Abstract
Purpose: the pathophysiologic mechanisms explaining differences in clinical outcomes following COVID-19 are not completely described. This study aims to investigate antibody responses in critically ill patients with COVID-19 in relation to inflammation, organ failure and 30-day survival. Methods: All patients with PCR-verified COVID-19 and gave consent, and who were admitted to a tertiary Intensive care unit (ICU) in Sweden during March–September 2020 were included. Demography, repeated blood samples and measures of organ function were collected. Analyses of anti-SARS-CoV-2 antibodies (IgM, IgA and IgG) in plasma were performed and correlated to patient outcome and biomarkers of inflammation and organ failure. Results: A total of 115 patients (median age 62 years, 77% male) were included prospectively. All patients developed severe respiratory dysfunction, and 59% were treated with invasive ventilation. Thirty-day mortality was 22.6% for all included patients. Patients negative for any anti-SARS-CoV-2 antibody in plasma during ICU admission had higher 30-day mortality compared to patients positive for antibodies. Patients positive for IgM had more ICU-, ventilator-, renal replacement therapy- and vasoactive medication-free days. IgA antibody concentrations correlated negatively with both SAPS3 and maximal SOFA-score and IgM-levels correlated negatively with SAPS3. Patients with antibody levels below the detection limit had higher plasma levels of extracellular histones on day 1 and elevated levels of kidney and cardiac biomarkers, but showed no signs of increased inflammation, complement activation or cytokine release. After adjusting for age, positive IgM and IgG antibodies were still associated with increased 30-day survival, with odds ratio (OR) 7.1 (1.5–34.4) and 4.2 (1.1–15.7), respectively. Conclusion: In patients with severe COVID-19 requiring intensive care, a poor antibody response is associated with organ failure, systemic histone release and increased 30-day mortality.
Keywords: COVID-19, SARS-CoV-2, critical care, antibody response, NET, histones
1. Background
The ongoing COVID-19 pandemic, caused by the novel SARS-CoV-2 virus, has caused millions of deaths worldwide and left the healthcare system in many countries in the worst crisis for decades. Since the virus phenotype is novel for humans, no patients have previous antibodies specific for the virus, creating a situation where, in theory, all humans are susceptible for infection and severe disease. Despite this, the clinical course of SARS-CoV-2 infection varies substantially, from asymptomatic carriers to severe multiple organ dysfunction syndrome (MODS) and death, probably explained by individual variations in the immune response.
Several risk factors, both for the development of severe disease but also for death, have been identified [1]. Age is the strongest risk factor, but several others such as male sex, cardiovascular disease, obesity, chronic obstructive pulmonary disease (COPD), Alzheimer’s disease and genetic predisposition are now known to increase the risk of poor outcomes following COVID-19 disease [2,3,4,5,6]. Even if part of the increased risk is due to physiologic fragility, for example, very old patients have lower cardiopulmonary reserve to cope with a pneumonia regardless of the causative agent, the immune response to the infection is likely of great importance [7,8]. Several studies have described the immune response during COVID-19 and defined differences between patients developing severe disease and patients with asymptomatic or mild disease [9,10]. However, somewhat conflicting results concerning antibody responses have been presented, perhaps reflecting sampling site, varying cohorts or the timing of blood sampling [11,12,13,14,15,16]. It appears that an adequate, early response from the innate immune system, including expression of type I interferons (IFN), is important for reducing viral replication, allowing the slower adaptive immune system to become fully activated [17]. SARS-CoV-2 has the ability to suppress the expression of type I IFN, and hence inhibit the innate immune response to the virus [18]. A delayed innate immune response might also lead to a longer activation time for the adaptive immune response, since the two systems are dependent on each other for optimal function. We hypothesised that a delayed or absent adaptive immune response in critically ill patients with COVID-19 would cause higher mortality and organ failure. Several groups have also reported that SARS-CoV-2 can induce neutrophil extracellular trap (NET) formation in neutrophiles and that NET-formation could be part of the immunopathology in severe COVID-19 [19,20,21,22,23,24,25].
Our group previously reported that a weak anti-SARS-CoV-2 antibody response was associated with increased mortality in a small cohort of intensive care patients with COVID-19 disease [26]. The aim of this study is to describe the effects of an impaired antibody response, with regard to 30-day survival, organ failure and activation of other parts of the immune system, identifying key pathophysiological mechanisms in a large group of intensive care patients.
2. Materials and Methods
2.1. Study Design
This single centre, prospective observational investigation is a sub-study of the PronMed-study, approved by the Swedish National Ethical Review Agency (EPM; No. 2020-01623). Informed consent was obtained either by the patient or by a next-of-kin if the patient was unable to receive information due to their clinical status. The Declaration of Helsinki and its subsequent revisions were followed. The protocol of the study was registered a priori (ClinicalTrials ID: NCT04316884). STROBE guidelines were followed for reporting.
2.2. Data Collection
All patients admitted to the central intensive care unit at Uppsala University Hospital during the first wave of the pandemic in 2020, with suspected COVID-19 infection, were screened for inclusion.
Background characteristics of the patients were obtained through patients’ electronic medical records. Clinical data were collected prospectively daily. Blood samples were taken at ICU admission and three times per week during the time patients were treated in the ICU. Simplified Acute Physiology Score 3 (SAPS3) on admission and daily Sequential Organ Failure Assessment (SOFA) score were calculated prospectively [27,28]. Acute kidney injury (AKI) was diagnosed according to the Kidney Disease: Improving Global Outcome (KDIGO) creatinine criteria [29].
2.3. Plasma Analyses
Peripheral blood from patients with COVID-19 was collected into EDTA- and citrate-containing tubes and plasma was separated using centrifugation at 3000× g for 10 min. After separation, all plasma samples were stored at −80 °C.
