Abstract
Increased sensitivity to light is common after concussion. Viewing a flickering light can also produce uncomfortable somatic sensations like nausea or headache. We examined effects evoked by viewing a patterned, flickering screen in a cohort of 81 uninjured youth athletes and 84 concussed youth. We used Multiple correspondence analysis and identified two primary dimensions of variation: the presence or absence of visually evoked effects and variation in the tendency to manifest effects that localized to the eyes (e.g., eye watering) versus more generalized neurological effects (e.g., headache). Based on these two primary dimensions, we grouped participants into three categories of evoked symptomatology: no effects, eye-predominant effects, and brain-predominant effects. A similar proportion of participants reported eye-predominant effects in the uninjured (33.3%) and concussed (32.1%) groups. By contrast, participants who experienced brain-predominant effects were almost entirely from the concussed group (1.2% of uninjured, 35.7% of concussed). The presence of brain-predominant effects was associated with a higher concussion symptom burden and reduced performance on visio-vestibular tasks. Our findings indicate that the experience of negative constitutional, somatic sensations in response to a dynamic visual stimulus is a salient marker of concussion and is indicative of more severe concussion symptomatology. We speculate that differences in visually evoked effects reflect varying levels of activation of the trigeminal nociceptive system.
Keywords: biomarkers, pediatric brain injury, sensory function, traumatic brain injury
Introduction
Visual symptoms and eye movement abnormalities are common after head trauma1 and have been explored as physiological biomarkers for concussion. As an example, dynamic pupillary responses are increased in children after concussion and have been proposed as an objective biomarker.2,3 Similarly, deficiencies in saccadic eye movements, vestibulo-ocular reflex (VOR), and visual motion sensitivity (VMS) are all associated with concussion.4–7
It is also well-established that light sensitivity can be enhanced after concussion8 and thus is a standard question on concussion symptom inventories.9–11 Visually evoked effects offer an intriguing avenue for concussion assessment—including evaluations that extend beyond effects localized to the eye. High contrast patterned and flickering light is uncomfortable to view and can induce multiple somatic sensations12; however, the somatic effects evoked by intense visual stimuli have not been assessed as a marker for concussion.
In migraine—for which light sensitivity is a cardinal symptom—visual discomfort and somatic sensations from flickering light are common interictal complaints.13,14 These stimuli are also associated with enhanced activity in the visual cortex in migraine15,16 and photophobia acutely after concussion.17 The effects evoked by patterned flickering light may serve as a biomarker and provide a window into the neural pathways involved in concussion.
The purpose of this investigation was to determine whether a patterned flickering visual stimulus discriminated between uninjured athletes and concussed youth based on the characteristics of the signs and symptoms provoked.
Methods
Participants
We performed a retrospective analysis of youth between 13 and 20 years old participating in a broader prospective study through the Children's Hospital of Philadelphia (CHOP) Minds Matter Concussion Program from February 2018 to October 2021. Uninjured participants were recruited through the sports teams of a local Philadelphia-area school. They had the opportunity to participate in clinical assessments in association with their sports season. Concussed participants were recruited from the same high school or from the CHOP Minds Matter Concussion Program.
Concussion diagnosis was determined by a trained sports medicine pediatrician according to the Consensus Statement on Concussion in Sports.18 All concussed participants” data were collected within 28 days of their concussion. Assent and consent were obtained from participants and guardians, respectively, and the study was approved by the CHOP Institutional Review Board in accordance with the Declaration of Helsinki.
Participants were prescreened to ensure normal or corrected to normal binocular and monocular visual acuity using a Snellen visual acuity chart at 10 feet. Fifty-nine participants (20 uninjured, 39 concussed) wore corrective lenses during testing. Age, sex, race, ethnicity, and concussion and migraine history (self and family) were self-reported for uninjured participants and abstracted from the medical record for concussed participants.
Data collection sessions
Data were collected at a single recording session from uninjured youth as part of a pre- or post-season sports evaluation and from concussed youth during clinical visits for concussion management. Trained research staff conducted clinical assessments in either the athletic training room at the high school, primarily for uninjured athletes, or the sports medicine office, primarily for concussed participants. In the case where more than one recording session was collected, only the first was used for this analysis.
Symptom inventory
Once before testing, all participants completed the Post-Concussion Symptom Inventory (PCSI), a scale validated in youth for measuring concussion symptoms.9 The question on feeling different since the concussion was excluded from the total PCSI score because this question was not applicable to the uninjured group.
Visio-vestibular assessment
All participants underwent visio-vestibular examination (VVE) before assessment of visually evoked effects to assess (1) smooth pursuit for five repetitions, (2) horizontal and vertical saccades for 30 repetitions, (3) horizontal and vertical VOR for 30 repetitions, (4) VMS for five repetitions, and (5) near point convergence, with abnormal defined as a break point where vision becomes double at greater than 6 cm. For all other metrics, abnormality was defined as provocation of symptoms limiting the number of repetitions the participant was able to complete. For more detailed information on the VVE, refer to our previous work, which has shown it to be reliable and specific for concussion across multiple clinical settings.7,19–22
Visually evoked effects
Participants viewed a wide field 85% contrast checkerboard with a pattern reversal rate of two reversals per second. Stimuli were presented for five continuous 20 sec blocks. The full description of the visual stimulus conditions can be found in our previous report.23 Eye movements were assessed during and after each recording session. While examining eye movements, experimenters also documented the presence or absence of the following physical signs of visual discomfort: eyes watering, eyes reddening, eyes slowing, circular eye movements, or “other signs.”
