Abstract
Selection of the denitrifying community by plant roots (i.e., increase in the denitrifier/total heterotroph ratio in the rhizosphere) has been reported by several authors. However, very few studies to evaluate the role of the denitrifying function itself in the selection of microorganisms in the rhizosphere have been performed. In the present study, we compared the rhizosphere survival of the denitrifying Pseudomonas fluorescens YT101 strain with that of its isogenic mutant deficient in the ability to synthesize the respiratory nitrate reductase, coinoculated in nonplanted or planted soil. We demonstrated that under nonlimiting nitrate conditions, the denitrifying wild-type strain had an advantage in the ability to colonize the rhizosphere of maize. Investigations of the effect of the inoculum characteristics (density of the total inoculum and relative proportions of mutant and wild-type strains) on the outcome of the selection demonstrated that the selective effect of the plant was expressed only during the phase of bacterial multiplication and that the intensity of selection was dependent on the magnitude of this phase. Moreover, application of the de Wit replacement series technique to our results suggests that the advantage of the wild-type strain was maximal when the ratio between the two strains in the inoculum was close to 1:1. This work constitutes the first direct demonstration that the presence of a functional structural gene encoding the respiratory nitrate reductase confers higher rhizosphere competence to a microorganism.
Denitrification is considered to be an important soil process, since it influences (i) the functioning of ecosystems by controlling the global soil N budget and the balance between the mineral nitrogen forms (and, consequently, the nitrogen nutrition of plants) and (ii) the quality of the atmosphere by producing nitrogen oxides and, especially, N2O, which is involved in the terrestrial greenhouse effect (10, 16) and affects the chemistry of O3 in the upper troposphere and lower stratosphere (9). Most of the numerous studies on this process have dealt with how biotic and/or abiotic parameters regulate the denitrifying activity. However, the denitrifying activity is an integrative measurement depending, among other variables, on the density and the distribution of denitrifiers. The distribution of denitrifiers in physicochemically heterogeneous environments basically results from (i) multiplication of denitrifiers—most being heterotrophs—which essentially depends on the availability of assimilable organic substrates and (ii) selection of denitrifiers within the heterotrophic microflora (i.e., increase in the denitrifier/total heterotroph ratio). With regard to the first aspect, several authors reported an increase in the density of denitrifiers in the rhizosphere and attributed this observation to the carbon compounds exudated by roots (14, 17, 19, 20). Few authors have demonstrated that the denitrifier/total heterotrophic microflora ratio may be modified by plants (3, 4, 11). However, these works did not demonstrate that the ability to dissimilate nitrate or nitrite is actually responsible for the selection of the dissimilating community in the rhizosphere. Indeed, other characteristics associated with denitrification could be responsible for this selection. The best way to evaluate the role of denitrification in the adaptative and/or competitive advantage for root colonization is the use of mutants affected in the ability to perform the various steps of the denitrifying pathway. Using this approach, Philippot et al. (15) demonstrated that inactivation (by Tn5 insertion) of the structural gene encoding the cd1-type nitrite reductase decreased the ability of a Pseudomonas fluorescens strain to colonize the rhizosphere. Recent acquisition of a respiratory nitrate reductase mutant by allelic exchange of the narG gene in the same strain (8) allowed for an extension of these studies to the first step of the denitrifying pathway (the most energetic one).
The purposes of the present work were (i) to evaluate the role of nitrate reduction in the ability of a Pseudomonas fluorescens strain to colonize roots of maize grown in a nonsterilized soil and (ii) to evaluate the effects of the inoculum characteristics (i.e., density of the total inoculum and relative proportions of mutant and wild-type [WT] strains) on the outcome of the selection exerted by the plant.
MATERIALS AND METHODS
Soil.
