Abstract
In recent decades, many new and exciting findings have paved the way to the better understanding of plant responses in various environmental changes. Some major areas are focused on role of phytohormone during abiotic stresses. Salicylic acid (SA) is one such plant hormone that has been implicated in processes not limited to plant growth, development, and responses to environmental stress. This review summarizes the various roles and functions of SA in mitigating abiotic stresses to plants, including heating, chilling, salinity, metal toxicity, drought, ultraviolet radiation, etc. Consistent with its critical roles in plant abiotic tolerance, this review identifies the gaps in the literature with regard to the complex signalling network between SA and reactive oxygen species, ABA, Ca2+, and nitric oxide. Furthermore, the molecular mechanisms underlying signalling networks that control development and stress responses in plants and underscore prospects for future research on SA concerning abiotic-stressed plants are also discussed.
Keywords: abiotic stress, reactive oxygen species, salicylic acid, signalling
1. Introduction
Salicylic acid (SA) is a phytohormone that plays multifaceted signalling roles in mediating plant growth, development, and defences to environmental stresses [1,2]. It is a simple beta-hydroxy phenolic acid that was firstly isolated from willow, and its name was derived from the Latin word “salix”. The amount of SA across different plant species ranges from 0.1 to 10 µg g−1 fresh weight, and most SA is stored in methylated and/or glucosylated forms [3]. In plants, SA can be synthesized via two distinct enzymatic pathways: the phenylalanine ammonia-lyase (PAL) and the isochorismate synthases (ICS) pathway, which both require the primary metabolite chorismate [4]. The PAL pathway mainly takes place in the cytoplasm. Firstly, the phenylalanine is converted into cinnamic acid by PAL; then, the side chain of cinnamic acid is decarboxylated to form benzoic acid; and finally, the benzoic acid undergoes 2-hydroxylation to form SA. This pathway has been confirmed by silencing PAL genes in pathogen-attacked Arabidopsis, which resulted in a 90% reduction of basal PAL activity and exhibited a 50% decrease in SA production [5]. Another pathway mainly occurs in the chloroplasts, mediated by ICS, which directly catalyses the conversion of chorismate into isochorismate. According to this pathway, SA is generated from chorismate by the synthesis of ICS1 and ICS2 [6]. Loss of ICS1 suppresses the pathogen-induced SA accumulation in SA-deficient mutants, sid2 [7], whereas loss of both ICS genes results in further reduction in the biosynthesis of SA [6].
SA is best known as a defence hormone. The first report on SA signalling was involved in plant immunity in 1979, which described that the application of aspirin (acetyl-SA) in virus-susceptible tobacco conferred resistance against tobacco mosaic virus [8]. During the early 1990s, studies with transgenic plants revealed how SA is perceived and synthesised under pathogen attack [9]. Recently, increasing evidence has shown that SA plays an important role in mediating plant responses to various abiotic stresses, including chilling [10], drought [11], thermogenesis [12], osmotic stress [13], and metal toxicity [14]. Although SA has been confirmed as an important signalling molecule for the regulation of reactive oxygen species (ROS) production in plants [15], most of the literature so far has found that pre-treatment with an appropriate level of SA may induce an acclimation effect on all kinds of abiotic stresses [14,16,17]. Indeed, the signalling roles of SA depend on many factors, including plant species, application mode, the exogenous and endogenous levels of SA, as well as stress faced by the plants [16]. Although recent studies have unravelled some of the molecular mechanisms’ signalling networks that control plant development and stress responses, a few regarding them are still unknown. This review starts with the literature on the role of SA in the protection of plants against abiotic stresses (including heat, chilling, salinity, metal toxicity, drought, ozone, pesticide, and ultraviolet radiation), followed by a proposal of possible mechanisms and prospects for research on SA in abiotic-stressed plants.
2. Functions of SA in Mitigating Abiotic Stresses
2.1. Heat
Global warming is causing a serious threat to plant growth and food security. Heat stress disturbs plant cellular homeostasis, retards development, and causes sterility and reduced yield [18]. It has been reported that the application of exogenous SA enhances rice yield under high-temperature conditions [19], while inhibiting the synthesis of SA markedly reduced the level of thermotolerance in pea plants [20]. Furthermore, the biosynthesis of SA was increased under heat stress, as observed in many plant species, such as mustard [21], creeping bentgrass [22], grape [23], and melon [24]. These findings indicate that SA signalling is involved in heat acclimation in plants (See Table 1).
Table 1.
The collected references of SA on heat tolerance in plants.
| SA | Heat Treatment | Plant Species | Main Responses | Reference | |
|---|---|---|---|---|---|
| Type of SA | SA Treatment | ||||
| 0–0.5 mM | 38 °C for 72 h | Alfalfa (Medicago sativa L.) | I, II, III | [12] | |
| 0–1.5 mM | T(max) ≥ 35 °C for 128 d | Rice (Oryza sativa L.) | I, II, III | [19] | |
| 100 µM | 38 °C for 24 h | Grapevine (Vitis vinifera L.) | I, II | [23] | |
| 1 mM | 40 °C for 24 h | Cucumis sativa L. | I, II, III, V | [26] | |
| 100 µM | 43 °C for 5 h | Grape (Vitis vinifera L.) | I, III, VII | [27] | |
| 0.01% | 15–35 °C for 60 d | wheat (Triticum aestivum L.) | I, II, III | [28] | |
| 1 mM | 42 °C for 36 h | Tomato (Solanum lycopersicum L.) | I, II, III, V | [29] | |
| 0.5 mM | 40 °C for 6 h | Wheat (Triticum aestivum L.) | I, II, III, Ethylene formation | [30] | |
| 100 µM | 45 °C for 3 h or 6 h | Grape plants (Vitis vinifera L.) | I, Plasma membrane H+-ATPase, Ca2+-ATPase | [31] | |
| 10 µM | 40 °C for 1 h | etr-1, nahG mutants | I, II | [32] | |
| 0–1 mM | 38 °C for 16 h | npr1 mutant, NahG plants, cpr5 mutants | I, V, VII, VI, PR genes | [33] | |
| 0–1 mM | 40 °C for 30 min | Lycopersicon esculentum L. | I, VII, Membrane permeability | [34] | |
| 1 mM | 41 °C for 2 h | Rice (Oryza sativa L.) | I, VII | [36] | |
| 100 µM | 45 °C for 3 h | Pea (Pisum sativum L.) | I, VI, Expression of PIP2-PLC+ | [37] | |
| 100 µM | 44 °C for 3 h | Grape plants (Vitis vinifera L.) | I, II, V, PM-Ca2+ATPase, V-Ca2+ ATPase | [40] | |
| Endogenous SA | 37 °C for 2 h | Pea (Pisum sativun L.) | I, II, VII, VI | [20] | |
| 45 °C for 1 h | Mustard (Sinapis alba L.) | I, II | [21] | ||
| 50 °C for 20 s | Melon (Cucumis melo L.) | I, Up-chitinase1 gene | [24] | ||
| 40 °C for o–48 h | Maize (Zea mays L.) | I, III, VII, VI, II, III, ABA, and IAA | [39] | ||
I, Growth; II, Antioxidant system; III, Photosynthesis; IV, SA-induced genes; V, Electrolyte leakage; VI, Endogenous free SA; VII, Heat shock proteins.
Photosystem II, which functions as an electron transport chain in chloroplasts, is one of the most thermosensitive structures in plants [25]. A study found that spraying 0.25 mM SA onto alfalfa leaves for 5 days ameliorated the heat damage to PSII and photosynthetic efficiency [12]. This may be because SA improves the antioxidant system and chlorophyll fluorescence [26], thus maintaining the thermo-stability of the electron donor and reaction centres of PSII [27]. Heat stress also disturbs osmotic potential and destroys plasma membranes, thereby leading to ion leakage in plant cells. The application of SA can enhance free proline content, which plays a key role in the osmoregulation of plant cells. This phenomenon has been widely observed in wheat [28], cucumber [26], and tomato [29,30]. Furthermore, spraying 100 mM SA on grape leaves stabilized the activity of the proton pumps in membranes, including H+- and Ca2+-ATPase, which may be another important mechanism for maintaining the integrity of the membrane under heat stress [31]. Activities of SA contribute to better regulation of stomatal aperture along with photosynthetic apparatus, such as PSII and Rubisco activity, and thus increase the capacity of photosynthesis when subjected to stressful temperature conditions [30].
Transcriptome analysis of plants has revealed SA signalling of heat-stress-responsive genes during thermotolerance, such as NPR1 (non-expresser of pathogenesis-related), HSPs (heat shock proteins), MBF1c (multiprotein bridging factor 1c), TGA, and PR-1 (pathogenesis-related protein 1) [22,32]. Exogenous application of SA induces the synthesis of heat shock proteins (HSPs), the proteins chiefly responsible for defence against heat stress, as noted in Arabidopsis thaliana plants [33], tomato [34,35], and rice [36]. However, a study with transgenic Arabidopsis obtained the inconsistent results that SA failed to affected the expression of Hsp [33], indicating the molecular mechanism still needs to be further investigated. Endogenous free SA stimulated the production of PIP2-phospholipase C of pea, a lipid-associated enzyme involved in intracellular signalling, in response to heat treatment. In response to heat stress, the pea plant elevated the synthesis of SA initially, which then signalled the production of PIP2-phospholipase C, a lipid-associated enzyme involved in intracellular signalling [37]. SA also increases the expression of the chitinase-1 gene in melons under heat shock [24]. Furthermore, cross–talk between SA and other plant signallings, such as H2S, Ca2+, IAA, and ABA, has also been reported [38,39,40]. For example, treatment with SA increases the activity of L-cysteine desulfhydrase, a key enzyme in H2S biosynthesis, indicating that H2S might be a downstream signalling molecule in SA-induced heat tolerance [38].
2.2. Chilling
Chilling injury is one of the main limitations in the growth and productivity of tropical and subtropical crops. The regulatory role of SA in defending against chilling stress has been reported in many plant species, such as maize [41], mountain rye [42], watermelon [43], beans [44], wheat [10,45], and barley [46]. Furthermore, low temperatures induced the accumulation of endogenous SA in Arabidopsis thaliana and wheat plants, which further confirmed the relationship between SA and cold stress responses [45,47] (See Table 2).
Table 2.
The collected references of SA on chilling tolerance in plants.
| SA Treatment | Chilling Treatment | Plant Species | Main Responses | Reference | |
|---|---|---|---|---|---|
| Type | Treatment | ||||
| Exogenous SA | 100 µM | 4 °C for 7 d | Wheat (Triticum aestivum L.) | II, IV | [10] |
| 0.5 mM | 2 °C for 2 d | Maize (Zea mays L.) | I, II, III, Ethylene | [41] | |
| 0–100 mg kg−1 | 10 °C for 12 h–15 h; 15 °C for 12 h | Mountain rye (secale montanum) | I | [42] | |
| 0–1mM | 10/5 °C for 7 d | Watermelon (Citrullus lanatus) | I, II, III, IV, V, SA biosynthesis | [43] | |
| 0.1 mM | 15 °C for 0–30 d | Bean (Phaseolusvulgaris L.) | I, II, III, Phytohormone | [44] | |
| 0.1 mM | 7/5 °C for 0– 28 d | Barley (Hordeum vulgare) | II, Apoplastic proteins | [46] | |
| 5 °C for 36 d | NahG, npr1 mutant, cpr1 mutant | I, II, VI | [47] | ||
| 0.5 mM | 5 °C for 3 d | Banana (Musa acuminata coll) | II | [48] | |
| 1 mM | 5 °C for 10 d | Banana fruits (Musa AAA group.) | I, II, V | [49] | |
| 0–4 mM | 4 °C for 21 d | Anthurium andraeanum | I, II, V, VIII | [50] | |
| 0–3 mM | 1 °C for 50 d | Bamboo shoots | I, II, V, VIII | [51] | |
| 2 mM | −0.5– 4.5 °C for 28 d | Lemon (Citrus limon L.) | I, II, V, VIII | [52] | |
| 2 mM | −0.5–4.5 °C for 28 d | Lemon (Citrus limon L.) | II, VII, IV | [53] | |
| 7 mM | 2 °C for 50 d | Cucumber (Cucumis sativus L. | I, II, III, V, VI, VIII | [54] | |
| 200 µM | 4 °C for 25 d | Pepper (C. annuum L.) | I, II, V, Fatty acids metabolism | [55] | |
| 0–1 mM | 0 °C for 28 d | Peach (Prunus persica L.) | I, II, VII | [56] | |
| 1 mM | 0 °C for 35 d | Peach fruit (Prunus persica Batsch) | II, Polyamine contents | [57] | |
| 0–1.0 mM | 0 °C for 0–84 d | Pomegranates (Punica granatum L.) | I, II, Primary metabolism | [58] | |
| 0–2.0 mM | 2 °C for 90 d | Pomegranates (Punica granatum) | I, II, V | [59] | |
| 0–2.5 mM | 1 °C for 0–60 d | Plums (Prunus salicina Lindl.) | I, II, V, Ethylene | [60] | |
| 0–0.5 mM | 20 °C for 8 d | Muskmelon (Cucumis melo L.) | I, II | [61] | |
| 0.5 mM | 2.5 °C for 1–4 d | Maize, Cucumber, Rice | I, II, V | [64] | |
| 0.1 mM | 0 °C for 14 d | Pepper (C. annuum L.) | I, IV | [65] | |
| 0–3 mM | 0 °C for 2–4 d | Bean (P. vulgaris L.) | I, II, III, IV, Soluble sugars | [67] | |
| 0–5 mM | 4 °C for 2 d | Acha inchi (Plukenetia volubilis) | II, Soluble sugars | [70] | |
| 0–1 mM | 15/10, 10/5, 5/3 °C for 0–45 d | Wheat (Triticum aestivum L) | I, II | [72] | |
| 0–0.5 mM | 4 °C for 2 w–4 w | Tomato (Lycopersicon esculentum L.) | I, IV | [73] | |
| 2 mM | 4 °C for 21 d | Anthurium cut flowers | I, II, Fatty acid metabolism | [74] | |
| Endogenous SA | 4 °C for 0–21 d | Wheat (Triticum aestivum L.) | Stress-protective proteins, Phytohormone | [45] | |
I, Growth; II, Antioxidant system; III, Photosynthesis; IV, SA-induced genes; V, Electrolyte leakage; VI, Endogenous free SA; VII, Heat shock proteins; VIII, Phenolic metabolism.