At the end of the recording session, participants were asked if they experienced provocation or worsening of the following symptoms: dizziness, headache, nausea, eye pain, eye fatigue, or other symptoms. No participants displayed circular eye movements, eye slowing, and no “other signs” were reported, so these were not included in data analysis. Other symptoms that were entered via free text included “eyes tired” (4), ”eyes foggy” (1), “eye strain” (1), “blurry vision,” “blurriness,” or “eye blur” (2), “double vision” (1), “eyes dry” (2), “feeling out of it” (1). Because the “other symptoms” responses were heterogenous and the same symptoms were not reported frequently, these symptoms were excluded from data analysis.
Data analysis
All analyses were performed using custom written code in Matlab (Mathworks, Natick, MA).24
Demographic data
Demographics were compared between the uninjured and concussed groups using a Fisher exact test for percentage data, and Kruskal-Wallis testing for interval data. A p value <0.05 was used as the threshold for significance throughout. Concussion history, migraine history, and migraine family history were also compared, because some studies25,26 (although not all27) indicate these are predictors for prolonged concussion symptoms. The median was reported for age (in years), days post-injury, and individual and total PCSI scores. All values were reported with 95% confidence intervals (CIs) derived by bootstrap analysis.
Analysis of visually evoked effects
Multiple correspondence analysis (MCA) was used across all participants to reduce the dimensionality of seven visually evoked effects: dizziness, headache, nausea, eyes watering, eye fatigue, eyes reddening, and eye pain. The MCA reduces the dimensionality of categorical data in Euclidean space,28 which is analogous to principal component analysis for quantitative data. Factor loadings represent the geometrical distance between the presence or absence of a visually evoked effect, with 0 being the center point of the variation. The code used to perform MCA was based on the indicator matrix, adapted from publicly available code.29
The first two dimensions represented (1) the presence or absence of visually evoked effects and (2) whether effects were eye- or brain-predominant. These two primary dimensions were used to separate participants into three visually evoked effects categories: (1) no effects, defined as a dimension 1 factor loading of -1 or less, (2) eye-predominant effects defined as a dimension 1 factor loading of greater than -1 and dimension 2 factor loading of 0.1 or less, and (3) brain-predominant effects defined as a dimension 1 factor loading greater than -1 and a dimension 2 factor loading of greater than 0.1.
These cutoffs are based on factor loadings of the individual visually evoked effects (Supplementary Table S1). The combined absence of all visually evoked effects is equal to a dimension 1 factor loading of -1.5, and the presence of one visually evoked effect could yield a dimension 1 loading as low as -0.7; therefore, a cutoff of -1 separated the no effects from eye- and brain-predominant categories effectively. The score of 0.1 was chosen to separate eye-predominant from brain-predominant effects because “eye pain” had a dimension 2 factor loading slightly above 0.
The percentage of participants per visually evoked effects category was compared between uninjured and concussed groups using cross-tabulation with chi-square statistical testing. Because of differences in PCSI and VVE between concussed and uninjured participants based on 95% CIs, statistical analysis for differences between visually evoked effects categories was conducted on the concussed group only. A Kruskal-Wallis test was used to compare visually evoked effects categories across PCSI and VVE with H test estimated by chi-square distribution. Multiple comparisons with the Tukey method were used to determine significance between individual visually evoked effects categories.
Results
Data from 81 uninjured participants and 84 concussed participants were included in analysis. Uninjured and concussed groups did not differ significantly in age, biological sex, or racial/ethnic identity (Table 1). Our cohort was similar in demographics to that of the state of Pennsylvania,30 although the demographics were notable for an underrepresentation of participants who identify as Hispanic. Compared with uninjured youth, a greater percentage of concussed youth reported a history of previous concussion (27.1% vs. 44.0%; p = 0.03) and family history of migraine (9.9% vs. 34.5%; p = 5.3e−4). Concussed youth reported significantly higher PCSI scores (2.0 vs. 25.5; H = 69.9, p = 6.4e−17).
Table 1.
Demographics of Uninjured and Concussed Groups
| Uninjured | Concussed | Stats | |
|---|---|---|---|
| Participants (% Female) | 81 (51.9%) | 84 (59.5%) | p = 0.34 |
| Median age (Range) | 16 (13 – 19) | 16 (13 – 20) | H = 1.3, p = 0.26 |
| Race/Ethnicity | PA demographics 2018 | ||
| Non-Hispanic White | 64 (80.2%) | 64 (76.1%) | 76.1% |
| Non-Hispanic Black | 7 (8.6%) | 7 (8.3%) | 10.8% |
| Hispanic | 3 (3.7%) | 1 (1.2%) | 7.6% |
| Non-Hispanic Asian | 2 (2.5%) | 3 (3.6%) | 3.6% |
| More than 1 race | 4 (4.9%) | 0 (0.0%) | 1.7% |
| Other | 0 (0.0%) | 3 (10.7%) | 0.2% |
| Median days post-injury | – | 12 [1 – 28] | – |
| Concussion history | 22 (27.1%) | 37 (44.0%) | p = 0.03 |
| Migraine history | 5 (6.2%) | 11 (13.1%) | p = 0.19 |
| Median total PCSI score | 2.0 (1.0 – 3.0) | 25.5 (19.5 – 41.0) | H = 69.9, p = 6.4e−17 |
Fisher exact test was used to compare percentages, and Kruskal-Wallis test was used to compare age, days post-injury, and Post-Concussion Symptom Inventory (PCSI) scores. PA, Pennsylvania.
