Abstract
Interventional Radiology (IR) has experienced an exponential growth in recent years. Technological advances of the last decades have made it possible to use new treatments on a larger scale, with good results in terms of safety and effectiveness. In musculoskeletal field, painful bone metastases are the most common target of IR palliative treatments; however, in selected cases of bone metastases, IR may play a curative role, also in combination with other techniques (surgery, radiation and oncology therapies, etc.). Primary malignant bone tumors are extremely rare compared with secondary bone lesions: osteosarcoma, Ewing sarcoma, and chondrosarcoma are the most common; however, the role of interventional radiology in this fiels is marginal. In this review, the main techniques used in interventional radiology were examined, and advantages and limitations illustrated. Techniques of ablation (Radiofrequency, Microwaves, Cryoablation as also magnetic resonance imaging-guided high-intensity focused ultrasound), embolization, and Cementoplasty will be described. The techniques of ablation work by destruction of pathological tissue by thermal energy (by an increase of temperature up to 90 °C with the exception of the Cryoablation that works by freezing the tissue up to −40 °C). Embolization creates an ischemic necrosis by the occlusion of the arterial vessels that feed the tumor. Finally, cementoplasty has the aim of strengthening bone segment weakened by the growth of pathological tissue through the injection of cement. The results of the treatments performed so far were also assessed and presented focused the attention on the management of bone metastasis.
Keywords: interventional radiology, bone metastases, tumour embolization, thermal ablation, magnetic resonance imaging-guided high-intensity focused ultrasound, electrochemotherapy
1. Introduction
Interventional radiology deals, in the context of radiodiagnostics, with more invasive diagnostic and therapeutic procedures than the execution of diagnostic examinations, for example with computed tomography (CT) and magnetic resonance imaging (MRI). It involves a surgical-like approach but with a minimally invasive profile because the procedures are usually performed by specific needles and/or through vessels.
IR in malignant bone lesions is mainly aimed at the treatment of bone metastases due to the clear epidemiological prevalence compared to primary tumors.
The incidence of metastatic bone disease is approximately 280,000 new cases per year [1]. Bone is the third most common site of metastatic disease after lungs and liver. Bone metastasis (BM) is the major cause of morbidity in patients with cancer. Apart from pain, complications like pathological fractures and spinal cord compression may occur, worsening patients’ quality of life and prognosis. Fifty percent of the painful symptomatology experienced by patients with cancer originates from BM [1,2,3,4]. In case of tumoral extension to neural structures, pain may be radicular (exacerbated by percussion or palpation) and/or mechanical (exacerbated by movement) [5,6]. In advanced stages of the disease, pain can become intolerable and refractory to conventional therapies, causing walking disability, psychological and functional impact, and significantly impairing quality of life [2,3,4,7]. The approach to the patient with BM must be strictly multidisciplinary with the involvement of all professional figures (oncologist, radiotherapist, orthopedic, and pain therapy specialist) to offer the patient the best treatments. In the decision making for surgical and/or medical treatment, life expectancy of patients with metastatic bone disease is the most important factor; a short life expectancy suggests the use of less invasive procedures, that bring lower complication rates and shorter rehabilitation times [7]. In this context, interventional radiology procedures play an important primary or complementary role in the management of BMs [8,9,10], especially in terms of palliation of pain and treatment of fractures. Short life expectancies more often require palliative rather than curative regimens. Therefore, rapid pain relief has become a priority. Several conventional treatment options have been described, including opioids, hormone therapy, chemotherapy, radiation therapy, and surgery, all of which have side effects and contraindications. Radiation therapy (RT) remains the gold standard, but up to 20% of patients do not respond; moreover, the greatest reported benefit is received only after 5–20 weeks from completion of treatment [6,11,12]. Over the last two decades, percutaneous image-guided interventional techniques have emerged with satisfactory results in the management of painful bone metastases. The thermal ablation techniques allow loco-regional tumor ablation while cementoplasty stabilizes the bone segments weakened by pathological tissue [13,14,15]. These techniques can also be used with a combined approach [16,17,18]. Percutaneous bone ablation is a minimally invasive treatment offering several advantages. It is repeatable, does not need skin exposure, as in the case of radiotherapy and has no interference with systemic treatments.
Aim of our study was to evaluate, through the review of the recent literature, the efficacy and duration of pain and local tumor control using interventional radiology procedures in the treatment of both primary and metastatic bone tumors.
Primary bone tumors are relatively rare compared with secondary (skeletal metastatic) disease [19,20,21]. They represent less than 1% of all cancers in adults. Usually, IR in this field is reserved to non operable patients or adiuvant treatments.
2. Interventional Radiology—Techniques
The most common techniques for management of patients with metastatic bone disease include embolization and thermal ablation therapies (Table 1).
Table 1.
Techniques of IR and their applications.
| Interventional Radiology Techniques | ||||
|---|---|---|---|---|
| Techniques | Type | Aim of the Technique | Indications | Curative Treatment |
| Chemical ablation | Ethanol, Doxycycline, Polidocanol (detergent sclerosant, STS (detergent sclerosant) | Chemical-based ablation |
|
|
| Embolization | Nano-particles | Minimizing blood loss |
|
|
| RFA | Energy-based ablations (thermoablation) | Ablation techniques (inducing tumoral necrosis) needle driven |
|
√ |
| MWA | Energy-based ablations (thermoablation) | Ablation techniques (inducing tumoral necrosis) needle driven |
|
√ |
| CA | Energy-based ablations (thermoablation) | Ablation techniques (inducing tumoral necrosis) needle driven |
|
√ |
| MRgFUS | Energy-based ablations (thermoablation) | Ablation techniques (inducing tumoral necrosis) needleless technique |
|
√ |
| Cementoplasty | Cementoplasty (PMMA) including free-hand injection, vertebroplasty, kyphoplasty | Mechanical stabilization techniques |
|
|
| Osteosynthesis | Intramedullary nails and screws | Mechanical stabilization techniques |
|
|
-
1.
Embolization
Embolization is performed for the treatment of bone lesions either alone or in combination with other techniques [2,3,7]. It reduces hypervascularization of the lesions by injection of embolic agents into the vessels, causing necrosis of the tumors. The embolic agents can be used for preoperative transarterial embolization (TAE) and for palliative cases. Nano-particles are most used agents for bone devascularization. The choice of particle diameter (40–1200 micron) depends on vessel size and desired distal embolization [5,22]. This procedure is performed in the angio-suite. A peripheral artery is cannuled (most commonly femoral or radial artery). Navigating through the arterial circulation, the lesion is reached and the arterial vessels that feeds the lesion are embolized.
Indications
TAE can be used in case of hyper-vascular BM for the following scenarios:
To reduce blood deficit during surgery; in this case, TAE should be executed within 3 days from surgery to lower the risk of tumor revascularization [3,4].
To lower pain and natural bleeding of tumors that cannot gain benefit from surgical or percutaneous therapy.
To reduce tumor vascularization before percutaneous ablation and reduce the heat/cold-sink effect.
-
2.
Thermal ablation
Percutaneous thermal ablation was introduced in the clinical practice as a palliative treatment of painful bone metastases in the early 2000 [23,24]. It is defined “percutaneous” because the energy pass through the skin, the soft tissues and the bone using a needle or a needleless technique (magnetic resonance imaging-guided high-intensity focused ultrasound MRgFUS). The most used techniques include radiofrequency thermal ablation (RFA), cryoablation (CA), laser and microwaves thermal ablation (MWA) [7]. The main advantage is the possibility to create an ablation area of known and reproducible size. Ethanol injection is a precursor of thermal ablation and is considered a technique of chemical ablation: through direct injection of alcohol into the lesion, it produces tumor necrosis directly through cellular dehydration and indirectly through vascular thrombosis and tissue ischaemia. This technique does not allow full control of the ablation, since the diffusion of ethanol is not fully predictable or reproducible [7].