Complete blood cell counts (CBC), plasma C-reactive protein (CRP), procalcitonin, IL-6, fibrin D-dimer, troponin I and N-terminal pro-brain natriuretic peptide (NT-pro-BNP), kidney function tests (plasma creatinine and cystatin C), liver function tests (plasma bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP)) were performed in the hospital central laboratory. CBC was analysed on a Sysmex XN instrument (Sysmex, Kobe, Japan) while plasma CRP, ferritin, troponin I, kidney and liver markers were analysed on an Architect ci16200 (Abbott Laboratories, Abbott Park, IL, USA). IL-6 was measured by a commercial sandwich ELISA kit (D6050, R&D Systems, Minneapolis, MN, USA). IgA, IgG and IgM antibodies against SARS-CoV-2 Spike-1 protein were quantified by FluoroEnzymeImmunoassay (FEIA), Phadia AB, Uppsala, Sweden. The analyses were performed on the last sample obtained during the stay at the ICU but within 30 days from symptoms onset to maximise the probability to discover plasma-antibodies. The lower limit of detection was 5 and 20 ug/L for IgA and IgM, respectively, and 10 U/L for IgG.
Cytokine and complement analyses are described in detail in the Supplementary Material.
SARS-CoV-2 RNA in plasma was determined by reverse transcription qPCR as previously described [30]. For qualitative and quantitative detection of viral RNA, we used the 2019-nCoV N1 reagent set from the published protocol from the Center for Disease Control (CDC) of the United States [31]. For quantitative analysis, the ISO 13485 certified molecular standard Quantitative Synthetic SARS-CoV-2 RNA: ORF, E, N (VR-3276SD, American Tissue Type Collection) was used as external calibrator. The reaction showed linearity over 6 orders of magnitude with 109 copies/mL and 300 copies/mL as the upper and lower limits of quantitative detection, respectively. The viral RNA analyses were performed at samples taken between day 1 and day 7 in the ICU.
2.4. Histone Analyses
The presence of histones was determined via a semi-quantitative Western blotting method as previously described [32,33]. In short, plasma was diluted 10 times and separated via SDS-PAGE gel electrophoresis (4–15% gradient gel), and transferred to a PVDF membrane (Bio-Rad Laboratories, Hemel Hempstead, UK) using semi-dry blotting. After blocking, the membranes were incubated overnight with a primary rabbit anti-histone H3 antibody (1:10,000, sc-8654-R, Santa Cruz Biotechnology, Heidelberg, Germany), followed by 1 h incubation with a secondary biotin-conjugated donkey anti-rabbit IgG antibody (1:10,000, ab97083, Abcam, Cambridge, UK), and 30 min with a streptavidin-biotin complex (1:500, Vectastain, Vector Laboratories, Burlingame, CA, USA). Histone H3 bands were visualised by the WesternBright ECL substrate (Advansta, San Jose, CA, USA) on the iBright CL1500 Imaging System (ThermoFisher Scientific, Waltham, MA, USA). The band densities were quantified by iBright Analysis Software, compared to known standard concentrations of purified calf thymus H3 (Roche, Basel, Switzerland).
2.5. Statistics
Categorical variables are presented as number of observations (percentage of total number of observations) and continuous variables as medians and interquartile range (IQR). Comparison between dichotomous variables were made with Pearson’s Chi2-test or Fischer’s exact test as appropriate. Continuous variables were compared with the Mann–Whitney U test. Correlation between antibody levels and SAPS3/SOFA were assessed with Spearman correlation. Analyses of survival in relation to whether patients were positive or negative for antibodies were further assessed with multiple logistic regression while controlling for age. For calculations and figures, SPSS Statistics software, version 23 (IBM) was used. p < 0.05 was considered significant.
3. Results
Between 13 March and 28 September 2020, 125 patients were included. After the exclusion of patients without verified COVID-19 infection, patients where no blood samples were obtained and patients where the diagnoses of COVID-19 were considered a secondary finding, 115 patients were included in the final analyses. The vast majority (88%) of the patients in the study were admitted during March–May.
3.1. Patient Characteristics
The median age for all patients was 62 years and 77% were male (Table 1). Median time from onset of symptoms until ICU admission was 10 days. Ninety percent of the total cohort developed anti-SARS-CoV-2 antibodies during their time in the ICU. Eighty-nine patients (77%) were alive after 30 days. Five (4%) of the included patients had a known immune deficiency prior to admission, either due to immune suppressive treatment or disease (previous organ transplant, lymphoma or B-cell suppressive treatment). Two of these patients did not develop antibodies and two only expressed IgM and IgM + IgG, respectively. Four of these patients were alive at 30 days from ICU admission. Thus, 110 out of 115 patients had no known reason for impaired antibody responses. For the groups with negative vs. positive SARS-CoV-2 antibodies, there was no difference in median time from ICU admission to blood sampling for antibody analyses.
Table 1.
Patient characteristics.
| All Patients n = 115 |
Alive at 30 Days | |||
|---|---|---|---|---|
| Yes n = 89 |
No n = 26 |
|||
| Age | 62 (52–71) | 57 (51–67) | 73 (68–79) | |
| Male sex | 88 (77%) | 67 (75%) | 21 (81%) | |
| SAPS3 on ICU arrival | 53 (47–57) | 50.5 (46–56) | 60 (55–65) | |
| Days with symptoms on ICU arrival | 10 (8–12) | 10 (9–12) | 10 (8–12) | |
| BMI | 28.6 (25.6–33.2) | 28.8 (26.6–33.8) |
27.4 (23.9–30.8) | |
| Pulmonary disease | 29 (25%) | 21 (24%) | 8 (31%) | |
| Hypertension | 62 (54%) | 41 (46%) | 21 (81%) | |
| Diabetes | 32 (28%) | 24 (46%) | 8 (31%) | |
| Smoker | Ongoing | 7 (6%) | 5 (6%) | 2 (9%) |
| Previous | 20 (18%) | 15 (17%) | 5 (23%) | |
| Alive at 30 days | 89 (77%) | 89 (100%) | 0 (0%) | |
| IgG positive | 98 (85%) | 80 (90% | 18 (69%) | |
| IgA positive | 96 (83%) | 80 (90%) | 16 (62%) | |
| IgM positive | 103 (90%) | 85 (96%) | 18 (69%) | |
Results are expressed as n (%) or median (interquartile range, IQR). Abbreviations: BMI: Body mass index (kg/m2), Alive at 30 days: 30 days from ICU admission. Age counted in years. Antibody-positive: Before ICU discharge.