Visually evoked effects
The MCA of seven visually evoked effects was performed to identify underlying structures within the data. Close to half of the variability (44.7%) across participants was explained by the first two dimensions (Fig. 1a). The first dimension, which accounted for 26.5% of the variability, represents the absence (negative direction) or presence (positive direction) of visually evoked effects. Concussed participants had a higher mean dimension 1 factor loading compared with uninjured participants: 0.78 (95% CI: 0.33, 1.21) vs. -0.82 (95% CI: -1.03, -0.55) indicating concussed participants reported more visually evoked effects than uninjured participants.
FIG. 1.
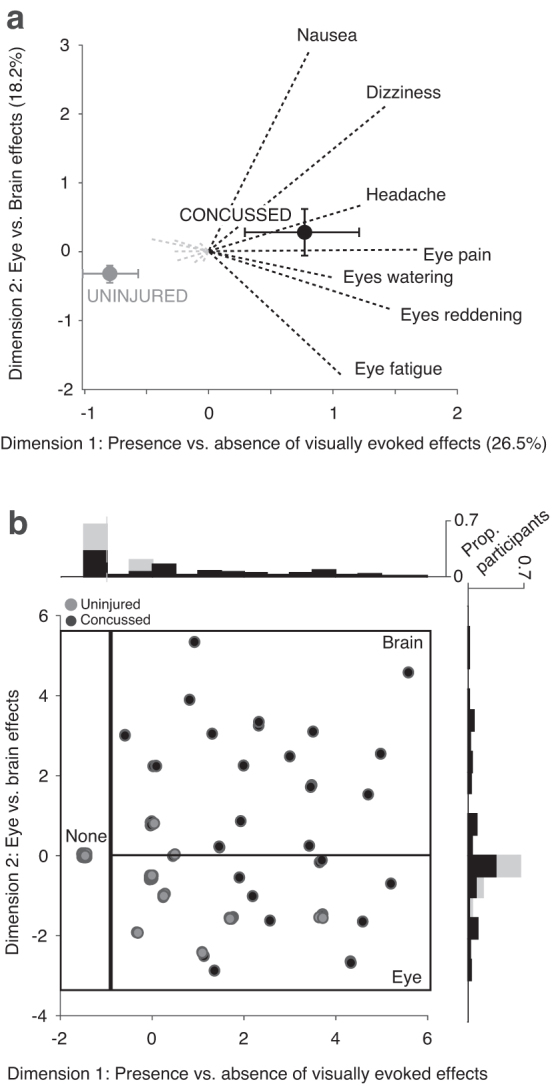
Multiple correspondence analysis (MCA) for visually evoked effects for uninjured and concussion groups. (a) MCA of visually evoked effects. Factor loadings represent the geometric distance between the presence (dotted black line) or absence (dotted gray line) of individual visually evoked effects, with 0 representing the midpoint of variation. Dimension 1 represents the absence (negative direction) or presence (positive direction) of visually evoked effects, and dimension 2 divides eye-related effects (negative direction) and brain-related effects (positive direction). Mean MCA factor loadings are shown for uninjured (gray) and concussed (black) youth. Error bars represent 95% confidence intervals by bootstrap analysis. (b) Dimension 1 and dimension 2 coefficients for individual uninjured (gray) and concussed (black) youth. Because a finite number of factor loadings are possible given the limited number of visually evoked effects, many of the individual participants overlap. This is captured by jittered data points and histograms depicting the proportion of participants with a given factor loading for dimension 1 (x-axis) and dimension 2 (y-axis). Boxes represent visually evoked effects categories.
The second dimension, which accounted for 18.2% of the variability, split effects qualitatively. Dizziness, headache, and nausea were associated with a larger positive factor loading, while eye fatigue, eyes watering, eyes reddening, and eye pain were associated with a smaller or negative factor loading. We adopt here the shorthand terms “eye effects” (negative values) and “brain effects” (positive values) to describe the two ends of variation along this second dimension revealed by the MCA.
Concussed participants had a higher mean dimension 2 score compared with uninjured participants: 0.29 (95% CI -0.07, 0.64) suggesting that the concussed participants overall reported more brain effects. Uninjured participants had a negative mean factor loading of -0.30 (95% CI -0.43, -0.19) suggesting that when visually evoked effects were present, they were more likely to be eye effects.
Brain-predominant visually evoked effects are associated with concussion and higher symptom burden
Visually evoked headache, dizziness, and nausea were unique to concussed youth (Table 2). Eye effects were reported more frequently in concussed compared with uninjured youth, but these effects were reported in both groups. The MCA dimension 1 and dimension 2 factor loadings were used to categorize participants into three categories: those who experienced (1) no effects, (2) eye-predominant effects, or (3) brain-predominant effects (Fig. 1b). There was some heterogeneity across concussed participants in these groups: 27% of those in the eye-predominant group also reported headache, and 50% of the brain-predominant group reported at least one eye effect (Table 2).
Table 2.