Thermal ablation causes a fast change in the temperature of cells, thus damaging their membranes and generating necrosis or coagulation or both. We can distinguish two types of techniques: “needle driven”, when a needle is inserted into the lesion to drive energy (RFA, MW, or CA) or needleless technique, like High Intensity Focused Ultrasound (HIFU), where an ultrasound beam passes through the tissue and is focused on the target causing the ablation; in this case no needles are used.
2.1. Radiofrequency Ablation
RFA is the most widely employed thermoablation method with proved high success rates, not only in the treatment of liver, lung, and kidney tumors, but also for the treatment of bone and soft tissue tumors, for skeletal issues and local tumor management [23]. RFA is usually employed in combination with CT for a rapid and correct placement and control of the ablation electrodes [9]. The procedure is painful, therefore patients are usually under general anesthesia or periferical blocks. RF probes with active tips able to create different sizes of ablation (from 0.5 to 4–5 cm) are typically positioned in the center of the tumor. If some critical structures (like nerves or spinal cord in case of vertebral lesions) are too close to the lesion, the area of ablation can be reduced. This may increase the risk of relapse or uncomplete treatment [6,10]. Before ablation, it is possible to obtain biopsy samples using a coaxial approach. Ablations are performed for a total of 6–10 min at a temperature of 80-to-95 °C, depending on the manufacturers. RFA can also be safely combined with cement injection (cementoplasty), with excellent results in pain palliation and bone stabilization at various skeletal sites, in particular the vertebral bodies [13,25,26,27,28,29]. When treating bone metastasis, the role of RFA is not only limited to pain palliation; the technique is in fact offering promising results also in local tumor management. In this case, the ablation area should exceed the whole lesion of some millimeters [13,27,30]. This last rule should be observed also in case of treating bone tumors others than bone metastasis. On the other hand, in case of benign lesions (osteoid osteoma OO, osteoblastoma OB, etc.) only the area covered by the lesion is treated.
Indications:
Benign tumors (OO, OB, desmoid tumors, chondroblastoma, and gigantocellular tumors of the bones GCTs [31,32,33,34]).
Malignant tumors (skeletal metastases, myeloma, soft tissue metastases, and plasmocytomas) also combined with cementoplasty [13,35,36,37].
Neurovascular diseases related to the musculoskeletal system: Use for palliative pain relief; targeting locoregional neural supply, neuromas, neuroendocrine metastases, hemangioendotheliomas, and intramuscular hemangioma [38,39].
2.2. Microwave Ablation (MWA)
MWA can induce effective coagulation quicker than other ablation techniques and spread out deeper thanks to the characteristics of the created energy. It is less time consuming and provides better results on deeper and larger lesions than other ablation techniques [40,41,42]. As in RFA, an ablation needle is introduced into the center of tumors and MWA is performed with variable energies and times (averagely, from 3 to 10 min) depending on the size of the lesion. Compared to other ablation techniques, the penetration of MWA is deeper, and more resistant to the effects of heat buildup and charring [43,44,45].
Indications:
Benign OO: MWA can reliably treat OO, with no recognized complications or recurrence; less evidence then RFA.
Malignant (skeletal metastases, multiple myeloma, soft tissue metastases, and plasmocytomas).
2.3. Cooling Techniques: Cryoablation
Cryoablation freezes the tumour core with extremely cold temperatures. The tip of the probe employed generates temperatures that reach −40 °C and even less. A −20 °C temperature induces cell destruction and trigger apoptosis and focal sudden cellular ischemia. The gas used to trigger freezing is argon or helium (both inert gases) delivered through small cryoprobes to induce rapid freezing and thawing of target tissues according to the Joule–Thompson effect [46,47,48]. CT and MRI are preferred as techniques of guidance because it is possible to monitor the area of ablation that is called “ice ball” to ensure a full coverage of the tumor and minimizing complications to the surrounding vital structures [49,50].
Indications:
Benign: Extra-abdominal desmoid tumors, OO, OB, Aneurismal Bone Cists, primary bone tumors, neuroma and arteriovenous malformations (AVMs) and chondroblastoma.
Malignant: Skeletal metastases (even osteoblastic and sclerotic; myeloma, soft tissue metastases, plasmocytomas, and in-transit melanoma metastases.
Neurovascular: Palliative; neuroendocrine metastases, hemangioendotheliomas, and benign neural tumors such as Morton’s neuroma.
2.4. Non-Percutaneous Thermal Ablation Procedures: High-Intensity Focused Ultrasound Ablation (HIFU)
HIFU uses high intensity focused ultrasound beams, that act as vehicle of energy and burn the lesion in depth. Combined with MRI, the technique is called Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound (MRgFUS) [43,51]. MRgFUS is a totally non-invasive method of ablation able to reach temperatures up to 90 °C, resulting in coagulative necrosis [52,53]. So far, this technique has been applied in the treatment of diseases involving bladder, prostate, uterine tumors, and bone metastases. MRgFUS is a safe and effective non-invasive treatment option for pain-inducing metastatic bone lesions refractory to radiation therapy with >70% of patients experiencing variable pain reduction [54,55]. Some limits are represented by presence of thick cortical bones (being an obstacle to the propagation of the ultrasound beam), patients with great mass index increasing the distance between the skin surface and the lesion, and lesions situated <1 cm from the skin increasing the risk of skin burns. In real time during the procedure, it is possible to measure the temperatures reached in the focal zone of ablation and verify whether more energy is needed to perform the procedure successfully [56,57,58].
Indications:
Benign: OO and OB can be successfully treated with MRgFUS with complete pain relief and no morbidity; extra-abdominal desmoid tumors.
Malignant: metastases and/or multiple myeloma, plasmocytoma, and other focal myeloproliferative disorders
2.5. Stabilizers
2.5.1. Cementoplasty
The procedure consists of the injection of polymethyl methacrylate (PMMA) within a vertebra or another bone segment fractured or weakend by pathological tissue. The cement strengthens the bone and reduces mechanic pain. PMMA is injected once the needle is positioned in the bone lesion (using fluoroscopy or CT). The consistency of the cement increases slighthly and hardens after approximately 10 min during the polymerization phase. This is accompanied by an exothermic reaction with the temperature peak increasing up to 75 °C in the middle of the treated bone [59,60]. Vertebroplasty is currently the most adequate local treatment to quickly stabilize vertebral fractures or vertebrae at risk of fracture, to reduce pain and improve quality of life [61,62]. Cementoplasty is contraindicated in patients with coagulation problems, extensive osteolytic destruction, especially on the posterior border of the target vertebral body, poor general conditions, periodic presence of local or systemic infections, allergy to bone cement, and asymptomatic vertebral compression [63,64,65,66]. Because vertebroplasty is only aimed at treating the pain and consolidating the weight-bearing bone, other specific tumor treatments should be performed in combination for tumor management [13,67,68,69], in particular ablation for bone tumors.
The major indications for vertebroplasty in oncological settings are:
Patients with severe pain and neurological damage caused by spinal lesions, and resistance to conventional treatments.
Patients with spinal instability caused by spinal lesions within the vertebral body.
Patients with contraindications to open surgery.