3.2. Survival and Organ Dysfunction
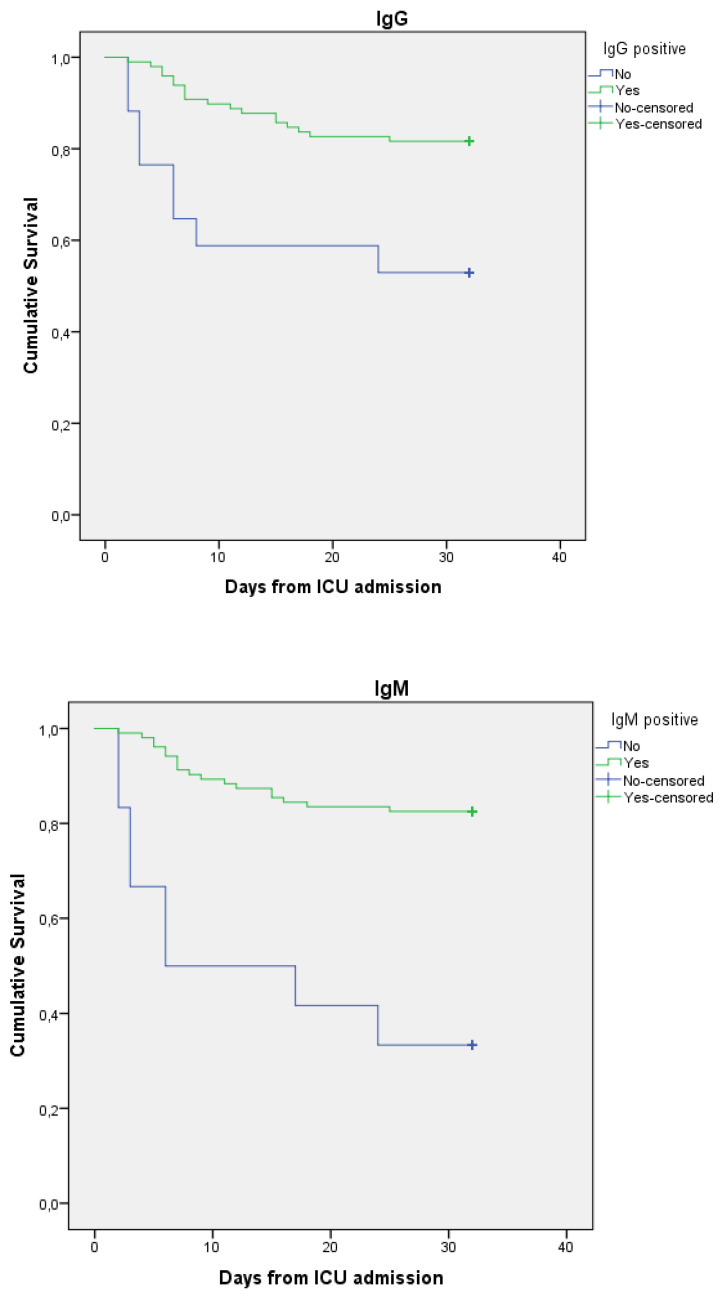
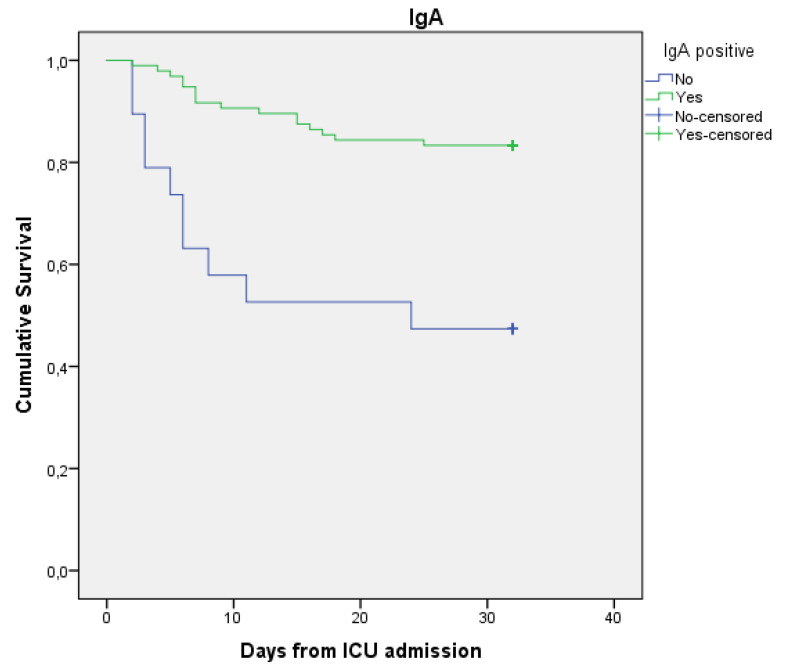
Fifty-nine percent of the included patients were treated with mechanical ventilation and 15% received renal replacement therapy. Patients positive for anti-SARS-CoV2 antibodies had higher 30-day survival (which was the main outcome in the present analysis) compared to patients negative for antibodies (30-day survival for IgM 83% vs. 33%, IgG 82% vs. 53% and IgA 83% vs. 47%). As a complementary analyses, 90-day survival was analysed. This confirmed the findings with higher survival rates in the patient groups positive for anti-SARS-CoV2 antibodies. (Figure 1 and Table 2).
Figure 1.
Kaplan–Meier curves describing survival after hospital admission for patients positive vs. negative for SARS-CoV-2 antibodies in plasma.
Table 2.
