Abstract
Background: Patients with heart failure represent a vulnerable population for COVID-19 and are prone to having worse prognoses and higher fatality rates. Still, the clinical course of the infection is dynamic, and complication occurrence in particular in patients with heart failure is fairly unpredictable. Considering that individual components of the C2HEST (C2: Coronary Artery Diseases (CAD)/Chronic obstructive pulmonary disease (COPD); H: Hypertension; E: Elderly (Age ≥ 75); S: Systolic HF; T: Thyroid disease) are parallel to COVID-19 mortality risk factors, we evaluate the predictive value of C2HEST score in patients with heart failure (HF) Material and Methods: The retrospective medical data analysis of 2184 COVID-19 patients hospitalized in the University Hospital in Wroclaw between February 2020 and June 2021 was the basis of the study. The measured outcomes included: in-hospital mortality, 3-month and 6-month all-cause-mortality, non-fatal end of hospitalization, and adverse in-hospital clinical events. Results: The heart failure cohort consists of 255 patients, while 1929 patients were assigned to the non-HF cohort. The in-hospital, 3-month, and 6-month mortality rates were highest in the HF cohort high-risk C2HEST stratum, reaching 38.61%, 53.96%, and 65.36%, respectively. In the non-HF cohort, in-hospital, 3-month, and 6-month mortalities were also highest in the high-risk C2HEST stratum and came to 26.39%, 52.78%, and 65.0%, respectively. An additional point in the C2HEST score increased the total death intensity in 10% of HF subjects (HR 1.100, 95% CI 0.968–1.250 p = 0.143) while in the non-HF cohort, the same value increased by 62.3% (HR 1.623, 95% CI 1.518–1.734 p < 0.0001). Conclusions: The C2HEST score risk in the HF cohort failed to show discriminatory performance in terms of mortality and other clinical adverse outcomes during hospitalization. C2HEST score in the non-HF cohort showed significantly better performance in terms of predicting in-hospital and 6-month mortality and other non-fatal clinical outcomes such as cardiovascular events (myocardial injury, acute heart failure, myocardial infarction, cardiogenic shock), pneumonia, sepsis, and acute renal injury.
Keywords: heart failure, COVID-19, SARS-CoV-2, outcomes, C2HEST score, mortality, prediction
1. Introduction
Since the outbreak of the global COVID-19 pandemic, numerous risk factors of SARS-CoV-2 infection severity have been described [1,2]. Even though several scoring systems [3] have been proposed, the clinical course among individuals with COVID-19 is still unpredictable. Therefore, the relevant need for a more accurate evaluation of potential outcomes to support clinical decisions and, consequently, to avoid unnecessary resource consumption is a crucial point in preventing public health collapse.
Patients with congestive heart failure due to advanced age and coexisting comorbidities constitute a particularly challenging subpopulation experiencing worse outcomes, including increased mortality. In addition, in the course of SARS-CoV-2 infection, they face low cardiovascular reserve, increased metabolic demand, uncontrolled immune response [4], and thromboembolic issues [5]. Due to limited resources, all additional diagnostic and prognostic features seem to be advisable to support life-saving interventions and improve decision-making processes regarding hospital admission and an inpatient referral.
Originally, the C2HEST score was proposed as a scoring system to allow the stratification of the risk of developing atrial fibrillation (AF) in the general population [6]. However, recently, Liang et al. [7] demonstrated that C2HEST score could also predict adverse clinical outcomes, including death and hospitalization, among patients with heart failure. Considering that individual components of the C2HEST (C2: CAD/COPD; H: Hypertension; E: Elderly (Age ≥ 75); S: Systolic HF; T: Thyroid disease) are parallel to COVID-19 mortality risk factors, juxtaposing it with the outcomes of recently published studies [8,9,10] suggesting that this simple scoring system has the ability to predict outcomes in several COVID-19 subpopulations, we designed this study to evaluate the predictive value of C2HEST score in patients with heart failure (HF).
2. Materials and Methods
2.1. Study Population
The study population consisted of 2184 patients hospitalized in the University Hospital in Wroclaw between February 2020 and June 2021 with confirmed infection of SARS-CoV-2 virus (diagnosis was based on results of reverse transcription-polymerase chain reaction (RT-PCR) for viral RNA from nasopharyngeal swab specimens). The study (COronavirus in Lower Silesia (COLOS) study) had the approval of the local ethics committee (No: KB-444/2021). Due to the retrospective, observational character of the study, written informed consent for participation was waived.
Anonymized medical data of all study subjects were preanalyzed and assigned to one of the two study cohorts. For the first study arm, we incorporated patients with a history of HF diagnosed prior to index hospitalization. Diagnosis of heart failure was based on the European Society of Cardiology (ESC) guidelines [11]. The second study arm was composed of patients without a previous diagnosis of HF.
For all patients recruited to both study cohorts, we evaluated the C2HEST value. C2HEST score analysis was performed based on original variables; coronary artery disease (1 point), chronic obstructive pulmonary disease (1 point), hypertension (1 point), elderly (age ≥ 75 years, 2 points), systolic HF (2 points), and thyroid disease (1 point). Depending on the calculated score, subjects were allocated to one of three strata: low-risk, 0 or 1 point; medium-risk, 2 or 3 points; and high-risk, 4 or more points.
2.2. Endpoints and Outcomes
The primary clinical outcome was defined as in-hospital, 3-month, and 6-month all-cause mortality. Additionally, data regarding other clinical outcomes focused on the in-hospital period were collected. The measured outcomes included: end of hospitalization other than death (discharge, deterioration, or recovery with transfer to another hospital), level of respiratory support, shock, sepsis, systemic inflammatory response syndrome (SIRS), acute kidney injury, acute liver dysfunction, pneumonia, myocardial injury, acute heart failure, stroke, pulmonary embolism, and all-type symptomatic bleeding.
2.3. Statistical Analysis
R language version 4.0.4 with additional packages [12,13,14,15] pROC and time-ROC, coin, survival, and odds ratio was used by professional academic statisticians to perform data analysis. A significance value level was set at 0.05. Descriptive data regarding categorical variables were presented as numbers and percentages, while numerical variables were presented as mean with standard deviation, range (minimum–maximum), and the number of non-missing values. Chi-square and Omnibus tests were used in the case of categorical variables which exceeded 5 expected cases in each group. The Fisher exact test was used for subjects with fewer cell counts. Welch’s ANOVA was performed to analyze continuous variables to adjust for unequal variances among the risk-strata and a sample size large sufficient for the appropriateness of asymptotic results. In the case of continuous variables, the Games–Howell variant of Tukey correction was used as a part of a post hoc analysis. The post hoc test, for categorical variables, was analogous to the omnibus test. Therefore, it was used in subgroups with a Bonferroni correction. In-hospital mortality along with all-cause mortality were right-censored data. Therefore, time-dependent ROC analysis with inverse probability of censoring weighting (IPCW) was used to estimate them. The time-dependent area under the curve (AUC) was used to assess the C2HEST score. The log-rank test was used as a part of the confirmation of differences in survival curves among risk strata.
The Grambsch–Therneau test was used to verify proportional hazard assumption. The Cox proportional hazard model was used to perform an analysis of the hazard ratio (HR) in the C2HEST score, its components, and the risk strata. The logistic regression model was used during an analysis of secondary outcomes due to their dichotomic nature. Classical ROC analysis with an AUC measure was performed to assess predictive capability. Odds ratio (OR) was presented as a size effect for the influence of the C2HEST score, its components, and risk strata.
3. Results
3.1. Baseline Demographical and Clinical Features of the Studied Population
The baseline demographic characteristics, along with a past medical history of subjects allocated to both study cohorts, were presented in Table 1. The heart failure group consisted of 255 mainly male patients (144 (56.47%)). In this study cohort, no subjects were allocated to the low-risk stratum. This fact is related to the fundamentals of the C2HEST score—the previous diagnosis of systolic HF results in increasing the value of a score up to 2 points and automatically allocates all patients with HF to the medium- or high-risk strata. The vast majority of patients in the HF arm were assigned to the high-risk stratum (n= 202), while the medium-risk stratum consisted of 53 patients. In total, 1929 patients were assigned to the non-HF cohort. Among all C2HEST risk strata in the study, the most numerous was the low-risk non-HF, with 1417 participants; medium-risk consisted of 439 subjects, and high-risk consisted of 72 patients.
Table 1.