2.5.2. Percutaneous Osteosynthesis
Percutaneous osteosynthesis consists in the insertion of screws for the fixation of minimally displaced or non-displaced fractures, and consolidation of fractures [70,71]. The indication is reserved to cancer patients, who are not candidates for surgery and have limited life expectancy [72]. The procedure is performed under fluoroscopic guidance or CT, with planning of the screw trajectory and skin entry points. The procedure takes approximately two hours, therefore general anaesthesia is usually performed. Surgery is preferred to percutaneous osteosynthesis whenever possible since the long-term effectiveness of the latter is still to be investigated [73,74].
2.6. General Requirements Needing Careful Analysis before IR Procedures
Despite the goal of treatment, metastatic bone disease does not require biopsy before interventional sessions, unless:
-
-
The primary cancer is unknown;
-
-
More than two primary tumors are suspected;
-
-
Specimen analysis is crucial to adjust systemic therapy.
In these cases, a biopsy can be easily performed prior to each percutaneous procedure [75].
Before undergoing IR procedures, each patient should have normal recent laboratory tests including blood cells count, coagulation tests (prothrombin time, activated partial prothrombin time and international normalized ratio) and normal kidney function. In case of known or suspected local or systemic infection, any type of IR procedure should be postponed until the infection is over. Each IR bone procedure needs a strictly sterile environment and prophylactic intra-operative antibiotic coverage (1 g cefazolina ev).
2.7. Follow-Up after IR Procedures
Clinical and radiological follow-up should be scheduled on a regular basis according to the aim and type of treatment. It is important that radiological follow-up be performed by dedicated radiologists having at least basic knowledge in the field of extra-vascular IR.
Despite the goal of treatment, clinical evaluation should assess any change in pain experienced by the patient as compared to the pre-treatment status. The most applied test is the 0–10 points VAS. Furthermore, patient independence in daily life activities and quality of life should be evaluated by applying dedicated tests like the Functional Independence Measure or the Karnofsky Performance Score. Additionally, some authors also evaluate the consumption of analgesics including opioids [12,76] limited by side effects such as constipation, sedation, and nausea.
In case of palliative treatments, clinical evaluation may be sufficient (unless unexpected adverse events are suspected) and clinical and radiological follow-up should be adapted according to the specific local evolution of the treated lesion. For instance, clinical suspicion of secondary fracture needs careful investigation with imaging exams (X-ray or CT). No contrast medium administration is needed if CT scan is applied.
On the other hand, radiological assessment is mandatory to evaluate the results of curative treatment by depicting any residual viable tumor. In this perspective, contrast- enhanced imaging (CT, MR, or PET-CT) is needed to exclude any tumor size increase and contrast medium uptake, as compared to baseline imaging. The techniques of choice are MR and PET-CT.
3. Literature Search Strategy and Results—Interventional Strategies in Bone Metastases
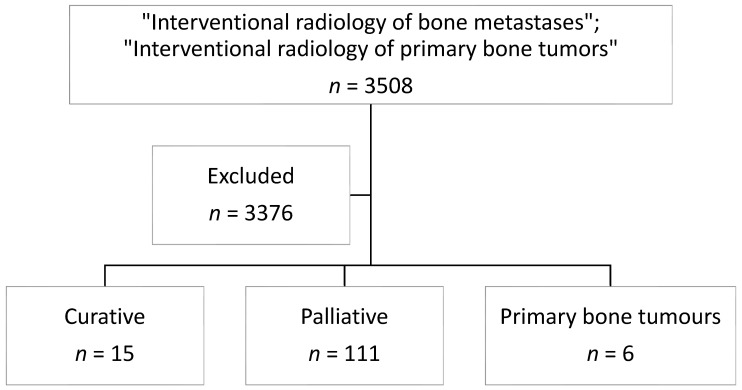
Research was conducted on PubMed library with the following key words “Interventional radiology of primary bone tumour” and “Interventional radiology of bone metastases”. Filters applied were publication years between 1990 and 2022 and other languages except English. Duplicate results were excluded. Abstracts were excluded. In the initial screening, 3508 articles were identified. We excluded 3376 articles because the treatments were not performed percutaneously. We also excluded meta-analysis and systematic reviews. The resulting 132 articles were divided into three groups: curative treatment (n = 15), palliative (n = 111), and primary bone tumor (n = 6). A flowchart summarizing study selection is shown in Figure 1. Baseline characteristics of included studies are summarized in Table 2, Table 3, Table 4 and Table 5. The analysis was performed following the Quality Improvement guidelines for bone metastasis management issued by the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), indicating that interventional treatments not only offer local tumor control, but also effective pain palliation [75]. All the results of our research are listed in the Table 2, Table 3 and Table 4; the most recent results are discussed in each section.
Figure 1.
Flowchart summarizing the selection of studies in the literature.
Table 2.
Results of curative ablation.
| Author | Journal | Year | Ablation Modality | Title | No. of Patients/(no. of Tumors) | % Local Control | Survival Rate Overall Survival (%/Years; Median Month | Follow-Up (Months) | % Complications |
|---|---|---|---|---|---|---|---|---|---|
| Cazzato R. et al. [77] | Diagn Interv Imaging | 2021 | RFA, CA | Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control | 23 | 7 (30%) | NR | 21 | 22 |
| Cazzato R. et al. [28] | International Journal of Hyperthermia | 2018 | RFA + CA | Percutaneous image-guided ablation of bone metastases: local tumor control in oligometastatic patients | 46 | 71.7% 1 year | 95.4% 2 years | 34 | 10 |
| Ma Y. et al. [78] | Cardiovasc Intervent Radiol | 2018 | CA, RFA, MWA | Percutaneous Image-Guided Ablation in the Treatment of Osseous Metastases from Non-small Cell Lung Cancer | 45 (76) | 68% 1 year | NR | NR | 2.6 |
| Vaswani D. et al. [79] | Cardiovasc Intervent Radiol | 2018 | RFA, CA | Radiographic Local Tumor Control and Pain Palliation of Sarcoma Metastases within the Musculoskeletal System with Percutaneous Thermal Ablation | 13 (13) | 100% 1 year | NR | 12 | 5 |
| Gardner C. et al. [15] | J Bone Joint Surg Am | 2017 | CA | Cryoablation of Bone Metastases from Renal Cell Carcinoma for Local Tumor Control | 40 (50) | 41/50 (82%) | 31 (77/1 year 26/5 years) | 35 | 4 (8) |
| Erie A. et al. [80] | J Vasc Interv Radiol | 2017 | RFA, CA | Retrospective Review of Percutaneous Image-Guided Ablation of Oligometastatic Prostate Cancer: A Single-Institution Experience | 16 (18) | 15 (83%) | 100/2 years | 27 | 0 |
| Aubry S. et al. [81] | Eur Radiol | 2017 | MWA | Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours | 13 (16) | 4 (36.3%) | NR | 12 | 0 |
| Wallace A. et al. [13] | AJNR Am J Neuroradiol | 2016 | CA | Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation | 56 (92) | 79% 1 year | NR | NR | 4.3 |
| Tomasian A. et al. [82] | AJNR Am J Neuroradiol | 2016 | CA | Spine Cryoablation: Pain Palliation and Local Tumor Control for Vertebral Metastases | 14 (31) | 30 (96.7%) | NR | 10 | 0 |
| Wallace A. et al. [13] | AJNR Am J Neuroradiol | 2016 | RFA | Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation | NR (55) | 70% 1 year | NR | 7.9 | 0 |
| Barral M. et al. [30] | Cardiovasc Intervent Radiol | 2016 | RFA, CRIO, MWA | Percutaneous Thermal Ablation of Breast Cancer Metastases in Oligometastatic Patients | 79/114;18/NR | 83/1 year; 76/2 years | 98.3/1 year, 95.5/2 years | 18.4 | 12 (10.5) |
| Deschamps F. et al. [83] | Eur Radiol | 2014 | RFA, CA | Thermal ablation techniques: a curative treatment of bone metastases in selected patients? | 89 (122) | 67% 1 year | 91/1 year | 22.8 | 11 (9) |
| McMenomy B. et al [84]. | J Vasc Interv Radiol | 2013 | CA | Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission | 40 (52) | 45 (87%) | 91/1 year, 84/2 years | 21 | 2 (5) |
| Littrup P. et al. [85]. | J Vasc Interv Radiol | 2013 | CA | Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes | 126/251 | 225 (90%) | NR | 11 | 5 (2.3) |
| Bang H. et al. [48]. | J Vasc Interv Radiol | 2012 | CA | Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: initial survival, local control, and cost observations | 8 (18) | 17 (94%) | NR | 11 | 2 (11) |
| Bang H. et al. [86]. | J Vasc Interv Radiol | 2012 | CA | Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation | 27 (48) | 47 (97%) | NR | 16 | 1 (2) |
NR not reported, CA cryoablation, RFA radiofrequency ablation, MWA Microwave ablation.