Comparison between patients with or without anti-SARS-CoV-2 antibodies.
| All Patients | Iga Positive | p | Igm Positive | p | Igg Positive | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes n = 96 |
No n = 19 |
Yes n = 103 |
No n = 12 |
Yes n = 98 |
No n = 17 |
|||||
| Alive at 30 days | 89 (77%) | 80 (83%) | 9 (47%) | 0.002 | 85 (83%) | 4 (33%) | 0.001 | 80 (82%) | 9 (53%) | 0.02 |
| Alive at 90 days | 84 (73%) | 78 (79%) | 6(38%) | 0.001 | 82 (79%) | 2 (18%) | <0.001 | 79 (79%) | 5 (33%) | 0.01 |
| Male sex | 88 (77%) | 79 (82%) | 9 (47%) | 0.002 | 83 (81%) | 5 (42%) | 0.007 | 79 (81%) | 9 (53%) | 0.03 |
| Thrombotic events | 15 (13%) | 15 (16%) | 0 (0%) | n.s. | 15 (15%) | 0 (0%) | n.s | 15 (15%) | 0 (0%) | n.s. |
| Critical illness | 15 (13%) | 14 (15%) | 1 (5%) | n.s. | 13 (13%) | 2 (17%) | n.s | 14 (14%) | 1 (6%) | n.s. |
| Secondary infection | 58 (51%) | 51 (54%) | 7 (37%) | n.s. | 52 (51%) | 6 (50%) | n.s | 50 (52%) | 8 (47%) | n.s. |
| Vasoactive medication | 75 (65%) | 65 (68%) | 10 (53%) | n.s. | 67 (65%) | 8 (67%) | n.s | 65 (66%) | 10 (59%) | n.s. |
| Invasive ventilation | 68 (59%) | 62 (65%) | 6 (32%) | 0.007 | 61 (59%) | 7 (58%) | n.s | 60 (61%) | 8 (47%) | n.s. |
| Renal replacement therapy | 17 (15%) | 16 (17%) | 1 (5%) | n.s. | 15 (15%) | 2 (17%) | n.s | 16 (16%) | 1 (6%) | n.s. |
| AKI | 68 (62%) | 57 (62%) | 11 (65%) | n.s. | 61 (62%) | 7 (64%) | n.s | 60 (63%) | 8 (62%) | n.s. |
| Severe AKI | 19 (17%) | 17 (18%) | 2 (12%) | n.s. | 18 (18%) | 1 (9%) | n.s | 19 (20%) | 0 (0%) | n.s. |
| SARS-CoV-2 Plasma | 57 (64%) | 53 (64%) | 4 (67%) | n.s. | 52 (63%) | 5 (83%) | n.s | 50 (62%) | 7 (88%) | n.s. |
| Days with symptoms on ICU arrival | 10 (8–12) | 10 (8–12) | 10 (8–13) | n.s. | 10 (9–12) | 9 (7–11) | n.s. | 10 (9–12) | 9 (7–11) | n.s. |
| BMI | 28.6 (25.6–33.2) |
29.0 (26.6–33.4) |
26.4 (22.9–29.2) |
n.s. | 28.6 (25.6–32.8) |
28.7 (26.4–38.3) |
n.s. | 28.7 (26.2–33.4) |
26.9 (22.9–32.3) |
n.s. |
Data are expressed as n (%) or median (interquartile range, IQR). Statistically significant differences between groups marked in red. Antibody-positive: Before ICU discharge. Abbreviations: Alive at 30 days: 30 days from ICU admission. AKI: Acute kidney injury. Severe AKI: AKI ≥ stage III. SARS-CoV-2 plasma: Patients with SARS-CoV-2 virus detected in plasma. BMI: Body mass index (kg/m2). n.s.: Not significant. Groups compared with Z-test or Mann-Whiney U test.
Patients positive for IgM also had more ICU-free days, ventilator-free days, renal replacement-free days and vasoactive medication-free days. For IgG and IgA, antibody-positive patients had more renal replacement-free days. (Table 3)
Table 3.
Organ support in relation to positivity for anti-SARS-CoV-2 antibodies.
| IgM Positive | ||||
| Total | Yes n = 103 |
No n = 12 |
p | |
|---|---|---|---|---|
| ICU-free days | 17 (0–24) | 18 (0–24) | 0 (0–0) | 0.002 |
| RRT-free days | 30 (15–30) | 30 (28–30) | 0 (0–15) | <0.001 |
| Ventilator-free days | 24 (6–30) | 25 (15–30) | 0 (0–11) | 0.002 |
| Vasoactive-free days | 25 (15–30) | 26 (19–30) | 0 (0–19) | 0.002 |
| Lowest p/f-ratio | 78.8 (69.8–95.3) | 78.8 (69.8–95.3) | 76.5 (66.0–105.5) | n.s. |
| SARS-CoV-2 plasma (copies/mL) | 0 (0–800) | 0 (0–800) | 600 (0–1100) | n.s. |
| IgG positive | ||||
| Total | Yes n = 98 |
No n = 17 |
p | |
| ICU-free days | 17 (0–24) | 18 (0–23) | 0 (0–26) | n.s. |
| RRT-free days | 30 (15–30) | 30 (28–30) | 0 (0–30) | 0.012 |
| Ventilator-free days | 24 (6–30) | 25 (12–30) | 11 (0–30) | n.s. |
| Vasoactive-free days | 25 (15–30) | 26 (19–30) | 19 (0–30) | n.s. |
| Lowest p/f-ratio | 78.8 (69.8–95.3) | 78.0 (69.8–94.5) | 89.3 (68.3–105.8) | n.s. |
| SARS-CoV-2 plasma (copies/mL) | 0 (0–800) | 0 (0–800) | 450 (0–2000) | n.s. |
| IgA positive | ||||
| Total | Yes n = 96 |
No n = 19 |
p | |
| ICU-free days | 17 (0–24) | 18 (0–23) | 0 (0–27) | n.s. |
| RRT-free days | 30 (15–30) | 30 (26–30) | 30 (0–30) | 0.039 |
| Ventilator-free days | 24 (6–30) | 24 (12–30) | 21 (0–30) | n.s. |
| Vasoactive-free days | 25 (15–30) | 26 (19–30) | 22 (0–30) | n.s. |
| Lowest p/f-ratio | 78.8 (69.8–95.3) | 78.0 (69.0)–93.0) | 89.3 (70.5–113.3) | n.s. |
| SARS-CoV-2 plasma (copies/mL) | 0 (0–800) | 0 (0–800) | 150 (0–2600) | n.s. |
Data are expressed as median (interquartile range, IQR). Statistically significant differences between groups marked in red. Antibody-positive: Before ICU discharge. Abbreviations: RRT: Renal replacement therapy. Vasoactive-free days: Days without vasoactive treatment. p/f-ratio: mmHg/FiO2 SARS-CoV-2 plasma: Viral copies in plasma.