The soil used was a permanent pasture silt loam from the region of Lyon, France, air dried and sieved to a particle size of less than 2 mm. The properties of the soil were as follows: clay, 31.4%; loam, 36.4%; sand, 32.2%; pH 7.5; organic C, 2.69%; total N, 0.35%; water holding capacity, 0.4 g g−1.
Organisms and growth conditions.
Pseudomonas fluorescens YT101 (WT) is a natural, rifampin-resistant clone of strain AK-15 isolated from a loam soil from the Kellog Biological Station, Kalmazoo County, Mich. (24). Strain LP59JG (Nar− mutant) was obtained from strain YT101 by allelic exchange of a gentamicin resistance gene in the narG gene encoding the catalytic subunit of the respiratory nitrate reductase (8). The isogenic character of the mutant LP59JG was confirmed by molecular, biochemical, and immunological assays. Moreover, the similar growth rates of the WT strain and the Nar− mutant under aerobic conditions (with oxygen as the sole electron acceptor) and the total absence of growth of the mutant under anaerobic conditions with nitrate as the sole electron acceptor (8) validate the use of the biological models for the present study.
Strains were grown at 28°C under agitation in Luria-Bertani (LB) medium supplemented with rifampin (50 μg ml−1) for the WT strain and rifampin (50 μg ml−1) plus gentamicin (50 μg ml−1) for the Nar− mutant.
Plant experiments.
The effect of plant roots was assayed by using microcolumn systems previously described by Steinberg et al. (18) and Philippot et al. (15). Briefly, 10 g (nonplanted treatment) or 8 g (planted treatment) of nonsterile soil was introduced into 10-ml syringes (microcolumn) fitted with a cotton wick. Each microcolumn was introduced into a test tube containing a KNO3 solution (10 mM) in distilled water. The cotton wick allowed provision of sufficient water and nitrate to maintain constant soil moisture and nonlimiting nitrate conditions in the two treatments (nonplanted and planted soil) during the entire experiment. This experimental design does not allow adjustment of soil moisture to different values but does allow its maintenance at around 90% of the water-holding capacity for both treatments. For planted soil, maize seeds were allowed to germinate for 24 h on moistened filter papers at 28°C in the dark before being placed on the soil. Then, an additional 2 g of soil was added to the microcolumns in order to cover the seeds. For preparation of inocula, strains were cultured separately in 40 ml of LB medium. After 24 h of growth, bacterial cells were collected by centrifugation at 5,500 × g for 15 min. The two strains were mixed in different relative proportions (50, 20, or 80% of Nar−) in appropriate quantities of KNO3 solution (10 mM) to achieve total (WT + Nar−) densities of 103, 104, or 107 cells g of dry soil−1 in the microcolumns. Then, 4 ml of each of the WT + Nar− mutant mixtures was inoculated into the microcolumns. The proportion of 50% Nar− mutants was associated with the three cell densities, while the proportions of 20 and 80% Nar− mutants were associated only with 104 cells g of dry soil−1.
Microcolumns were incubated in a growth room under the following conditions: photoperiod, 15 h of light (700 microeinsteins m−2 s−1) and 9 h of dark; temperature and humidity, 25°C and 70 to 80% during the day and 19°C and 90% during the night, respectively.
At set times, three planted and three nonplanted microcolumns were used for bacterial enumeration, water content determination, denitrifying activity measurements, and NO3− and NO2− concentration measurements. NO3− measurements were performed in order to maintain concentrations between 20 and 100 μg of NO3−-N g of dry soil−1 by adjusting the NO3− concentration in the test tubes containing the remaining microcolumns. During all experiments, NO2− accumulation was never observed in soil.
Denitrifying activity measurements.
At 3, 7, and 10 days, three noninoculated nonplanted and three noninoculated planted microcolumns were used to compare the denitrifying activities in the presence and absence of the plant. The cylinders of soil were extracted from each microcolumn and transferred (after cutting the aerial part of the plant for the planted systems) into 150-ml plasma flasks. The flasks were then sealed with rubber stoppers. In each flask, 15 ml of atmosphere was replaced by 15 ml of C2H2 in order to ensure N2O reductase inhibition. After 3 days of incubation at 25°C, gas samples were analyzed for N2O with a gas chromatograph equipped with an electron capture detector.