Table 3.
Results of palliative ablation and combined of the treatment of bone fracture and impending fracture, main results.
| Author | Journal | Year | Ablation Modality | Title | No. of Patients (No. of Tumors) | No. (%) of Patients with Reduced Pain |
Mean Pain Score Change | Survival Rate Overall Survival (%/Years; Median Month) | Follow-Up (Months) | % Major Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Arrigoni F. et al. [87] | Radiol Med | 2022 | CA | CT-guided cryoablation for management of bone metastases: a single center experience and review of the literature | 28 | 100% | 6.9–3.5 (3.4/10) | 100% 3 months | 3 | 0 |
| Wang F. et al. [16] | Skeletal Radiol | 2022 | RFA + PVP | The combination of radiofrequency ablation and vertebroplasty shows advantages over single vertebroplasty in treating vertebral neoplastic lesions | 35 | NR | 8.46–1.7 (6.76/10) | NR | 6 | 0 |
| Jennings J. et al. [88] | Radiol Imaging Cancer | 2021 | CA | Cryoablation for Palliation of Painful Bone Metastases: The MOTION Multicenter Study | 66 | 74% | 7.3–3.7 (3.6/10) | NR | 6 | 4.6 |
| Madani K. et al. [89] | Support Care Cancer | 2021 | RFA + PVP | Combined local treatments for vertebral metastases with limited epidural extension | 18 (24) | 66.7% | 7.3–2 (5.3/10) | 73% 2 years | 16.7 | 0 |
| Kastler A. et al. [90] | Medicina (Kaunas) | 2021 | RFA + PVP | Bipolar Radiofrequency Ablation of Painful Spinal Bone Metastases Performed under Local Anesthesia: Feasibility Regarding Patient’s Experience and Pain Outcome | 25 | 83% | 8.4–1.8 (6.6/10) | 100% 1 year | 12 | 0 |
| Pusceddu C. et al. [91] | Curr Oncol | 2021 | PVP + RFA | The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases | 35 (41) | NR | 5.7–0.9 (4.8/10) | 72% 1 year | 19 | 0 |
| Levy J. et al. [14] | J Vasc Interv Radiol | 2020 | RFA | Radiofrequency Ablation for the Palliative Treatment of Bone Metastases: Outcomes from the Multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 Patients | 100 (134) | 100% | 8.2–3.5 (4.7/10) | 70% 6 months | 6 | 4 |
| Jiao D. et al. [18] | Acad Radiol | 2020 | MWA + PC | Simultaneous C-arm Computed Tomography-Guided Microwave Ablation and Cementoplasty in Patients with Painful Osteolytic Bone Metastases: A Single-center Experience | 30 (42) | 100% | 7.4–1.3 (6.1/10) | 66.7 1 year | 12 | 0 |
| Yang Y. et al. [92] | Cryobiology | 2020 | CA | Retrospective analysis of CT-guided percutaneous cryoablation for treatment of painful osteolytic bone metastasis | 26 (36) | 94.4% | 7.1–1.8 (5.3/10) | 100% 6 months | 6 | 11,5 |
| Yang X. et al. [93] | Eur J Radiol | 2020 | PVP | Vesselplasty using the Mesh-Hold™ bone-filling container for the treatment of pathological vertebral fractures due to osteolytic metastases: A retrospective study | 63 (105) | 97.4% | 8.2–2.1 (6.1/10) | 66.7% 1 year | 4–30 months | 16.2 cement leakage rate;1.9 paravertebral vein embolism |
| De Marini P.et al. [94] | Int J Hyperthermia | 2020 | RFA + CA | Percutaneous image-guided thermal ablation of bone metastases: a retrospective propensity study comparing the safety profile of radio-frequency ablation and cryo-ablation | 274 | NR | NR | 99,64% 1 year | 18.5 | 2.5 |
| Deib G. et al. [95] | AJR Am J Roentgenol | 2019 | MWA + PVP | Percutaneous Microwave Ablation and Cementoplasty: Clinical Utility in the Treatment of Painful Extraspinal Osseous Metastatic Disease and Myeloma | 65 (77) | NR | 6.32–2 (4,32/10) | 90.7% 1 year | 6 | 0 |
| Tanigawa N. et al. [96] | Cardiovasc Intervent Radiol | 2018 | RFA | Phase I/II Study of Radiofrequency Ablation for Painful Bone Metastases: Japan Interventional Radiology in Oncology Study Group 0208 | 33 (33) | 69.7% | 6–1.2 (4.8/10) | 97% 1 year | 12 | 12 |
| Prologo J. et al. [97] | Skeletal Radiol | 2014 | RFA | Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience | 50 (54) | 94% | 8–3 (5/10) | 100% 3 months | 3 | 8 |
| Pusceddu C. et al. [76] | J Vasc Interv Radiol | 2013 | MWA | Treatment of bone metastases with microwave thermal ablation | 18 (21) | 100% | 5.6–0.45 (5.15/10) | 100% 3 months | 3 | 0 |
| Rossi G. et al. [98] | Radiol Med | 2013 | TAE using N-2-butyl cyanoacrylate (NBCA) | Embolisation of bone metastases from renal cancer | 107 (163) | 96% | NR | 10 months | 48 | Post embolisation syndrome 9.2%; Transient paraesthesias 25%; 1 intraprocedural tear of the left L3 artery and iliopsoas haemorrhage |
NR not reported, CA cryoablation, RFA radiofrequency ablation, MWA Microwave ablation, HIFU High-intensity focused ultrasound, TAE Transarterial embolization, PVP Percutaneous vertebroplasty, PC percutaneous cementoplasty.
Table 4.