Low temperatures are effective for the storage of fruits and vegetables, but they may also cause chilling injury. SA as a highly efficient buffering agent against cold stress has been widely demonstrated in many fruits. For example, spraying 0.5 mM SA changed H2O2 metabolisms and increased the chilling tolerance of banana seedlings [48,49]. Similar results have been reported in cut flowers [50], bamboo shoots [51], as well as fruits in lemons [52,53], cucumbers [54], bell peppers [55], peaches [56,57], pomegranates [58,59], and plums [60].
A study also reported that SA exposure alleviated chilling injury on the seed germination of mountain rye, musk melon, and bean plants [42,44,61]. This may be because SA activates protein synthesis, such as that of 20S proteasome, and simulates the activity of enzymes, such as pentose phosphate, gluconeogenesis, and glycolysis, which together release the plant from the quiescent state [62]. Meanwhile, Zhao et al. (2021) found that treatment with 1 µM SA alleviates chilling injury in peach fruit through enhancing the total soluble sugars together with related genes expression (SPS4, NINV2, SuSy2, and SUT1) and cold-response genes expression (DREB1A and DREB2A) [63]. SA has also been shown to play a key role in growth development (including phonological aspects such as photosynthesis, respiration, and osmolytes synthesis) under cold stress. For example, SA treatment promotes the chilling tolerance of shoots in maize, rice, and cucumber, which is accompanied by an increase in the activities of glutathione reductase (GR) and guaiacol peroxidase [64]. Spraying SA maintained photosynthesis and chloroplast construction and mitigated chilling damage in grape leaves [40]. Methyl SA alleviated cold-injury-induced oxidative damage on sweet peppers by activating the alternative oxidase [65] gene expression, the key oxidase enzymes involved in electron transfer [66]. The synthesis of soluble sugars and proline might also be involved in SA signalling in Phaselous vulgaris [67] because these osmolytes can maintain the composition and ratio of fatty acids in membranes due to antioxidant (form of NADH or NADPH) and osmotic adjustment [68], thus stabilizing the overall structure of cells, which is a prerequisite for cold tolerance [52,69,70]. Furthermore, it was reported that exogenous SA (1mM) promoted chilling tolerance in cucumber plants by upregulating the cold signalling pathway (ICE1, CBF1, and COR47) and genes related to SA metabolism (PAL, ICS, and SABP2) [71].
Proteomic studies have gradually uncovered the relationship between SA and proteins expressed under low-temperature stress. Treatment with SA has been reported to reduce ice nucleation and induce anti-freezing protein, which inhibits ice crystal formation in plant cells [72]. Treatment with 0.01 mM methyl SA induced pathogenesis-related protein expression and increased the cold tolerance of tomatoes [73]. Introduction of 2 mM SA enhanced the γ-aminobutyric acid shunt pathway of anthurium-cut flowers during storage at 4 °C, providing sufficient ATP content to cope with the oxidative damage induced by the low temperature [74]. Treatment with 2 mM SA reduced chilling injury in lemons by increasing the production of HSPs [53]. Furthermore, it was reported that exogenous SA (1 mM) improved chilling tolerance in cucumber plants by upregulating the cold signalling pathway (ICE1, CBF1, and COR47) and genes related to SA metabolism (PAL, ICS, and SABP2) [71].
However, a study using an SA-deficient mutant, NahG, showed a higher growth rate than wild-type plants under cold stress. After being stored at a temperature of 5 °C for a 2-month-long cultivation, NahG plants displayed 2.7-fold larger biomass than the wild-type plant Col-0, while the free SA levels of NahG were only 5% of Col-0. This indicates that SA-mediated cold tolerance still needs to be studied further, in particular, regarding SA homeostasis in plants [47].
2.3. Salinity
When grown in saline soils, plants may suffer from superabundant ion and osmotic stress, leading to ion imbalance and toxicity in plant cells [75]. It has been found that salt stress can cause a decrease in SA content in plants, such as Iris hexagona [76], tomato [77], and soybean [78], whereas the application of SA increased tolerance to salt toxicity in many plant species, such as pepper [13], cucumber [79], and soybean [80] (See Table 3).
Table 3.
The collected references of SA on salinity tolerance in plants.
| SA Treatment | Salinity | Plant Species | Main Responses | Reference | |
|---|---|---|---|---|---|
| Type | Treatment | ||||
| Exogenous SA | 1 mM | 0–150 Mm for 10 d | Capsicum annuum | I, II, III, Phenolic content | [13] |
| 200 µM | 100 mM for 4 h | Tomato (Lycopersicon esculentum Mill.) | I, II | [77] | |
| 1 mM | 0–10 dS m−1 | Soybean (Glycine max L.) | I, II, III, VI | [80] | |
| 0–1.0 mM | 40 Mm for 56 d | Maize (Zea mays L.) | I, II, III, VI | [81] | |
| 1.5 mM | 0–15 dS m−1 for 900 d | Saffron | I, II, III, VI | [82] | |
| 0–0.5 mM | 2.51 g kg−1 for 17 d | Leymus chinensis Trin.Tzvel. | I, II, V, VI | [83] | |
| 1 mM | 0–100 mM | Soybean (Glycine max L.) | I, III, VI, H+-ATPase | [84] | |
| 50 µM | 100 mM for 14 d | rbohD mutant, gork1-1 mutant | I, V, VI | [85] | |
| 0.5 mM | 100 mM for 15 d | Mungbean (Vigna radiata L.) | I, II, III, VI, Ethylene | [88] | |
| 0.05 mM | 2% for 24 h | Wheat (Triticum aestivum L.) | I, II, Phytohormones | [89] | |
| 0.5 mM | 250 mM for 72 h | Wheat (Triticum aestivum L.) | I, II, IV | [93] | |
| 0–0.1 mM | 100 mM for 7 d | Solanum lycopersicum | II, GST genes | [94] | |
| Endogenous SA | 0–400 mM for 0–48 h | Iris hexagona walter | Phytohormones | [76] | |
| 0–140 mM for 80 d | Soybean (Glycine max L.) | I, III, Phytohormones | [78] | ||
| 0–100 mM for 0–7 d | Cucumber (Cucumis sativus L.) | I, II, VI, Phytohormones | [79] | ||
| 100 mM for 15 d | NahG plants | I, II, Stress-induced genes | [90] | ||
| 0–300 mM for 14 d | nahG, npr1-1, snc1/nahG mutants | I, II, V | [92] | ||
I, Growth; II, Antioxidant system; III, Photosynthesis; IV, SA-induced genes; V, Electrolyte leakage; VI, Ion uptake.
SA is an important regulator of influx and efflux of Na+. For instance, addition of SA to soil alleviated salt toxicity in maize by decreasing Na+ accumulation [81]. Exogenous foliar application of 1.5 mM SA reduced osmotic stress and improved the aerial K+/Na+ ratio of saffron under saline conditions [82]. Soaking seeds of Leymus chinensis in SA solution lowered osmotic damage on the plasma membrane by accumulating K+ and Ca2+ [83]. SA-signalled K+ accumulation might be due to the activation of H+-ATPase in the membrane [84], which occurs via guard cells outwardly rectifying K+ channel (GORK), as noted in Arabidopsis thaliana under salt stress [85]. The increase in Ca2+ influx in the cytoplasm may activate the transport system of Na+/H+ in the plasma membrane, which is mediated by the salt overly sensitive (SOS) signalling pathway [86]. Furthermore, the application of SA has been shown to maintain the membrane integrity by regulating compatible metabolites such as proline and soluble sugars. Irrigation of the solution with 1 mM SA into soil increased proline content and sustained membrane integrity of pepper cells [13]. Exogenous SA increases proline, soluble carbohydrates, and proteins contents in soybean leaves, thereby adjusting the water content of cells [84]. Pre-treatment of SA might induce a pre-adaptive response through a transient increase in H2O2 level, which may act as a second messenger to “set up” the plant to defend the following salt stress that may occur. Pre-treatment with SA enhances the activities of antioxidant enzymes in plants, which in turn decreases stress-induced oxidative stress, as has been noted in Leymus chinensis [83] and Iris pseudacorus [87]. The signalling role of SA is also cross-linked with ABA, glycinebetaine, and ethylene (ET), as they are closely correlated with the synthesis of stress proteins and maintenance of leaf water potential [88,89].
However, a transgenic investigation revealed a negative result while comparing wild-type Arabidopsis thaliana and an SA-deficient mutant (NahG) under salt stress. Wild-type plants showed extensive necrosis in shoots when grown in a 100 mM NaCl medium. Under the same conditions, NahG plants were more tolerant and retained green leaves [90]. Subsequent research on Arabidopsis thaliana transgenic mutants or lines further demonstrated that the high level of SA (snc1, 15 fold higher than wild-type) [91] in plants increased salt-induced damage, while a low level of SA contributed to the tolerance of plants to salt toxicity [92]. It seems as though the excessive levels of SA in plants may aggravate the oxidative burden induced by salt stress, as SA is a key signalling compound during the regulation of the antioxidant system [93,94].
The molecular basis for salinity amelioration associated with SA positively regulates the antioxidant genes, especially for GST-gene family members [16]. Pre-treatment with SA reinforced the antioxidant defence systems and mitigated the negative effects of salt stress in barley (Hordeum vulgare L.) [95]. Exposure of SA alleviated the salinity stress in Solanum lycopersicum by regulating the expression of SlGSTT2, SlGSTT3, and SlGSTF4 [94]. Exogenous application of SA improved the salt tolerance in Triticum aestivum due to the enhancement of transcript levels and activities of ascorbate (AsA)-GSH pathway enzymes antioxidant genes, such as GPX1, GPX2, DHAR, GR, GST1, GST2, MDHAR, and GS [93].
2.4. Metal Toxicity
Metal phytotoxicity has been a major subject of current plant biology research. Heavy metals can be absorbed easily by plant roots, transported into shoots, and cause various visible toxic symptoms, such as growth retardation, leaf chlorosis, wilting, and cell death. The beneficial role of SA in defence against metal toxicity has been reported in a wide range of plant species [96,97]. For instance, application of SA improved the growth and photosynthetic abilities in Pb-stressed rice [98], Cu-stressed Phaseolus vulgaris [99], and Ni-stressed mustard [100]. Recently, the co-reaction of SA with other promoters has also been evaluated. For example, combination exposure of SA and plant-growth-promoting bacteria reduced the Cr-induced oxidative damage in maize [101]. SA in combination with kinetin or calcium ameliorated Ni and Pb stress in Phaseolus vulgaris plants [102]. The combined supplementation of melatonin and SA effectively detoxified As toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants [103] (See Table 4).
Table 4.
The collected references of SA on heavy meatal tolerance in plants.
| SA Treatment | Heavy Meatal Treatment | Plant Species | Main Responses | Reference | ||
|---|---|---|---|---|---|---|
| Type | Treatment | Type | Treatment | |||
| 0.1 mM | Pb | 0–0.26 mM for 18 d | Rice (Oryzu sutivu L.) | I, II, III | [98] | |
| 1 mM | Cu | 0–0.2 mM for 10 d | Bean seedlings | I, II, III | [99] | |
| 0.01 mM | Ni | 0–150 µM for 7 d | Mustard (Brassica juncea L.) | I, II, III, VIII, V | [100] | |
| 100 µM | Cr | 50 mg kg−1 for 7 d | Maize (Zea mays L.) | I, II, III, IV | [101] | |
| 0.1 mM | Ni, Pb | 2.5 mM Ni, 0.5 mM Pb for 45 d | Bean (Phaseolus vulgaris L.) | I, II, III, VIII | [102] | |
| 0.5 mM | As | 50 µM | Pepper | I, II, III, IV, VI | [103] | |
| 500 µM | Cd | 0–2.80 mg L−1 for 14 d | Maize (Zea mays L.) | I, II, III, IV | [105] | |
| 500 µM | 2.80 mg L−1 for 12 d | Barley (Hordeum vulgare) | I, II, III, IV, V, VI, VII | [106] | ||
| 500 µM | 0–112 mg kg−1 for 56 d | Wheat (Triticum aestivum L.) | I, II, III, IV | [108] | ||
| 500 µM | 0–5.60 mg L−1 for 7 d | Kentucky bluegrass | I, II, III, IV, V | [109] | ||
| 500 µM | 2.80 mg L−1 for 10 d | Barley (Hordeum vulgare) | I, II, III, IV, V, VI, VII | [111] | ||
| 500 µM | 0–100 mg kg−1 | Hemp (Cannabis sativa L.) | I, II, III, IV | [112] | ||
| 300 mg L−1 | 0–1.0 mM for 40 d | Radish (Raphanus sativus) | I, IV | [115] | ||
| 50 µM | 0–300 mg kg−1 for 14 d | Oilseed rape (Brassica napus) | II, III, V, VI | [116] | ||
| 0–0.1 mM | 0–6 mg kg−1 for 7 d | Soybean (Glycine max L.) | I, III, IV, V | [118] | ||
| 10 µM | 5.6 mg L−1 for 6 d | Rice (Oryza sativa L.) | I, II, IV | [119] | ||
| 0.1 mM | 0–1500 μM for 15 d | Rice (Oryza sativa L.) | I, IV, V | [122] | ||
| 10 µM | 5.60 mg L−1 for 6 d | Rice (Oryza sativa L.) | I, II | [124] | ||
| 0–500 mM | 5.6 mg L−1 for 5 d | Soybean (Glycine max L.) | II, III, IV, V, VI, VII | [128] | ||
| 0–200 µM | 44.8 mg kg−1 for10 d | Melon (Cucumis melo L.) | I, II, III | [129] | ||
| 10 µM | 150 µM | Rice (Oryza sativa L.) | I, II, III | [130] | ||
| 500 µM | 40 mg kg−1 for 6 d | Soybean (Glycine max L.) | I, II, III | [131] | ||
| 200 µM | 11.2 mg L−1 for 14 d | Ryegrass (Lolium perenne L.) | I, II, III, VI | [132] | ||
| 0–500 µM | 50 µM for 12 d | Bean (Ricinus communis L.) | I, III, IV | [134] | ||
| Endogenous SA | Cd | 0–16.8 mg L−1 for 7 d | snc1, npr1−1, nahG, snc1/nahG mutants | I, II, III | [113] | |
| 0.56 mg L−1 for 12 d | Sid2 mutants | I, II, III, IV, V, VI, VII | [114] | |||
| 0.5 mM | NahG plants | II, IV | [117] | |||
| 50 µM for 7d | nahG, npr1-1, snc1 mutants | I, II, VII | [120] | |||
| 5.6 mg L−1 for 7 d | NahG, snc1 mutants | I, II, III, IV, VII | [133] | |||
I, Growth; II, Antioxidant system; III, Photosynthesis; IV, Heavy meatal uptake; V, Ion uptake; VI, Phytochelatins; VII, SA- or heavy meatal-induced genes; VIII, Electrolyte leakage.