Percentage of Participants Reporting Visually Evoked Effects by Uninjured and Concussed Groups across All Participants and Participants Separated by Visually Evoked Effects Categories
| Visually evoked effect | Total |
None |
Eye |
Brain |
||||
|---|---|---|---|---|---|---|---|---|
| Uninjured (n = 81) | Concussed (n = 84) | Uninjured (n = 53) | Concussed (n = 27) | Uninjured (n = 27) | Concussed (n = 27) | Uninjured (n = 1) | Concussed (n = 30) | |
| Nausea | 0 (0%) | 8 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (27%) |
| Dizziness | 0 (0%) | 12 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (40%) |
| Headache | 1 (1%) | 27 (32%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (26%) | 1 (100%) | 20 (67%) |
| Eye pain | 3 (4%) | 20 (24%) | 0 (0%) | 0 (0%) | 3 (11%) | 12 (44%) | 0 (0%) | 8 (27%) |
| Eye watering | 20 (25%) | 32 (38%) | 0 (0%) | 0 (0%) | 20 (74%) | 21 (78%) | 0 (0%) | 11 (37%) |
| Eye reddening | 8 (10%) | 18 (21%) | 0 (0%) | 0 (0%) | 8 (30%) | 14 (52%) | 0 (0%) | 2 (7%) |
| Eye fatigue | 3 (4%) | 7 (8%) | 0 (0%) | 0 (0%) | 3 (11%) | 7 (26%) | 0 (0%) | 0 (0%) |
The percentage of uninjured and concussed participants within each effect category differed (Fig. 2a; χ2 = 35.5, p = 1.9e−8). Most uninjured participants reported no effects (65.4%), with fewer reporting eye-predominant effects (33.3%), and only one participant reporting brain-predominant effects (1.2%). There were similar percentages of concussed participants across the three categories (no effects 32.1%; eye-predominant 32.1%; brain-predominant 35.7%).
FIG. 2.
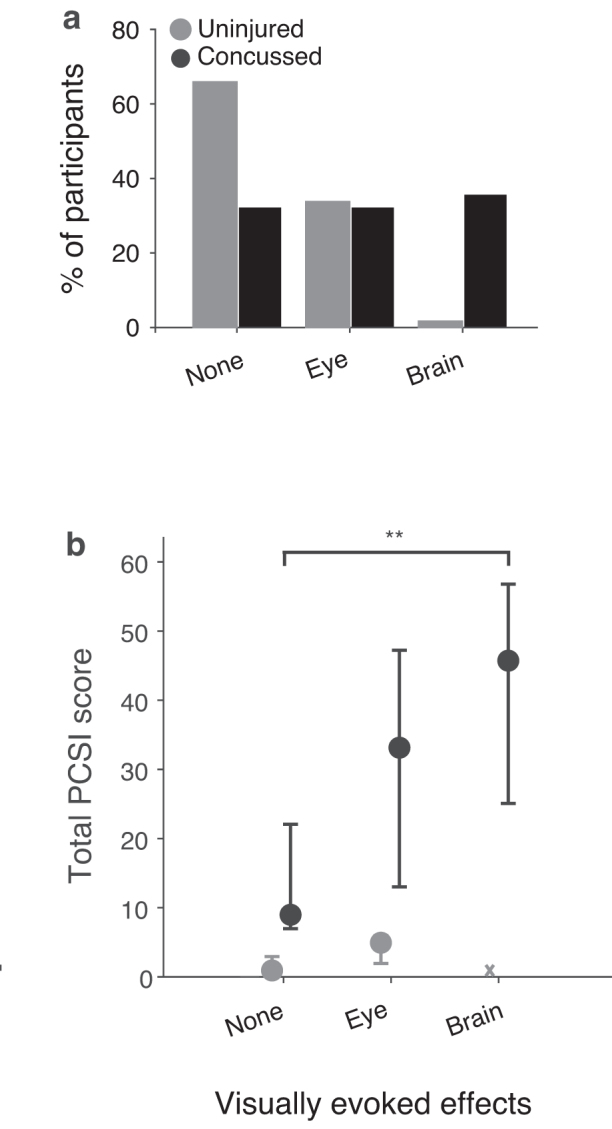
(a) Comparison of % of uninjured (gray) and concussed (black) youth by visually evoked effects category. (b) Median total Post-Concussion Symptom Inventory (PCSI) values by visually evoked effects category for uninjured and concussion youth. Error bars represent 95% confidence intervals by bootstrap analysis. “X” indicates the single uninjured participant in the brain-predominant effects category. Multiple comparisons using the Tukey method found a significant difference in total PCSI scores between no effects and brain-predominant effects categories (**p = 3.0e−4).
The PCSI scores differed significantly across the visually evoked effects category for concussed participants (χ2 = 15.39, p = 4.5e−4). The median PCSI score (95% CI) of concussed participants was 9.0 (7.0, 13.0) for no effects, 33.0 (13.0, 47.0) for eye-predominant effects, and 45.5 (25.0, 56.5) for brain-predominant effects (Fig. 2b). Analysis of individual PCSI question scores showed that multiple symptoms were elevated in the brain-predominant effects group to a greater extent than the other two groups. Symptoms that were elevated (95% CIs excluded zero) for the brain-predominant effects group included headache, light sensitivity, sound sensitivity, balance, dizziness, fatigue, drowsiness, slowed thinking, answering slowly, difficulty concentrating, mentally foggy, and visual problems (Supplementary Fig. S1).
There were no significant differences in migraine history, migraine family history, age, days post-injury, or biological sex across visually evoked effects categories for concussed youth (Supplementary Table S2). Concussed youth with no effects were more likely to have had a history of concussion than those in the eye-predominant effects and brain-predominant effects groups (χ2 = 9.4, p = 9.1e−3).