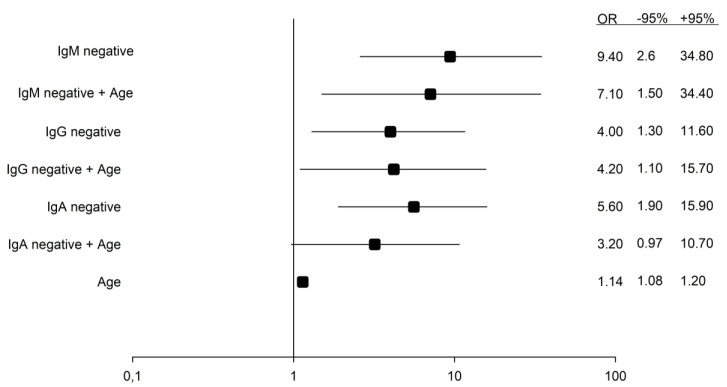
In a simple logistic regression model, odds ratios (confidence interval, CI) for 30-day survival were 9.4 (2.6–34.8), 4.0 (1.3–11.6) and 5.6 (1.9–15.9) for patients positive for IgM, IgG and IgA, respectively. When adjusting for age in a multiple logistic regression model, positive tests for IgM and IgG antibodies were still correlated with higher OR for 30-day survival, whereas no difference between the groups were seen for IgA. (Figure 2)
Figure 2.
Odds ratios for death in single and multiple logistic regression models with antibody negativity and patient age as independent variables. Increasing age (counted in years) is associated with higher mortality rates.
In the correlation analyses, IgA antibody concentrations correlated negatively with both SAPS3 (r = −0.233, p = 0.013) and maximal SOFA score (r = −0.231, p = 0.014). No correlation was seen between IgG and SAPS3 or SOFA whereas the levels of IgM antibodies correlated negatively with SAPS3 (r = −0.231, p = 0.014).
3.3. Plasma Biomarkers in Relation to Antibody Response
Next, we analysed plasma biomarkers and their relation to antibody response. Clinical chemistry tests and blood cell counts analysed during ICU care were extracted from the patients’ medical records, and ICU entry and peak values were calculated.
To corroborate the association between antibody response and organ support, biomarkers of organ failure were compared among antibody-negative and -positive patients. Significant associations were observed for kidney (Creatinine, Cystatin C) and cardiac biomarkers (NT-proBNP). A prominent activation of the coagulation system with elevated D-dimer and platelet counts is an important feature of severe COVID-19 [34]. However, antibody-negative subjects had significantly lower platelet counts, and a non-significant trend towards lower D-dimer levels was observed. Furthermore, antibody-negative patients had lower CRP levels, indicating an attenuation of systemic inflammation in patients with an impaired antibody response.
To further characterise the immune response in relation to antibody positivity, biomarkers of the innate immune system, including cytokines, systemic histone release and complement activation, and white blood cell differential counts were analysed.
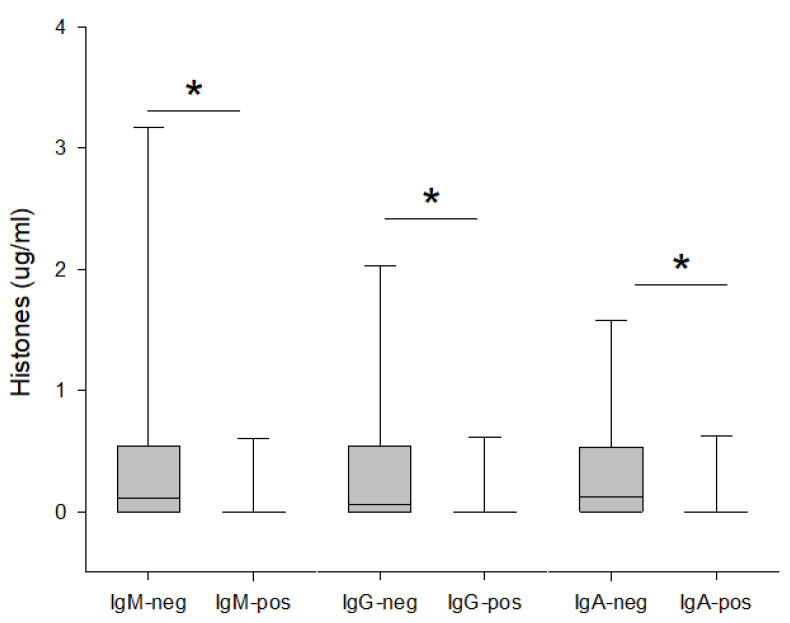
Antibody-negative patients had significantly higher plasma levels of extracellular histones on day 1 compared to antibody-positive patients, and elevated extracellular histone levels were observed irrespective of the antibody class (Figure 3). We could not find strong associations between antibody levels in plasma and any of the analysed cytokines (Supplementary Material).
Figure 3.
Concentration of Histones H3 in plasma in relation to positivity/negativity for SARS-CoV-2 antibodies in plasma. Antibody-positive: Before ICU discharge. Statistically significant differences between groups are marked by *.
Complement factors and activation markers were analysed in plasma samples taken upon ICU admission (Supplementary Material). There was no difference in the degree of complement activation between antibody-positive and -negative patients, as measured by C3a, C3d and sC5b9 levels in plasma. In contrast, IgG and IgA antibody-negative patients had significantly lower circulating levels of intact complement factor C3 and factor B.
4. Discussion
The main finding of this study is that a poor antibody response is associated with increased mortality in patients with severe SARS-CoV-2-infection. Furthermore, patients with poor antibody response have higher rates of organ failure based on SAPS3 and SOFA score, even if the correlations are weak and should be interpreted with caution, and also based on the duration of organ support such as renal replacement therapy and vasoactive medication.
These clinical associations were corroborated by biomarker analyses, where the strongest associations were seen for kidney function and cardiac biomarkers. In particular, antibody-negative patients had strongly elevated NT-proBNP levels during ICU care, which could indicate an increased rate of circulatory failure in agreement with a trend towards longer duration of vasoactive support in these patients.