Enumeration.
To investigate the dynamics of the total (WT + Nar−) population and the evolution of the proportion of Nar− mutants in the total population, enumeration was made at 0, 3, 7, 10, and 14 days. Bacteria were extracted by blending whole samples (soil or soil plus roots) for 1.5 min in 100 ml of sterile NaCl solution (8 g liter−1) with a Waring blender. Appropriate dilutions of the soil suspensions were spread on LB agar supplemented with rifampin (50 μg ml−1) for enumeration of the total (WT + Nar−) inoculant. The colonies were then transferred by velvet replication on LB agar supplemented with rifampin (50 μg ml−1) plus gentamicin (50 μg ml−1) for enumeration of Nar− mutants. Cycloheximide (200 μg ml−1) was added to LB agar to prevent fungal growth. Between 30 and 300 CFU of bacteria per plate were counted after 24 h of incubation at 28°C. For each enumeration, three replicates of the appropriate dilution were analyzed. The use of the velvet replica technique allows accurate statistical analysis of the observed proportions. Background counts of Lyon soil on LB agar with 50 μg of rifampin ml−1 indicated a naturally rifampin-resistant population lower than 102 CFU g−1. It was previously verified that no loss of the rifampin or gentamicin resistance marker occurred by comparison of counts on selective LB agar plates (rifampin or rifampin plus gentamicin) and nonselective LB agar plates after 2 weeks of incubation of sterile soil inoculated with both strains (15).
Statistical treatment of data.
For population values, the homogeneity of nine estimations (3 microcosms × 3 replicates) was tested by one-way analysis of variance at each time for each treatment. Since no significant difference between these estimates was observed, means and their confidence intervals were calculated from the nine values (3 microcosms × 3 replicates). Means in planted and nonplanted soil were compared at each time by using the Student t test at the 5% significance level.
For proportion values, the homogeneity of the nine estimations (3 microcosms × 3 replicates) was tested by using the χ2 test at each time for each treatment. Since no significant difference between these estimates was observed, proportions and their confidence intervals were calculated with pooled samples. Proportions in planted and nonplanted soil were compared at each time by using the χ2 test for the pooled samples. The results were summarized and discussed by the de Wit replacement series technique (6): the plot of the proportion of mutants at the end of the experiment measured against the proportion of mutants at the beginning of the experiment (i.e., in the inoculum).
RESULTS
Comparison of the denitrifying activities of planted and nonplanted soil.
The values of denitrifying activity were 0.27 (standard deviation [SD], 0.36) and 2.73 (SD, 0.96) μg of N2O-N g of dry soil−1 at 3 days, 0.42 (SD, 0.29) and 2.85 (SD, 0.29) μg of N2O-N g of dry soil−1 at 7 days, and 0.13 (SD, 0.14) and 5.97 (SD, 1.01) μg of N2O-N g of dry soil−1 at 10 days for nonplanted and planted soil, respectively. This shows that, during our experiments, the potential for denitrification (measured under aerobiosis and nonlimiting nitrate conditions) was at least 7 times higher in planted soil than in nonplanted soil.
Evolution of the total (WT + Nar−) inoculated P. fluorescens population in planted and nonplanted soil.