Concussion and brain-predominant visually evoked effects are associated with worse performance on saccadic, VOR, and VMS repetitions
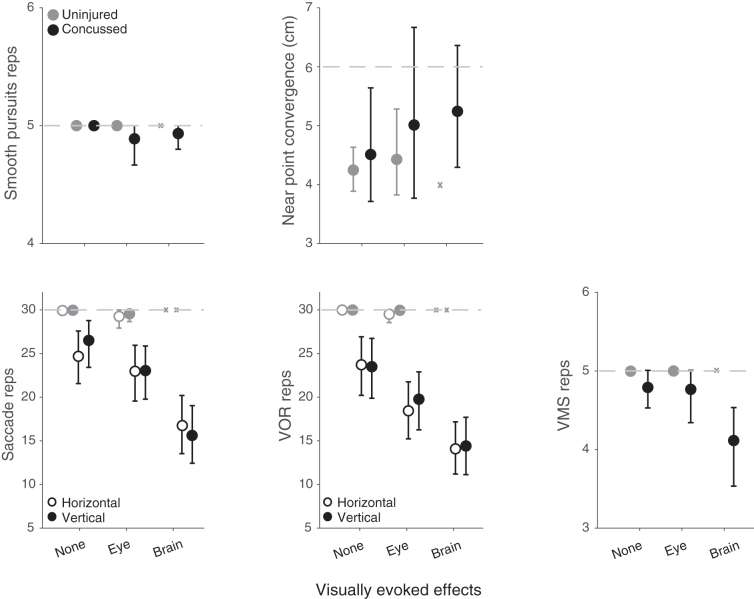
Concussed youth performed fewer repetitions than uninjured youth on multiple VVE metrics (Fig. 3). There was a significant effect of visually evoked effects category in concussed youth on horizontal (H = 11.4, p = 3.4e−3) and vertical (H = 20.0, p = 4.4e−5) saccades, horizontal (H = 13.5, p = 1.2e−3) and vertical (H = 12.8, p = 1.7e−3) VOR, and VMS (H = 7.3, p = 0.026) repetitions, but not smooth pursuit repetitions (H = 0.97, p = 0.62) or near-point convergence (H = 1.6, p = 0.44). Concussed youth who experienced brain-predominant effects displayed the worst performance on multiple VVE tasks.
FIG. 3.
Mean smooth pursuit repetitions, near perfect convergence (NPC), horizontal and vertical saccade repetitions, horizontal and vertical vestibulo-ocular reflex (VOR) repetitions, and visual motion sensitivity (VMS) repetitions for uninjured (gray) and concussed (black) youth. Light gray dotted line represents target number of repetitions, or threshold for NPC insufficiency based on visio-vestibular examination criteria. Error bars represent 95% confidence intervals by bootstrap analysis. “X” indicates the single uninjured participant in the brain-predominant effects category.
Discussion
Our study identified a relationship between the experience of negative somatic effects evoked by flickering light, the presence of concussion, and other characteristics of concussive injury among a cohort of concussed and uninjured adolescents. We found that participants exposed to patterned flickering light differ along two primary axes: the presence or absence of evoked effects and the degree to which these effects reflect predominantly eye-localized or more constitutional “brain” effects.
Unsurprisingly, uninjured participants were most likely to experience no effects. Nearly a third of the uninjured and concussed groups experienced eye-predominant effects. The similar proportion in both groups suggests that such effects may not be directly related to concussion, but rather evidence of a common pre-morbid sensitivity. This is consistent with the finding that 24% of uninjured neurologically normal children presenting to an emergency department were found to have abnormalities in visio-vestibular testing.22
Brain-predominant effects were uniquely associated with concussion. These effects were also associated with a greater symptom burden including multiple physical, fatigue, and cognitive post-concussion symptoms. Those with brain-predominant effects also showed the greatest deficits on visio-vestibular tasks that produced rapidly changing visual and/or vestibular input, like a flickering visual stimulus. Interestingly, the specificity and sensitivity of saccadic and VOR repetitions for identifying concussion has been optimized at 20 repetitions.7 We measured 30 repetitions for both tasks and found that only the brain-predominant group consistently had a mean repetition count of less than 20 before symptoms halted testing.
While there is some inherent ambiguity in separating visually evoked effects into categories because their origins likely involve complex and intertwined neural pathways, the clusters are intriguing. Eye pain, tearing, and conjunctival injection have been associated with trigemino-vascular and trigemino-autonomic reflexes that involve trigeminal nerve afferents and brainstem nuclei.8 Visually evoked eye effects may not require further activation beyond the reflex arch.
The presence of multiple symptoms across different domains and impaired performance on multiple VVE tasks supports the idea that visually evoked brain effects reflect broader neurological dysfunction. Indeed, constitutional somatic sensations including headache, dizziness, and nausea involve multiple cortical and subcortical brain regions31–33 and thus likely require contributions from both bottom-up and top-down processes.
Communication between trigeminal reflexes and thalamocortical circuitry is bidirectional: activation of trigeminal afferents provides input to thalamocortical circuits,8 which has been used to explain the multiple sensory and cognitive symptoms accompanying migrainous headache.34 Thalamocortical circuits can also promote activation of trigeminal nociceptive pathways through disruption of inhibitory descending pain modulation and cortical spreading depression, which are also both proposed to underlie post-traumatic headache.35–38
Activation of trigeminal nociceptive pathways has been extensively studied in migraine pathogenesis and may help explain why there is symptom overlap between migraine and concussion.26,27,39 Indeed, multiple studies have proposed a migrainous concussion phenotype that has been associated with high symptom burden and prolonged recovery.40–44 The brain-predominant group in our study reported elevation of headache and light and sound sensitivity, which are cardinal features of migraine.45 In addition, fatigue, dizziness, and cognitive complaints are common in individuals with chronic migraine.46,47
We did not find a significant difference in distribution of visually evoked effects categories for youth with a family or personal history of migraine. The higher percentage of participants with a family history of migraine in the concussed participant group, however, may suggest these individuals were predisposed to having symptoms necessitating concussion evaluation. Alternatively, high symptom burden and impairment on VVE in the brain effects category may simply reflect more widespread neural dysfunction as opposed to a particular concussion phenotype. Indeed, one of the most consistent predictors of prolonged recovery is overall symptom burden,27,47 and high correlation across all symptoms is present even in studies that identified concussion phenotypes.44,48
Our study included youth who were between one and 28 days post-concussion, yet biological mechanisms of concussion, including post-traumatic trigeminal sensory sensitivity, likely evolve within the first days to weeks.49,50 Interestingly, we did not find a relationship between days post-concussion and visually evoked effects (Table 2). Further research is needed to elucidate the relationship between migraine, concussion time course, and visually evoked effects.