Cadmium is one of the most toxic and widespread heavy metals in the world [104]. It is the typical toxic metal that can induce representative symptoms in plants, such as replacing and inactivating essential elements, destroying protein structure, and interfering with photosynthesis, respiration, and cell division [14]. A wide range of plant species have shown that SA is deeply involved in promoting Cd tolerance during processes such as plant growth, element assimilation, Cd translocation, photosynthesis, and senescence [14]. Therefore, this review on the topic of metal toxicity is focused on the interaction of SA and Cd in plants.
The phytotoxicity of cadmium (Cd) is a major subject of current plant biology research. Recent studies have shown that the synthesis of SA in plants is markedly promoted by Cd stress. For example, after 25 μM Cd treatment, the bound SA of maize was 10 times higher than that of untreated plants [105]. Similar phenomena have been observed in barley [106] and Pisum sativum [107]. Studies on a wide range of plant species have shown that SA is deeply involved in promoting Cd tolerance, including in plant growth, element assimilation, Cd translocation, photosynthesis, and senescence [14].
The mediation of SA in Cd tolerance has been noted in all plant developmental stages [3]. Pre-soaking seeds with SA improved the germination rate of bluegrass and wheat when subjected to Cd toxicity [108,109]. This might be because SA upregulated protein (superoxide dismutase, NAC domain-containing protein, pathogenesis-related protein) [62] synthesis and degraded the stored proteins (storage proteins 7S, albumin 2, α-cruciferin 12S seed storage protein, aminopeptidase) [110] during seed maturation. Furthermore, SA is cross-linked with the synthesis of ABA-regulated proteins, such as dehydrins, late embryogenesis abundant proteins, and HSPs, during seed germination [62]. Furthermore, exogenous exposure to SA mitigated the inhibitory effects of Cd toxicity on growth. Pre-treatment of rice roots with 10 μM SA for 24 h significantly reversed the growth-inhibitory effect of Cd stress on day 6 compared to Cd treatment alone [81]. Cd treatment reduced the dry weight of barley seedlings by approximately 35%, whereas pre-treatment with SA significantly alleviated these inhibitory effect [106]. The effect of SA on Cd phytotoxicity is dose–dependent. No or negative effects were observed in high-SA treatments in a few plants when subjected to Cd stress, such as ryegrass [111], hemp [112], and bluegrass [109]. Furthermore, equivocal conclusions have been reported in transgenic Arabidopsis thaliana plant sand mutants. Excessive SA in snc1 mutants aggravated Cd-induced inhibition, whereas SA depletion in nahG was mitigated by Cd toxicity [113]. However, another SA-deficient phenotype, sid2 mutants, showed accentuated symptom when subjected to Cd stress [114].
The role of SA in Cd uptake and translocation signalling remains controversial. Foliar spraying of SA significantly decreased Cd uptake in radish plants [115]. Studies of bluegrass [109] and oilseed rape [116] also reported this finding. In contrast, the barley seedlings pre-treated with SA failed to impede Cd influx into vacuoplasts and mesophyll [106]. Studies with SA-deficient mutants, such as sid2 and NahG, reported that SA did not influence Cd assimilation either in shoots or roots [115,117], and the simultaneous application of SA and Cd further increased Cd assimilation in soybean [118]. Rice roots immersed in SA experienced Cd translocation from Cd-treated parts to the Cd-untreated parts, as studied using a split-root system [119], which indicated that SA might not mediate an avoidance mechanism in Cd uptake in plants. The Cd translocation in plant roots mediated by SA might be associated with cell wall modification since the expression levels of pectin methylesterase inhibitor-encoding genes in nahG were dramatically higher than wild-type plants [120].
As SA plays an important role in regulating the activity of H+-ATPase in the plasma membrane [121], it can stabilize the optimal nutritional status of plants under Cd toxicity. Many studies have reported that SA treatment maintains the balance of ion (including K, Ca, Fe, Mn, Mg, and Zn) uptake under Cd stress, as has been reported in ryegrass [111], rice [122], bluegrass [109], and oilseed rape [116]. Treatment with SA alone may inhibit K absorption in roots [123], whereas under Cd stress, SA stimulated the K, Fe, and Mg uptake of the SA-deficient mutant, sid2 [114]. SA is also involved in S assimilation in barley and sid2 mutants [106,114].
The beneficial role of SA was always observed in which SA treatment was performed in advance of the Cd stress. Pre-treatment of SA can “set up” the antioxidant system and then induce the resistance. For instance, pre-treatment of SA initially increased H2O2 accumulation in rice roots. Correspondingly, the level of antioxidant system, including non-protein thiols (NPT), GSH, and ascorbic acid (AsA), and the activities of antioxidant enzymes were all elevated compared with the non-SA-exposed roots under Cd stress [119,124]. Pre-treatment of SA in acid form (SA) mainly increased the activities of antioxidant enzymes, whereas the salt form (NaSA) mainly influenced the GSH-related redox of Cd-stressed maize seedlings [125]. SA enhanced the expression levels of StSABP2, StAPX, and StSOD in potato and decreased Cd-induced oxidative damage [126]. Application of SA upregulated the expression of OsPCS1 and OsHMA3 while downregulating the OsNRAMP2 gene in Cd-exposed rice seedlings [127].
It has been reported that SA regulates plant photosynthesis through RuBisCO, redox homeostasis, light acclimation, and stomatal switch [3]. SA treatment mitigated chlorophyll destruction in soybean [128] and oilseed rape [116]. In contrast, SA deficiency aggravates Cd-induced damage on chlorophyll [114]. Under Cd stress, SA exposure significantly improved the photosynthetic yield in barley [106], increased the Fv/Fm (Fv: variable fluorescence, Fm maximal fluorescence) of melon [129], and relieved energy transfer from PSII to PSI [130]. SA application also recovered carotenoid synthesis, strengthened stomatal closure, and inhibited the activities of chlorophyll-degrading enzymes [112,131,132]. However, abnormal levels of SA also have a negative effect on photosynthesis. For example, SA deficiency upregulated the photosynthetic electron transport-related genes (PETM (phosphatidylethanolamine N-methyltransferase) and PETE1 (plastocyanin 1)) under Cd stress [133]. Pre-treatment of castor bean leaves with 500 µM SA aggravated Cd injury during photosynthesis, which might have been associated with an increase in stomatal limitation [109,134].
2.5. Other Stresses
2.5.1. Drought
During drought stress, plants have elevated SA levels, as noted in many plant species, such as barley [135], Phillyrea angustifolia [136], and Salvia officinalis [137]. The alleviation of drought injury by SA goes along with the hardening of the antioxidant system, increasing relative water and proline contents and regulating other phytohormones [1,138]. For example, pre-treatment of SA cleared the drought-induced superoxide radical with enhancement of the expression of redox regulating genes and increased proline content with its synthesis-related genes [139] (See Table 5).
Table 5.
The collected references of SA on other stress tolerances in plants.
| SA Treatment | Stress Treatment | Plant Species | Main Responses | Reference | ||
|---|---|---|---|---|---|---|
| Type | Treatment | Type | Treatment | |||
| Exogenous SA | 1–50 mg L−1 | 10 mg L−1 CLO, 20 mg L−1 DFN, 10 mg L−1 DFZ; 10 d | Cucumber (Cucumis sativus L.) | II, III | [87] | |
| 0.5 mM | Drought | Water deficit for 0–15 d | Brassica napus | I, II, Phytohormone | [139] | |
| 0.2 g kg−1 | Water deficit | Sweet basil (Ocimum basilicum) | I, II, III | [143] | ||
| 0–3 mM | Water deficit for 30 d | Rosmarinus officinalis L. | I, Oil compounds | [144] | ||
| 0.01 mM | Water deficit for 46 d | tomato (Lycopersicon esculentum L.) | II, III, V | [146] | ||
| 1 mM | Water deficit for 5 d | Maize (Zea mays L.) | I, II, III, ABA | [147] | ||
| 100 µM | Ozone | 100–150 µg L−1, 5 h d−1, 130 d | Rice (Oryza sativa L.) | I, II, III, VI | [156] | |
| 0–2 mM | Pesticide | 750 g kg −1 Mancozeb, 2 mL L−1 Termite kill, 350 g L−1 Anchor, for 24 h | Vigna radiata (L.) | I, II, III | [161] | |
| 0–1 mM | 6.6 mM thiram for 1–11 d | Solanum lycopersicum Mill. | I, II, III, Pesticide detoxification genes | [167] | ||
| 1 mg kg−1 | 1 mg kg−1 THIM, 1 mg kg−1 HMI, 1 mg kg−1 CAP | Cucumber (Cucumis sativus L.) | VI, Pesticides metabolism | [168] | ||
| Endogenous SA | Drought | Water deficit for 100 d | Phillyrea angustifolia L. | II, III | [136] | |
| Water deficit for 27 d | Salvia officinalis L. | III, JA | [137] | |||
| Ozone | 0.20 µL L−1 for 6 h | Nicotiana tabacum | IV | [150] | ||
| 0.20 µL L−1 for 12 h | etr1, ein2, npr1, eds5, sid2 mutants, NahG plants | IV | [151] | |||
| 120 µg L−1, 5 h d−1,0–36 d | Salvia officinalis | I, II, III, Phytohormones | [153] | |||
| 120 µM.m−2.s−1 | NahG plants | I, II, Cell death, Phytohormones | [154] | |||
| Ultravioletradiation | 200 µM.m−2.s−1 24 h | Nicotiana tabacum L. | IV | [150] | ||
I, Growth; II, Antioxidant system; III, Photosynthesis; IV, SA-induced genes; V, Electrolyte leakage; VI, Ion uptake.
SA treatments effectively ameliorated the negative effects of drought through not only improving the photosynthetic performance and membrane permeability but also enhancing the activity of antioxidant enzymes. For instance, foliar application of SA substantially decreased the ROS and MDA contents of maize under drought stress [140]. Application of SA at 100 mM enhanced antioxidant enzymatic activities together with other physio-biochemical traits, such as membrane stability, chlorophyl content, and photosynthetic rates in wheat under drought stress [141]. When sprayed with SA at 0.5 mM, wheat seedlings effectively increased the activities of antioxidant enzymes (SOD, CAT, and PPO) to alleviate the drought-stress-induced damage effects [142]. Foliar spray of SA in sweet basil significantly promoted the plant growth and relative water contents under water-deficit conditions [143]. Spraying 2 mM SA into the leaves of Rosmarinus officinalis L. increased the production of essential oil under the mild drought stress (60% field capacity) [144]. Treatment with SA protected tomato plants from drought stress, mainly by maintaining membrane stability and activities of carbonic anhydrase that directly affect the rate of photosynthetic CO2 fixation [145,146]. Pre-treatment with SA reduced damage to the cell membranes and increased ABA content in the leaves of barley and maize, suggesting that there is cross-talk between SA and ABA during drought stress [135,147].
2.5.2. Ozone
Ozone is a powerful oxidising agent that reacts with lipids and proteins in plant cells and causes oxidative damage [148,149]. SA deficiency in NahG plants is sensitive to the ozone, whereas ozone exposure stimulates SA accumulation and promotes virus resistance in tobacco [150]. Further evidence has shown that enhanced accumulation of SA by ozone stress is through the ICS pathway [151]. SA controls ET production of Salvia officinalis during ozone exposure by balancing cell redox and shrinking chlorosis formation in leaves [152]. However, abnormal levels of SA cause greater ozone injury either in deficiency or superfluousness. Many deficient genotypes, such as Cvi-0, NahG, npr1, eds5, and sid2, are sensitive to the ozone stress [153]. Exogenous SA application decreased the stomatal conductance, chlorophyll content, and Mg assimilation of rice under ozone stress [154]. Recently, an interesting study was conducted to test whether the O3-induced cell death is regulated through SA, JA, or ethylene. The global and targeted analysis of transcriptional changes in single, double, and triple mutants mainly showed that the basal SA levels are essential for plants to defend against ROS-induced cell death, which is in conjunction with ethylene and JA signalling [155,156] (See Table 5).