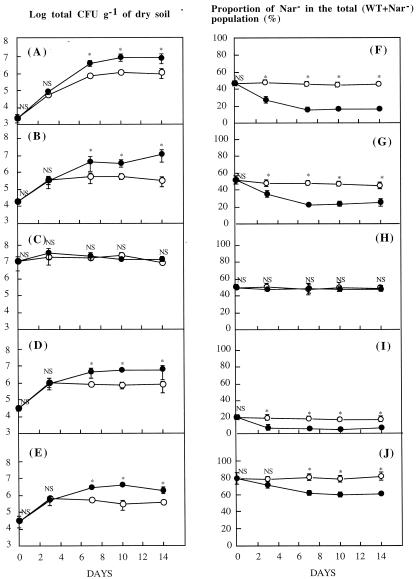
Figure 1A, B, D, and E show that when the inoculum densities were 103 and 104 cells of g dry soil−1, the dynamics of the total (WT + Nar−) population consisted of an increase until day 7 followed by a stabilization from day 7 to day 14. From the beginning of the experiment to day 3, no significant difference was observed between the planted and nonplanted treatments. Then, the dynamics systematically and significantly differed between the two treatments, and the levels of stabilization were about 106 and 107 cells g of dry soil−1 in nonplanted and planted soil, respectively. When the initial inoculum was 107 cells g of dry soil−1 (Fig. 1C), the total population remained at the initial value until the end of the experiment, and no significant difference between planted and nonplanted soil was observed during the whole period dynamics (14 days).
FIG. 1.
Dynamics of the total P. fluorescens inoculated population (WT + Nar− mutant) (left) and of the proportion of Nar− mutants (right) in nonplanted (open circles) and planted (solid circles) soils. Characteristics of the inoculum are as follows (density of inoculum and proportion of Nar− mutants): A and F, 103 cells g of dry soil−1 and 50%; B and G, 104 cells g of dry soil−1 and 50%; C and H, 107 cells g of dry soil−1 and 50%; D and I, 104 cells g of dry soil−1 and 20%; E and J, 104 cells g of dry soil−1 and 80%; Bars denote SD. (Symbols without visible bars indicate that the SD was smaller than the size of the circles.) ∗, significant difference at P = 0.05 between populations or proportions. NS, nonsignificant difference.
Evolution of the proportion of Nar− mutants in the total (WT + Nar−) population in planted and nonplanted soil: influence of the inoculum characteristics.
Figure 1F, G, H, I, and J show that in all nonplanted microcolumns, the proportion of Nar− mutants in the total (WT + Nar−) population remained constant and equal to the proportion of the inoculum during the entire experiment: about 50% in Fig. 1F, G, and H; about 20% in I; and about 80% in J.
When the inoculum densities were 103 and 104 cells g of dry soil−1 (Fig. 1F, G, I, and J), the proportion of Nar− mutants in the total (WT + Nar−) population was significantly lower in planted soil than in nonplanted soil after day 3 (except in Fig. 1J, where the values were not significantly different at day 3). Then, at day 7, the proportion of Nar− mutants in the planted soil remained constant until the end of experiment at the following densities: about 20% when the density of the total inoculum was 103 cells g of dry soil−1 (with 50% of Nar− mutants) (Fig. 1F); about 25% when the density of the total inoculum was 104 cells g of dry soil−1 (with 50% Nar− mutants) (Fig. 1G); about 5% when the density of the total inoculum was 104 cells g of dry soil−1 (with 20% Nar− mutants) (Fig. 1I); and about 60% when the density of the total inoculum was 104 cells g of dry soil−1 (with 80% Nar− mutants) (Fig. 1J).
A radically different trend was observed when the density of the total (WT + Nar−) inoculum was 107 cells g of dry soil−1 (with 50% Nar− mutants) (Fig. 1H). In this case, no significant difference in the evolution of the proportion of Nar− mutants was observed between the planted and nonplanted treatments (the proportion of Nar− mutants remained roughly constant and equal to the proportion of the inoculum during the entire experiment for planted and nonplanted soil).