Conclusions and Future Directions
This study demonstrates an intriguing relationship between brain-predominant visually evoked effects, symptom burden, and reduced visio-vestibular performance after concussion. Although the study was limited by retrospective design, these strong associations warrant further exploration. The presence of visually evoked effects has great potential as an inexpensive, easily implemented, real-time psychophysical metric that could be used on the sideline of a sports field, the emergency department, or outpatient setting to supplement other concussion assessments.
Prospective longitudinal studies are needed to validate this tool. It is not known whether visually evoked effects are a prognostic indicator of prolonged recovery or whether they can predict treatment response to different therapies. In addition, it should be determined whether other neurological conditions like chronic migraine are associated with visually evoked brain effects. It may be that visually evoked brain effects are a biomarker of broad activation of the trigeminal nociceptive pathway across a range of neurological conditions.
Supplementary Material
Acknowledgments
The authors would like to thank Daniele Fedonni, Fairuz Mohammed, Olivia Podolak, and Anne Mozel who were instrumental in data collection and organization that made this work possible.
Funding Information
This work was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health R01NS097549, the Pennsylvania Department of Health SAP100077078, the American Academy of Neurology Clinical Research Scholarship (to C.P.G.), the International Headache Academy Research Fellowship (to C.P.G.), the Minds Matter Frontier Program grant from the Children's Hospital of Philadelphia, and Department of Defense Grant W81XWH-151-0447 (to G.K.A.).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Heitger, M.H., Jones, R.D., Macleod, A.D., Snell, D.L., Frampton, C.M., and Anderson, T.J. (2009). Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132, 2850–2870. [DOI] [PubMed] [Google Scholar]
- 2. Master, C.L., Podolak, O.E., Ciuffreda, K.J., Metzger, K.B., Joshi, N.R., McDonald, C.C., Margulies, S.S., Grady, M.F., and Arbogast, K.B. (2020). Utility of pupillary light reflex metrics as a physiologic biomarker for adolescent sport-related concussion. JAMA Ophthalmol. 138, 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsu, J., Stec, M., Ranaivo, H.R., Srdanovic, N., and Kurup, S.P. (2021). Concussion alters dynamic pupillary light responses in children. J. Child Neurol. 36, 195–202. [DOI] [PubMed] [Google Scholar]
- 4. Taghdiri, F., Chung, J., Irwin, S., Multani, N., Tarazi, A., Ebraheem, A., Khodadadi, M., Goswami, R., Wennberg, R., Mikulis, D., Green, R., Davis, K., Tator, C., Eizenman, M., and Tartaglia, M.C. (2018). Decreased number of self-paced saccades in post-concussion syndrome associated with higher symptom burden and reduced white matter integrity. J. Neurotrauma 35, 719–729. [DOI] [PubMed] [Google Scholar]
- 5. Hunfalvay, M., Roberts, C.M., Murray, N., Tyagi, A., Kelly, H., and Bolte, T. (2019). Horizontal and vertical self-paced saccades as a diagnostic marker of traumatic brain injury. Concussion 4, CNC60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cochrane, G.D., Christy, J.B., Almutairi, A., Busettini, C., Swanson, M.W., and Weise, K.K. (2019). Visuo-oculomotor function and reaction times in athletes with and without concussion. Optom. Vis. Sci. 96, 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Storey, E.P., Corwin, D.J., McDonald, C.C., Arbogast, K.B., Metzger, K.B., Pfeiffer, M.R., Margulies, S.S., Grady, M.F., and Master, C.L. (2021). Assessment of saccades and gaze stability in the diagnosis of pediatric concussion. Clin. J. Sport Med. [Epub ahead of print; DOI: 10.1097/JSM.0000000000000897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Digre, K.B., and Brennan, K.C. (2012). Shedding light on photophobia. J. NeuroOphthalmol. 32, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlow, K.M., Crawford, S., Stevenson, A., Sandhu, S.S., Belanger, F., and Dewey, D. (2010). Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 126, E374–E381. [DOI] [PubMed] [Google Scholar]
- 10. Novak, Z., Aglipay, M., Barrowman, N., Yeates, K.O., Beauchamp, M.H., Gravel, J., Freedman, S.B., Gagnon, I., Gioia, G., Boutis, K., Burns, E., Ledoux, A.A., Osmond, M.H., and Zemek, R.L. (2016). Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 170, e162900. [DOI] [PubMed] [Google Scholar]
- 11. Randolph, C., Millis, S., Barr, W.B., McCrea, M., Guskiewicz, K.M., Hammeke, T.A., and Kelly, J.P. (2009). Concussion symptom inventory: An empirically derived scale for monitoring resolution of symptoms following sport-related concussion. Arch. Clin. Neuropsychol. 24, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkins, A.J. (1995). Visual Stress. Oxford Psychology Series. Oxford University Press: New York. [Google Scholar]