Baseline characteristics after C2HEST risk stratification in the HF and non-HF study cohorts.
| Variables, Units (N) (HF/Non-HF) |
Low Risk (0–1) |
Medium Risk (2–3) |
High Risk (≥4) |
t-Test | OMNIBUS p Value |
p Value for Post Hoc Analysis |
|||
|---|---|---|---|---|---|---|---|---|---|
| Min–Max (N) or n/N (% of Risk Category) |
Min–Max (N) or n/N (% of Risk Category) |
Min–Max (N) or n/N (% of Risk Category) |
|||||||
| HF | Non-HF | HF | Non-HF | HF | Non-HF | HF | Non-HF | ||
| Demographics | |||||||||
|
Age, years (255/1929) |
51.11 ± 15.9 17–74 (1417) |
63.38 ± 9.76 36–74 (53) |
77.03 ± 11.1 29–100 (439) |
77.71 ± 10.39 38–100 (202) |
81.04 ± 4.93 73–94 (72) |
<0.0001 c | <0.0001 | <0.0001 a,b,c | |
|
Male gender (255/1929) |
735/1417 (51.87%) |
33/53 (62.26%) |
175/439 (39.86%) |
111/202 (54.95%) |
28/72 (38.89%) |
0.4236 c | <0.0001 |
<0.0001 a 0.1274 b <0.0001 c |
|
|
BMI kg/m2 (69/485) |
28.28 ± 5.07 15.36–49.38 (397) |
29.36 ± 6.9 20.89–46.71 (17) |
29.27 ± 5.36 18.59–47.75 (73) |
28.12 ± 6.14 17.28–48.21 (52) |
26.67 ± 4.71 16.41–34.89 (15) |
0.5129 c | 0.146 | N/A | |
|
Obesity (BMI ≥ 30 kg/m2) (69/485) |
132/397 (33.25%) |
8/17 (47.06%) |
30/73 (41.1%) |
17/52 (32.69%) |
4/15 (26.67%) |
0.7294 c | 0.3259 | N/A | |
|
Cigarette smoking never previous current (255/1929) |
1337/1417 (94.35%) 46/1417 (3.25%) 34/1417 (2.4%) |
38/53 (71.7%) 8/53 (15.09%) 7/53 (13.29%) |
393/439 (90.14%) 27/439 (6.19%) 16/439 (3.67%) |
162/202 (80.2%) 24/202 (11.88%) 16/202 (7.92%) |
56/72 (78.87%) 12/72 (16.9%) 3/72 (4.23%) |
0.3396 c | <0.0001 |
0.0238 a
<0.0001 b 0.0334 c |
|
| Comorbidities | |||||||||
|
Hypertension (255/1929) |
415/1417 (29.29%) |
36/53 (67.92%) |
321/439 (73.12%) |
179/202 (88.61%) |
70/72 (97.22%) |
0.0005 c | <0.0001 | <0.0001 a,b,c | |
|
DM (255/1929) |
208/1417 (14.68%) |
20/53 (37.74%) |
126/439 (28.77%) |
94/202 (46.77%) |
24/72 (33.39%) |
0.4209 c | <0.0001 |
<0.0001 a 0.0028 b 0.879 c |
|
|
Dyslipidemia (172/653) |
289/417 (69.3%) |
26/34 (76.47%) |
148/199 (74.37%) |
117/138 (84.78%) |
31/37 (83.78%) |
0.3661 c | 0.1034 | N/A | |
|
Atrial fibrillation/flutter (255/1929) |
49/1417 (3.46%) |
22/53 (41.51%) |
84/439 (19.13%) |
112/202 (55.45%) |
23/72 (31.94%) |
0.0982 c | <0.0001 |
<0.0001 a,b 0.061 c |
|
|
Previous coronary revascularization (255/1929) |
6/1417 (0.42%) |
4/53 (7.55%) |
33/439 (7.52%) |
89/202 (44.06%) |
22/72 (30.56%) |
<0.0001 c | <0.0001 | <0.0001 a,b,c | |
|
Previous myocardial infarction (255/1929) |
11/1417 (0.78%) |
4/53 (7.55%) |
59/439 (13.44%) |
88/202 (43.56%) |
29/72 (40.28%) |
<0.0001 c | <0.0001 | <0.0001 a,b,c | |
|
Heart failure (255/1929) |
0/1417 (0%) |
53/53 (100%) |
0/439 (0%) |
202/202 (100%) |
0/72 (0%) |
<0.0001 c | <0.0001 | <0.0001 a,b,c | |
|
Moderate/severe valvular heart disease or previous valve heart surgery (255/1929) |
13/1417 (0.92%) |
16/53 (30.19%) |
16/439 (3.64%) |
48/202 (23.76%) |
3/72 (4.17%) |
0.434 c | 0.0002 |
0.0007 a 0.1157 b 1.0 c |
|
|
Peripheral artery disease (255/1929) |
26/1417 (1.83%) |
6/53 (11.32%) |
25/439 (5.69%) |
37/202 (18.32%) |
6/72 (8.33%) |
0.3151 c | <0.0001 |
0.0002 a 0.0104 b 1.0 c |
|
|
Previous stroke/TIA (255/1929) |
47/1417 (3.32%) |
6/53 (11.32%) |
53/439 (12.07%) |
47/202 (23.27%) |
11/72 (15.28%) |
0.0859 c | <0.0001 |
<0.0001 a 0.0002 b 1.0 c |
|
|
Chronic kidney disease (255/1929) |
70/1417 (4.94%) |
14/53 (26.42%) |
56/439 (12.76%) |
78/202 (38.61%) |
13/72 (18.06%) |
0.1375 c | <0.0001 |
<0.0001 a,b 0.9041 c |
|
|
Hemodialysis (255/1929) |
19/1417 (1.34%) |
4/53 (7.55%) |
16/439 (3.64%) |
17/202 (8.42%) |
2/72 (2.78%) |
1.0 c | 0.0078 |
0.0121 a 0.8097 b 1.0 c |
|
|
Asthma (255/1929) |
54/1417 (3.81%) |
3/53 (5.66%) |
17/439 (3.87%) |
7/202 (3.47%) |
4/72 (5.56%) |
0.4379 c | 0.6782 | N/A | |
|
COPD (255/1929) |
6/1417 (0.42%) |
0/53 (0%) |
25/439 (5.69%) |
29/202 (14.36%) |
15/72 (20.83%) |
0.0072 c | <0.0001 |
<0.0001 a,b
0.0003 c |
|
|
Hypothyroidism (255/1929) |
76/1417 (5.36%) |
0/53 (0%) |
68/439 (15.49%) |
33/202 (16.34%) |
31/72 (43.06%) |
0.0004 c | <0.0001 | <0.0001 a,b,c | |
|
Hyperthyroidism (255/1929) |
4/1417 (0.28%) |
0/53 (0%) |
10/439 (2.28%) |
7/202 (3.47%) |
0/72 (0%) |
||||
Continuous variables are presented as mean ± SD, range (minimum–maximum), and number of non-missing values. Categorized variables are presented as a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: CAD—coronary artery disease, OMNIBUS—analysis of variance, N—valid measurements, n—number of patients with parameter above cut-off point, SD—standard deviation, BMI—body mass index, DM—diabetes mellitus, TIA—transient ischemic attack, COPD—chronic obstructive pulmonary disease, N/A—not applicable, a—low risk vs. medium risk, b—low risk vs. high risk, c—medium risk vs. high risk. Bold text—statistically significant values.
In both study cohorts, an increase in the C2HEST risk stratum resulted in more advanced subject age and a higher prevalence of comorbidities. Differences between study cohorts were regarding the prevalence of diabetes, atrial fibrillation, valvular heart disease, peripheral artery disease, stroke/TIA, and chronic kidney disease.
In particular, the non-HF cohort revealed significant differences between C2HEST risk groups in terms of treatment applied before admission to the hospital. Detailed data are presented in Supplementary Table S1.
Analysis of baseline patient-reported symptoms and vital signs (Table 2) revealed significant differences among prevalence, systolic blood pressure, oxygen saturation (SpO2) in room air, crackles, wheezing, pulmonary congestion, and peripheral oedema in the non-HF cohort, whereas no differences were observed in the HF cohort.
Table 2.