2.5.3. Pesticide
Some chemical pesticides, such as herbicide, also directly induced the oxidative damage in plants, as observed in cucumber, pistachio plants (Pistacia vera L.), and barley [157,158,159,160]. The injury caused by paraquat (a kind of herbicide) continuously generates superoxide in the chloroplasts of plant cells, motivates redox reaction chains, generates various forms of ROS, and leads to oxidative damage [158]. Transgenic NahG in rice plants causes SA deficiency, with lower glutathione (GSH) content showing great sensitivity to paraquat exposure [158]. SA significantly increases enzymatic parameters and photosynthetic pigments of Vigna radiata when exposed to fungicide (mancozeb), insecticide (chlorpyrifos), and herbicide (metribuzin) [161]. Pre-treatment with 1 mM SA triggers the activity and expression of pesticide detoxification enzymes (GSTs: glutathione S-transferases; a carbon-monoxide-bound enzyme, P450 (absorption band at 450 nm)) in thiram-treated leaves [162]. Treatment with 1 mM SA promotes the degradation of pesticides and blocks their accumulation in cucumber [163] (See Table 5).
2.5.4. Ultraviolet Radiation
UV radiation a key environmental signal that influences plant growth and development and can reduce disease and pest incidence [164]. However, because it is beyond the capacity of sunlight utilisation in plants, excessive exposure can directly induce the ROS production, adversely affects photosynthesis, and damage cell membranes and proteins [161]. It has been shown that SA counteracted the UV-A-, UV-B-, and UV-C-induced oxidative stress on pepper through activating antioxidant enzymes such as POD, APX, CAT, and GR [165]. Furthermore, UV radiation activated SA defences and then enhanced the tomato resistance to pathogen attack in the JA-deficient genotype [166]. Similar to ozone, UV radiation induces SA accumulation in tobacco, which is accompanied by higher activity of benzoic acid 2-hydroxylase, a key enzyme in the catalysis of SA biosynthesis [150]. It has been found that exogenous SA alleviates the damaging effects of UV irradiance in many plant species such as blue grass, soybean, and maize [167,168]. The possible roles may include increase in anthocyanin and α-tocopherol content, photochemical efficiency, and activities of antioxidant enzymes (See Table 5).
3. Possible Mechanisms of SA in Mitigating Abiotic Stresses
Intensive research has shown that all abiotic stressors increase the level of endogenous SA, indicating that this simple molecule is involved in stress signalling in plants [16,169,170]. The regulatory roles of SA are mediated by various physiological processes, including growth development, photosynthesis, ion assimilation, respiration, antioxidant system, and cross-talk with other hormones [3]. The first report on the SA signalling is that it affects ROS production and then provokes pathogenesis-related1 (PR1) expression under pathogenic attack [171]. This discovery sparked the further studies on the complex signalling network between SA and ROS in plants [172]. Thus, the primary mechanism of SA reviewed here is its defensive role through redox signalling.
3.1. Redox Signalling
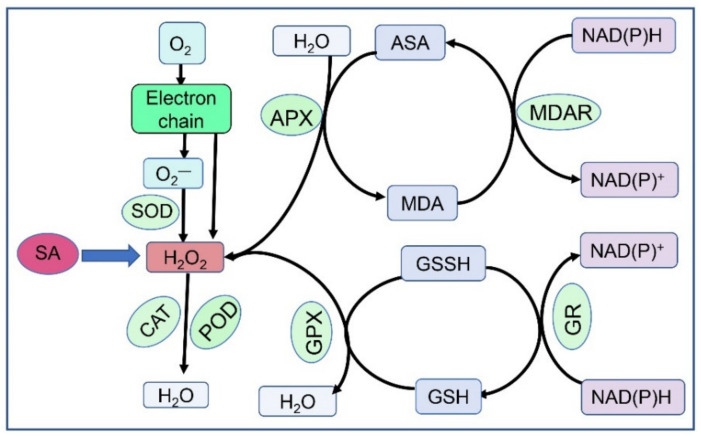
ROS are defined as the inevitable by-products of electron transfer in mitochondria, chloroplasts, and other energy-generating sites of plant cells [173]. Owing to their strong oxidisability, they can interfere with most biochemical metabolic processes, such as enzyme activity, membrane permeability, DNA stability, and protein synthesis. Under normal conditions, ROS are detoxified and maintained at equilibrium by the antioxidant defence system [174]. This system has experienced very complex evolutionary processes for 2.7 billion years. It is estimated that at least 152 genes in plants are involved in this highly dynamic and redundant network, which develops enzymatic and non-enzymatic compounds and encodes ROS-producing and -scavenging proteins [175] (See Figure 1). In many cases, the capacity to cease production of ROS is an important indicator of plant tolerance. Furthermore, low concentrations of ROS are successfully utilised by plants as a leading signalling pathway in physiological metabolic processes, such as growth development, hormone signalling, programmed cell death, cell cycle, and biotic and abiotic stress responses under normal and stress conditions [176].
Figure 1.
Brief pathways for reactive oxygen species scavenging in plants.
Many studies have uncovered the relationship between SA and ROS in plant signal transduction. Upon pathogen attack, tobacco immediately launches the synthesis of SA, which inhibits catalase activity and leads to H2O2 burst. Then, the SA-induced H2O2 acts as a second messenger to induce defending proteins and initiate SAR [177]. Since H2O2 is an essential signal in plants against abiotic stresses, the SA-induced H2O2 may enable plants to resist subsequent abiotic stresses. This phenomenon has been especially noted in heavy metal experiments either by the modes of pre-soaking, spraying, or hydroponic exposure prior to metal stress [14]. For example, pre-treatment with SA initially induced H2O2 accumulation in rice roots, which was accompanied by an increase in the levels of antioxidant molecules and the activities of antioxidant enzymes. The strengthened antioxidant system led to a decrease in oxidative injury caused by the Cd stress [124].
During the long-term evolutionary process, plants have evolved many types of ROS-scavenging enzymes, including catalases (CATs) [175]. CATs are the most efficient H2O2-scavenging enzymes that can rapidly dismutate H2O2 into H2O in plant cells. Under biotic stresses, cross-talk between SA and ROS suggests that SA inhibits CAT, thus creating a self-amplifying loop of ROS production, triggering PR1 expression, and inducing SAR [171]. Detailed analyses of tobacco showed that SA acted as an electron donor for the peroxidative cycle of CAT. The self-amplifying loop of the SA-H2O2 cycle leads to the continual accumulation of SA induced by ROS in plants [15]. When SA increased to a high level (100 mM), it siphoned CAT down to the slow peroxidative reaction and then induced the damaging levels of H2O2 [9]. In mitochondria, the SA-triggered H2O2 burst occurs due to the blockage of the electron flow from substrate dehydrogenated to the ubiquinone pool [178]. Under Cd toxicity, the SA-enhanced H2O2 in rice leaves was visualised using DAB staining [179]. SA pre-treatment at an acceptable level decreased CAT activity in salt-stressed tomatoes, freezing-stressed wheat [72], and Cd-stressed rice [124]. However, this effect is not always obvious and depends on the species used, the application mode, and environmental conditions. CAT inhibition was recovered in Bermuda grass after SA exposure on day 12 under freezing stress [180]. It has also been found that SA enhances CAT activity in soybean [181] and maize [182]. Treatment with 0.5 mM SA in maize significantly inhibited the activity of the CAT1 but failed to affect CAT2 activity under chilling stress [183].
Ascorbate peroxidase (APX) is another important H2O2-scavenging enzyme that participates in the ascorbate–glutathione cycle and has a higher affinity for H2O2 than CAT. Supplementation with SA enhances the activity of APX in wheat and soybean under Cd and waterlogging stress, respectively [184]. Pre-treatment with SA did not affect APX activity of bentgrass plants after heating for the first 24 h, but after that, APX activity was maintained at a significantly higher level than that in controls [185]. It has been reported that the inhibition of APX activity is SA dependent when plants suffer a pathogen attack [186]. However, a previous study on tobacco found that SA acted on a slow-reducing substrate and did not inhibit ascorbate oxidation by APX [57].
GSH is one of the most powerful molecules in plants and plays a role in the reduction of oxidative stress and detoxification of heavy metals by chelation. Many genetic studies have reported that GSH biosynthesis is linked to endogenous SA signalling. High levels of SA were found in catalase-deficient mutants (cat2) together with the upregulation of GSH [187]. Compared to cat2, the cat2 atrbohF mutant with low SA content attenuated the synthesis of GSH [188]. Similar results were also found in other SA-deficient mutants, such as sid2, npr1−1, and mpk4-1 [189,190]. Serine acetyltransferase is an important precursor enzyme that catalyses cysteine formation by GSH. A few studies with Arabidopsis thaliana mutants have shown that both genetics and exogenous SA increased the specific activity of SAT and GSH contents [191]. The enzymes related to GSH metabolism include glutathione synthetase (GSHS), GR [192], glutathione S-transferases (GST), and glutathione peroxidase (GPX). Endogenous SA regulates the LcGSHS transcript and leads to a higher GSH content and Cd tolerance in Cd-stressed Arabidopsis thaliana [193]. The synthesis of GR in Arabidopsis thaliana is mediated through the SA signalling pathway, and SA deficiency decreases the GR transcription of sid2 and lowers GSH levels [194]. Photooxidative stress in the chloroplasts of Arabidopsis thaliana was alleviated by SA along with the depletion of GPX [195]. The expression of the promoter regions of GST genes, such as osgstu4, osgstu3, as-1, and Gnt35, was simulated by SA, suggesting that SA has class-specific functions for this enzyme [196].
Although the benefits of SA signalling have been thoroughly studied, few studies have reported ambiguous results. SA treatment mitigated Cd toxicity in barley but failed to affect the activity of antioxidant enzymes [84]. High levels of SA promote the generation of H2O2 in leaves [15]. The results of studies on SA mutants are still contradictory. High SA levels in snc1 mutants generated a large amount of ROS, whereas SA deficiency in NahG lowered Cd-induced oxidative stress [91]. However, this finding is in contrast to the case of sid2 mutants, in which Cd-induced oxidative damage was aggravated by the SA deficiency [197].
3.2. Cross-Talk with Other Plant Hormones
Besides of ROS, other plant hormones are involved in the SA signal transduction pathway of plants [198]. Most studies have observed a relationship between ABA and SA levels under stress. Treatment with SA induces ABA concentrations in barley and tomato [199]. Exposure of Arabidopsis thaliana leaves to ABA inhibits SA transduction both upstream and downstream through the SAR signalling pathway, and this suppressive effect is not related to jasmonate (JA)/ET-mediated signalling [200]. Similarly, salt stress increases the content of JA and ABA but decreases the levels of IAA, gibberellic acid (GA), and SA in Iris hexagona and soybean [76,78]. Insect feeding caused a strong accumulation of JA-specific mRNA transcripts, such as GmBPI1, GmKTI1, and GmAAT, but did not influence the free SA or SA-marker gene transcripts accumulation [201]. Drought stress increases the levels of SA and ABA in Brassica napus, and the effect on ABA is more pronounced [194]. However, the signalling role of SA might be stronger than that of ABA because the inhibition of SA biosynthesis leads to serious heating damage compared to the inhibition of ABA biosynthesis [202]. It seems that the biosynthesis of ABA is a downstream signalling event associated with SA sensing. Treatment with SA in salt-stressed tomato resulted in ABA accumulation in both root and leaf tissues together with upregulation of some ABA biosynthesis genes, such as SlZEP1, SlNCED1, SlAO1, and SlAO2 [203]. In pea plants, the activity of SA glucosyl transferase may be inhibited by ABA, thus enhancing the concentrations of free SA [204].
Calcium (Ca2+) is a key messenger in plants that can induce various defence responses against stress. SA-induced stomatal closure is associated with ABA signalling, and this process is mediated by Ca2+/Ca2+-dependent protein kinases (CPK) in cpk3-2 and cpk6-1 mutants but not in the Ca2+-independent protein kinase Open Stomata1 (OST1) ost1-3 mutant [205]. It was also observed that SA triggered the Ca2+-sensing receptor in chloroplast thylakoid membranes of Arabidopsis thaliana [135]. Calmodulin, a Ca2+-binding messenger protein, transduces Ca2+ signals by binding Ca2+ and then modifying the target proteins. The biosynthesis of SA is regulated by calmodulin-binding-protein (CBP60g) via the activation of isochorismate synthase 1 (ICS1) [206]. Recent studies in Arabidopsis thaliana have shown that the SA-signalled plant immunity is associated with calmodulin-binding transcription activators (CAMTA) [207].
Similar to the function of SA, nitric oxide plays a crucial role in controlling redox homeostasis in plant responses to abiotic stresses [208]. The application of SA and SNP (NO donor) significantly improved the heat-stress tolerance of hyacinth bean and Ni tolerance of finger millet [209]. Under As toxicity, the increase in NO concentration in rice is induced by SA through the enhancement of nitrate reductase activity [210]. SA increased As tolerance in maize by activating the antioxidant defence system, but this effect was completely negated when NO synthesis was blocked [211]. Furthermore, NO may act as a downstream signalling molecule that participates in SA-signalled cell wall construction, which could impede Cd influx in Cd-stressed rice seedlings [212]. Both NO and SA are involved in the signal transduction of stomatal closure, and the increase in NO levels is dependent on SA-induced NO synthase-like enzymes [213].
3.3. Mitogen-Activated Protein Kinase
Mitogen-activated protein kinase (MAPK) is a type of protein kinase that is specific to the threonine and amino acids serine, which is involved in cell functions and cellular responses to a diverse array of stimuli [214]. MPK3, MPK4, and MPK6 kinases are the main mediators of plant responses to biotic and abiotic stresses. Studies on Arabidopsis thaliana have shown that SA is involved in transmitting MAPKs cascade signalling [215]. Compared with the wild-type, approximately 50% of the basal expression level of AtMPK3 was noted in the SA-deficient mutants with low activity of AtMPK3 [216]. A 48-kD MAPKs in tobacco was identified by SA activation since it preferentially phosphorylates myelin basic protein (MBP) [217]. Conversely, MAPK regulated the levels of SA in stressed plants [218]. It was reported that SA treatment increased the TaMAPK4 transcripts in wheat under an avirulent race of pathogen attack, whereas knockdown the TaMAPK4 gene downregulated the SA accumulation [219]. Meanwhile, StMKK1 protein negatively regulated SA-related signalling pathways in defence against pathogens in potato [220]. Mpk4 mutant accumulated excessive levels of SA, but this was not the reason for its extreme dwarf phenotype, as knocking down the ICS1 gene (SA synthesis) did not revert mpk4-impaired growth [221]. Furthermore, the accumulation of MPK4 might also be related to SA-regulated redox homeostasis, but this mechanism is still unknown and further study [222].