Results of palliative ablation and combined of the treatment of bone fracture and impending fracture.
| Author | Journal | Year | Ablation Modality | Title | No. of Patients(no. of Tumors) |
|---|---|---|---|---|---|
| Cazzato R. et al. [77] | Diagn Interv Imaging | 2021 | RFA/CA | Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control follow-up | 23 |
| Pusceddu C. et al. [17] | Medicina (Kaunas) | 2021 | MWA + PC | Combined Microwave Ablation and Osteosynthesis for Long Bone Metastases | 11 |
| Campanacci L. et al. [99] | Eur J Surg Oncol | 2021 | ECT | Operating procedures for electrochemotherapy in bone metastases: Results from a multicenter prospective study on 102 patients | 102 |
| Koirala N. et al. [100] | Skeletal Radiol | 2020 | PC | Percutaneous reinforced osteoplasty for long bone metastases: a feasibility study | 15 |
| Giles S. et al. [101] | J Vasc Interv Radiol | 2019 | HIFU | Comparison of Imaging Changes and Pain Responses in Patients with Intra- or Extraosseous Bone Metastases Treated Palliatively with Magnetic Resonance-Guided High-Intensity-Focused Ultrasound | 21 |
| Sundararajan S. et al. [102] | J Oncol | 2019 | TAE+CRIO | Sequential Interventional Management of Osseous Neoplasms via Embolization, Cryoablation, and Osteoplasty cooling system | 15 |
| Tian Q. et al. [103] | Korean J Radiol | 2019 | PSP | Percutaneous Sacroplasty for Painful Sacral Metastases Involving Multiple Sacral Vertebral Bodies: Initial Experience with an Interpedicular Approach | 10 |
| Liu H. et al. [104] | Cardiovasc Intervent Radiol | 2019 | POP | Application of Percutaneous Osteoplasty in Treating Pelvic Bone Metastases: Efficacy and Safety | 126 |
| Cazzato R. et al. [105] | Int J Hyperthermia | 2018 | RFA | Low-power bipolar radiofrequency ablation and vertebral augmentation for the palliative treatment of spinal malignancies | 11 (11) |
| Chen Z. et al. [106] | Orthop Surg | 2018 | HIFU | Evaluation of Quality of Life Using EORTC QLQ-BM22 in Patients with Bone Metastases after Treatment with Magnetic Resonance Guided Focused Ultrasound | 26 |
| Khan M. et al. [107] | AJNR | 2018 | MWA | Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma | 69 (102) |
| Couraud G. et al. [108] | J bone oncol | 2018 | PC | Evaluation of short-term efficacy of extraspinal cementoplasty for bone metastasis: a monocenter study of 31 patients. | 31 |
| Fares A. et al. [109] | J Egypt Natl Canc Inst | 2018 | PC | Combined percutaneous radiofrequency ablation and cementoplasty for the treatment of extraspinal painful bone metastases: a prospective study. J Egypt Natl Canc | 30 |
| Bertrand A. et al. [110] | J Ther Ultrasound | 2018 | HIFU | Focused ultrasound for the treatment of bone metastases: effectiveness and feasibility | 17 |
| Ma Y. et al. [78] | Cardiovasc Intervent Radiol | 2018 | RFA/CA/MWA | Percutaneous Image-Guided Ablation in the Treatment of Osseous Metastases from Non-small Cell Lung Cancer | 45 |
| Vaswani D. et al. [79] | Cardiovasc Intervent Radiol | 2018 | CA + PC | Radiographic Local Tumor Control and Pain Palliation of Sarcoma Metastases within the Musculoskeletal System with Percutaneous Thermal Ablation | 41 |
| Coupal T. et al. [111] | Pain Physician | 2017 | TAE | The Hopeless Case? Palliative Cryoablation and Cementoplasty Procedures for Palliation of Large Pelvic Bone Metastases | 48 |
| Pusceddu C. et al. [112] | Skeletal Radiol | 2017 | PC | CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture | 27 |
| Reyad R. et al. [113] | Diagn Interv Imaging | 2017 | PVP | Thick cement usage in percutaneous vertebroplasty for malignant vertebral fractures at high risk for cement leakage | 77 |
| Motta A. et al. [114] | Eur J Radiol | 2017 | CA | Feasibility of percutaneous cryoablation of vertebral metastases under local anaesthesia in ASAIII patients | 11 |
| McArthur T. et al. [115] | Curr Probl Diagn Radiol | 2017 | CA | Percutane Image-Guided Cryoablation of Painful Osseous Metastases: A Retrospective Single-Center Review | 16 |
| Baagla S. et al. [116] | Cardiovasc Intervent Radiol | 2016 | RFA | Multicenter Prospective Clinical Series Evaluating Radiofrequency Ablation in the Treatment of Painful Spine Metastases | 50 (69) |
| Tomasian A. et al. [82] | AJNR Am J Neuroradiol | 2016 | CA | Spine Cryoablation: Pain Palliation and Local Tumor Control for Vertebral Metastases | 14 (31) |
| Facchini G. et al. [117] | Eur J Orthop Surg Traumatol | 2016 | TAE | Palliative embolization for metastases of the spine | 164 |
| Pusceddu C. et al. [118] | Cardiovasc Intervent Radiol | 2016 | MWA + PC | Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture follow-up | 35 (37) |
| Anzidei M. et al. [119] | Radiol Med | 2016 | HIFU | Magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: role of apparent diffusion coefficient (ADC) and dynamic contrast enhanced (DCE) MRI | 23 |
| Wang F. et al. [120] | Pain Physician | 2016 | TAE | Sequential Transarterial Embolization Followed by Percutaneous Vertebroplasty Is Safe and Effective in Pain Management in Vertebral Metastases | 25 |
| Chen F. et al. [121] | Oncol Lett | 2016 | RFA | Percutaneous kyphoplasty for the treatment of spinal metastases | 282 |
| Susa M. et al. [47] | BMC Cancer | 2016 | CA | CT guided cryoablation for locally recurrent or metastatic bone and soft tissue tumor: initial experience | 9 |
| Bianchi G. et al. [122] | World J Surg | 2016 | ECT | Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial | 29 |
| Jiao D. et al. [123] | Oncotarget | 2016 | RFA | Radiofrequency ablation versus 125I-seed brachytherapy for painful metastases involving the bone | 79 |
| Joo B. et al. [124] | Yonsei Med J | 2015 | HIFU | Pain palliation in patients with bone metastases using magnetic resonance-guided focused ultrasound with conformal bone system: a preliminary report | 5 |
| Wallace A. et al. [125] | J Neurooncol | 2015 | RFA | Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases | 72 (110) |
| Wei Z. et al. [126] | Skeletal Radiol | 2015 | MWA | Computed tomography-guided percutaneous microwave ablation combined with osteoplasty for palliative treatment of painful extraspinal bone metastases from lung cancer | 26 (33) |
| Di Staso M. et al. [127] | PLoS One | 2015 | CA + RTA | Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: Propensity matching analysis in 175 patients | 175 |
| Cazzato R. et al. [128] | Eur J Surg Oncol | 2015 | RFA/PC/CA | Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer | 25 (49) |
| Tian Q. et al. [129] | J Vasc Interv Radiol | 2014 | RFA + pop | Combination radiofrequency ablation and percutaneous osteoplasty for palliative treatment of painful extraspinal bone metastasis: a single-center experience | 38 |
| Kastler A. et al. [130] | J Vasc Interv Radiol | 2014 | MWA | Microwave thermal ablation of spinal metastatic bone tumors | 17 (20) |
| Hurwitz M. et al. [131] | J Natl Cancer Inst | 2014 | HIFU | Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results | 112/NR versus 35/NR |
| Alemann G. et al. [132] | J Palliat Med | 2014 | RFA | Treatment of painful extraspinal bone metastases with percutaneous bipolar radiofrequency under local anesthesia: feasibility and efficacy in twenty-eight cases | 28 |
| Botsa E. et al. [133] | Ann Palliat Med | 2014 | RFA/MWA | CT image guided thermal ablation techniques for palliation of painful bone metastases | 45 |
| Li Z. et al. [134] | Chin Med J (Engl) | 2014 | PVP | Kyphoplasty versus vertebroplasty for the treatment of malignant vertebral compression fractures caused by metastases: a retrospective study | 80 |
| Deschamps F. et al. [83] | Eur Radiol | 2014 | RFA + CA | Thermal ablation techniques: a curative treatment of bone metastases in selected patients? | 89 |
| Li F. et al. [135] | Pathol Oncol Res. | 2014 | CA | An Effective Therapy to Painful Bone Metastases: Cryoablation Combined with Zoledronic Acid | 56 |
| Sun G. et al. [136] | Eur Radiol | 2014 | PC | Cementoplasty for man- aging painful bone metastases outside the spine | 51 |