Patient-reported symptoms, vital signs, and abnormalities measured during physical examination at hospital admission in the HF and non-HF study cohorts after C2HEST risk stratification.
| Variables, Units (N) (HF/Non-HF) |
Low-Risk (0–1) |
Medium-Risk (2–3) |
High-Risk (≥4) |
t-Test | OMNIBUS p Value |
p Value for Post Hoc Analysis |
|||
|---|---|---|---|---|---|---|---|---|---|
| Min–Max (N) or n/N (% of Risk Category) |
Min–Max (N) or n/N (% of Risk Category) |
Min–Max (N) or n/N (% of Risk Category) |
|||||||
| HF | Non-HF | HF | Non-HF | HF | Non-HF | HF | Non-HF | ||
| Patient-Reported Symptoms | |||||||||
|
Cough (255/1929) |
455/1417 (31.11%) |
13/53 (24.53%) |
111/439 (25.28%) |
54/202 (26.73%) |
15/72 (20.83%) |
0.8814 c | 0.0053 |
0.0238 a 0.181 b 1.0 c |
|
|
Dyspnea (255/1929) |
569/1417 (40.16%) |
32/53 (60.38%) |
174/439 (39.64%) |
112/202 (55.45%) |
34/72 (47.22%) |
0.6249 c | 0.4661 | N/A | |
|
Chest pain (255/1929) |
102/1417 (7.2%) |
8/53 (15.09%) |
26/439 (5.92%) |
21/202 (10.4%) |
6/72 (8.33%) |
0.4741 c | 0.5872 | N/A | |
|
Hemoptysis (255/1929) |
9/1417 (0.64%) |
0/53 (0%) |
2/439 (0.46%) |
4/202 (1.98%) |
0/72 (0%) |
0.5831 c | 1.0 | N/A | |
|
Smell dysfunction (255/1929) |
61/1417 (4.3%) |
1/53 (1.89%) |
9/439 (2.05%) |
3/202 (1.49%) |
2/72 (2.78%) |
1.0 c | 0.0731 | N/A | |
|
Taste dysfunction (255/1929) |
49/1417 (3.46%) |
2/53 (3.77%) |
8/439 (1.82%) |
3/202 (1.49%) |
4/72 (5.56%) |
0.2782 c | 0.0851 | N/A | |
|
Abdominal pain (255/1929) |
103/1417 (7.27%) |
2/53 (3.77%) |
24/439 (5.47%) |
14/202 (6.93%) |
3/72 (4.17%) |
0.5358 c | 0.3319 | N/A | |
|
Diarrhea (255/1929) |
75/1417 (5.3%) |
2/53 (3.77%) |
31/439 (7.06%) |
14/202 (6.93%) |
5/72 (6.94%) |
0.5358 c | 0.325 | N/A | |
|
Nausea/Vomiting (255/1929) |
57/1417 (4.02%) |
0/53 (0%) |
27/439 (6.15%) |
11/202 (5.45%) |
3/72 (4.17%) |
0.127 c | 0.1724 | N/A | |
| Measured Vital Signs | |||||||||
|
Body temperature °C (139/1046) |
37.07 ± 0.88 34.4–40.5 (809) |
37.07 ± 1.19 35.2–40.0 (26) |
36.91 ± 0.87 35.0–40.0 (209) |
36.9 ± 0.81 35.2–40.0 (113) |
37.1 ± 1.02 36.0–40.0 (28) |
0.4907 c | 0.0797 | N/A | |
|
Heart rate beats/minute (228/1444) |
86.41 ± 15.63 48–160 (1045) |
84.96 ± 17.79 54–120 (47) |
84.01 ± 16.31 50–160 (340) |
84.67 ± 19.71 36–170 (181) |
85.03 ± 15.78 54–140 (59) |
0.923 c | 0.1793 | N/A | |
|
Respiratory rate breaths/minute (48/270) |
18.35 ± 5.78 12–50 (204) |
18.0 ± 4.33 14–28 (12) |
18.8 ± 5.68 12–45 (56) |
19.92 ± 6.42 12–50 (36) |
17.1 ± 4.23 12–24 (10) |
0.2538 c | 0.5575 | N/A | |
|
Systolic blood pressure (231/1438) |
130.72 ± 21.28 60–240 (1040) |
126.85 ± 25.82 80–200 (46) |
135.24 ± 25.16 50–270 (339) |
134.71 ± 25.6 70-205 (185) |
133.85 ± 21.83 86-210 (59) |
0.0685 c | 0.0102 |
0.008 a 0.534 b 0.898 c |
|
| Diastolic blood pressure (231/1430) | 78.55 ± 12.68 40-150 (1037) |
77.65 ± 14.27 50-110 (46) |
78.11 ± 13.6 40-157 (334) |
74.75 ± 15.24 40-120 (185) |
78.98 ± 15.16 51-143 (59) |
0.2267 c | 0.8443 | N/A | |
|
SpO2 in room air, % (FiO2 = 21%) (161/1101) |
92.84 ± 7.13 48-100 (814) |
89.08 ± 11.85 50-99 (37) |
89.79 ± 9.29 50-100 (244) |
90.1 ± 9.39 50-99 (124) |
90.4 ± 5.48 74-98 (43) |
0.6345 c | <0.0001 |
<0.0001 a 0.019 b 0.824 c |
|
| Abnormalities Detected during Physical Examination | |||||||||
|
Crackles (255/1929) |
154/1417 (10.87%) |
13/53 (24.53%) |
86/439 (19.59%) |
56/202 (27.72%) |
10/72 (13.89%) |
0.7701 c | <0.0001 |
<0.0001 a 1.0 b 0.9737 c |
|
|
Wheezing (255/1929) |
94/1417 (6.62%) |
7/53 (13.21%) |
49/439 (11.16%) |
52/202 (25.74%) |
17/72 (23.61%) |
0.0813 c | <0.0001 |
0.0079 a
<0.0001 b 0.019 c |
|
|
Pulmonary congestion (255/1929) |
184/1417 (12.99%) |
19/53 (35.85%) |
86/439 (19.59%) |
66/202 (32.67%) |
12/72 (16.67%) |
0.785 c | 0.0025 |
0.0024 a 1.0 b,c |
|
|
Peripheral oedema (255/1929) |
76/1417 (5.36%) |
14/53 (26.42%) |
46/439 (10.48%) |
44/202 (21.78%) |
9/72 (12.5%) |
0.5947 c | 0.0002 |
0.0011 a 0.0551 b 1.0 c |
|
Continuous variables are presented as mean ± SD, range (minimum–maximum), and number of non-missing values. Categorized variables are presented as a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: SD—standard deviation, CAD—coronary artery disease, OMNIBUS—analysis of variance, N—valid measurements, n—number of patients with parameter above cut-off point, SBP-systolic blood pressure, DBP—diastolic blood pressure, a—low risk vs. medium risk, b—low risk vs. high risk, c—medium risk vs. high risk. Bold text—statistically significant values.
The detailed characteristics of the laboratory parameters measured during admission and discharge from the hospital in both study cohorts are pooled in Supplementary Table S2. At the time of admission, the decrease in hemoglobin levels correlated with the increase of risk in C2HEST score in both study arms. Interestingly, there were no significant differences between risk groups in HF and non-HF arms in terms of arterial blood gases (ABG) and acid–base balance parameters nor in the admission level of inflammatory markers (leucocytes, CRP, procalcitonin, IL-6). At admission, the non-HF high-risk stratum was characterized by more pronounced laboratory features of renal failure (higher level of creatinine and urea, along with lower eGFR). Additionally, in the non-HF cohort, admission levels of cardiac injury markers (TnI and NT-proBNP) rose together with the level of risk in the C2HEST score.
3.2. Treatment Applied during Hospitalization
All differences in applied therapy during the hospitalization period between the C2HEST group among both study cohorts are pooled in Supplementary Table S3. Subjects in non-HF cohorts in the higher C2HEST stratum were prone to receiving antimicrobial treatment. On the other hand, convalescent plasma was less frequently used in this subpopulation of patients. No statistically significant differences in applied therapy between all risk strata were observed in the HF cohort.
No significant differences in respiratory support were observed in the HF cohort. In the non-HF cohort, the assignment to a specific C2HEST stratum score correlated with the advancement of respiratory support applied during the hospitalization C2HEST. Along with increasing C2HEST score value, prevalence of noninvasive ventilation increased, whereas the oxygenation parameters from the period of qualification for advanced respiratory support decreased. On the other hand, the frequency of implementation of invasive ventilation decreased in parallel with the rise in C2HEST score value. No differences in either study cohort were shown regarding the need for urgent coronary revascularization procedures during the index hospitalization, need for catecholamine administration, nor for use of hemodialysis (Table 3).
Table 3.