4. Conclusions and Prospects
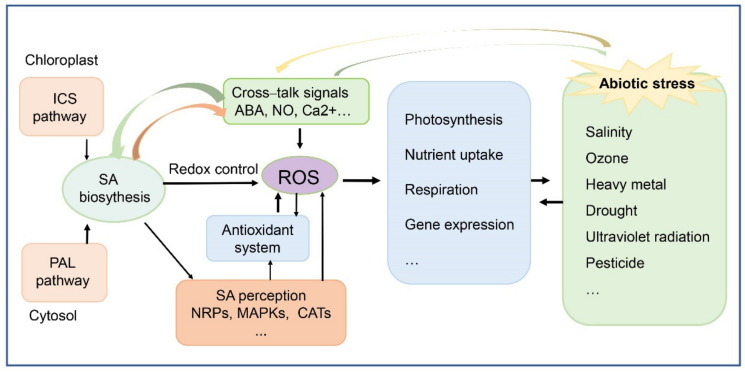
Increasing evidence has shown that SA acts as the primary signalling hormone in the defence against various abiotic stresses in plants. Different application modes of SA, including soaking seeds, hydroponic cultivation, and spraying on leaves, have demonstrated the protective roles. SA contributes to stress alleviation through the regulation of photosynthesis and physiological processes during growth development, and the possible mechanisms might be as follows: (1) SA indirectly regulates the antioxidant system by generating H2O2, which acts as the second messenger to induce various responses to face the abiotic stresses; (2) SA directly protects the cytomembrane and maintains the integrity of organelles by scavenging the free radicals; (3) SA takes part in the complex signal transduction network in coordination with other phytohormones, such as ABA, Ca2+, MAPK, and NO; and (4) under conditions of metal toxicity, SA might strengthen the cell wall and decrease the influx of heavy metals into the aerial parts (Figure 2).
Figure 2.
Modulation of SA signalling in plant tolerance under abiotic stress.
Considering the complex defending roles and integration of the signalling web, dissecting the genetic network of SA in plants is a major challenge for the future studies. The transcriptome analyses coupled with metabolomic and proteomic analyses of SA in plants are essential for future studies. A few gaps exist in the signalling roles of SA under abiotic stress and understanding them requires more detailed works. For instance, how is exogenous SA absorbed by plants, and how does it affect the level of endogenous SA? How does the partial exposure of SA “set up” the whole defence system of plants? Besides SABP and NPRs, are there any other hormone receptors or gene expressions involved in the network of SA signalling pathways? How do plants accurately control the balance between endogenous levels of SA and ROS? Integration of research on SA with the development of bioinformatics tools will provide a system-level understanding of the defence roles of SA in plants under abiotic stress.
Abbreviations
| AOX | Alternative oxidase |
| AsA | Acetylsalicylic acid |
| APX | Ascorbate peroxidase |
| ABA | Abscisic acid |
| BA2H | Benzoic acid-2-hydroxylase |
| CATs | Catalases |
| Cd | Cadmium |
| DHAR | Dehydroascorbate reductase |
| DREB | Dehydration-responsive element-binding protein |
| ET | Ethylene |
| GA | Gibberellic acid |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| GS | Glutathione synthetase |
| GSH | Glutathione |
| GORK | Guard cell outward rectifying K+ channel |
| GSHS | Glutathione synthetase |
| GSSH | Glutathione persulfide |
| GST | Glutathione S-transferases |
| HSPs | Heat shock proteins |
| ICS | Isochorismate synthases |
| CPK | Ca2+/Ca2+-dependent protein kinases |
| JA | Jasmonate |
| MDA | Malondialdehyde |
| MDHAR | Monodehydroascorbate reductase |
| MAPK | Mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NPR | Nonexpresser of pathogenesis-related |
| NINV2 | Neutral invertase 2 |
| POD | Peroxidase |
| PAL | Phenylalanine ammonia-lyase |
| PR1 | Pathogenesis-related protein 1 |
| PETM | Phosphatidylethanolamine N-methyltransferase |
| PETE1 | Plastocyanin 1 |
| SOD | Superoxide dismutase |
| SOS | Salt overly sensitive |
| ROS | Reactive oxygen species |
| SAR | Systemic acquired resistance |
| SuSy | Sucrose synthase |
| SPS | Sucrose phosphate synthase |
| SUT | Sucrose transporter |
| SlZEP | Zeaxanthin epoxidase1 |
| SlNCED | 9-cis-epoxycarotenoid dioxygenase |
| SlAO | Aldehyde oxidases |
| SA | Salicylic acid |
| SABP | Salicylic acid binding protein |
Author Contributions
Writing—original draft preparation, J.L. and G.Q.; conceptualization, G.Q.; writing—review and editing, J.L., B.G. and C.L.; visualization, H.L.; supervision, Q.F. and B.G.; validation, X.C. and Y.L.; funding acquisition, B.G. and J.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Department of Science and Technology of Zhejiang Province, China (2015C03020 and 2019C02008), Natural Science Foundation of China (41001184 and 42007120), Geological Exploration Fund of Zhejiang Province (2020006), and State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arif Y., Sami F., Siddiqui H., Bajguz A., Hayat S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020;175:104040. doi: 10.1016/j.envexpbot.2020.104040. [DOI] [Google Scholar]
- 2.Ding P., Ding Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020;25:549–565. doi: 10.1016/j.tplants.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Rivas-San Vicente M., Plasencia J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Li X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019;50:29–36. doi: 10.1016/j.pbi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey D.M.A., Vlot A.C., Wildermuth M.C., Klessig D.F. Salicylic acid biosynthesis and metabolism. Arab. Book/Am. Soc. Plant Biol. 2011;9:e0156. doi: 10.1199/tab.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcion C., Lohmann A., Lamodière E., Catinot J., Buchala A., Doermann P., Métraux J.-P. Characterization and biological function of the Isochorismate Synthase2 gene of Arabidopsis. Plant Physiol. 2008;147:1279–1287. doi: 10.1104/pp.108.119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 8.White R. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- 9.Malamy J., Carr J.P., Klessig D.F., Raskin I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 10.Ignatenko A., Talanova V., Repkina N., Titov A. Exogenous salicylic acid treatment induces cold tolerance in wheat through promotion of antioxidant enzyme activity and proline accumulation. Acta Physiol. Plant. 2019;41:1–10. doi: 10.1007/s11738-019-2872-3. [DOI] [Google Scholar]
- 11.Mohi-Ud-Din M., Talukder D., Rohman M., Ahmed J.U., Jagadish S., Islam T., Hasanuzzaman M. Exogenous Application of Methyl Jasmonate and Salicylic Acid Mitigates Drought-Induced Oxidative Damages in French Bean (Phaseolus vulgaris L.) Plants. 2021;10:2066. doi: 10.3390/plants10102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wassie M., Zhang W., Zhang Q., Ji K., Cao L., Chen L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.) Ecotoxicol. Environ. Saf. 2020;191:110206. doi: 10.1016/j.ecoenv.2020.110206. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Abass Ahanger M., Alshaya H., Latief Jan B., Yerramilli V. Salicylic acid mitigates salt induced toxicity through the modifications of biochemical attributes and some key antioxidants in capsicum annuum. Saudi J. Biol. Sci. 2022;29:1337–1347. doi: 10.1016/j.sjbs.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo B., Liu C., Liang Y., Li N., Fu Q. Salicylic acid signals plant defence against cadmium toxicity. Int. J. Mol. Sci. 2019;20:2960. doi: 10.3390/ijms20122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao M.V., Paliyath G., Ormrod D.P., Murr D.P., Watkins C.B. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes (salicylic acid-mediated oxidative damage requires H2O2) Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horváth E., Szalai G., Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007;26:290–300. doi: 10.1007/s00344-007-9017-4. [DOI] [Google Scholar]
- 18.Bita C., Gerats T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Duan L., He H., Li Y., Li X., Liu D., Wang J., Jin G., Huang S. Application of Exogenous KH2PO4 and Salicylic Acid and Optimization of the Sowing Date Enhance Rice Yield Under High-Temperature Conditions. J. Plant Growth Regul. 2022;41:1–15. doi: 10.1007/s00344-021-10399-y. [DOI] [Google Scholar]
- 20.Pan Q., Zhan J., Liu H., Zhang J., Chen J., Wen P., Huang W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 2006;171:226–233. doi: 10.1016/j.plantsci.2006.03.012. [DOI] [Google Scholar]
- 21.Dat J.F., Foyer C.H., Scott I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998;118:1455–1461. doi: 10.1104/pp.118.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkindale J., Hall J.D., Knight M.R., Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L.-J., Li S.-H. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006;48:137–144. doi: 10.1007/s10725-005-6146-2. [DOI] [Google Scholar]
- 24.Widiastuti A., Yoshino M., Hasegawa M., Nitta Y., Sato T. Heat shock-induced resistance increases chitinase-1 gene expression and stimulates salicylic acid production in melon (Cucumis melo L.) Physiol. Mol. Plant Pathol. 2013;82:51–55. doi: 10.1016/j.pmpp.2013.01.003. [DOI] [Google Scholar]
- 25.Čajánek M., Štroch M., Lachetova I., Kalina J., Spunda V. Characterization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. J. Photochem. Photobiol. B Biol. 1998;47:39–45. doi: 10.1016/S1011-1344(98)00197-3. [DOI] [Google Scholar]
- 26.Shi Q., Bao Z., Zhu Z., Ying Q., Qian Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006;48:127–135. doi: 10.1007/s10725-005-5482-6. [DOI] [Google Scholar]
- 27.Wang L.-J., Fan L., Loescher W., Duan W., Liu G.-J., Cheng J.-S., Luo H.-B., Li S.-H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010;10:34. doi: 10.1186/1471-2229-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afzal I., Akram M., Rehman H., Rashid S., Basra S. Moringa leaf and sorghum water extracts and salicylic acid to alleviate impacts of heat stress in wheat. S. Afr. J. Bot. 2020;129:169–174. doi: 10.1016/j.sajb.2019.04.009. [DOI] [Google Scholar]
- 29.Jahan M.S., Wang Y., Shu S., Zhong M., Chen Z., Wu J., Sun J., Guo S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019;247:421–429. doi: 10.1016/j.scienta.2018.12.047. [DOI] [Google Scholar]
- 30.Khan M.I.R., Iqbal N., Masood A., Per T.S., Khan N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013;8:e26374. doi: 10.4161/psb.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Zhang J., Liu H., Huang W. Salicylic acid or heat acclimation pre-treatment enhances the plasma membrane-associated ATPase activities in young grape plants under heat shock. Sci. Hortic. 2008;119:21–27. doi: 10.1016/j.scienta.2008.06.027. [DOI] [Google Scholar]
- 32.Larkindale J., Knight M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke S.M., Mur L.A., Wood J.E., Scott I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004;38:432–447. doi: 10.1111/j.1365-313X.2004.02054.x. [DOI] [PubMed] [Google Scholar]
- 34.Cronjé M.J., Bornman L. Salicylic acid influences Hsp70/Hsc70 expression in Lycopersicon esculentum: Dose-and time-dependent induction or potentiation. Biochem. Biophys. Res. Commun. 1999;265:422–427. doi: 10.1006/bbrc.1999.1692. [DOI] [PubMed] [Google Scholar]
- 35.Snyman M., Cronjé M. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 2008;59:2125–2132. doi: 10.1093/jxb/ern075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang P.-F.L., Jinn T.-L., Huang W.-K., Chen Y., Chang H.-M., Wang C.-W. Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. Plant Sci. 2007;172:64–75. doi: 10.1016/j.plantsci.2006.07.017. [DOI] [Google Scholar]
- 37.Liu H.-T., Huang W.-D., Pan Q.-H., Weng F.-H., Zhan J.-C., Liu Y., Wan S.-B., Liu Y.-Y. Contributions of PIP2-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. J. Plant Physiol. 2006;163:405–416. doi: 10.1016/j.jplph.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Li Z.-G., Xie L.-R., Li X.-J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015;177:121–127. doi: 10.1016/j.jplph.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Dinler B., Demir E., Kompe Y. Regulation of auxin, abscisic acid and salicylic acid levels by ascorbate application under heat stress in sensitive and tolerant maize leaves. Acta Biol. Hung. 2014;65:469–480. doi: 10.1556/ABiol.65.2014.4.10. [DOI] [PubMed] [Google Scholar]
- 40.Wang L.-J., Li S.-H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006;170:685–694. doi: 10.1016/j.plantsci.2005.09.005. [DOI] [Google Scholar]
- 41.Janda T., Szalai G., Tari I., Paldi E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 1999;208:175–180. doi: 10.1007/s004250050547. [DOI] [Google Scholar]
- 42.Ansari O., Sharif-Zadeh F. Does Gibberelic acid (GA), Salicylic acid (SA) and Ascorbic acid (ASc) improve Mountain Rye (Secale montanum) seeds germination and seedlings growth under cold stress. Int. Res. J. Appl. Basic Sci. 2012;3:1651–1657. [Google Scholar]
- 43.Cheng F., Lu J., Gao M., Shi K., Kong Q., Huang Y., Bie Z. Redox signaling and CBF-responsive pathway are involved in salicylic acid-improved photosynthesis and growth under chilling stress in watermelon. Front. Plant Sci. 2016;7:1519. doi: 10.3389/fpls.2016.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gharib F., Hegazi A. Salicylic acid ameliorates germination, seedling growth, phytohormone and enzymes activity in bean (Phaseolus vulgaris L.) under cold stress. J. Am. Sci. 2010;6:675–683. [Google Scholar]
- 45.Kosová K., Prášil I.T., Vítámvás P., Dobrev P., Motyka V., Floková K., Novák O., Turečková V., Rolčik J., Pešek B. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012;169:567–576. doi: 10.1016/j.jplph.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Mutlu S., Karadağoğlu Ö., Atici Ö., Nalbantoğlu B. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol. Plant. 2013;57:507–513. doi: 10.1007/s10535-013-0322-4. [DOI] [Google Scholar]