| Callstrom M. et al. [137] | Cancer | 2013 | CA | Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial | 61 (69) |
| Napoli A. et al. [138] | invest Radiol | 2013 | HIFU | Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound | 18 (18) |
| Anselmetti G. et al. [139] | Pain Physician | 2013 | PVP | Percutaneous vertebral augmentation assisted by PEEK implant in painful osteolytic vertebral metastasis involving the vertebral wall: experience on 40 patients | 40 |
| Trumm C. et al. [140] | Skeletal Radiol | 2013 | PVP | CT fluoroscopy-guided vertebral augmentation with a radiofrequency-induced, high-viscosity bone cement (StabiliT(®)): technical results and polymethylmethacrylate leakages in 25 patients | 25 |
| Kastler A. et al. [141] | Pain Med | 2013 | MWA | Analgesic effects of microwave ablation of bone and soft tissue tumors under local anesthesia | 15 (25) |
| Masala S. et al. [60] | Neuroradiology. | 2012 | CA | Combined use of percutaneous cryoablation and vertebroplasty with 3D rotational angiograph in treatment of single vertebral metastasis: Comparison with vertebroplasty. | 23 |
| Iannessi A. et al. [142] | Diagn Interv Imaging | 2012 | PC | Percutaneous cementoplasty for the treatment of extraspinal painful bone lesion: a prospective study. | 20 |
| Rossi G. et al. [143] | Radiol Med | 2011 | TAE | Selective arterial embolisation for bone tumours: experience of 454 cases | 365 (454) |
| Masala S. et al. [144] | Singapore Med J | 2011 | RFA + VP/CA + VP | Percutaneous ablative treatment of metastatic bone tumours: visual analogue scale scores in a short-term series | 30 |
| Thacker P. et al. [46] | AJR Am J Roentgenol. | 2011 | CA | Palliation of painful metastatic disease involving bone with imaging-guided treatment: Comparison of patients’ immediate response to radiofrequency ablation and cryoablation | 36 |
| Masala S. et al. [145] | Skeletal Radiol. | 2011 | CA | Metabolic and clinical assessment of efficacy of cryoablation therapy on skeletal masses by 18F-FDG positron emission tomography/computed tomography (PET/CT) and visual analogue scale (VAS): Initial experience | 20 |
| Masala S. et al. [146] | Support Care Cancer | 2011 | PC | Percutaneus osteoplasty in the treatment of extraspinal painful multiple myeloma lesions | 39 |
| Rossi G. et al. [5] | J Vasc Interv Radiol | 2011 | TAE | Selective embolization with N-butyl cyanoacrylate for metastatic bone disease | 243 (309) |
| Dupuy D. et al. [25] | Cancer | 2010 | RFA | Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial follow-up | 55 (55) |
| Kashima M. et al. [147] | AJR Am J Roentgenol | 2010 | RFA | Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma | 40 |
| Liberman B.et al. [54] | Ann Surg Oncol | 2009 | HIFU | Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study | 31 (32) |
| Carrafiello G. et al. [148] | Radiol Med | 2009 | RFA | Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: preliminary experience | 10 |
| Delpla A. et al. [149] | Cardiovasc Intervent Radiol | 2009 | PVP | Preventive Vertebroplasty for Long-Term Consolidation of Vertebral Metastases | 100 |
| Thanos L. et al. [150] | Skeletal Radiol | 2008 | RFA | Radiofrequency ablation of osseous metastases for the palliation of pain | 30 |
| Gianfelice D. et al. [151] | Radiology | 2008 | HIFU | Palliative treatment of painful bone metastases with MR imaging--guided focused ultrasound | 11-dic |
| Basile A. et al. [152] | Radiol Med | 2008 | RFA + PC | Cementoplasty in the management of painful extraspinal bone metastases: our experience | 13 |
| Anselmetti G. et al. [153] | Cardiovasc Intervent Radiol | 2008 | PC | Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients MDCT features, and treatment with RFA | 50 |
| Trumm C. et al. [154] | J Vasc Interv Radiol | 2008 | PVP | CT fluoroscopy-guided percutaneous vertebroplasty for the treatment of osteolytic breast cancer metastases: results in 62 sessions with 86 vertebrae treated | 53 |
| Tuncali K. et al. [155] | AJR Am J Roentgenol | 2007 | CA + HIFU | MRI-guided percutaneous cryotherapy for soft-tissue and bone metastases: initial experience | 22 |
| Catane R. et al. [55] | Ann Oncol | 2007 | HIFU | MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases--preliminary clinical experience | 113 |
| Forauer A. et al. [156] | Acta Oncol | 2007 | TAE | Selective palliative transcatheter embolization of bony metastases from renal cell carcinoma | 21 |
| Callstrom M. et al. [157] | Radiology | 2006 | CA | Painful metastases involving bone: percutaneous image-guided cryoablation--prospective trial interim analysis | 14 |
| Barragán-Campos H. et al. [69] | Radiology | 2006 | PVP | Percutaneous vertebroplasty for spinal metastases: complications | 117 |
| Weber C. et al. [158] | Rofo | 2006 | PVP | [CT-guided vertebroplasty and kyphoplasty: comparing technical success rate and complications in 101 cases] | 69 (101) |
| Callstrom M. et al. [159] | Oncology | 2005 | RFA | Percutaneous ablation: safe, effective treatment of bone tumors | 14 |
| Cai H. et al. [160] | Ai Zheng | 2005 | RFA + VP/CA + VP | [Treatment effect of percutaneous vertebroplasty combined with interventional chemotherapy on vertebral metastases] | 75 |
| Masala S. et al. [161] | In Vivo | 2005 | PVP | MRI and bone scan imaging in the preoperative evaluation of painful vertebral fractures treated with vertebroplasty and kyphoplasty | 30 |
| Guzman R. et al. [162] | Eur Spine J | 2005 | TAE | Preoperative transarterial embolization of vertebral metastases | 24 |
| Goetz M. et al. [37] | J Clin Oncol | 2004 | RFA | Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study | 43 (43) |
| Masala S. et al. [163] | J Chemother | 2004 | PVP | Vertebroplasty and kyphoplasty in the treatment of malignant vertebral fractures | 33 |
| Poggi G. et al. [164] | Anticancer Res | 2003 | RFA | Percutaneous ultrasound-guided radiofrequency thermal ablation of malignant osteolyses | 5 |
| Eustatia-Rutten C. et al. [165] | J Clin Endocrinol Metab | 2003 | TAE | Outcome of palliative embolization of bone metastases in diferentiated thyroid carcinoma | 16 |
| Fourney D. et al. [166] | J Neurosurg | 2003 | PVP | Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients | 56 |
| Winking M. et al. [167] | Ger Med Sci | 2003 | PVP | PMMA vertebroplasty in patients with malignant vertebral destruction of the thoracic and lumbar spine | 22 |
| Alvarez L. et al. [168] | Eur Spine J | 2003 | PVP | Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life | 21 |
| Hierholzer J. et al. [169] | J Vasc Interv Radiol | 2003 | PC | Percutaneous osteoplasty as a treatment for painful malignant bone lesions of the pelvis and femur | 5 |
| Grönemeyer D. et al. [170] | Cancer J | 2002 | RFA | Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode | 10 |
| Chatziioannou A. et al. [171] | Eur Radiol | 2000 | TAE | Preoperative embolization of bone metastases from renal cell carcinoma | 26 |
| Barr J. et al. [172] | Spine (Phila Pa 1976) | 2000 | PVP | Percutaneous vertebroplasty for pain relief and spinal stabilization | 47 |
| Martin J. et al. [173] | Bone | 1999 | PVP | Vertebroplasty: clinical experience and follow-up results | 40 |
| Sun S. et al. [174] | J Vasc Interv Radiol | 1998 | TAE | Bone metastases from renal cell carcinoma: preoperative embolization | 16 |
| Weill A. et al. [175] | Radiology | 1996 | PVP | Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement | 37 |
| Cotten A. et al. [176] | Radiology | 1996 | PVP | Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up | 37 |
| Breslau J. et al. [177] | J Vasc Interv Radiol | 1995 | TAE | Preoperative embolization of spinal tumors | 14 |
| Corcos G. et al. [178] | Spine (Phila Pa 1976) | 1995 | PVP | Cement leakage in percutaneous vertebroplasty for spinal metastases: a retrospective evaluation of incidence and risk factors | 56 |
CA cryoablation, RFA radiofrequency ablation, MWA Microwave ablation, HIFU High-intensity focused ultrasound, TAE Transarterial embolization, PVP Percutaneous vertebroplasty, PC percutaneous cementoplasty, ECT eletrochemotherapy.