Applied treatment and procedures.
| Variables, Units (N) (HF/Non-HF) |
Low-Risk (0–1) n/N |
Medium-Risk (2–3) n/N |
High-Risk (≥4) n/N |
t-Test | OMNIBUS p Value |
p Value for Post Hoc Analysis |
|||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
|||||||
| HF | Non-HF | HF | Non-HF | HF | Non-HF | HF | Non-HF | ||
| Applied Treatment and Procedures | |||||||||
|
The most advanced respiratory support applied during the hospitalization no oxygen (255/1928) |
0.4126 c | <0.0001 |
0.0007 a 0.0012 b 0.2081 c |
||||||
| 741/1417 | 19/53 | 183/439 | 61/202 | 28/72 | |||||
| (52.37%) | (35.85%) | (41.78%) | (30.2%) | (38.89%) | |||||
|
low flow oxygen support (255/1928) |
|||||||||
| 451/1417 | 19/53 | 169/439 | 90/202 | 34/72 | |||||
| (31.87%) | (35.85%) | (38.58%) | (44.55%) | (47.22%) | |||||
|
high flow nasal cannula noninvasive ventilation (255/1928) |
|||||||||
| 65/1417 | 3/53 | 36/439 | 21/202 | 6/72 | |||||
| (4.59%) | (5.66%) | (8.22%) | (10.4%) | (8.33%) | |||||
|
invasive ventilation (255/1928) |
|||||||||
| 141/1417 | 8/53 | 41/439 | 21/202 | 1/72 | |||||
| (9.96%) | (15.09%) | (9.36%) | (10.4%) | (1.39%) | |||||
|
Oxygenation parameters from the period of qualification for advanced respiratory support: SpO2, % (87/544) |
90.63 ± 7.88 50–100 (410) |
88.33 ± 8.67 72–98 (12) |
86.31 ± 9.83 55–99 (121) |
84.63 ± 10.31 59–99 (75) |
91.08 ± 5.01 81–98 (13) |
0.2002 c | 0.0004 |
<0.0001 a 0.948 b 0.022 c |
|
|
Therapy with catecholamines (255/1928) |
131/1417 (9.24%) |
7/53 (13.21%) |
38/439 (8.66%) |
38/202 (18.81%) |
4/72 (5.56%) |
0.4532 c | 0.5456 | N/A | |
|
Coronary revascularization or/and an indication for coronary revascularization (255/1928) |
10/1417 (0.71%) |
4/53 (7.55%) |
8/439 (1.82%) |
8/202 (3.96%) |
0/72 (0%) |
0.2796 c | 0.0927 | N/A | |
|
Hemodialysis (255/1928) |
46/1417 (3.25%) |
5/53 (9.43%) |
8/439 (1.82%) |
12/202 (5.94%) |
0/72 (0%) |
0.3601 c | 0.1196 | N/A | |
Continuous variables are presented as: mean ± SD, range (minimum–maximum), and number of non-missing values. Categorized variables are presented as: a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: CAD—coronary artery disease, OMNIBUS—analysis of variance, N–valid measurements, n—number of patients with parameter above cut-off point, SD—standard deviation, N/A—not applicable, a—low risk vs. medium risk, b—low risk vs. high risk, c—medium risk vs. high risk. Bold text–statistically significant values.
3.3. Association C2HEST Score with Results and Mortality
The in-hospital—and then the 3-month and 6-month—mortality rates were the highest in the high-risk HF cohort C2HEST stratum, reaching 65.36%, 53.96%, and 38.61%, respectively. Interestingly, in this study cohort, significant differences in mortality rates between the medium-risk stratum and high-risk stratum were observed only for in-hospital mortality. Regarding the post-discharge period, no similar relationship was observed. All data regarding short and long-term mortality were pooled in Table 4. In the non-HF cohort, in-hospital, 3-month, and 6-month mortality were also highest in the high-risk C2HEST stratum and came to 26.39%, 52.78%, and 65.0%, respectively. In this study, arm differences between low-risk vs. high-risk and low-risk vs. medium-risk C2HEST groups were statistically significant.
Table 4.
Total and in-hospital all-cause mortality in the C2HEST risk strata in diabetes and non-diabetes cohorts.
| Variables, Units (N) (HF/Non-HF) |
Low-Risk (0–1) |
Medium-Risk (2–3) |
High-Risk (≥4) |
t-Test | OMNIBUS p Value |
p Value for Post Hoc Analysis |
|||
|---|---|---|---|---|---|---|---|---|---|
| n/N (% of Risk Category) |
n/N (% of Risk Category) |
n/N (% of Risk Category) |
|||||||
| HF | Non-HF | HF | Non-HF | HF | Non-HF | HF | Non-HF | ||
| All-Cause Mortality Rate | |||||||||
|
In-hospital mortality (255/1928) |
119/1417 (8.4%) |
11/53 (20.75%) |
99/439 (22.55%) |
78/202 (38.61%) |
19/72 (26.39%) |
0.0235 c | <0.0001 |
<0.0001 a,b 1.0 c |
|
|
3-month mortality (255/1928) |
202/1417 (14.26%) |
23/53 (43.4%) |
175/439 (39.86%) |
109/202 (53.96%) |
38/72 (52.78%) |
0.2242 c | <0.0001 |
<0.0001 a,b 0.1604 c |
|
|
6-month mortality (220/1270) |
214/867 (24.68%) |
24/41 (58.54%) |
184/343 (53.64%) |
117/179 (65.36%) |
39/60 (65.0%) |
0.5212 c | <0.0001 |
<0.0001 a,b 0.4074 c |
|
Continuous variables are presented as mean ± SD, range (minimum–maximum), and number of non-missing values. Categorized variables are presented as a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: CAD—coronary artery disease, OMNIBUS—analysis of variance, N—valid measurements, n—number of patients with parameter above cut-off point, a—low risk vs. medium risk, b—low risk vs. high risk, c—medium risk vs. high risk; Bold text—statistically significant values.
3.4. The All-Cause Mortality Discriminatory Performance of the C2HEST Score
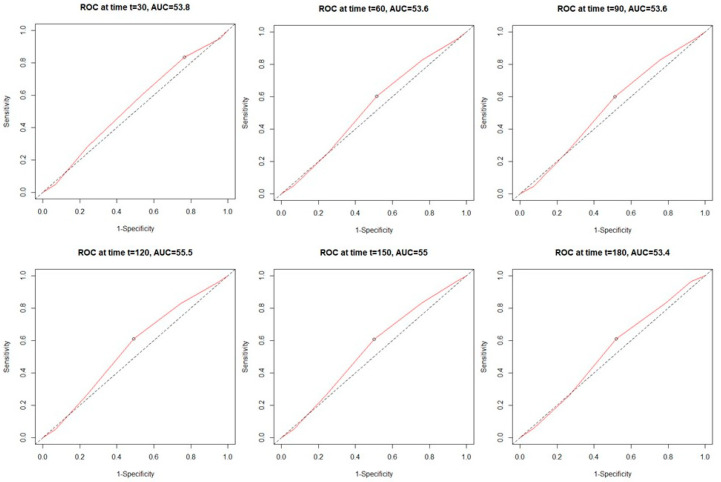
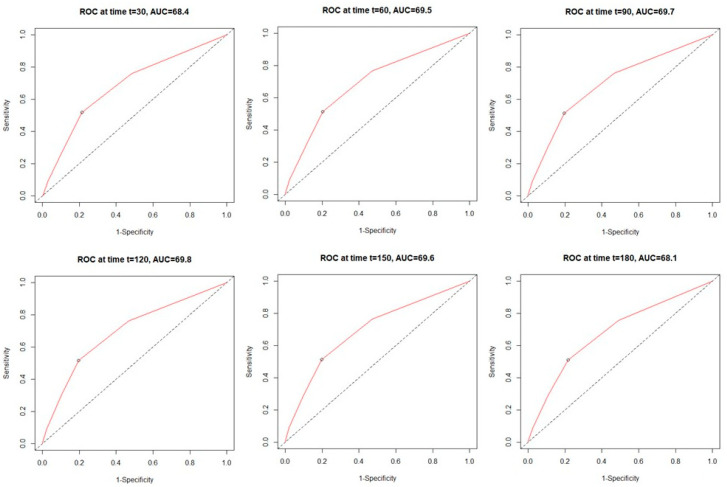
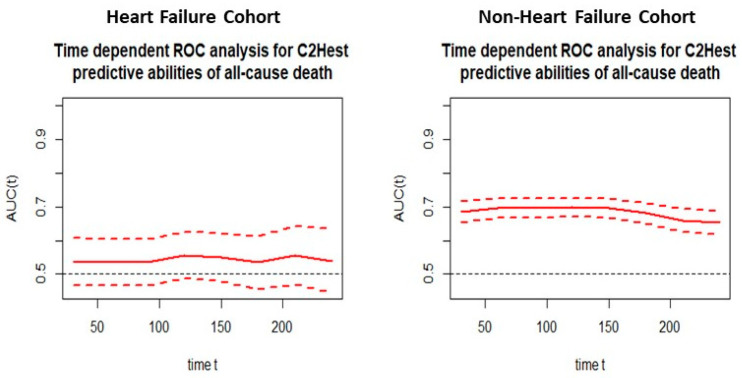
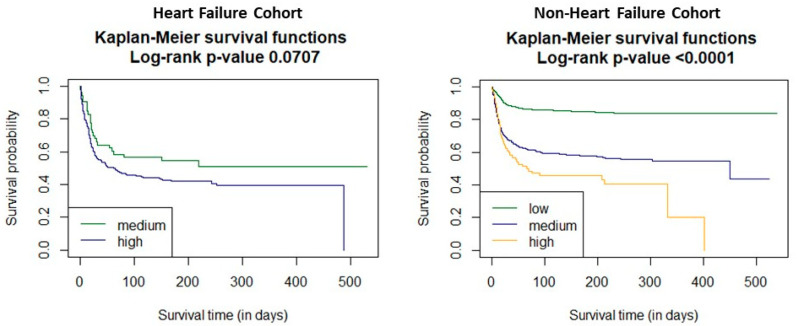
Analysis of the time-dependent receiver operating characteristic (ROC) in both study cohorts revealed higher sensitivity of the C2HEST scale in the non-HF cohort compared to HF subjects. (Figure 1 and Figure 2). C2HEST predicting AUC in the HF cohort failed to predict all-cause mortality [16]. In the non-HF cohort, the AUC value was significantly higher. Analysis of the ACU values of the HF cohort vs. the non-HF cohort in different periods revealed that at 1-month, AUC = 53.8 vs. 68.4%; 3-month AUC = 53.6 vs. 69.7%; 6-month AUC = 53.4 vs. 68.1%. The “all-cause death” time-dependent AUC for the C2HEST score was presented in Figure 3. Additionally, Kaplan–Meier survival curves for the C2HEST strata were presented in Figure 4. The p value for the log-rank test was <0.0001. We have observed differences in estimated survival probability in both study cohorts. Practically, starting from admission time, subjects in the HF cohorts were less likely to survive COVID-19 compared to the non-HF cohort.