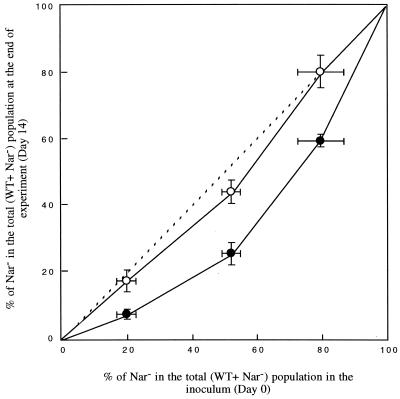
de Wit plot.
The experimental design could be viewed as a de Wit replacement series (6). Figure 2 shows a graphical summary obtained by plotting the proportion of Nar− mutants at the end of the experiment against the proportion of Nar− mutants in the inoculum. In nonplanted soil, the observed relation was close to the null hypothesis of the de Wit representation, i.e., a similar proportion of Nar− mutants at the end of the experiment and in the inoculum (intracompetition = intercompetition). A slight divergence from the null hypothesis was observed only when the initial ratio between the two strains was 1:1. In planted soil, the observed relation shows that intercompetition was higher than intracompetition: the WT strain had the advantage for all the tested proportions of Nar− mutants in the total inoculum, but its advantage was higher (maximal divergence from the null hypothesis) when the initial ratio between the two strains was 1:1.
FIG. 2.
Plot of the proportion of Nar− mutants in the total (WT + Nar−) population at the end of the experiment against the proportion of Nar− mutants in the inoculum in nonplanted (open circles) and planted (solid circles) soils. The dotted line represents the de Wit expected curve for the null hypothesis (intracompetition = intercompetition). Bars denote the SD of the proportions.
DISCUSSION
The major result of this study was to demonstrate that the presence of a functional structural gene encoding the dissimilative nitrate reductase confers to the P. fluorescens YT101 strain an advantage in its ability to colonize the rhizosphere of maize. This result was obtained by comparing the evolution of the proportion of an isogenic Nar− mutant in a total (Nar− mutant plus the corresponding WT) population in the presence and absence of the selective factor (the plant in this study). Such an approach is generally considered to be the most accurate to demonstrate the role of a given microbial function (i.e., the selective value of the corresponding genes) on the environmental competence of a microorganism. Using the same P. fluorescens strain, Philippot et al. (15) reported that the presence of plant roots is able to lower the survival ability of a Tn5 mutant affected in the nirS gene (encoding the cd1-type nitrite reductase) compared to that of the WT denitrifying strain. They concluded that the presence of a functional nirS gene conferred a selective advantage in the rhizosphere of maize. The results of the present study improve our understanding of the importance of denitrifying ability for competition of microorganisms in the rhizosphere by (i) demonstrating the role of the narG gene (encoding the catalytic subunit of the respiratory nitrate reductase) in the rhizosphere competence of the studied strain and (ii) investigating to what extent the outcome of the selection exerted by the plant depends on the population dynamics of the studied strain. Measurements of denitrifying activity demonstrated that conditions in the planted systems were significantly more conducive for denitrification than conditions in the nonplanted ones. This was an absolute prerequisite to ensure that the experimental systems used were satisfactorily adapted to reach our objectives.
Influence of plants and of the density of the total (WT + Nar−) inoculum on the dynamics of the total P. fluorescens population.
