mental health comorbidities at a much higher rate compared to those who were hospitalized without COVID-19 and those with COVID-19 without hospitalization. Since, veterans have a higher comorbidity burden to begin with, any additional increase in the incidence of physical and mental health sequelae resulting from COVID-19 is a concerning finding that needs to inform policy and healthcare system changes within the VHA and beyond.
Acknowledgments
This study was supported using data from the VA COVID-19 Shared Data Resource. This work was supported using resources and facilities of the Department of Veterans Affairs (VA) Informatics and Computing Infrastructure (VINCI), VA HSR RES 13-457.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11123390/s1.
Author Contributions
Conceptualization, N.P. and J.S.B.; methodology, N.P. and B.D.; software, B.D.; validation, N.P., J.S.B. and B.D.; formal analysis, B.D.; data curation, B.D.; writing—original draft preparation, N.P.; writing—review and editing, N.P., B.D., J.S.B.; visualization, B.D.; supervision, N.P.; project administration, N.P.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of McGuire VA Medical Center (protocol ID 02599 and date of approval 07/07/2020).
Informed Consent Statement
Patient consent was waived due to retrospective cohort analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisicaro F., Di Napoli M., Liberto A., Fanella M., Di Stasio F., Pennisi M., Bella R., Lanza G., Mansueto G. Neurological Sequelae in Patients with COVID-19: A Histopathological Perspective. Int. J. Environ. Res. Public Health. 2021;18:1415. doi: 10.3390/ijerph18041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan B.-H., Liu J.-M., Gui Y., Wu S., Suo J.-L., Li Y.-C. Neurological involvement in the respiratory manifestations of COVID-19 patients. Aging. 2021;13:4713–4730. doi: 10.18632/aging.202665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roshdy A., Zaher S., Fayed H., Coghlan J.G. COVID-19 and the Heart: A Systematic Review of Cardiac Autopsies. Front. Cardiovasc. Med. 2020;7:626975. doi: 10.3389/fcvm.2020.626975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar J., Gary J., Reagan-Steiner S., Estetter L.B., Tong S., Tao Y., Denison A.M., Lee E., DeLeon-Carnes M., Li Y., et al. Evidence of SARS-CoV-2 Replication and Tropism in the Lungs, Airways and Vascular Endothelium of Patients with Fatal COVID-19: An Autopsy Case-Series. J. Infect. Dis. 2021;223:752–764. doi: 10.1093/infdis/jiab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson K.M., Starkebaum G.A., Reiber G.E. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122:93–100. doi: 10.1177/003335490712200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall M., Kilpatrick K.E., Pendergast J.F., Jones K.R., Vogel W.B. Differences in patient characteristics between Veterans Administration and community hospitals. Implications for VA planning. Med. Care. 1987;25:1099–1104. doi: 10.1097/00005650-198711000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Kazis L.E., Miller D.R., Clark J., Skinner K., Lee A., Rogers W., Spiro A., 3rd, Payne S., Fincke G., Selim A., et al. Health-related quality of life in patients served by the Department of Veterans Affairs: Results from the Veterans Health Study. Arch. Intern. Med. 1998;158:626–632. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]
- 10.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62,354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Zhu L.Y., Ma Y.F., Bo H.X., Deng H.B., Cao J., Wang Y., Wang X.J., Xu Y., Lu Q.D., et al. Association of insomnia disorder with sociodemographic factors and poor mental health in COVID-19 inpatients in China. Sleep Med. 2020;75:282–286. doi: 10.1016/j.sleep.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Writing Committee for the COMEBAC Study Group. Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., Gasnier M., Lecoq A.-L., Meyrignac O., et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA. 2021;325:1525. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borah P., Deb P.K., Chandrasekaran B., Goyal M., Bansal M., Hussain S., Shinu P., Venugopala K.N., Al-Shar’i N.A., Deka S., et al. Neurological Consequences of SARS-CoV-2 Infection and Concurrence of Treatment-Induced Neuropsychiatric Adverse Events in COVID-19 Patients: Navigating the Uncharted. Front. Mol. Biosci. 2021;8:627723. doi: 10.3389/fmolb.2021.627723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi R.B., Post E.P., Sun H., Pomerantz A., Saxon A.J., Piette J.D., Maynard C., Arnow B., Curtis I., Fihn S.D., et al. Prevalence, Comorbidity, and Prognosis of Mental Health Among US Veterans. Am. J. Public Health. 2015;105:2564–2569. doi: 10.2105/AJPH.2015.302836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichter B., Norman S., Haller M., Pietrzak R.H. Physical health burden of PTSD, depression, and their comorbidity in the U.S. veteran population: Morbidity, functioning, and disability. J. Psychosom. Res. 2019;124:109744. doi: 10.1016/j.jpsychores.2019.109744. [DOI] [PubMed] [Google Scholar]
- 17.Hall K.S., Beckham J.C., Bosworth H.B., Sloane R., Pieper C.F., Morey M.C. PTSD is negatively associated with physical performance and physical function in older overweight military veterans. J. Rehabil. Res. Dev. 2014;51:285–296. doi: 10.1682/JRRD.2013.04.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sher L. The impact of the COVID-19 pandemic on suicide rates. QJM. 2020;113:707–712. doi: 10.1093/qjmed/hcaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y.-M., Shang Y.-M., Song W.-B., Li Q.-Q., Xie H., Xu Q.-F., Jia J.-L., Li L.-M., Mao H.-L., Zhou X.-M., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., Zhou X., Liu X., Huang X., Yuan S., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent J., Aklilu A., Yamamoto Y., Simonov M., Li F., Biswas A., Ghazi L., Greenberg J.H., Mansour S.G., Moledina D.G., et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function After Hospital Discharge Among Patients With and Without COVID-19. JAMA Netw. Open. 2021;4:e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., Paranjpe I., Somani S., Richter F., Miotto R., et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo J., Spittle D.A., Newnham M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax. 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 24.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middeldorp S., Coppens M., Van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopinathannair R., Merchant F.M., Lakkireddy D.R., Etheridge S.P., Feigofsky S., Han J.K., Kabra R., Natale A., Poe S., Saha S.A., et al. COVID-19 and cardiac arrhythmias: A global perspective on arrhythmia characteristics and management strategies. J. Interv. Card. Electrophysiol. 2020;59:329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sala S., Peretto G., De Luca G., Farina N., Campochiaro C., Tresoldi M., Dagna L., Zangrillo A., Gulletta S., Della Bella P. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin. Electrophysiol. 2020;43:891–893. doi: 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manolis A.S., Manolis T.A., Apostolopoulos E.J., Papatheou D., Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc. Med. 2020;30:451–460. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajaj J.S., Garcia-Tsao G., Biggins S.W., Kamath P.S., Wong F., McGeorge S., Shaw J., Pearson M., Chew M., Fagan A., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: Multicentre matched cohort. Gut. 2021;70:531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.