- 47.Scott I.M., Clarke S.M., Wood J.E., Mur L.A. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004;135:1040–1049. doi: 10.1104/pp.104.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang G., Wang C., Sun G., Wang Z. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 2003;50:9–15. doi: 10.1016/S0098-8472(02)00109-0. [DOI] [Google Scholar]
- 49.Khademi O., Ashtari M., Razavi F. Effects of salicylic acid and ultrasound treatments on chilling injury control and quality preservation in banana fruit during cold storage. Sci. Hortic. 2019;249:334–339. doi: 10.1016/j.scienta.2019.02.018. [DOI] [Google Scholar]
- 50.Aghdam M.S., Jannatizadeh A., Sheikh-Assadi M., Malekzadeh P. Alleviation of postharvest chilling injury in anthurium cut flowers by salicylic acid treatment. Sci. Hortic. 2016;202:70–76. doi: 10.1016/j.scienta.2016.02.025. [DOI] [Google Scholar]
- 51.Luo Z., Wu X., Xie Y., Chen C. Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. Food Chem. 2012;131:456–461. doi: 10.1016/j.foodchem.2011.09.007. [DOI] [Google Scholar]
- 52.Siboza X.I., Bertling I., Odindo A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon) J. Plant Physiol. 2014;171:1722–1731. doi: 10.1016/j.jplph.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Siboza X.I., Bertling I., Odindo A.O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.] Sci. Hortic. 2017;225:659–667. doi: 10.1016/j.scienta.2017.07.023. [DOI] [Google Scholar]
- 54.Zhang Y., Zhang M., Yang H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015;174:558–563. doi: 10.1016/j.foodchem.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 55.Ge W., Zhao Y., Kong X., Sun H., Luo M., Yao M., Wei B., Ji S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020;327:127057. doi: 10.1016/j.foodchem.2020.127057. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Chen S., Kong W., Li S., Archbold D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006;41:244–251. doi: 10.1016/j.postharvbio.2006.04.010. [DOI] [Google Scholar]
- 57.Cao S., Hu Z., Zheng Y., Lu B. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 2010;58:93–97. doi: 10.1016/j.postharvbio.2010.05.010. [DOI] [Google Scholar]
- 58.Sayyari M., Castillo S., Valero D., Díaz-Mula H.M., Serrano M. Acetyl salicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol. Technol. 2011;60:136–142. doi: 10.1016/j.postharvbio.2010.12.012. [DOI] [Google Scholar]
- 59.Sayyari M., Babalar M., Kalantari S., Serrano M., Valero D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009;53:152–154. doi: 10.1016/j.postharvbio.2009.03.005. [DOI] [Google Scholar]
- 60.Luo Z., Chen C., Xie J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’plum fruit. Postharvest Biol. Technol. 2011;62:115–120. doi: 10.1016/j.postharvbio.2011.05.012. [DOI] [Google Scholar]
- 61.Kaur S., Gupta N. Effect of proline and salicylic acid on germination and antioxidant enzymes at different temperatures in Muskmelon (Cucumis melo L.) seeds. J. Appl. Nat. Sci. 2017;9:2165–2169. doi: 10.31018/jans.v9i4.1504. [DOI] [Google Scholar]
- 62.Rajjou L., Belghazi M., Huguet R., Robin C., Moreau A., Job C., Job D. Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol. 2006;141:910–923. doi: 10.1104/pp.106.082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y., Song C., Brummell D.A., Qi S., Lin Q., Bi J., Duan Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021;358:129867. doi: 10.1016/j.foodchem.2021.129867. [DOI] [PubMed] [Google Scholar]
- 64.Kang H.M., Saltveit M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 2002;115:571–576. doi: 10.1034/j.1399-3054.2002.1150411.x. [DOI] [PubMed] [Google Scholar]
- 65.Wu S., Zhang X., Sun Y., Wu Z., Li T., Hu Y., Su D., Lv J., Li G., Zhang Z. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS, TEM–EDS, and XAFS. Environ. Sci. Technol. 2015;49:14036–14047. doi: 10.1021/acs.est.5b03659. [DOI] [PubMed] [Google Scholar]
- 66.Fung R.W., Wang C.Y., Smith D.L., Gross K.C., Tian M. MeSA and MeJA increase steady-state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.) Plant Sci. 2004;166:711–719. doi: 10.1016/j.plantsci.2003.11.009. [DOI] [Google Scholar]
- 67.Soliman M.H., Alayafi A.A., El Kelish A.A., Abu-Elsaoud A.M. Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot. Stud. 2018;59:6. doi: 10.1186/s40529-018-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couée I., Sulmon C., Gouesbet G., El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- 69.Gusta L.V., Wisniewski M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013;147:4–14. doi: 10.1111/j.1399-3054.2012.01611.x. [DOI] [PubMed] [Google Scholar]
- 70.Luo Y., Su Z., Bi T., Cui X., Lan Q. Salicylic acid improves chilling tolerance by affecting antioxidant enzymes and osmoregulators in sacha inchi (Plukenetia volubilis) Braz. J. Bot. 2014;37:357–363. doi: 10.1007/s40415-014-0067-0. [DOI] [Google Scholar]
- 71.Fu X., Feng Y.-Q., Zhang X.-W., Zhang Y.-Y., Bi H.-G., Ai X.-Z. Salicylic Acid Is Involved in Rootstock–Scion Communication in Improving the Chilling Tolerance of Grafted Cucumber. Front. Plant Sci. 2021;12:1228. doi: 10.3389/fpls.2021.693344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taşgín E., Atící Ö., Nalbantoğlu B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003;41:231–236. doi: 10.1023/B:GROW.0000007504.41476.c2. [DOI] [Google Scholar]
- 73.Ding C.-K., Wang C., Gross K.C., Smith D.L. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- 74.Aghdam M.S., Naderi R., Malekzadeh P., Jannatizadeh A. Contribution of GABA shunt to chilling tolerance in anthurium cut flowers in response to postharvest salicylic acid treatment. Sci. Hortic. 2016;205:90–96. doi: 10.1016/j.scienta.2016.04.020. [DOI] [Google Scholar]
- 75.Zhao C., Zhang H., Song C., Zhu J.-K., Shabala S. Mechanisms of plant responses and adaptation to soil salinity. Innovation. 2020;1:100017. doi: 10.1016/j.xinn.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y., Mopper S., Hasenstein K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001;27:327–342. doi: 10.1023/A:1005632506230. [DOI] [PubMed] [Google Scholar]
- 77.Molina A., Bueno P., Marín M.C., Rodríguez-Rosales M.P., Belver A., Venema K., Donaire J.P. Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. N. Phytol. 2002;156:409–415. doi: 10.1046/j.1469-8137.2002.00527.x. [DOI] [PubMed] [Google Scholar]
- 78.Hamayun M., Khan S.A., Khan A.L., Shinwari Z.K., Hussain J., Sohn E.-Y., Kang S.-M., Kim Y.-H., Khan M.A., Lee I.-J. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. [(accessed on 10 June 2022)];Pak. J. Bot. 2010 42:3103–3112. Available online: https://www.pakbs.org/pjbot/PDFs/42(5)/PJB42(5)3103.pdf. [Google Scholar]
- 79.Chojak-Koźniewska J., Linkiewicz A., Sowa S., Radzioch M., Kuźniak E. Interactive effects of salt stress and Pseudomonas syringae pv. lachrymans infection in cucumber: Involvement of antioxidant enzymes, abscisic acid and salicylic acid. Environ. Exp. Bot. 2017;136:9–20. doi: 10.1016/j.envexpbot.2017.01.004. [DOI] [Google Scholar]
- 80.Farhangi-Abriz S., Ghassemi-Golezani K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018;147:1010–1016. doi: 10.1016/j.ecoenv.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 81.Gunes A., Inal A., Alpaslan M., Eraslan F., Bagci E.G., Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007;164:728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Feizi H., Moradi R., Pourghasemian N., Sahabi H. Assessing saffron response to salinity stress and alleviating potential of gamma amino butyric acid, salicylic acid and vermicompost extract on salt damage. S. Afr. J. Bot. 2021;141:330–343. doi: 10.1016/j.sajb.2021.04.036. [DOI] [Google Scholar]
- 83.Hongna C., Leyuan T., Junmei S., Xiaori H., Xianguo C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021;188:104498. doi: 10.1016/j.envexpbot.2021.104498. [DOI] [Google Scholar]
- 84.Ghassemi-Golezani K., Farhangi-Abriz S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 2018;166:18–25. doi: 10.1016/j.ecoenv.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 85.Jayakannan M., Bose J., Babourina O., Rengel Z., Shabala S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013;64:2255–2268. doi: 10.1093/jxb/ert085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun J., Wang M.J., Ding M.Q., Deng S.R., Liu M.Q., Lu C.F., Zhou X.Y., Shen X., Zheng X.J., Zhang Z.K. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010;33:943–958. doi: 10.1111/j.1365-3040.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 87.Liu T., Li T., Zhang L., Li H., Liu S., Yang S., An Q., Pan C., Zou N. Exogenous salicylic acid alleviates the accumulation of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus L.) Ecotoxicol. Environ. Saf. 2021;208:111654. doi: 10.1016/j.ecoenv.2020.111654. [DOI] [PubMed] [Google Scholar]
- 88.Khan M.I.R., Asgher M., Khan N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol. Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 89.Shakirova F.M., Sakhabutdinova A.R., Bezrukova M.V., Fatkhutdinova R.A., Fatkhutdinova D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003;164:317–322. doi: 10.1016/S0168-9452(02)00415-6. [DOI] [Google Scholar]
- 90.Borsani O., Valpuesta V., Botella M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001;126:1024–1030. doi: 10.1104/pp.126.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X., Clarke J.D., Zhang Y., Dong X. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant-Microbe Interact. 2001;14:1131–1139. doi: 10.1094/MPMI.2001.14.10.1131. [DOI] [PubMed] [Google Scholar]
- 92.Hao L., Zhao Y., Jin D., Zhang L., Bi X., Chen H., Xu Q., Ma C., Li G. Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil. 2012;354:81–95. doi: 10.1007/s11104-011-1046-x. [DOI] [Google Scholar]
- 93.Li G., Peng X., Wei L., Kang G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene. 2013;529:321–325. doi: 10.1016/j.gene.2013.07.093. [DOI] [PubMed] [Google Scholar]
- 94.Csiszár J., Horváth E., Váry Z., Gallé Á., Bela K., Brunner S., Tari I. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 2014;78:15–26. doi: 10.1016/j.plaphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Torun H., Novák O., Mikulík J., Pěnčík A., Strnad M., Ayaz F.A. Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.) Sci. Rep. 2020;10:13886. doi: 10.1038/s41598-020-70807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yadav V., Arif N., Kováč J., Singh V.P., Tripathi D.K., Chauhan D.K., Vaculík M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021;159:100–112. doi: 10.1016/j.plaphy.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 97.Huihui Z., Xin L., Zisong X., Yue W., Zhiyuan T., Meijun A., Yuehui Z., Wenxu Z., Nan X., Guangyu S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020;195:110469. doi: 10.1016/j.ecoenv.2020.110469. [DOI] [PubMed] [Google Scholar]
- 98.Jing C., Cheng Z., Li L.-P., Sun Z.-Y., Pan X.-B. Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 2007;19:44–49. doi: 10.1016/S1001-074260007-2. [DOI] [PubMed] [Google Scholar]
- 99.Zengin F. Exogenous treatment with salicylic acid alleviating copper toxicity in bean seedlings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014;84:749–755. doi: 10.1007/s40011-013-0285-4. [DOI] [Google Scholar]
- 100.Zaid A., Mohammad F., Wani S.H., Siddique K.M. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 2019;180:575–587. doi: 10.1016/j.ecoenv.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 101.Islam F., Yasmeen T., Arif M.S., Riaz M., Shahzad S.M., Imran Q., Ali I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016;108:456–467. doi: 10.1016/j.plaphy.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Khalil R., Haroun S., Bassyoini F., Nagah A., Yusuf M. Salicylic acid in combination with kinetin or calcium ameliorates heavy metal stress in Phaseolus vulgaris plant. J. Agric. Food Res. 2021;5:100182. doi: 10.1016/j.jafr.2021.100182. [DOI] [Google Scholar]
- 103.Kaya C., Sarıoglu A., Ashraf M., Alyemeni M.N., Ahmad P. The combined supplementation of melatonin and salicylic acid effectively detoxifies arsenic toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants. Environ. Pollut. 2022;297:118727. doi: 10.1016/j.envpol.2021.118727. [DOI] [PubMed] [Google Scholar]
- 104.Huybrechts M., Cuypers A., Deckers J., Iven V., Vandionant S., Jozefczak M., Hendrix S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019;20:3971. doi: 10.3390/ijms20163971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krantev A., Yordanova R., Janda T., Szalai G., Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008;165:920–931. doi: 10.1016/j.jplph.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 106.Metwally A., Finkemeier I., Georgi M., Dietz K.-J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003;132:272–281. doi: 10.1104/pp.102.018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Metwally A., Safronova V.I., Belimov A.A., Dietz K.-J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005;56:167–178. doi: 10.1093/jxb/eri017. [DOI] [PubMed] [Google Scholar]