When the densities of total (WT + Nar−) P. fluorescens inocula were 103 and 104 cells g of dry soil−1, an increase followed by a stabilization of the population was observed (Fig. 1A, B, D, and E). Under these conditions, the level of stabilization in the nonplanted soil was 106 cells g of dry soil−1. For the same strain and the same soil but under gnotobiotic conditions (i.e., the conditions defining the carrying capacity stricto sensu of the soil for the strain used), the level of stabilization observed by Philippot et al. (15) was about 109 cells g of dry soil−1 (i.e., 3 orders of magnitude higher). This means that the biotic components of the soil (through competition and/or predation processes) drastically decreased its ability to receive the introduced P. fluorescens strain but nevertheless allowed the strain to multiply and maintain itself in the soil during at least the 2 weeks of the experiments. This suggests that irrespective of its denitrifying abilities, P. fluorescens YT101 shows high adaptative and competitive potentialities in the studied soil and thus can be considered as a denitrifier that exhibits good competitive abilities as an aerobic heterotroph (12, 13). The presence of a plant improved these potentialities, as shown by the level of stabilization, which was about 1 order of magnitude higher in planted than in nonplanted microcolumns. Thus, approximately 106 and 107 cells g of dry soil−1 might be considered to be the carrying capacities of the studied strain in the nonplanted and planted soil, respectively (lato sensu, i.e., under conditions in which both physicochemical and biotic components can interfere with the survival of the introduced strain). One may assume that the introduced strain must systematically stabilize at these densities, whatever the density of inoculum. Indeed, several studies demonstrated that the carrying capacity of a given soil for a given strain can be considered as a constant and that the dynamics of the strain decrease or increase to reach the carrying capacity (2, 5, 21). As expected, the population in the planted soil remained constant until the end of the experiment, when the density of the WT + Nar− P. fluorescens inoculum was 107 cells g of dry soil−1, corresponding to the carrying capacity of the planted soil (Fig. 1C). However, the population of the nonplanted soil did not decrease to the carrying capacity (106 cells g of dry soil−1) but remained constant and equal to the inoculum density. A possible explanation is that the limited duration of the experiment (14 days) would not allow us to observe the decrease in P. fluorescens density to the carrying capacity, which may be a slow process, especially in silt loam soils (22).
Influence of the density of the total (WT + Nar−) inoculum on the outcome of the selection exerted by the plant.
Another major result of our work was to demonstrate that the selective effect of the plant was expressed only during the phase of cell multiplication (when such phase occurred) of the introduced P. fluorescens population and that the intensity of the selection is dependent on the magnitude of the phase of multiplication. In order to base our interpretations on treatments differing in one single parameter, only the experiments involving the same proportion (50%) of mutants in the inoculum are discussed (Fig. 1F, G, and H).
When the densities of WT + Nar− P. fluorescens inocula were 103 and 104 cells g of dry soil−1, the proportion of Nar− mutants in the total population decreased in the planted soils until day 7 (Fig. 1F and G), i.e., during the phase of cell multiplication. From day 7 to the end of the experiment, while the WT + Nar− population was stabilized at the value of the carrying capacity (Fig. 1A and B), the proportion of Nar− mutants remained constant. Moreover, the differences observed in the evolution of the proportions of Nar− mutants between planted and nonplanted soil (Fig. 1F and G) were higher when the total population increase was about 4 orders of magnitude (103 to 107 cells g of dry soil−1 [Fig. 1A]) than when it was only about 3 orders of magnitude (104 to 107 cells g of dry soil−1 [Fig. 1B]). The absolute requirement of a cell multiplication phase to induce a plant selection of the WT strain was clearly confirmed by the fact that, when no cell multiplication phase occurred (inoculum density of 107 cells g of dry soil−1 [Fig. 1C]), no selection was observed (Fig. 1H).
Influence of the proportion of mutants in the WT + Nar− inoculum on the outcome of the selection exerted by the plant.