Figure 1.
The time-dependent receiver operating characteristic (ROC) for all-cause mortality in the heart failure cohort.
Figure 2.
The time-dependent receiver operating characteristic (ROC) for all-cause mortality in the non-heart-failure cohort.
Figure 3.
Time-dependent ROC analysis for the C2HEST predictive abilities of all-cause death in both study cohorts.
Figure 4.
The survival curves for the C2HEST strata in both study cohorts, estimated by Kaplan–Meier function.
We performed Cox model analysis regarding the predictive value of C2HEST score in terms of mortality in both study cohorts. In the overall model with uncategorized value of C2HEST, additional points in the C2HEST score were related to an increase of the total death intensity in 10% of HF subjects, while in the non-HF subpopulation, that value was 62.3% (Table 5 and Table 6). In the categorized model, the change from the medium to the high category in the heart failure cohort increased death expectation by 46.5%. On the other hand, transfer from the low-risk to the medium-risk stratum increased the all-cause death intensity by three times, while the shift from low-risk to high-risk increased the probability of death by almost five times (Table 5 and Table 6).
Table 5.
The total all-cause death hazard ratios for C2HEST risk stratification in the heart failure cohort.
| Total Death | |||
|---|---|---|---|
| Overall | HR | 95%CI | p Value |
| 1.100 | 0.968–1.250 | 0.143 | |
| Risk Strata | |||
| Medium-Risk vs. High-Risk | 1.465 | 0.951–2.259 | 0.085 |
Table 6.
The total all-cause-death Hazard Ratios for C2HEST risk stratification in non-HF cohort.
| Total Death | |||
|---|---|---|---|
| Overall | HR | 95%CI | p Value |
| 1.623 | 1.518–1.734 | <0.0001 | |
| Risk Strata | |||
| Low-Risk vs. Medium-Risk | 3.414 | 2.811–4.148 | <0.0001 |
| Low-Risk vs. High-Risk | 4.953 | 3.570–6.873 | <0.0001 |
The associations of individual C2HEST score components with mortality in both study cohorts are presented in Table 7 and Table 8. The highest prognostic value for all-cause death in both study groups was noticed for age (1.4743 in HF subjects vs. 3.211 in men). Interestingly, among all individual C2HEST score components in the HF cohort, only age over 75 and thyroid diseases were associated with a significant change in HR for death. However, in the non-HF cohort, CAD, COPD, age over 75, and hypertension increased the HR for death.
Table 7.
Associations of individual C2HEST score components with mortality in the HF cohort.
| Component | HR | CI Min. | CI Max. | p Value | |
|---|---|---|---|---|---|
| All-cause mortality | Coronary artery disease | 1.1759 | 0.8376 | 1.6509 | 0.3492 |
| COPD | 1.3432 | 0.8127 | 2.2201 | 0.2498 | |
| Age > 75 | 1.4743 | 1.0561 | 2.0581 | 0.0226 | |
| Thyroid disease | 0.5794 | 0.3476 | 0.9658 | 0.0363 | |
| Hypertension | 0.9133 | 0.5895 | 1.4151 | 0.6849 | |
| HFrEF | NA | NA | NA | NA |
Abbreviations: COPD—chronic obstructive pulmonary disease, HFrEF—heart failure with reduce ejection fraction.
Table 8.
Associations of individual C2HEST score components with mortality in non-HF cohort.
| Component | HR | CI Min. | CI Max. | p Value | |
|---|---|---|---|---|---|
| All-cause mortality | Coronary artery disease |
1.8775 | 1.4009 | 2.5162 | <0.0001 |
| COPD | 1.6793 | 1.0969 | 2.5707 | 0.017 | |
| Age > 75 | 3.2112 | 2.6317 | 3.9183 | <0.0001 | |
| Thyroid disease | 0.8555 | 0.6201 | 1.1804 | 0.3421 | |
| Hypertension | 1.3936 | 1.1383 | 1.7062 | 0.0013 | |
| HFrEF | NA | NA | NA | NA |
Abbreviations: COPD—chronic obstructive pulmonary disease, HFrEF—heart failure with reduce ejection fraction.
In order to assess whether the original cut-off values of C2HEST score risk (the low/medium/high-risk categories for 0–1/2–3/ ≥ 4 points, respectively) are the best possible stratification system for both study cohorts, we evaluated the difference in Kaplan–Meier survival curves, and all the possible C2HEST intervals were analyzed. Additionally, the log-rank statistics was calculated (Supplementary Tables S4 and S5). In terms of the HF cohort categories, the values of 0–3/4–6/7–8 points (the low/medium/high-risk categories, respectively) were revealed to be characterized by better separation than was generally accepted. Meanwhile, for non-HF cohort values, 0/1/2–8 points (the low/medium/high-risk categories, respectively) showed the highest separation accuracy.
3.5. Association of C2HEST Score with Non-Fatal Outcomes
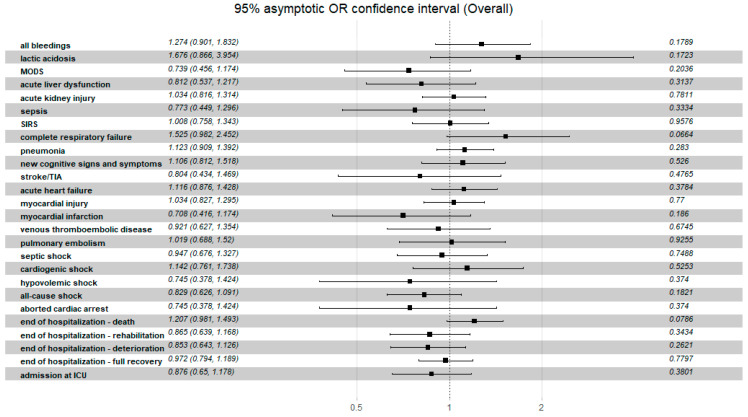
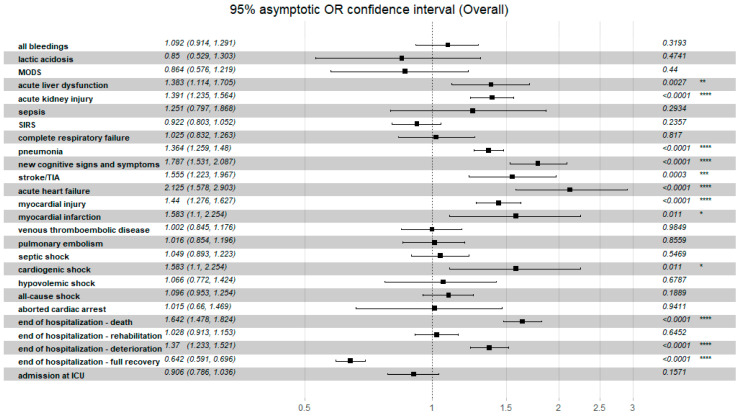
All the data regarding the relationship of clinical non-fatal events and the C2HEST risk strata in both study cohorts are presented in the Table 9 and Figure 5 and Figure 6. In the heart failure cohort, none of the study’s secondary endpoints showed significant differences in prevalence among the original C2HEST score risk strata. In the non-HF cohort assignment to C2HEST, higher risk strata were correlated with a higher probability of clinical deterioration (transfer to another hospital) and lower probability for full recovery (discharge to home). Additionally, subjects with higher C2HEST score values were more prone to experience some cardiovascular complications (including myocardial injury, acute heart failure episode, stroke/TIA) during a hospitalization period. Moreover, high C2HEST scores in the non-HF subpopulation were associated with a higher probability of pneumonia, sepsis, and acute kidney injury.
Table 9.