The strongest signal in our cohort for the associations with outcome is seen for IgM antibodies and it is reasonable to believe that this is due to the natural course of the B-cell development in the germinal centre (GC) reaction. Additionally, IgM, in its pentameric form, has the highest complement-activating capacity among all immunoglobulin subclasses, thus it has a very high neutralising capacity [35].
There was no significant difference in the concentration of viral copies in blood between the analysed groups, although patients negative for antibodies in this study did have numerically higher levels of viral copies in blood. The non-significance might reflect a lack of power in the study, and it would be interesting to analyse this in a larger group of ICU patients. However, we have previously described a lack of strong association between viremia and organ failure in COVID-19 [30,36].
As the production of antibodies is a result of a close and simultaneous collaboration between the innate and adaptive immune systems, alterations in the innate immune system due to age, gender and/or genetic variations will skew the adaptive responses in different directions [37]. It has been previously described that COVID-19 patients who develop a mild disease responds with a fast antibody response of short duration. Patients with severe disease instead have a slower antibody response with a longer duration [9]. Measurable antigen-specific antibodies in plasma from patients are a result of a successful GC-reaction. When it comes to viral infections, the GC-reaction is dependent on antigen-specific T-cells [38]. T-cell studies on patients with mild versus severe COVID-19 have in both cases shown a robust specific T-cell response (both CD4+ and CD8+ T-cells) but the T-cell phenotypes (e.g., cytokine production and dominant T-cell subset) differ between the two disease severity groups [39,40]. It is thus plausible that differences in T-cell responses would mirror any difference in priming of the GC-reaction and hence the antibody response. From the results in our study, it seems that among patients developing severe the disease who require intensive care, patients with a higher antibody response have a better chance of survival, suggesting that an adequate response from both T- and B-cells is of great importance in the defence against SARS-CoV-2 infections. The individual differences in the total immune response to the virus causing COVID-19 might be due to several, not mutually exclusive, mechanisms, e.g., it is described that the SARS-CoV-2 virus can inhibit the initial innate immune response through suppression of type I IFN [41]. This can lead to a slower activation of the adaptive immune system. In patients with an inherent poor type I IFN-response this effect can give rise to a greater initial viral replication causing more severe disease [42]. Thus, the slow activation of the adaptive immune system may reflect an initial suboptimal innate immune response.
This hypothesis is supported by our biomarker analysis, which by several measures indicated a lower degree of inflammation in antibody-negative subjects, possibly due to an unknown inborn or acquired immune defect.
Our complement analyses revealed no differences in complement activation between antibody-negative and -positive subjects, but they demonstrated significantly lower circulating levels of intact complement protein C3 and Factor B in antibody-negative patients. These factors are well-known to display an acute phase response pattern and are elevated during systemic inflammation, hence lower plasma levels are agreement with an attenuated inflammatory response in antibody-negative subjects. Furthermore, a recent study on bronkoalveolar lavage in patients with severe COVID-19 describes a more aggravated local immune response in the lung, suggesting plasma analyses might be misleading in compement analyses [43].
Another hypothesis is that patients with a suboptimal adaptive immune response, instead, develop a more powerful non-specific innate immune response, partly supported by previous research [44], with increased neutrophil activation and a powerful activation of the complement system [45]. We could not find any sign of increased complement activation or cytokine release in patients with poor antibody response, but those patients had more circulating cell-free histones. SARS-CoV-2 is able to induce neutrophil extracellular trap (NET) formation in healthy neutrophils, suggesting a role for NETs in driving cytokine release, respiratory failure, and microvascular injury in COVID-19 [19,20]. In this study, patients that did not express anti-SARS-CoV-2 antibodies in the ICU had increased extracellular histones in their blood at ICU admission, which may signal systemic neutrophil activation and NET formation [21]. Although we only measured histone H3, this could be seen as a proxy for all other histones. However, it is possible that the increased levels of histones are a marker of unspecific cell damage and are not linked to NET formation. Previous research shows that the number of neutrophils and markers of NETosis are elevated in severe COVID-19 patients [22], and a link has been suggested between NETosis and poor outcome [23], indicating a crucial contribution of NETs to the severity of the COVID-19 disease [24,25]. An association has also been described between anti-SARS-CoV-2 IgA2, NET formation and poor outcome [46]. As lymphopenia and inadequate T-cell responses are found to be predictors of COVID-19 severity, [47] our findings of an association between poor antibody response and amount of histones in blood in ICU patients suggests a link between poor adaptive immune activation and NET formation. Furthermore, patients with impaired antibody responses and increased number of free histones also have lower thrombocyte values. This could be an indirect sign of platelet consumption in NET-containing microthrombi as neutrophil-platelet infiltration has been observed in pulmonary autopsies from COVID-19 patients [48].
Our findings that a poor antibody response is associated with a poor outcome in ICU patients with COVID-19 could have clinical implications. If we could identify patients that are likely to have a slow antibody response early in the disease process, these might be the patients benefiting the most from immune-modulating treatment, as studies, in which patients treated as early as 72 h after COVID-19 diagnosis with convalescent plasma or monoclonal anti-SARS-CoV-2 antibodies, have shown [49,50]. In addition, specific organ failure, such as AKI, is common in severe COVID-19 and may cause long-term effects in surviving patients [51,52]. Together with common clinical variables, these data may prove useful in improving recognition of patients at risk of extra pulmonary organ failure.
The strength of this study is the large number of intensive care patients included and the high quality of clinical data prospectively collected in combination with analyses of both antibodies, cytokines, and components of the complement system. The major weakness is that samples for antibody analyses were not taken on the same day as for the other analyses; instead, we chose to analyse the last available blood sample taken in the ICU. The reason for this was to maximise the chance to have samples taken after the patients had developed an antibody response and hopefully also switched antibody class. This might have impacted the results, but there was no difference between the compared groups in median time from admission to sample day.
5. Conclusions
This study confirms that a weak anti-SARS-CoV-2 antibody response in intensive care patients with COVID-19 is associated with increased 30-day mortality and our results suggest that this may be due to multiple organ dysfunction and NETosis.