The advantage of the WT P. fluorescens strain in the rhizosphere was always observed, whatever the proportions of mutants in the inoculum, unless a cell multiplication phase was not occurring. In order to base our interpretations on treatments differing in one single parameter, only the experiments involving the same density of total (WT + Nar−) inoculum (104 cells g of dry soil−1) are discussed (Fig. 1G, I, and J). The general trend observed in the evolution of the proportion of Nar− mutants in the planted microcolumns was similar for the different tested proportions of mutants in the inoculum (50, 20, and 80%): a decrease until day 7 (corresponding to the cell multiplication phase) followed by a stabilization until the end of the experiment. In order to assess the effect of the proportion of mutants in the inoculum on the intensity of the selection, we used the de Wit replacement series technique as a representation of the competition between the two strains. This technique (initially devoted to plant ecology [7] and further applied to the analysis of fungal and bacterial competition [1, 23]) confirms that (i) the soil conditions existing in the nonplanted treatments did not induce a marked discrimination between the two strains, and (ii) in the planted treatments, the WT strain had an advantage for all the tested proportions of Nar− mutants in the inoculum (Fig. 2). These observations are in agreement with denitrifying activity measurements, which showed that, under the experimental conditions used in this study (especially soil moisture), the planted systems were significantly more conducive for denitrification than the nonplanted ones. Since the maximal distance from the curve representing the null hypothesis was obtained for an initial ratio of 1:1, application of this technique also suggests that the intensity of the selection exerted by roots was minimized when the initial ratio diverged from 1:1. The fact that a slight advantage of the WT strain was also observed in the case of an initial ratio of 1:1 in the nonplanted soil (where conditions allowed a small but detectable amount of denitrifying activity) supports the predominance of the 1:1 condition in discriminating between the strains.
It is important to underline that our results cannot be interpreted only in terms of competition restricted to the two strains studied. Indeed, in order to more realistically mimic what could occur in natura, our experiments were performed with nonsterile soil. Under these conditions, the preexisting soil microflora may obviously interact with both inoculated strains. The unexpected observation that the intensity of the selection was not inversely correlated to the proportion of the disadvantaged strain (Nar−) in the inoculum may result from this complex network of interactions.
Several studies of the indigenous soil microflora have demonstrated that denitrifying bacteria predominantly occur near or inside the roots (3, 4, 11) and proposed that the function of denitrification itself may constitute a significant advantage for root colonization. This assumption has now clearly been proved for one P. fluorescens strain (either for the role of nitrite reductase [15] or for the role of nitrate reductase [this study]), but generalization to other denitrifiers or a fortiori to the whole denitrifying community in a given soil remains to be investigated.
ACKNOWLEDGMENTS
We are very grateful to Agnès Richaume for helpful comments on the manuscript. We also thank Nadia Salin and Amandine Tabouret for technical assistance.
REFERENCES
- 1.Adee S R, Pfender W F, Hartnett D C. Competition between Pyrenophora tritici-repentis and Septoria nodorum in the wheat leaf as measured with de Wit replacement series. Phytopathology. 1990;80:1177–1182. [Google Scholar]
- 2.Benett R A, Lynch J M. Colonization potential of bacteria in the rhizosphere. Curr Microbiol. 1981;6:137–138. [Google Scholar]
- 3.Clays-Josserand A, Ghiglione J F, Philippot L, Lemanceau P, Lensi R. Effect of soil type and plant species on the fluorescent pseudomonads nitrate dissimilating community. Plant Soil. 1999;209:275–282. [Google Scholar]