Clinical non-fatal events and hospitalization outcomes in the C2HEST risk strata in heart failure and non-failure cohorts.
| Variables, Units (N) (HF/Non-HF) |
Low-Risk (0–1) |
Medium-Risk (2–3) |
High-Risk (≥4) |
t-Test | OMNIBUS p Value |
p Value for Post Hoc Analysis |
|||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
Mean ± SD Min–Max (N) or n/N (% of Risk Category) |
|||||||
| HF | Non-HF | HF | Non-HF | HF | Non-HF | HF | Non-HF | ||
| Hospitalization | |||||||||
|
Duration of hospitalization, days (255/1928) |
11.48 ± 13.66 1–131 (1417) |
13.34 ± 9.8 1–39 (53) |
13.16 ± 14.0 1–124 (439) |
16.02 ± 14.62 1–87 (202) |
16.25 ± 19.08 1–121 (72) |
0.1162 c | 0.0154 | 0.072 a 0.098 b 0.389 c |
|
|
Admission at ICU (255/1928) |
150/1417 (10.59%) |
7/53 (13.21%) |
31/439 (7.06%) |
25/202 (12.38%) |
8/72 (2.78%) |
1.0 c | 0.0125 | 0.1118 a 0.1589 b 0.7983 c |
|
|
End of hospitalization death (255/1928) |
119/1417 (8.4%) |
11/53 (20.75%) |
99/439 (22.55%) |
78/202 (38.61%) |
19/72 (26.39%) |
0.0606 c | <0.0001 |
<0.0001 a,b 0.9339 c |
|
|
discharge to home–
full recovery |
993/1417 (70.08%) |
23/53 (43.4%) |
197/439 (44.87%) |
75/202 (37.13%) |
20/72 (38.89%) |
||||
| transfer to another hospital—worsening | 139/1417 (9.81%) |
12/53 (22.64%) |
85/439 (19.36%) | 25/202 (12.38%) |
19/72 (26.39%) |
||||
| transfer to another hospital—in recovery | 166/1417 (11.71%) |
7/53 (13.21%) |
58/439 (13.21%) |
24/202 (11.88%) |
6/72 (8.33%) |
||||
|
Aborted cardiac arrest (255/1928) |
15/1417 (1.06%) |
1/53 (1.89%) |
2/439 (0.46%) |
5/202 (2.48%) |
1/72 (1.59%) |
1.0 c | 0.3613 | N/A | |
|
Shock (255/1928) |
108/1417 (7.62%) |
8/53 (15.09%) |
39/439 (8.66%) |
30/202 (14.85%) |
3/72 (4.17%) |
1.0 c | 0.3999 | N/A | |
|
Hypovolemic shock (255/1928) |
22/1417 (1.55%) |
1/53 (1.89%) |
6/439 (1.37%) |
5/202 (2.48%) |
1/72 (1.39%) |
1.0 c | 1.0 | N/A | |
|
Cardiogenic shock (255/1928) |
7/1417 (0.49%) |
3/53 (5.66%) |
8/439 (1.82%) |
13/202 (6.44%) |
1/72 (1.39%) |
1.0 c | 0.0196 |
0.036 a 0.9839 b 1.0 c |
|
|
Septic shock (255/1928) |
88/1417 (6.21%) |
4/53 (7.55%) |
26/439 (5.92%) |
20/202 (9.9%) |
2/72 (2.78%) |
0.793 c | 0.576 | N/A | |
|
Venous thromboembolic disease (255/1928) |
83/1417 (5.86%) |
5/53 (9.43%) |
25/439 (5.69%) |
13/202 (6.44%) |
2/72 (2.78%) |
0.5451 c | 0.6649 | N/A | |
|
Pulmonary embolism (255/1928) |
78/1417 (5.86%) |
4/53 (7.55%) |
24/439 (5.69%) |
13/202 (6.44%) |
2/72 (2.78%) |
0.2498 c | 0.98 | N/A | |
|
Myocardial infarction (255/1928) |
8/1417 (0.56%) |
3/53 (5.66%) |
7/439 (0.59%) |
7/202 (3.47%) |
1/72 (1.39%) |
0.4379 c | 0.078 | N/A | |
|
Myocardial injury (185/989) |
113/678 (16.67%) |
15/39 (38.46%) |
83/266 (31.2%) |
70/146 (47.95%) |
17/45 (37.78%) |
0.3816 c | <0.0001 |
<0.0001 a 0.0023 b 1.0 c |
|
|
Acute heart failure (255/1928) |
8/1417 (0.56%) |
11/53 (20.75%) |
11/439 (2.51%) |
42/202 (20.79%) |
4/72 (5.56%) |
1.0 c | <0.0001 |
0.004 a 0.0056 b 0.7389 c |
|
|
Stroke/TIA (255/1928) |
18/1417 (1.27%) |
3/53 (5.66%) |
16/439 (3.64%) |
4/202 (1.98%) |
3/72 (4.17%) |
0.1591 c | 0.0023 |
0.0099 a 0.2315 b 1.0 c |
|
|
New cognitive signs and symptoms (255/1928) |
38/1417 (2.68%) |
7/53 (13.21%) |
44/439 (10.02%) |
22/202 (10.89%) |
10/72 (13.89%) |
0.8183 c | <0.0001 |
<0.0001 a 0.0002 b 0.9175 c |
|
|
Pneumonia (255/1928) |
682/1417 (48.13%) |
35/53 (66.04%) |
270/439 (61.5%) |
141/202 (69.8%) |
45/72 (62.5%) |
0.7184 c | <0.0001 |
<0.0001 a 0.0717 b 1.0 c |
|
|
Complete respiratory failure (60/216) |
57/121 (47.11%) |
5/10 (50.0%) |
41/78 (52.56%) |
36/50 (72.0%) |
7/17 (41.18%) |
0.2632 c | 0.6146 | N/A | |
|
SIRS (255/1860) |
142/1352 (10.5%) | 8/53 (15.09%) |
34/436 (7.8%) |
27/202 (13.43%) |
9/72 (12.5%) |
0.9297 c | 0.1981 | N/A | |
|
Sepsis (118/766) |
9/576 (1.56%) |
3/24 (12.5%) |
4/159 (2.52%) |
7/94 (7.45%) |
0/31 (0%) |
0.4228 c | 0.0077 | 0.3098 a 0.0256 b 0.9164 c |
|
|
Acute kidney injury (255/1928) |
110/1417 (7.76%) |
9/53 (16.98%) |
58/439 (13.21%) |
47/202 (23.27%) |
12/72 (16.67%) |
0.4252 c | 0.0003 |
0.0022 a 0.0409 b 1.0 c |
|
|
Acute liver dysfunction (239/1735) |
30/1256 (2.39%) |
5/50 (10.0%) |
17/415 (4.1%) |
11/189 (5.82%) |
3/64 (4.69%) |
0.338 c | 0.0951 | N/A | |
|
Multiple organ dysfunction syndrome (255/1928) |
21/1417 (1.48%) |
4/53 (7.55%) |
4/439 (0.91%) |
8/202 (3.96%) |
0/72 (0%) |
0.2796 c | 0.5482 | N/A | |
|
Lactic acidosis (55/190) |
9/105 (8.57%) |
0/10 (0%) |
5/69 (7.25%) |
8/45 (17.78%) |
0/16 (0%) |
0.3263 c | 0.7588 | N/A | |
|
Bleedings (255/1928) |
64/1417 (4.52%) |
2/53 (3.77%) |
23/439 (5.24%) |
21/202 (10.4%) |
4/72 (5.56%) |
0.1802 c | 0.6717 | N/A | |
Continuous variables are presented as mean ± SD, range (minimum–maximum), and number of non-missing values. Categorized variables are presented as a number with a percentage. Information about the numbers with valid values is provided in the left column. Abbreviations: CAD—coronary artery disease, OMNIBUS—analysis of variance, N—valid measurements, n—number of patients with parameter above cut-off point, SD—standard deviation, ICU—intensive care unit, TIA —transient ischemic attack, SIRS—systemic inflammatory response syndrome, N/A—not applicable, a—low risk vs. medium risk, b—low risk vs. high risk, c—medium risk vs. high risk. Bold text—statistically significant values.
Figure 5.
The overall odds ratio for the discriminatory performance of the C2HEST score on clinical non-fatal events in the HF cohort. Abbreviations: MODS—multiple organ dysfunction syndrome, TIA—transient ischemic attack, SIRS—systemic inflammatory response syndrome.
Figure 6.
The overall odds ratio for the discriminatory performance of the C2HEST score on clinical non-fatal events in the non-HF cohort. Abbreviations: MODS—multiple organ dysfunction syndrome, TIA—transient ischemic attack, SIRS—systemic inflammatory response syndrome. Significance code: * < 0.05; ** < 0.01; *** < 0.001; **** < 0.0001.