- 108.Agami R.A., Mohamed G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013;94:164–171. doi: 10.1016/j.ecoenv.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 109.Guo Q., Meng L., Mao P.-C., Jia Y.-Q., Shi Y.-J. Role of exogenous salicylic acid in alleviating cadmium-induced toxicity in Kentucky bluegrass. Biochem. Syst. Ecol. 2013;50:269–276. doi: 10.1016/j.bse.2013.05.002. [DOI] [Google Scholar]
- 110.Diaz-Vivancos P., Barba-Espín G., Hernández J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013;32:1491–1502. doi: 10.1007/s00299-013-1473-7. [DOI] [PubMed] [Google Scholar]
- 111.Bai X., Dong Y., Kong J., Xu L., Liu S. Effects of application of salicylic acid alleviates cadmium toxicity in perennial ryegrass. Plant Growth Regul. 2015;75:695–706. doi: 10.1007/s10725-014-9971-3. [DOI] [Google Scholar]
- 112.Shi G., Cai Q., Liu Q., Wu L. Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol. Plant. 2009;31:969–977. doi: 10.1007/s11738-009-0312-5. [DOI] [Google Scholar]
- 113.Tao S., Sun L., Ma C., Li L., Li G., Hao L. Reducing basal salicylic acid enhances Arabidopsis tolerance to lead or cadmium. Plant Soil. 2013;372:309–318. doi: 10.1007/s11104-013-1749-2. [DOI] [Google Scholar]
- 114.Guo B., Liu C., Li H., Yi K., Ding N., Li N., Lin Y., Fu Q. Endogenous salicylic acid is required for promoting cadmium tolerance of Arabidopsis by modulating glutathione metabolisms. J. Hazard. Mater. 2016;316:77–86. doi: 10.1016/j.jhazmat.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 115.Raza S.H., Shafiq F. Exploring the role of salicylic acid to attenuate cadmium accumulation in radish (Raphanus sativus) [(accessed on 10 June 2022)];Int. J. Agric. Biol. 2013 15:547–552. Available online: http://www.fspublishers.org/published_papers/6491_..pdf. [Google Scholar]
- 116.Ali E., Maodzeka A., Hussain N., Shamsi I.H., Jiang L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2015;75:641–655. doi: 10.1007/s10725-014-9966-0. [DOI] [Google Scholar]
- 117.Zawoznik M.S., Groppa M.D., Tomaro M.L., Benavides M.P. Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci. 2007;173:190–197. doi: 10.1016/j.plantsci.2007.05.004. [DOI] [Google Scholar]
- 118.Drazic G., Mihailovic N. Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci. 2005;168:511–517. doi: 10.1016/j.plantsci.2004.09.019. [DOI] [Google Scholar]
- 119.Guo B., Liang Y., Zhu Y. Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J. Plant Physiol. 2009;166:20–31. doi: 10.1016/j.jplph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Q., Gu C., Sun Y., Li G., Li L.-L., Hao L. Root defense in salicylic acid-altering Arabidopsis plants in responses to cadmium stress. J. Plant Growth Regul. 2021;40:1764–1776. doi: 10.1007/s00344-020-10233-x. [DOI] [Google Scholar]
- 121.Gordon L., Minibayeva F., Rakhmatullina D., Alyabyev A., Ogorodnikova T., Loseva N., Valitova Y. Heat production of wheat roots induced by the disruption of proton gradient by salicylic acid. Thermochim. Acta. 2004;422:101–104. doi: 10.1016/j.tca.2004.04.032. [DOI] [Google Scholar]
- 122.Fatima R.N., Javed F., Wahid A. Salicylic Acid Modifies Growth Performance and Nutrient Status of Rice (Oryza sativa) under Cadmium Stress. Int. J. Agric. Biol. 2014;16:1083–1090. doi: 10.12692/ijb/6.4.177-183. [DOI] [Google Scholar]
- 123.Harper J.R., Balke N.E. Characterization of the inhibition of K+ absorption in oat roots by salicylic acid. Plant Physiol. 1981;68:1349–1353. doi: 10.1104/pp.68.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo B., Liang Y.C., Zhu Y.G., Zhao F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007;147:743–749. doi: 10.1016/j.envpol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 125.Gondor O.K., Pál M., Darkó É., Janda T., Szalai G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.) PLoS ONE. 2016;11:e0160157. doi: 10.1371/journal.pone.0160157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Q., Wang G., Wang Y., Yang D., Guan C., Ji J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 2019;172:317–325. doi: 10.1016/j.ecoenv.2019.01.078. [DOI] [PubMed] [Google Scholar]
- 127.Majumdar S., Sachdev S., Kundu R. Salicylic acid mediated reduction in grain cadmium accumulation and amelioration of toxicity in Oryza sativa L. cv Bandana. Ecotoxicol. Environ. Saf. 2020;205:111167. doi: 10.1016/j.ecoenv.2020.111167. [DOI] [PubMed] [Google Scholar]
- 128.Noriega G., Caggiano E., Lecube M.L., Cruz D.S., Batlle A., Tomaro M., Balestrasse K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals. 2012;25:1155–1165. doi: 10.1007/s10534-012-9577-z. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Xu S., Yang S., Chen Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.) Protoplasma. 2015;252:911–924. doi: 10.1007/s00709-014-0732-y. [DOI] [PubMed] [Google Scholar]
- 130.Yotsova E.K., Dobrikova A.G., Stefanov M.A., Kouzmanova M., Apostolova E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018;30:57–70. doi: 10.1007/s40626-018-0102-9. [DOI] [Google Scholar]
- 131.Li X., Ma L., Bu N., Li Y., Zhang L. Effects of salicylic acid pre-treatment on cadmium and/or UV-B stress in soybean seedlings. Biol. Plant. 2014;58:195–199. doi: 10.1007/s10535-013-0375-4. [DOI] [Google Scholar]
- 132.Wang Q., Liang X., Dong Y., Xu L., Zhang X., Kong J., Liu S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013;32:721–731. doi: 10.1007/s00344-013-9339-3. [DOI] [Google Scholar]
- 133.Wang Y.-Y., Wang Y., Li G.-Z., Hao L. Salicylic acid-altering Arabidopsis plant response to cadmium exposure: Underlying mechanisms affecting antioxidation and photosynthesis-related processes. Ecotoxicol. Environ. Saf. 2019;169:645–653. doi: 10.1016/j.ecoenv.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 134.Liu C., Guo J., Cui Y., Lü T., Zhang X., Shi G. Effects of cadmium and salicylic acid on growth, spectral reflectance and photosynthesis of castor bean seedlings. Plant Soil. 2011;344:131–141. doi: 10.1007/s11104-011-0733-y. [DOI] [Google Scholar]
- 135.Bandurska H. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005;27:379–386. doi: 10.1007/s11738-005-0015-5. [DOI] [Google Scholar]
- 136.Munne-Bosch S., Penuelas J. Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta. 2003;217:758–766. doi: 10.1007/s00425-003-1037-0. [DOI] [PubMed] [Google Scholar]
- 137.Abreu M.E., Munné-Bosch S. Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 2008;64:105–112. doi: 10.1016/j.envexpbot.2007.12.016. [DOI] [Google Scholar]
- 138.Safari M., Mousavi-Fard S., Rezaei Nejad A., Sorkheh K., Sofo A. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2021;19:969–984. doi: 10.1007/s13762-020-03092-2. [DOI] [Google Scholar]
- 139.Lee B.-R., Islam M.T., Park S.-H., Jung H.-i., Bae D.-W., Kim T.-H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019;157:1–10. doi: 10.1016/j.envexpbot.2018.09.013. [DOI] [Google Scholar]
- 140.Shemi R., Wang R., Gheith E.-S., Hussain H.A., Hussain S., Irfan M., Cholidah L., Zhang K., Zhang S., Wang L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021;11:3195. doi: 10.1038/s41598-021-82264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ahmad A., Aslam Z., Naz M., Hussain S., Javed T., Aslam S., Raza A., Ali H.M., Siddiqui M.H., Salem M.Z. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE. 2021;16:e0260556. doi: 10.1371/journal.pone.0260556. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Khalvandi M., Siosemardeh A., Roohi E., Keramati S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon. 2021;7:e05908. doi: 10.1016/j.heliyon.2021.e05908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Damalas C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019;246:360–365. doi: 10.1016/j.scienta.2018.11.005. [DOI] [Google Scholar]
- 144.Abbaszadeh B., Layeghhaghighi M., Azimi R., Hadi N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crops Prod. 2020;144:111893. doi: 10.1016/j.indcrop.2019.111893. [DOI] [Google Scholar]
- 145.Tiwari A., Kumar P., Singh S., Ansari S. Carbonic anhydrase in relation to higher plants. Photosynthetica. 2005;43:1–11. doi: 10.1007/s11099-005-1011-0. [DOI] [Google Scholar]
- 146.Hayat S., Hasan S.A., Fariduddin Q., Ahmad A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008;3:297–304. doi: 10.1080/17429140802320797. [DOI] [Google Scholar]
- 147.Tayyab N., Naz R., Yasmin H., Nosheen A., Keyani R., Sajjad M., Hassan M.N., Roberts T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE. 2020;15:e0232269. doi: 10.1371/journal.pone.0232269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wedow J.M., Ainsworth E.A., Li S. Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem. Sci. 2021;46:992–1002. doi: 10.1016/j.tibs.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 149.Oksanen E., Pandey V., Pandey A., Keski-Saari S., Kontunen-Soppela S., Sharma C. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013;177:189–200. doi: 10.1016/j.envpol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 150.Yalpani N., Enyedi A.J., León J., Raskin I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta. 1994;193:372–376. doi: 10.1007/BF00201815. [DOI] [Google Scholar]
- 151.Ogawa D., Nakajima N., Tamaoki M., Aono M., Kubo A., Kamada H., Saji H. The isochorismate pathway is negatively regulated by salicylic acid signaling in O3-exposed Arabidopsis. Planta. 2007;226:1277–1285. doi: 10.1007/s00425-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 152.Pellegrini E., Trivellini A., Cotrozzi L., Vernieri P., Nali C. Plant Hormones under Challenging Environmental Factors. Springer; Berlin, Germany: 2016. Involvement of Phytohormones in Plant Responses to Ozone; pp. 215–245. [Google Scholar]
- 153.Marchica A., Cotrozzi L., Lorenzini G., Nali C., Pellegrini E. Antioxidants and Phytohormones Act in Coordination to Regulate Sage Response to Long Term Ozone Exposure. Plants. 2022;11:904. doi: 10.3390/plants11070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rao M.V., Davis K.R. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313X.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- 155.Xu E., Vaahtera L., Brosché M. Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Mol. Plant. 2015;8:1776–1794. doi: 10.1016/j.molp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 156.Kittipornkul P., Treesubsuntorn C., Thiravetyan P. Effect of exogenous catechin and salicylic acid on rice productivity under ozone stress: The role of chlorophyll contents, lipid peroxidation, and antioxidant enzymes. Environ. Sci. Pollut. Res. 2020;27:25774–25784. doi: 10.1007/s11356-020-08962-3. [DOI] [PubMed] [Google Scholar]
- 157.Dubey P., Mishra A.K., Singh A.K. Comparative analyses of genotoxicity, oxidative stress and antioxidative defence system under exposure of methyl parathion and hexaconazole in barley (Hordeum vulgare L.) Environ. Sci. Pollut. Res. 2015;22:19848–19859. doi: 10.1007/s11356-015-5216-x. [DOI] [PubMed] [Google Scholar]
- 158.Homayoonzadeh M., Hosseininaveh V., Haghighi S.R., Talebi K., Roessner U., Maali-Amiri R. Evaluation of physiological and biochemical responses of pistachio plants (Pistacia vera L.) exposed to pesticides. Ecotoxicology. 2021;30:1084–1097. doi: 10.1007/s10646-021-02434-1. [DOI] [PubMed] [Google Scholar]
- 159.Huang Y., Adeleye A.S., Zhao L., Minakova A.S., Anumol T., Keller A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019;270:47–52. doi: 10.1016/j.foodchem.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 160.Homayoonzadeh M., Moeini P., Talebi K., Roessner U., Hosseininaveh V. Antioxidant system status of cucumber plants under pesticides treatment. Acta Physiol. Plant. 2020;42:1–11. doi: 10.1007/s11738-020-03150-9. [DOI] [Google Scholar]
- 161.Fatma F., Kamal A., Srivastava A. Exogenous application of salicylic acid mitigates the toxic effect of pesticides in Vigna radiata (L.) Wilczek. J. Plant Growth Regul. 2018;37:1185–1194. doi: 10.1007/s00344-018-9819-6. [DOI] [Google Scholar]
- 162.Nazish T., Huang Y.-J., Zhang J., Xia J.-Q., Alfatih A., Luo C., Cai X.-T., Xi J., Xu P., Xiang C.-B. Understanding paraquat resistance mechanisms in Arabidopsis thaliana to facilitate developing paraquat-resistant crops. Plant Commun. 2022;3:100321. doi: 10.1016/j.xplc.2022.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kusumi K., Yaeno T., Kojo K., Hirayama M., Hirokawa D., Yara A., Iba K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol. Plant. 2006;128:651–661. doi: 10.1111/j.1399-3054.2006.00786.x. [DOI] [Google Scholar]
- 164.Meyer P., Van de Poel B., De Coninck B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021;8:194. doi: 10.1038/s41438-021-00629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mahdavian K., Ghorbanli M., Kalantari K.M. Role of salicylic acid in regulating ultraviolet radiation-induced oxidative stress in pepper leaves. Russ. J. Plant Physiol. 2008;55:560–563. doi: 10.1134/S1021443708040195. [DOI] [Google Scholar]
- 166.Escobar Bravo R., Chen G., Grosser K., Van Dam N.M., Leiss K.A., Klinkhamer P.G. Ultraviolet radiation enhances salicylic acid-mediated defense signaling and resistance to Pseudomonas syringae DC3000 in a jasmonic acid-deficient tomato mutant. Plant Signal. Behav. 2019;14:e1581560. doi: 10.1080/15592324.2019.1581560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yüzbaşıoğlu E., Dalyan E. Salicylic acid alleviates thiram toxicity by modulating antioxidant enzyme capacity and pesticide detoxification systems in the tomato (Solanum lycopersicum Mill.) Plant Physiol. Biochem. 2019;135:322–330. doi: 10.1016/j.plaphy.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 168.Liu T., Yuan C., Gao Y., Luo J., Yang S., Liu S., Zhang R., Zou N. Exogenous salicylic acid mitigates the accumulation of some pesticides in cucumber seedlings under different cultivation methods. Ecotoxicol. Environ. Saf. 2020;198:110680. doi: 10.1016/j.ecoenv.2020.110680. [DOI] [PubMed] [Google Scholar]