Acknowledgments
We thank the study nurses Joanna Wessbergh and Elin Söderman for their expertise in compiling the study. We are also indebted to the biobank research assistants Erik Danielsson, Labolina Spång, Amanda Svensson and Philip Karlsson for organising the sample analysis and the Uppsala Intensive Care COVID-19 research group: Sara Bülow Anderberg, Tomas Luther, Katja Hanslin, Anna Gradin, Sarah Galien and Jacob Rosén for patient inclusion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11123419/s1, Methods describing analyses of cytokines end complement factors. Tables over complement factors and complement activation factors, clinical chemistry tests including inflammation and organ damage markers. Figure describing correlation of cytokine concentration and antibody concentration. Reference [53] is cited in the supplementary materials.
Author Contributions
R.L, B.K., K.A., A.F., M.H., M.L., G.A.F.N. and R.F. conceived the study. B.K., A.L., O.E., B.P., J.B.H., A.B. and S.F. analysed the blood samples. R.L., M.H., O.E. and R.F. performed data analysis. R.L. drafted the manuscript. All authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Informed consent was obtained either by the patient or by a next-of-kin if the patient was unable to receive information due to their clinical status.
Data Availability Statement
Individual level data is available from the authors on reasonable request as detailed at https://doi.org/10.17044/scilifelab.14229410.v1.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
SciLifeLab/KAW national COVID-19 research program project grant (KAW 2020.0182 and KAW 2020.0241) and from the Swedish Heart-Lung Foundation (20210089) to MH. The Swedish Research Council (2014-02569 and 2014-07606) and The Swedish Kidney Foundation (F2020-0054) to RF. The study was supported by the Netherlands Thrombosis Foundation (2016_01) and Thrombosestichting (2016-1) to GN. OE was supported by grants from the Göran Gustafsson Foundation, the Swedish Society of Medicine (SLS-943007) and the Swedish Research Council (2015-06429).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niemi M.E., Karjalainen J., Liao R.G., Neale B.M., Daly M., Ganna A., Pathak G.A., Andrews S.J., Kanai M., Veerapen K., et al. Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlström B., Frithiof R., Hultström M., Larsson I.M., Strandberg G., Lipcsey M. The swedish COVID-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol. Scand. 2021;65:525–533. doi: 10.1111/aas.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakanishi T., Pigazzini S., Degenhardt F., Cordioli M., Butler-Laporte G., Maya-Miles D., Bujanda L., Bouysran Y., Niemi M.E., Palom A., et al. Age-dependent impact of the major common genetic risk factor for COVID-19 on severity and mortality. medRxiv: The preprint server for health sciences. J. Clin. Investig. 2021;131 doi: 10.1172/JCI152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderberg S.B., Luther T., Berglund M., Larsson R., Rubertsson S., Lipcsey M., Larsson A., Frithiof R., Hultström M. Increased levels of plasma cytokines and correlations to organ failure and 30-day mortality in critically ill COVID-19 patients. Cytokine. 2021;138:155389. doi: 10.1016/j.cyto.2020.155389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson O., Hultström M., Persson B., Lipcsey M., Ekdahl K.N., Nilsson B., Frithiof R. Mannose-Binding Lectin is Associated with Thrombosis and Coagulopathy in Critically Ill COVID-19 Patients. Thrombosis and haemostasis. 2020;120:1720–1724. doi: 10.1055/s-0040-1715835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 8.Gradin A., Andersson H., Luther T., Anderberg S.B., Rubertsson S., Lipcsey M., Åberg M., Larsson A., Frithiof R., Hultström M. Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients. Cytokine. 2021;146:155589. doi: 10.1016/j.cyto.2021.155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsetti R., Zaffina S., Piano Mortari E., Terreri S., Corrente F., Capponi C., Palomba P., Mirabella M., Cascioli S., Palange P., et al. Different innate and adaptive immune response to SARS-CoV-2 infection of asymptomatic, mild and severe cases. Front. Immunol. 2020;11:610300. doi: 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5:eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowitdamrong E., Puthanakit T., Jantarabenjakul W., Prompetchara E., Suchartlikitwong P., Putcharoen O., Hirankarn N. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS ONE. 2020;15:e0240502. doi: 10.1371/journal.pone.0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlesinger T., Weißbrich B., Wedekink F., Notz Q., Herrmann J., Krone M., Sitter M., Schmid B., Kredel M., Stumpner J., et al. Biodistribution and serologic response in SARS-CoV-2 induced ARDS: A cohort study. PLoS ONE. 2020;15:e0242917. doi: 10.1371/journal.pone.0242917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K., Huang B., Wu M., Zhong A., Li L., Cai Y., Wang Z., Wu L., Zhu M., Li J., et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020;11:6044. doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham J.L., Virhammar J., Rönnberg B., Castro Dopico X., Kolstad L., Albinsson B., Kumlien E., Nääs A., Klang A., Westman G., et al. Anti-SARS-CoV2 antibody responses in serum and cerebrospinal fluid of COVID-19 patients with neurological symptoms. J. Infect. Dis. 2022;225:965–970. doi: 10.1093/infdis/jiab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Zhang L., Sang L., Ye F., Ruan S., Zhong B., Song T., Alshukairi A.N., Chen R., Zhang Z., et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020;130:5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dispinseri S., Secchi M., Pirillo M.F., Tolazzi M., Borghi M., Brigatti C., De Angelis M.L., Baratella M., Bazzigaluppi E., Venturi G., et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021;12:2670. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S., Butler-Laporte G., Nakanishi T., Morrison D.R., Afilalo J., Afilalo M., Laurent L., Pietzner M., Kerrison N., Zhao K., et al. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nat. Med. 2021;27:659–667. doi: 10.1038/s41591-021-01281-1. [DOI] [PubMed] [Google Scholar]
- 18.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathologySARS-CoV-2 directly triggers ACE-dependent NETs. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 22.Bülow Anderberg S., Lipcsey M., Hultström M., Eriksson A.K., Venge P., Frithiof R. Systemic Human Neutrophil Lipocalin Associates with Severe Acute Kidney Injury in SARS-CoV-2 Pneumonia. J. Clin. Med. 2021;10:4144. doi: 10.3390/jcm10184144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cani E., Dwivedi D.J., Liaw K.-L., Fraser D.D., Yeh C.H., Martin C., Slessarev M., Cerroni S.E., Fox-Robichaud A.A., Weitz J.I., et al. Immunothrombosis Biomarkers for Distinguishing Coronavirus Disease 2019 Patients From Noncoronavirus Disease Septic Patients with Pneumonia and for Predicting ICU Mortality. Crit. Care Explor. 2021;3:e0588. doi: 10.1097/CCE.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asif S., Frithiof R., Lipcsey M., Kristensen B., Alving K., Hultström M. Weak anti-SARS-CoV-2 antibody response is associated with mortality in a Swedish cohort of COVID-19 patients in critical care. Crit. Care. 2020;24:639. doi: 10.1186/s13054-020-03362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno R.P., Metnitz P.G.H., Almeida E., Jordan B., Bauer P., Campos R.A., Iapichino G., Edbrooke D., Capuzzo M., Le Gall J.-R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensiv. Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., Reinhart C.K., Suter P., Thijs L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 29.Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L. Section 2: AKI Definition. Kidney Int. Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Järhult J.D., Hultström M., Bergqvist A., Frithiof R., Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci. Rep. 2021;11:7163. doi: 10.1038/s41598-021-86500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC Research Use Only 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Primers and Probes. [(accessed on 1 March 2020)];2020 Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html.