4. Discussion
Patients with cardiovascular disease represent a vulnerable population for worse COVID-19 outcomes [17,18,19]. Considering that chronic heart failure (CHF) in modern societies involves more than 2% of the general population [20] and approximately 10% of the population over 70, a similarly high number is highly susceptible to unfavorable COVID-19 outcomes, including high hospitalization rate, longer duration of in-hospital treatment, ICU admission rate, and higher mortality rate. Even so, in these high-risk subjects, the clinical course of the infection remains dynamic and hardly predictable, particularly at the time of admission. Additionally, overlapping the clinical and radiological presentations provides additional difficulties in the correct triage of patients admitted to the hospital.
Accurately performed risk stratification in individual patients can provide adequate guidance, allowing for reasonable management of limited resources during a COVID-19 pandemic. Unfortunately, a simple, fast, well-validated scoring system dedicated to the HF subpopulation is still missing. Taking into account some encouraging data from our previous analyses concerning other subpopulations of COVID-19 patients [8,9,19], we decided to validate the C2HEST score system in terms of heart failure subjects.
According to the theoretical assumptions, the C2HEST score scale is well correlated with patient comorbidity rate in both study cohorts, but this relationship was more pronounced in the HF group and predicted a wide spectrum of cardiovascular disorders (Table 1). Not surprisingly, a reflection of this finding was the prevalence of specific, targeted pre-hospital treatment (ACEI, ARBN, β-blocker, diuretics, statins) applied in the HF cohort [20]. This relationship was not present in the non-HF cohort. Nevertheless, this therapy was associated with a reduction in mortality and rehospitalization in patients with cardiovascular disease, particularly those with heart failure and coronary artery disease. It is worth noting that in patients with active SARS-CoV-2 infections, some safety concerns are still rising, and an intense debate is ongoing on this matter [21,22,23]. Some recently published large-population studies on COVID-19 [22,24] have proven that the number of cardiovascular comorbidities appears to be independently associated with increased COVID-19-related death. It is worth noting that no relationship between commonly used CVD medications and increased risk of death due to COVID-19 was identified. Furthermore, some data suggest that specific cardiovascular drugs should be continued in order to reduce potential unfavorable cardiovascular events in the course of SARS-CoV-2 infection, particularly in subjects with heart failure.
In contrast to the earlier reports [25] suggesting that increased inflammatory response with subsequent increased production of inflammatory markers in patients with HF could potentially affect the coagulation cascade, induce an endothelial dysfunction, and hemodynamic imbalance [26,27] leading to decompensation of heart failure, thus increasing the rate of an unfavorable outcome [28], in our cohort study, no significant differences were observed regarding the levels of inflammatory markers on admission. A similar interesting observation was made for the non-HF cohort. Surprisingly, in the HF cohort, there were no differences between C2HEST score risk strata in terms of need for use of ventilation support during hospitalization. However, at the same time, in the non-HF cohort, we observed an increase in respiratory support parallel to the coexisting increase in the C2HEST score risk category.
In our study in the HF cohort, the C2HEST score risk failed to show discriminatory performance in terms of mortality (Figure 1 and Figure 3). It is likely that HF by itself is a strong risk factor for poor COVID-19 outcomes when hospitalization was required. The additional discriminants from the C2HEST score scale did not allow for an appropriate selection of patients with higher overall mortality. This is partially confirmed by the high in-hospital mortality in the HF cohort—approximately 38% in high-risk C2HEST score. Those data are additionally visualized in Kaplan–Maier curves for the heart failure cohort (Figure 4). A similar observation was previously made in terms of other COVID-19 strong risk factors (diabetes and coronary artery diseases) [9]. On the other hand, in the non-HF cohort, we observed significantly better performance of C2HEST scores in terms of morality discriminatory ability. Furthermore, in the HF cohort, we observed the inability of C2HEST to predict other non-fatal secondary outcomes during the hospitalization period. Similarly, in the non-HF subjects, C2HEST scores were able to predict in-hospital cardiovascular complications (such as myocardial injury, acute heart failure episode, stroke/TIA) as well as pneumonia, acute kidney injury, and sepsis.
Interestingly, so far, several prognostic scales for COVID-19 have been introduced, including the COVID-Gram Risk Score, the PRIEST score [29], and the Brescia COVID Severity Scale (BRCSS) [3]. Nevertheless, mostly due to their complexity (assessment based on laboratory assays, clinical data, and radiographic imaging), their implementation into everyday clinical practice as a routine triage tool is limited. Therefore, the data obtained in our study suggest that the C2HEST score is a useful triage tool that could be used in the general population and not only in strictly selected high-risk populations.
A growing body of evidence indicates that some diagnostic tools, including the lung ultrasound (LUC) may present some usefulness in diagnosis, optimization of treatment, and risk stratification in COVID-19 patients [30,31,32]. Therefore, a multidimensional assessment of risk factors for an unfavorable outcome of COVID-19, including the data from imaging diagnostics, could constitute an interesting approach. Combining the C2HEST risk score with LUC might be valuable and increase the discriminatory performance of the C2HEST score in predicting outcomes without an unnecessary increase in the complexity of scale. Nevertheless, further studies evaluating the value of such a modified C2HEST score scale are necessary.
5. Limitations
This study contains several limitations, including the study design—particularly its retrospective, single-center, non-randomized character. The homogeneous study population focused on hospitalized subjects without ambulatory subjects. Additionally, all hospitalizations were carried out in extraordinary circumstances—during the global COVID-19 pandemic. Therefore, the clinical outcomes were probably partially affected by limited resources. Additionally, the study protocol did not include a routine in-hospital assessment of the LVEF (mainly due to safety concern), and the TTE was performed only in deteriorating/decompensating subjects when needed. Therefore, the allocation to the HF cohort was made on the basis of past medical history—the diagnosis of HFrEF or HFmrEF was made based on the TTE performed prior to admission to the hospital. Therefore, we decided not to present the data collected during hospitalization based mostly on the deteriorating subjects, as they could not reflect the whole studied cohort.
6. Conclusions
The present study is the first to demonstrate the differences in the predictive value of the C2HEST score between patients with and without heart failure hospitalized due to COVID-19. The C2HEST score risk in the HF cohort failed to show discriminatory performance in terms of mortality and other clinical adverse outcomes during hospitalization. On the other hand, in the non-HF cohort, it revealed significantly better performance in predicting in-hospital and 6-month-mortality as well as other non-fatal clinical outcomes, including not only cardiovascular events (myocardial injury, acute heart failure, myocardial infract, carcinogenic shock) but also pneumonia, sepsis, and acute renal injury. Therefore, C2HEST score, as a relatively simple and easy-to-apply tool, might become a useful tool for risk stratification in the general population, but not in the strictly selected high-risk subpopulation with congestive heart failure.
Acknowledgments
The authors are grateful to all the staff and the patients at the study center who contributed to this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11123495/s1, Table S1: Baseline characteristics of the study cohort-treatment applied before hospitalization; Table S2: Laboratory parameters measured during the hospitalization in the studied cohort. Table S3: Therapies applied during the hospitalization in the studied cohort. Table S4: The Log-rank statistics for matching the C2HEST risk strata for in-hospital mortality in HF cohort. Table S5. The Log-rank statistics for matching the C2HEST risk strata for in-hospital mortality in non-HF cohort.
Author Contributions
Conceptualization, P.R., A.D., M.T., E.A.J. and K.M.; methodology, P.R., A.D., M.T., E.A.J. and K.M.; software, K.G. and K.K. (Krzysztof Kujawa); validation, K.G. and K.K. (Krzysztof Kujawa); formal analysis, P.R., K.G., K.K. (Krzysztof Kujawa), A.D., E.A.J. and K.M.; investigation, P.R., A.D., M.T., J.G., T.M., D.G., M.M., S.Z., T.S., J.D., A.S., A.Z.-Z., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), J.S., S.W., E.A.J. and K.M.; resources, P.R., A.D., M.T., J.G., T.M., D.G., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), M.M., S.Z., T.S., J.D., A.S., A.Z.-Z., J.S., S.W., E.A.J. and K.M.; data curation, P.R., A.D., M.T., J.G., T.M., D.G., B.A., K.K. (Krzysztof Kaliszewski), K.K.-P., A.M.-W., M.P. (Michał Pomorski), M.P. (Marcin Protasiewicz), M.M., S.Z., T.S., J.D., A.S., A.Z.-Z., J.S., S.W., E.A.J. and K.M.; writing—original draft preparation, P.R., A.D., M.T., E.A.J. and K.M.; writing—review and editing, P.R., A.D., E.A.J. and K.M.; visualization, K.G., K.K. (Krzysztof Kaliszewski), P.R., A.D., E.A.J. and K.M.; supervision, P.R., A.D., E.A.J. and K.M.; project administration, P.R., A.D., M.T., E.A.J. and K.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of Wroclaw Medical University, Wroclaw, Poland (Signature number: KB-444/2021).