Table 5.
Results of primary bone tumors.
| Author | Journal | Year | Ablation Modality | Title | No. of Patients/(No. of Tumors) | % Local Control Tumor Response (Complete or Partial Response) |
Mean Pain Score Change |
No. (%) of Patients with Reduced Pain |
Survival Rate Overall Survival (%/Years; Median Month | Follow-Up (Months) | % Major Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakatsuka A. et al. [179] | J Vasc Interv Radiol | 2016 | RFA | Safety and Clinical Outcomes of Percutaneous Radiofrequency Ablation for Intermediate and Large Bone Tumors Using a Multiple-Electrode Switching System: A Phase II Clinical Study | 20 | 88.9% | 3.6–1.5 (2.1/10) | 84.6% | 60.9% 1 year; 14.1 months | 12 | 0 |
| Yu W. et al. [180] | Surg Oncol | 2015 | HIFU | High-intensity focused ultrasound: noninvasive treatment for local unresectable recurrence of osteosarcoma | 27 | 85.2% | 2.04–0.32 (1.7/3) | NR | 21 months | 21 | 0 |
| Anselmetti G. et al. [181] | Cardiovasc Intervent Radiol | 2012 | PVP | Percutaneous vertebroplasty in multiple myeloma: prospective long-term follow-up in 106 consecutive patients | 106 | NR | 9–1 (8/10) | 90% | NR | 28.2 ± 12.1 months | 1.6% |
| Li C. et al. [182] | Cancer | 2010 | HIFU | Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound | 13 | 46.2%complete response; 38.4% partial response | 1.85–0.08 (1.77/3) | 100% | 38.5% 5 year; 43.0 months | 60 | 0 |
| Chen W. et al. [21] | Radiology | 2010 | HIFU | Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation follow-up | 80 | 86% | NR | NR | 50.5% 5 year | 60 | 50% |
| Li C. et al. [75] | Cancer Biol Ther | 2009 | HIFU | Osteosarcoma: Limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound | 7 | 42.9% complete response;42.9% partial response | NR | 100% | 71.4% 5 year; 68 months | 81 | 0 |
NR not reported, RFA radiofrequency ablation, HIFU High-intensity focused ultrasound, PVP percutaneous vertebroplasty.
3.1. Curative Treatment
Indications
According to guidelines of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), the curative treatment of bone metastases is defined as complete and definitive ablation of the tumor [75]. Candidate patients for these treatments are oligometastatic patients (<3 metastases, each <3 cm in size) with favorable prognostic factors such as young age, absence or limited destruction of cortical bone, absence of extraosseous metastases, good performance status and slow evolution of the underlying disease. Among oncological patients, few patients have these characteristics. Curative treatment can be achieved with percutaneous thermal ablation performed by means of RFA, MWA, or CA. To achieve effective local control, the margin of safety of tumor ablation must be at least 0.5–1 cm larger than the lesion. Combined treatment may be used, including embolization, but a technique of ablation should always be used to ensure the complete destruction of the tissue treated. After each treatment follows the assessment of local progression-free survival (LPFS), i.e., the time interval between locoregional treatment and the development of local tumor progression at the treated site; bone disease-free survival (BDFS—defined as the time interval between locoregional treatment and disease progression in the treated BM or another bone); disease-free survival (DFS—defined as the time interval between locoregional treatment and the time of any visceral or bone tumor progression). Finally, overall survival (OS).
Available evidence
Arrigoni et al. [87] retrospectively analyzed the results of 28 cryoablation procedures of BM. Of them, 11 patients were treated for local tumor control. In a mean follow-up of 22.4 months, it was recorded stability and/or reduction of lesion volume in 10 of 11 patients.
Cazzato et al. [77] reviewed from February 2009 to June 2020, 23 patients with sacral metastases undergoing RFA or CA with palliative or curative intent. In patients undergoing curative ablation (n = 7), local tumor progression occurred at 3 (43%) treated sites at a median follow-up of 17 months.
Ma et al. [78] performed a retrospective review (January 2011 to April 2016) of 76 BM in 45 patients treated with percutaneous ablation. A total of 48 out of 76 BM (63%) were treated with RFA, 35% (27/76) with CA, and 1.3% (1/76) with MWA. In 70% (53/76) of cases, an associated cementoplasty was performed. After 3 months, local tumor recurrence was documented in seven cases. No tumor progression in the treated area was documented at 6-month follow-up, and after 1 year, local tumor control for 17 tumors was 68% (17/25). There were no procedure-related complications for metastases treated with RFA, according to the Society of Interventional Radiology (SIR) guidelines. For metastases treated with CA, the complication rate was 7.4% (2/27).
A retrospective analysis by Vaswani et al. [79] of 64 bone metastases from sarcoma in 41 patients treated with ablation between December 2011 and August 2016 was performed. Two subgroups were treated: oligometastatic disease (n = 13) and extensively metastatic disease (n = 51). One hundred percent (10/10) of the treated lesions showed local tumor control in oligometastatic disease at 1 year. Three of thirteen ablated lesions were lost at follow-up at 3, 6, and 9 months, respectively.
Cazzato et al. [28] retrospectively reviewed 46 patients treated for BM from January 2008 and November 2017. Complications were observed in 20.4% of cases (n = 10;): out of whom, only two required interventional or surgical treatments; in the other cases, only postoperative pain (grade 2 complication) was observed. The mean follow-up was 34.1 ± 22 months (median 30.5; 95% confidence interval [CI], 27.6–40.7). Local tumor progression at the treated site was noted in 28.5% of cases (n = 14); 1- and 2-year survival was 76.8% and 71.7%, respectively. Only BM size >2 cm was associated with local tumor progression. BDFS was 71.7% and 53.1% at 1- and 2-year follow-up, respectively. At the same time interval, DFS was 86.3% and 61.5%, respectively. Finally, 1- and 2-year OS was 95.4%.