- 13. Yoshimoto, S., Garcia, J., Jiang, F., Wilkins, A.J., Takeuchi, T., and Webster, M.A. (2017). Visual discomfort and flicker. Vision Res. 138, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilkins, A., Nimmo-Smith, I., Tait, A., McManus, C., Della Sala, S. Tilley, A., Arnold, K., Barrie, M., and Scott, S. (1984). A neurological basis for visual discomfort. Brain 107, 989–1017. [DOI] [PubMed] [Google Scholar]
- 15. Chen, W.T., Wang, S.J., Fuh, J.L., Lin, C.P., Ko, Y.C., and Lin, Y.Y. (2011). Persistent ictal-like visual cortical excitability in chronic migraine. Pain 152, 254–258. [DOI] [PubMed] [Google Scholar]
- 16. Cucchiara, B., Datta, R., Aguirre, G.K., Idoko, K.E., and Detre, J. (2015). Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia 35, 585–592. [DOI] [PubMed] [Google Scholar]
- 17. Clark, J., Jacobs, B., Hasselfeld, K., Mangine, R., Ellis, J., Betz, B., Colosimo, A., and Divine, J. (2019). Visual evoked potential and voltage changes associated with acute concussion and frequency specific photophobia. Neurology 93, Suppl. 1, s9. [Google Scholar]
- 18. McCrory, P., Meeuwisse, W., Dvořák, J., Aubry, M., Bailes, J., Broglio, S., Cantu, R.C., Cassidy, D., Echemendia, R.J., Castellani, R.J., Davis, G.A., Ellenbogen, R., Emery, C., Engebretsen, L., Feddermann-Demont, N., Giza, C.C., Guskiewicz, K.M., Herring, S., Iverson, G.L., Johnston, K.M., Kissick, J., Kutcher, J., Leddy, J.J., Maddocks, D., Makdissi, M., Manley, G.T., McCrea, M., Meehan, W.P., Nagahiro, S., Patricios, J., Putukian, M., Schneider, K.J., Sills, A., Tator, C.H., Turner, M., and Vos, P.E. (2017). Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 51, 838–847. [DOI] [PubMed] [Google Scholar]
- 19. Roby, P.R., Metzger, K.B., McDonald, C.C., Corwin, D.J., Huber, C.M., Patton, D.A., Margulies, S.S., Grady, M.F., Master, C.L., and Arbogast, K.B. (2021). Pre- and post-season visio-vestibular function in healthy adolescent athletes. Phys. Sportsmed. 1–9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corwin, D.J., Arbogast, K.B., Swann, C., Haber, R., Grady, M.F., and Master, C.L. (2020). Reliability of the visio-vestibular examination for concussion among providers in a pediatric emergency department. Am. J. Emerg. Med. 38, 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corwin, D.J., Zonfrillo, M.R., Master, C.L., Arbogast, K.B., Grady, M.F., Robinson, R.L., Goodman, A.M., and Wiebe, D.J. (2014). Characteristics of prolonged concussion recovery in a pediatric subspecialty referral population. J. Pediatr. 165, 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corwin, D.J., Zonfrillo, M.R., Wiebe, D.J., Master, C.L., Grady, M.F., and Arbogast, K.B. (2018). Vestibular and oculomotor findings in neurologically-normal, non-concussed children. Brain Inj. 32, 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patterson Gentile, C., Joshi, N.R., Ciuffreda, K.J., Arbogast, K.B., Master, C., and Aguirre, G.K. (2021). Developmental effects on pattern visual evoked potentials characterized by principal component analysis. Transl. Vis. Sci. Technol. 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patterson Gentile, C. (2021, Nov 30), Visually evoked effects. https://github.com/pattersongentilelab/visuallyEvokedEffects (Last accessed February 22, 2022).
- 25. Kuczynski, A., Crawford, S., Bodell, L., Dewey, D., and Barlow, K.M. (2013). Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: a prospective cohort. Dev. Med. Child Neurol. 55, 636–641. [DOI] [PubMed] [Google Scholar]
- 26. Zemek, R., Barrowman, N., Freedman, S.B., Gravel, J., Gagnon, I., McGahern, C., Aglipay, M., Sangha, G., Boutis, K., Beer, D., Craig, W., Burns, E., Farion, K.J., Mikrogianakis, A., Barlow, K., Dubrovsky, A.S., Meeuwisse, W., Gioia, G., Meehan, W.P., Beauchamp, M.H., Kamil, Y., Grool, A.M., Hoshizaki, B., Anderson, P., Brooks, B.L., Yeates, K.O., Vassilyadi, M., Klassen, T., Keightley, M., Richer, L., De Matteo, C., Osmond, M.H., Xie, J., Chatfield, J., Dow, N., Papadimitropoulos, R., Levesque, T., Langford, C., Tran, T.T., Candice McGahern, Vanessa DiGirolamo, Mazza, J., Maryse Lagace, Cook, R., Fitzpatrick, E., Jessica MacIntyre, and Moore, J. (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025. [DOI] [PubMed] [Google Scholar]
- 27. Meehan, W.P., Mannix, R., Monuteaux, M.C., Stein, C.J., and Bachur, R.G. (2014). Early symptom burden predicts recovery after sport-related concussion. Neurology 83, 2204–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panagiotakos, D., and Pitsavos, C. (2004). Interpretation of epidemiological data using multiple correspondence analysis and log-linear models. J. Data Sci. 2, 75–86. [Google Scholar]
- 29. Trujillo-Ortiz, A. (2021). Multiple Correspondence Analysis Based on the Indicator Matrix. https://www.mathworks.com/matlabcentral/fileexchange/22154-multiple-correspondence-analysis-based-on-the-indicator-matrix (Last accessed February 22, 2022).