Informed Consent Statement
The routine data were collected retrospectively; therefore, written informed consent to participate in the study was not required. The Bioethics Committee approved the publication of anonymized data.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez-Abejón E., Herrera-Gómez F., Martín-García D., Tamayo E., Álvarez F.J. A Population-Based Registry Analysis on Hospitalized COVID-19 Patients with Previous Cardiovascular Disease: Clinical Profile, Treatment, and Predictors of Death. J. Cardiovasc. Dev. Dis. 2021;8:167. doi: 10.3390/jcdd8120167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duca A., Piva S., Foca E., Latronico N., Rizzi M. Calculated Decisions: Brescia-COVID Respiratory Severity Scale (BCRSS)/Algorithm. Emerg. Med. Pract. 2020;22:CD1–CD2. [PubMed] [Google Scholar]
- 4.Bader F., Manla Y., Atallah B., Starling R.C. Heart failure and COVID-19. Heart Fail. Rev. 2020;26:1–10. doi: 10.1007/s10741-020-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali M.A., Spinler S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021;31:143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y.G., Pastori D., Farcomeni A., Yang P.S., Jang E., Joung B., Wang Y.T., Guo Y.T., Lip G. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in Asian subjects: Derivation in 471,446 Chinese subjects, with internal validation and external application in 451,199 Korean subjects. Chest. 2019;155:510–518. doi: 10.1016/j.chest.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W., Wu Y., Xue R., Wu Z., Wu D., He J., Dong Y., Lip G.Y.H., Zhu W., Liu C. C2HEST score predicts clinical outcomes in heart failure with preserved ejection fraction: A secondary analysis of the TOPCAT trial. BMC Med. 2021;19:44. doi: 10.1186/s12916-021-01921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rola P., Doroszko A., Trocha M., Giniewicz K., Kujawa K., Skarupski M., Gawryś J., Matys T., Szahidewicz-Krupska E., Gajecki D., et al. Mortality Predictive Value of the C2HEST Score in Elderly Subjects with COVID-19—A Subanalysis of the COLOS Study. J. Clin. Med. 2022;11:992. doi: 10.3390/jcm11040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajecki D., Doroszko A., Trocha M., Giniewicz K., Kujawa K., Skarupski M., Gawryś J., Matys T., Szahidewicz-Krupska E., Rola P., et al. Usefulness of the C2HEST Score in Predicting the Clinical Outcomes of COVID-19 in Diabetic and Non-Diabetic Cohorts. J. Clin. Med. 2022;11:873. doi: 10.3390/jcm11030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rola P., Doroszko A., Trocha M., Giniewicz K., Kujawa K., Skarupski M., Gajecki D., Gawryś J., Matys T., Szahidewicz-Krupska E., et al. Sex-Dependent Differences in Predictive Value of the C2HEST Score in Subjects with COVID-19—A Secondary Analysis of the COLOS Study. Viruses. 2022;14:628. doi: 10.3390/v14030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 12.Therneau T. A Package for Survival Analysis in R. R Package Version 3.2-7. [(accessed on 14 June 2022)]. Available online: https://CRAN.R-project.org/package=survival.
- 13.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schratz P. R Package ‘oddsratio’: Odds Ratio Calculation for GAM(M)s & GLM(M)s, Version: 1.0.2. [(accessed on 14 June 2022)]. Available online: https://cran.r-project.org/web/packages/oddsratio/oddsratio.pdf.
- 15.Hothorn T., Hornik K., Van De Wiel M.A., Zeileis A. A lego system for conditional inference. Am. Stat. 2006;60:257–263. doi: 10.1198/000313006X118430. [DOI] [Google Scholar]
- 16.Li F., He H. Assessing the accuracy of diagnostic tests. Shanghai Arch. Psychiatry. 2018;30:207. doi: 10.11919/j.issn.1002-0829.218052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castagna F., Kataria R., Madan S., Ali S., Diab K., Leyton C., Arfaras-Melainis A., Kim P., Giorgi F., Vukelic S., et al. A History of Heart Failure Is an Independent Risk Factor for Death in Patients Admitted with Coronavirus 19 Disease. J. Cardiovasc. Dev. Dis. 2021;8:77. doi: 10.3390/jcdd8070077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villalba G.C., Amat-Santos I.J., Dueñas C., Otero D.L., Catala P., Aparisi A., López-Pais J., Antonio C.E.C., Candela J., Muiños P.A., et al. Impact of the presence of heart disease, cardiovascular medications and cardiac events on outcome in COVID-19. Cardiol. J. 2021;28:360–368. doi: 10.5603/CJ.a2021.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokolski M., Reszka K., Suchocki T., Adamik B., Doroszko A., Drobnik J., Gorka-Dynysiewicz J., Jedrzejczyk M., Kaliszewski K., Kilis-Pstrusinska K., et al. History of Heart Failure in Patients Hospitalized Due to COVID-19: Relevant Factor of In-Hospital Complications and All-Cause Mortality up to Six Months. J. Clin. Med. 2022;11:241. doi: 10.3390/jcm11010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziaeian B., Fonarow B.Z.G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labandeira-Garcia J.L., Labandeira C.M., Valenzuela R., Pedrosa M.A., Quijano A., Rodriguez-Perez A.I. Drugs Modulating Renin-Angiotensin System in COVID-19 Treatment. Biomedicines. 2022;10:502. doi: 10.3390/biomedicines10020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polverino F., Stern D.A., Ruocco G., Balestro E., Bassetti M., Candelli M., Cirillo B., Contoli M., Corsico A., D’Amico F., et al. Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO) Front. Cardiovasc. Med. 2020;7:585866. doi: 10.3389/fcvm.2020.585866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleksova A., Gagno G., Sinagra G., Beltrami A., Janjusevic M., Ippolito G., Zumla A., Fluca A., Ferro F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021;22:4526. doi: 10.3390/ijms22094526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey R., Rai D., Tahir M., Wahab A., Bandyopadhyay D., Lesho E., Laguio-Vila M., Fentanes E., Tariq R., Naidu S., et al. Prevalence of comorbidities and symptoms stratified by severity of illness amongst adult patients with COVID-19: A systematic review. Arch. Med Sci. Atheroscler. Dis. 2022;7:5–23. doi: 10.5114/amsad.2022.115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sisti N., Valente S., Mandoli G.E., Santoro C., Sciaccaluga C., Franchi F., Cameli P., Mondillo S., Cameli M. COVID-19 in patients with heart failure: The new and the old epidemic. Postgrad. Med. J. 2020;97:175–179. doi: 10.1136/postgradmedj-2020-138080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerotziafas G.T., Catalano M., Colgan M.-P., Pecsvarady Z., Wautrecht J.C., Fazeli B., Olinic D.-M., Farkas K., Elalamy I., Falanga A., et al. Guidance for the Management of Patients with Vascular Disease or Cardiovascular Risk Factors and COVID-19: Position Paper from VAS-European Independent Foundation in Angiology/Vascular Medicine. Thromb. Haemost. 2020;120:1597–1628. doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanek A., Fazeli B., Bartuś S., Sutkowska E. The Role of Endothelium in Physiological and Pathological States: New Data. BioMed Res. Int. 2018;2018:1098039. doi: 10.1155/2018/1098039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biegus J., Niewinski P., Josiak K., Kulej K., Ponikowska B., Nowak K., Zymlinski R., Ponikowski P. Pathophysiology of Advanced Heart Failure. Hear. Fail. Clin. 2021;17:519–531. doi: 10.1016/j.hfc.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Goodacre S., Thomas B., Sutton L., Burnsall M., Lee E., Bradburn M., Loban A., Waterhouse S., Simmonds R., Biggs K., et al. Derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19: The PRIEST observational cohort study. PLoS ONE. 2021;16:e0245840. doi: 10.1371/journal.pone.0245840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espersen C., Platz E., Alhakak A.S., Sengeløv M., Simonsen J., Johansen N.D., Davidovski F.S., Christensen J., Bundgaard H., Hassager C., et al. Lung ultrasound findings following COVID-19 hospitalization: A prospective longitudinal cohort study. Respir. Med. 2022;197:106826. doi: 10.1016/j.rmed.2022.106826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adatto N.L., Preisler Y., Shetrit A., Shepshelovich D., Hershkoviz R., Isakov O. Rapid 8-Zone Lung Ultrasound Protocol is Comparable to a Full 12-Zone Protocol for Outcome Prediction in Hospitalized COVID -19 Patients. J. Ultrasound Med. 2021;41:15849. doi: 10.1002/jum.15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tana C., Ricci F., Coppola M.G., Mantini C., Lauretani F., Campanozzi D., Renda G., Gallina S., Lugará M., Cipollone F., et al. Prognostic Significance of Chest Imaging by LUS and CT in COVID-19 Inpatients: The ECOVID Multicenter Study. Respiration. 2021;101:122–131. doi: 10.1159/000518516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.