- 4.Clays-Josserand A, Lemanceau P, Philippot L, Lensi R. Influence of two plant species (flax and tomato) on the distribution of nitrogen dissimilative abilities within fluorescent Pseudomonas spp. Appl Environ Microbiol. 1995;61:1745–1749. doi: 10.1128/aem.61.5.1745-1749.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compeau G, Al-Achi B J, Evangelia P, Plotsouka E, Levy S B. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988;54:2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit C T. On competition. Versl Landbouwkd Onderz. 1960;66:1–82. [Google Scholar]
- 7.de Wit C T, Tow G P, Ennik G C. Competition between legumes and grasses. Versl Landbouwk Onderz. 1966;687:1–30. [Google Scholar]
- 8.Ghiglione J F, Philippot L, Normand P, Lensi R, Potier P. Disruption of narG, the gene encoding the catalytic subunit of respiratory nitrate reductase, also affects nitrite respiration in Pseudomonas fluorescens YT101. J Bacteriol. 1999;181:5099–5102. doi: 10.1128/jb.181.16.5099-5102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graedel T E, Crutzen P J. Atmospheric change. An earth system perspective. New York, N.Y: W. H. Freeman and Company; 1992. [Google Scholar]
- 10.Intergovernmental Panel on Climate Change. Contribution of working group I to the second assessment report of the Intergovernmental Panel on Climate Change. In: Houghton J T, Meira Filho L G, Callander B A, Harris N, Kattenberg A, Maskell K, editors. Climate change 1995: the science of climate change. Cambridge, United Kingdom: Cambridge University Press; 1996. p. 572. [Google Scholar]
- 11.Linne Von Berg K H, Bothe H. The distribution of denitrifying bacteria in soil monitored by DNA-probing. FEMS Microbiol Ecol. 1992;86:331–340. [Google Scholar]
- 12.Murray R E, Parsons L L, Smith M S. Aerobic and anaerobic growth of rifampin-resistant denitrifying bacteria in soil. Appl Environ Microbiol. 1990;56:323–328. doi: 10.1128/aem.56.2.323-328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray R E, Parsons L L, Smith M S. Competition between two isolates of denitrifying bacteria added to soil. Appl Environ Microbiol. 1992;58:3890–3895. doi: 10.1128/aem.58.12.3890-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myrold D D, Tiedje J M. Establishment of denitrification capacity in soil: effects of carbon, nitrate, and moisture. Soil Biol Biochem. 1985;17:819–822. [Google Scholar]
- 15.Philippot L, Clays-Josserand A, Lensi R. Use of Tn5 mutants to assess the role of the dissimilatory nitrite reductase in the competitive abilities of two Pseudomonas strains in soil. Appl Environ Microbiol. 1995;61:1426–1430. doi: 10.1128/aem.61.4.1426-1430.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K A. Greenhouse gas fluxes between land surfaces and the atmosphere. Prog Phys Geogr. 1990;14:349–372. [Google Scholar]
- 17.Smith M S, Tiedje J M. The effect of roots on soil denitrification. Soil Sci Soc Am J. 1979;43:951–955. [Google Scholar]
- 18.Steinberg C, Gamard P, Faurie G, Lensi R. Survival and potential denitrifying activity of Azospirillum lipoferum and Bradyrhizobium japonicum inoculated into sterilized soil. Biol Fertil Soils. 1989;7:101–107. [Google Scholar]
- 19.Tiedje J M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. J. New York, N.Y: Wiley & Sons, Inc.; 1988. pp. 179–244. [Google Scholar]
- 20.Tiedje J M, Sextone A J, Myrold D D, Robinson J A. Denitrification: ecological niches, competition and survival. Antonie Leeuwenhoek. 1982;48:569–593. doi: 10.1007/BF00399542. [DOI] [PubMed] [Google Scholar]
- 21.van Dyke M I, Prosser J I. Effect of cell density and attachment on resuscitation in soil of starved Pseudomonas fluorescens MON787. FEMS Microbiol Ecol. 1998;26:63–70. [Google Scholar]
- 22.van Elsas J D, Dijkstra A F, Goevaert J M, van Veen J A. Survival of Pseudomonas fluorescens and Bacillus subtilis introduced into two soils with different texture in field microplots. FEMS Microbiol Ecol. 1986;38:151–160. [Google Scholar]
- 23.Wilson M, Lindow S E. Enhanced epiphytic coexistence of near-isogenic salicylate-catabolizing and non-salicylate-catabolizing Pseudomonas putida strains after exogenous salicylate application. Appl Environ Microbiol. 1995;61:1073–1076. doi: 10.1128/aem.61.3.1073-1076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye R W, Arunakumari A, Averill B A, Tiedje J M. Mutants of Pseudomonas fluorescens deficient in dissimilatory nitrite reduction are also altered in nitric oxide reduction. J Bacteriol. 1992;174:2560–2564. doi: 10.1128/jb.174.8.2560-2564.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]