- 30. ([Date unknown]). Pennsylvania State Data Center Research Brief. Available from: https://pasdc.hbg.psu.edu/sdc/pasdc_files/researchbriefs/June_2019.pdf.
- 31. Napadow, V., Sheehan, J.D., Kim, J., LaCount, L.T., Park, K., Kaptchuk, T.J., Rosen, B.R., and Kuo, B. (2013). The brain circuitry underlying the temporal evolution of nausea in humans. Cereb. Cortex 23, 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messina, R., Filippi, M., and Goadsby, P.J. (2018). Recent advances in headache neuroimaging. Curr. Opin. Neurol. 31, 379–385. [DOI] [PubMed] [Google Scholar]
- 33. Indovina, I., Riccelli, R., Chiarella, G., Petrolo, C., Augimeri, A., Giofrè, L., Lacquaniti, F., Staab, J.P., and Passamonti, L. (2015). Role of the insula and vestibular system in patients with chronic subjective dizziness: an fMRI study using sound-evoked vestibular stimulation. Front. Behav. Neurosci. 9, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashina, M., Hansen, J.M., Do, T.P., Melo-Carrillo, A., Burstein, R., and Moskowitz, M.A. (2019). Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 18, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashina, H., Porreca, F., Anderson, T., Mohammad Amin, F., Ashina, M., Winther Schytz, H., and Dodick, D.W. (2019). Post-traumatic headache: epidemiology and pathophysiological insights. Nat. Rev. Neurol. 15, 607–617. [DOI] [PubMed] [Google Scholar]
- 36. Ossipov, M.H., Morimura, K., and Porreca, F. (2014). Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 8, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bolay, H., Reuter, U., Dunn, A.K., Huang, Z., Boas, D.A., and Moskowitz, M.A. (2002). Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 8, 136–142. [DOI] [PubMed] [Google Scholar]
- 38. Ayata, C., and Lauritzen, M. (2015). Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 95, 953–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashina, H., Iljazi, A., Amin, F.M., Ashina, M., Lipton, R.B., and Schytz, H.W. (2020). Interrelations between migraine-like headache and persistent post-traumatic headache attributed to mild traumatic brain injury: a prospective diary study. J. Headache Pain 21, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau, B.C., Collins, M.W., and Lovell, M.R. (2012). Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery 70, 371–379. [DOI] [PubMed] [Google Scholar]
- 41. Kontos, A.P., Elbin, R.J., Schatz, P., Covassin, T., Henry, L., Pardini, J., and Collins, M.W. (2012). A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am. J. Sports Med. 40, 2375–2384. [DOI] [PubMed] [Google Scholar]
- 42. Ashina, H., Iljazi, A., Al-Khazali, H.M., Ashina, S., Jensen, R.H., Amin, F.M., Ashina, M., and Schytz, H.W. (2020). Persistent post-traumatic headache attributed to mild traumatic brain injury: deep phenotyping and treatment patterns. Cephalalgia 40, 554–564. [DOI] [PubMed] [Google Scholar]
- 43. Kamins, J., Richards, R., Barney, B.J., Locandro, C., Pacchia, C.F., Charles, A.C., Cook, L.J., Gioia, G., Giza, C.C., and Blume, H.K. (2021). Evaluation of posttraumatic headache phenotype and recovery time after youth concussion. JAMA Netw. Open 4, e211312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heyer, G.L., Young, J.A., Rose, S.C., McNally, K.A., and Fischer, A.N. (2016). Post-traumatic headaches correlate with migraine symptoms in youth with concussion. Cephalalgia 36, 309–316. [DOI] [PubMed] [Google Scholar]
- 45. International Headache Society. (2018). The international classification of headache disorders, 3rd edition. Cephalalgia 38, 1–211. [DOI] [PubMed] [Google Scholar]
- 46. Peres, M., Zukerman, E., Young, W., and Silberstein, S. (2002). Fatigue in chronic migraine patients. Cephalalgia 22, 720–724. [DOI] [PubMed] [Google Scholar]
- 47. Houts, C.R., Wirth, R.J., McGinley, J.S., Gwaltney, C., Kassel, E., Snapinn, S., and Cady, R. (2020). Content validity of HIT-6 as a measure of headache impact in people with migraine: a narrative review. Headache 60, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schilling, S., Mansour, A., Sullivan, L., Ding, K., Pommering, T., and Yang, J. (2020). Symptom burden and profiles in concussed children with and without prolonged recovery. Int. J. Environ. Res. Public Health 17, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Navratilova, E., Rau, J., Oyarzo, J., Tien, J., Mackenzie, K., Stratton, J., Remeniuk, B., Schwedt, T., Anderson, T., Dodick, D., and Porreca, F. (2019). CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia 39, 1762–1775. [DOI] [PubMed] [Google Scholar]
- 50. McCrea, M., Broglio, S.P., McAllister, T.W., Gill, J., Giza, C.C., Huber, D.L., Harezlak, J., Cameron, K.L., Houston, M.N., McGinty, G., Jackson, J.C., Guskiewicz, K., Mihalik, J., Brooks, M.A., Duma, S., Rowson, S., Nelson, L.D., Pasquina, P., Meier, T.B., Foroud, T., Katz, B.P., Saykin, A.J., Campbell, D.E., Svoboda, S.J., Goldman, J., and DiFiori, J. (2020). Association of blood biomarkers with acute sport-related concussion in collegiate athletes: findings from the NCAA and Department of Defense CARE Consortium. JAMA Netw. open 3, e1919771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.