- 169.Santisree P., Jalli L.C.L., Bhatnagar-Mathur P., Sharma K.K. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress. Wiley; Hoboken, NJ, USA: 2020. Emerging roles of salicylic acid and jasmonates in plant abiotic stress responses; pp. 342–373. [DOI] [Google Scholar]
- 170.Ahmad F., Singh A., Kamal A. Plant Signaling Molecules. Woodhead Publishing; Sawston, UK: 2019. Salicylic Acid–Mediated Defense Mechanisms to Abiotic Stress Tolerance; pp. 355–369. [DOI] [Google Scholar]
- 171.Rajendiran K., Ramanujam M. Alleviation of ultraviolet-B radiation-induced growth inhibition of green gram by triadimefon. Biol. Plant. 2003;46:621–624. doi: 10.1023/A:1024840301092. [DOI] [Google Scholar]
- 172.Del Valle J.C., Buide M.L., Whittall J.B., Valladares F., Narbona E. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS ONE. 2020;15:e0231611. doi: 10.1371/journal.pone.0231611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kosobryukhov A., Khudyakova A., Kreslavski V. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I. Spinger; Berlin, Germany: 2020. Impact of UV radiation on photosynthetic apparatus: Adaptive and damaging mechanisms; pp. 555–576. [DOI] [Google Scholar]
- 174.Kovacs E., Keresztes A. Effect of gamma and UV-B/C radiation on plant cells. Micron. 2002;33:199–210. doi: 10.1016/S0968-4328(01)00012-9. [DOI] [PubMed] [Google Scholar]
- 175.Hideg É., Jansen M.A., Strid Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013;18:107–115. doi: 10.1016/j.tplants.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Singh V.P., Kumar J., Singh M., Singh S., Prasad S.M., Dwivedi R., Singh M. Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul. 2016;78:79–91. doi: 10.1007/s10725-015-0076-4. [DOI] [Google Scholar]
- 177.Yan F., Liu Y., Sheng H., Wang Y., Kang H., Zeng J. Salicylic acid and nitric oxide increase photosynthesis and antioxidant defense in wheat under UV-B stress. Biol. Plant. 2016;60:686–694. doi: 10.1007/s10535-016-0622-6. [DOI] [Google Scholar]
- 178.Saleem M., Fariduddin Q., Castroverde C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021;168:381–397. doi: 10.1016/j.plaphy.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 179.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 180.Van Breusegem F., Vranová E., Dat J.F., Inzé D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001;161:405–414. doi: 10.1016/S0168-9452(01)00452-6. [DOI] [Google Scholar]
- 181.Mhamdi A., Van Breusegem F. Reactive oxygen species in plant development. Development. 2018;145:dev164376. doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- 182.He H., Van Breusegem F., Mhamdi A. Redox-dependent control of nuclear transcription in plants. J. Exp. Bot. 2018;69:3359–3372. doi: 10.1093/jxb/ery130. [DOI] [PubMed] [Google Scholar]
- 183.Horváth E., Janda T., Szalai G., Páldi E. In vitro salicylic acid inhibition of catalase activity in maize: Differences between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci. 2002;163:1129–1135. doi: 10.1016/S0168-9452(02)00324-2. [DOI] [Google Scholar]
- 184.Chen Z., Ricigliano J.W., Klessig D.F. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proc. Natl. Acad. Sci. USA. 1993;90:9533–9537. doi: 10.1073/pnas.90.20.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Durner J., Klessig D.F. Salicylic acid is a modulator of tobacco and mammalian catalases. J. Biol. Chem. 1996;271:28492–28501. doi: 10.1074/jbc.271.45.28492. [DOI] [PubMed] [Google Scholar]
- 186.Norman C., Howell K.A., Millar A.H., Whelan J.M., Day D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004;134:492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Szepesi Á. Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt-and osmotic stress. Acta Biol. Szeged. 2005;49:123–125. doi: 10.1111/j.1438-8677.2010.00344.x. [DOI] [Google Scholar]
- 188.Simaei M., Khavari-Nejad R.A., Saadatm S., Bernard F., Fahimi H. Effects of salicylic acid and nitric oxide on antioxidant capacity and proline accumulation in Glycine max L. treated with NaCl salinity. Afr. J. Agric. Res. 2011;6:3775–3782. doi: 10.5897/AJAR10.1088. [DOI] [Google Scholar]
- 189.Ahmad I., Ahmad T.K.A., Basra S.M., Hasnain Z., Ali A. Effect of seed priming with ascorbic acid, salicylic acid and hydrogen peroxide on emergence, vigor and antioxidant activities of maize. Afr. J. Biotechnol. 2012;11:1127–1137. doi: 10.5897/AJB11.2266. [DOI] [Google Scholar]
- 190.Hasanuzzaman M., Ahmed N., Saha T., Rahman M., Rahman K., Alam M.M., Rohman M.M., Nahar K. Exogenous Salicylic Acid and Kinetin Modulate Reactive Oxygen Species Metabolism and Glyoxalase System to Confer Waterlogging Stress Tolerance in Soybean (Glycine max L.) Plant Stress. 2022;3:100057. doi: 10.1016/j.stress.2022.100057. [DOI] [Google Scholar]
- 191.Larkindale J., Huang B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004;161:405–413. doi: 10.1078/0176-1617-01239. [DOI] [PubMed] [Google Scholar]
- 192.Durner J., Klessig D.F. Inhibition of ascorbate peroxidase by salicylic acid and 2, 6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA. 1995;92:11312–11316. doi: 10.1073/pnas.92.24.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kvaratskhelia M., George S.J., Thorneley R.N. Salicylic acid is a reducing substrate and not an effective inhibitor of ascorbate peroxidase. J. Biol. Chem. 1997;272:20998–21001. doi: 10.1074/jbc.272.34.20998. [DOI] [PubMed] [Google Scholar]
- 194.Chaouch S., Queval G., Vanderauwera S., Mhamdi A., Vandorpe M., Langlois-Meurinne M., Van Breusegem F., Saindrenan P., Noctor G. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE Synthase1 in a daylength-related manner. Plant Physiol. 2010;153:1692–1705. doi: 10.1104/pp.110.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chaouch S., Queval G., Noctor G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012;69:613–627. doi: 10.1111/j.1365-313X.2011.04816.x. [DOI] [PubMed] [Google Scholar]
- 196.Mateo A., Funck D., Mühlenbock P., Kular B., Mullineaux P.M., Karpinski S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006;57:1795–1807. doi: 10.1093/jxb/erj196. [DOI] [PubMed] [Google Scholar]
- 197.Petersen M., Brodersen P., Naested H., Andreasson E., Lindhart U., Johansen B., Nielsen H.B., Lacy M., Austin M.J., Parker J.E. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 198.Han Y., Chaouch S., Mhamdi A., Queval G., Zechmann B., Noctor G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013;18:2106–2121. doi: 10.1089/ars.2012.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Freeman J.L., Garcia D., Kim D., Hopf A., Salt D.E. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 2005;137:1082–1091. doi: 10.1104/pp.104.055293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guan C., Ji J., Jia C., Guan W., Li X., Jin C., Wang G. A GSHS-like gene from Lycium chinense maybe regulated by cadmium-induced endogenous salicylic acid and overexpression of this gene enhances tolerance to cadmium stress in Arabidopsis. Plant Cell Rep. 2015;34:871–884. doi: 10.1007/s00299-015-1750-8. [DOI] [PubMed] [Google Scholar]
- 201.Beyer S.F., Bel P.S., Flors V., Schultheiss H., Conrath U., Langenbach C.J. Disclosure of salicylic acid and jasmonic acid-responsive genes provides a molecular tool for deciphering stress responses in soybean. Sci. Rep. 2021;11:20600. doi: 10.1038/s41598-021-00209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chang C.C., Slesak I., Jordá L., Sotnikov A., Melzer M., Miszalski Z., Mullineaux P.M., Parker J.E., Karpinska B., Karpinski S. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 2009;150:670–683. doi: 10.1104/pp.109.135566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Moons A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal-and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003;553:427–432. doi: 10.1016/S0014-5793(03)01077-9. [DOI] [PubMed] [Google Scholar]
- 204.Garretón V., Carpinelli J., Jordana X., Holuigue L. The as-1 promoter element is an oxidative stress-responsive element and salicylic acid activates it via oxidative species. Plant Physiol. 2002;130:1516–1526. doi: 10.1104/pp.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sappl P.G., Oñate-Sánchez L., Singh K.B., Millar A.H. Proteomic analysis of glutathione S-transferases of Arabidopsis thaliana reveals differential salicylic acid-induced expression of the plant-specific phi and tau classes. Plant Mol. Biol. 2004;54:205–219. doi: 10.1023/B:PLAN.0000028786.57439.b3. [DOI] [PubMed] [Google Scholar]
- 206.Yasuda M., Ishikawa A., Jikumaru Y., Seki M., Umezawa T., Asami T., Maruyama-Nakashita A., Kudo T., Shinozaki K., Yoshida S. Antagonistic interaction between systemic acquired resistance and the abscisic acid–mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20:1678–1692. doi: 10.1105/tpc.107.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Park S.-H., Lee B.-R., Al Mamun M., Bae D.-W., Kim T.-H. Characterization of salicylic acid-and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021;186:104434. doi: 10.1016/j.envexpbot.2021.104434. [DOI] [Google Scholar]
- 208.Liu H.-T., Liu Y.-Y., Pan Q.-H., Yang H.-R., Zhan J.-C., Huang W.-D. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J. Exp. Bot. 2006;57:3337–3347. doi: 10.1093/jxb/erl098. [DOI] [PubMed] [Google Scholar]
- 209.Horváth E., Csiszár J., Gallé Á., Poór P., Szepesi Á., Tari I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015;183:54–63. doi: 10.1016/j.jplph.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 210.Prodhan M.Y., Munemasa S., Nahar M.N.-E.-N., Nakamura Y., Murata Y. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 2018;178:441–450. doi: 10.1104/pp.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nomura H., Komori T., Uemura S., Kanda Y., Shimotani K., Nakai K., Furuichi T., Takebayashi K., Sugimoto T., Sano S. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012;3:926. doi: 10.1038/ncomms1926. [DOI] [PubMed] [Google Scholar]
- 212.Sun T., Huang J., Xu Y., Verma V., Jing B., Sun Y., Orduna A.R., Tian H., Huang X., Xia S. Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol. Plant. 2020;13:144–156. doi: 10.1016/j.molp.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 213.Kim Y., Gilmour S.J., Chao L., Park S., Thomashow M.F. Arabidopsis CAMTA transcription factors regulate pipecolic acid biosynthesis and priming of immunity genes. Mol. Plant. 2020;13:157–168. doi: 10.1016/j.molp.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 214.Wei L., Zhang M., Wei S., Zhang J., Wang C., Liao W. Roles of nitric oxide in heavy metal stress in plants: Cross–talk with phytohormones and protein S-nitrosylation. Environ. Pollut. 2020;259:113943. doi: 10.1016/j.envpol.2020.113943. [DOI] [PubMed] [Google Scholar]
- 215.Rai K.K., Rai N., Rai S.P. Salicylic acid and nitric oxide alleviate high temperature induced oxidative damage in Lablab purpureus L plants by regulating bio-physical processes and DNA methylation. Plant Physiol. Biochem. 2018;128:72–88. doi: 10.1016/j.plaphy.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 216.Kotapati K.V., Palaka B.K., Ampasala D.R. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017;5:240–250. doi: 10.1016/j.cj.2016.09.002. [DOI] [Google Scholar]
- 217.Singh A.P., Dixit G., Kumar A., Mishra S., Kumar N., Dixit S., Singh P.K., Dwivedi S., Trivedi P.K., Pandey V. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.) Plant Physiol. Biochem. 2017;115:163–173. doi: 10.1016/j.plaphy.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 218.Kaya C., Ashraf M., Alyemeni M.N., Corpas F.J., Ahmad P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020;399:123020. doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- 219.Wang B., Song N., Zhang Q., Wang N., Kang Z. TaMAPK4 acts as a positive regulator in defense of wheat stripe-rust infection. Front. Plant Sci. 2018;9:152. doi: 10.3389/fpls.2018.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang H., Li F., Li Z., Cheng J., Chen X., Wang Q., Joosten M.H., Shan W., Du Y. Potato StMPK7 is a downstream component of StMKK1 and promotes resistance to the oomycete pathogen Phytophthora infestans. Mol. Plant Pathol. 2021;22:644–657. doi: 10.1111/mpp.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pan J., Guan M., Xu P., Chen M., Cao Z. Salicylic acid reduces cadmium (Cd) accumulation in rice (Oryza sativa L.) by regulating root cell wall composition via nitric oxide signaling. Sci. Total Environ. 2021;797:149202. doi: 10.1016/j.scitotenv.2021.149202. [DOI] [PubMed] [Google Scholar]
- 222.Agurla S., Sunitha V., Raghavendra A.S. Methyl salicylate is the most effective natural salicylic acid ester to close stomata while raising reactive oxygen species and nitric oxide in Arabidopsis guard cells. Plant Physiol. Biochem. 2020;157:276–283. doi: 10.1016/j.plaphy.2020.10.026. [DOI] [PubMed] [Google Scholar]