- 32.Wildhagen K.C., Wiewel M.A., Schultz M.J., Horn J., Schrijver R., Reutelingsperger C.P., van der Poll T., Nicolaes G.A. Extracellular histone H3 levels are inversely correlated with antithrombin levels and platelet counts and are associated with mortality in sepsis patients. Thromb. Res. 2015;136:542–547. doi: 10.1016/j.thromres.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Huckriede J., Vries F.D., Hultström M., Wichapong K., Reutelingsperger C., Lipcsey M., Garcia de Frutos P., Frithiof R., Nicolaes G.A. Histone H3 Cleavage in Severe COVID-19 ICU Patients. Front. Cell. Infect. Microbiol. 2021;11:694186. doi: 10.3389/fcimb.2021.694186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlowski C., Wagner T., Puranik A., Murugadoss K., Loscalzo L., Venkatakrishnan A., Pruthi R.K., Houghton D., O’Horo J.C., Morice W.G., et al. Inference from longitudinal laboratory tests characterizes temporal evolution of COVID-19-associated coagulopathy (CAC) eLife. 2020;9:e59209. doi: 10.7554/eLife.59209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czajkowsky D.M., Shao Z. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc. Natl. Acad. Sci. USA. 2009;106:14960–14965. doi: 10.1073/pnas.0903805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frithiof R., Bergqvist A., Järhult J.D., Lipcsey M., Hultström M. Presence of SARS-CoV-2 in urine is rare and not associated with acute kidney injury in critically ill COVID-19 patients. Crit. Care. 2020;24:587. doi: 10.1186/s13054-020-03302-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gubbels Bupp M.R., Potluri T., Fink A.L., Klein S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Victora G.D. SnapShot: The germinal center reaction. Cell. 2014;159:700–700.e1. doi: 10.1016/j.cell.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T., Sigal N., Feng Y., Bristow L., Tsang O.T.-Y., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermejo-Martin J.F., González-Rivera M., Almansa R., Micheloud D., Tedim A.P., Domínguez-Gil M., Resino S., Martín-Fernández M., Murua P.R., Pérez-García F., et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care. 2020;24:691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nossent E.J., Schuurman A.R., Reijnders T.D., Saris A., Jongerius I., Blok S.G., de Vries H., Duitman J., Noordegraaf A.V., Meijboom L.J., et al. Pulmonary Procoagulant and Innate Immune Responses in Critically Ill COVID-19 Patients. Front. Immunol. 2021;12:664209. doi: 10.3389/fimmu.2021.664209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen N.A.F., Grondman I., de Nooijer A.H., Boahen C.K., Koeken V.A.C.M., Matzaraki V., Kumar V., He X., Kox M., Koenen H.J.P.M., et al. Dysregulated Innate and Adaptive Immune Responses Discriminate Disease Severity in COVID-19. J. Infect. Dis. 2021;223:1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karawajczyk M., Douhan Håkansson L., Lipcsey M., Hultström M., Pauksens K., Frithiof R., Larsson A. High expression of neutrophil and monocyte CD64 with simultaneous lack of upregulation of adhesion receptors CD11b, CD162, CD15, CD65 on neutrophils in severe COVID-19. Ther. Adv. Infect. Dis. 2021;8:20499361211034065. doi: 10.1177/20499361211034065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staats L.A., Pfeiffer H., Knopf J., Lindemann A., Fürst J., Kremer A.E., Hackstein H., Neurath M.F., Muñoz L.E., Achenbach S., et al. IgA2 Antibodies against SARS-CoV-2 Correlate with NET Formation and Fatal Outcome in Severely Diseased COVID-19 Patients. Cells. 2020;9:2676. doi: 10.3390/cells9122676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Libster R., Marc G.P., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., et al. Early High-Titer Plasma Therapy to Prevent Severe COVID-19 in Older Adults. New Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luther T., Bülow-Anderberg S., Larsson A., Rubertsson S., Lipcsey M., Frithiof R., Hultström M. COVID-19 patients in intensive care develop predominantly oliguric acute kidney injury. Acta Anaesthesiol. Scand. 2020;65:364–372. doi: 10.1111/aas.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hultström M., Lipcsey M., Wallin E., Larsson I.-M., Larsson A., Frithiof R. Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit. Care. 2021;25:37. doi: 10.1186/s13054-021-03461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipcsey M., Persson B., Eriksson O., Blom A.M., Fromell K., Hultström M., Huber-Lang M., Ekdahl K.N., Frithiof R., Nilsson B. The Outcome of Critically Ill COVID-19 Patients Is Linked to Thromboinflammation Dominated by the Kallikrein/Kinin System. Front. Immunol. 2021;12:627579. doi: 10.3389/fimmu.2021.627579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual level data is available from the authors on reasonable request as detailed at https://doi.org/10.17044/scilifelab.14229410.v1.