Gardner et al. [15] retrospectively reviewed 40 patients with metastatic renal cell carcinoma who underwent cryoablation for BM between 2007 and 2014. Complications included five delayed fractures in four patients who were seen 1 month to 8 months after cryoablation. Only one of these delayed fractures was symptomatic and required treatment with cementoplasty for pain control. The overall local tumor control rate for metastases was 82% (41 of 50). The likelihood of local recurrence was 25-fold greater for patients with disseminated disease than for patients with oligometastatic disease (p = 0.001). Despite a local tumor control rate of 82%, 38 of the 40 patients had evidence of new metastases or disease progression to other sites at the end of the follow-up period, and the median time to progression was 6 months (95% CI, 4.0 to 11.0 months).
Eire et al. [80] retrospectively analyzed 16 patients with oligometastatic prostate cancer who underwent image-guided percutaneous ablation to treat 18 metastatic sites between 2009 and 2014. All but one were bone lesions; in the study a pelvic lymph node was included. Local tumor control was achieved in 15 out of 18 metastases (83%) at a median follow-up of 27 months after ablation. Local tumor recurrence occurred in three out of 18 metastases (17%). PFS rates at 12 and 24 months were 56% (95% CI, 30–76%) and 43% (95% CI, 19–65%), respectively. Among the 18 image-guided percutaneous ablations, no complications of grade 3 or higher according to the Common Terminology Criteria for Adverse Events were observed.
Wallace et al. [13] retrospectively reviewed 55 spinal bone metastases undergoing RFA and vertebral augmentation of bone metastases between April 2012 and July 2014. Five cases of residual tumor were documented within 3 months of treatment. In all five of these cases, there was also evidence of systemic disease progression. The radiographic control of local tumor at 3 months was 89%. The rate of radiographic tumor control 6 months after treatment was 74% (26/35) and after 1 year was 70% (21/30) and 67% (18/27) in cases of systemic progression of metastatic disease.
Deschamps et al. [83] retrospectively analyzed 122 patients who underwent thermal ablation of BM between September 2001 and February 2012. In multivariate analysis, factors associated with a lower risk of treatment failure were metachronous bone metastases (p = 0.004), no cortical bone erosion (p = 0.01), maximum diameter at baseline CT < 20 mm (p = 0.001), no nearby critical neurological structures (p = 0.002). The 1-year OS rates were 91% (95% CI: 80–97%) and 90% (95% CI: 75–97%) for patients in group 1 (in patients with oligometastatic disease) and group 2 (in patients with long life expectancy), respectively. For patients in group 1, the B-DFS was 64% (95% CI: 50–76%) 1 year after thermal ablation of all bone metastases. Major side effects occurred in 4 patients (3%) of the 122 thermal ablations performed: avascular necrosis of the femoral head, nerve root injury (2 patients), and stress cardiomyopathy (Tako–Tsubo) immediately after thermal ablation of a bone metastasis from a pheochromocytoma. Seven patients (8%) experienced SREs despite thermal ablation. Most of the SREs were fractures at the treated site. One patient experienced spinal cord compression that occurred 8 months after treatment failure of a bone metastasis located at T5.
3.2. Palliative Treatments
Indications
Palliative treatments can be offered to most patients with painful bone metastases (at least 6–7 on a VAS 0–10 scale). The goal is not complete ablation of the tumor but pain palliation, tumor size reduction (debulking), prevention of pathological fractures and/or decompression and debulking of tumors (especially for spine tumors protruding into the spinal canal). If pain management is necessary and there is no risk of fracture, only thermal ablation should be performed (such as RFA, MWA, CA, or MRgFUS). A combined percutaneous cementoplasty procedure is performed in case of fracture or impending fracture. Tumors with less than 1 cm from the critical structures like spinal cord or large blood vessels are a relative contraindication if these structures cannot be isolated and protected (with air, CO2, or glucose).
Available evidence
Arrigoni et al. [87] retrospectively evaluated 17 patients with single bone lesion treated by CA with a palliative intent. In a 3-month follow-up study, we recorded an overall reduction of pain (evaluated using a VAS 0–10 scale) between pre- and post-treatment from 7.4 to 4.5. No major complications were recorded.
From February 2016 to March 2018, Jenning et al. [88] performed a multicenter, prospective, single-arm study of 64 patients with painful metastatic bone disease, who were not candidates for or had not benefited from standard therapy. In an analysis of the 64 patients treated with CA, for whom complete follow-up data were available, response rates of pain over time ranged from 38% to 48% over the 24-week follow-up period from week 1 to 24 using the Brief Pain Inventory-Short Form (reference range 0 to 10 points). Response rates were similar among participants who had and had not undergone previous radiation per week. Opioid medication use decreased significantly in patients from week 4 to week 24, as did quality of life, which improved steadily over the first 6 months. Possibly related adverse events occurred in 22% (14 of 65) of cases. Three of the 65 participants (4.6%) each had a grade 3 or 4 serious adverse events (abdominal pain, hematoma, and skin burn or frostbite).
Levy et al. [14] analyzed one hundred and six patients between October 2017 and March 2019. After RFA, the patients experienced significant improvement in worst pain, average pain, pain interference, and quality of life. Before RFA, the mean worst pain score was 8.2. After ablation, worst pain improved significantly, with mean scores falling to 3.5 at 6 months (p < 0.0001). More than half of patients (59%) experienced immediate improvement, reporting a 2-point change in worst pain at targeted treatment sites 3 days after ablation. The mean 24-h morphine equivalent dose for all treated subjects at baseline was 61.0 mg and decreased to 50.4 mg at the 3-month visit.
Yang et al. [92] observed a total of 26 patients (with 36 metastatic bone lesions) who were treated with CA between May 2012 and June 2016. The overall pain response rate was 91.7%, 94.4%, 91.7%, and 94.4%, respectively, at 1 day, 1 month, 3 months, and 6 months after CA, with CR achieved at 22.2%, 41.7%, 36.1%, and 22.2%, respectively. Pain relapse occurred in four patients, one of whom had a pathologic fracture. Two of these patients were treated again with CA. Adverse events were observed in 3 out of 26 patients.
Thirty-three patients with metastatic bone tumors were enrolled by Tanigawa et al. [96]. No serious complications occurred during the study. Four patients showed adverse events, including one case each of grade 3 pain and grade 2 hypotension, and two grade 1 burns. The response rate was 69.7% (95% confidence interval: 51.3–84.4%). The dose of analgesics was reduced within 4 weeks in 13 patients but had to be increased in 2 patients. Clinical efficacy was assessed by intention-to-treat analysis and was 69.7%. Although this efficacy rate is lower than that reported in previous retrospective studies [7,20,21], it is acceptable for the present study because it was a prospective investigation. In addition, the changes in VAS demonstrate that the analgesic effects were both immediate and long-lasting.
Prologo et al. [97] retrospectively analyzed percutaneous CT-guided CA in the setting of painful musculoskeletal metastatic disease in 50 patients. There were statistically significant decreases in median VAS and narcotic use at both 24 h and 3 months. The median VAS score of all patients reported before surgery was 8 ± 1. At 24 h and 3 months after the procedure, the median VAS score decreased to 3 ± 1 (p < 0.000). Morphine equivalent values decreased to 85 ± 70 mg (p < 0.000) 24 h after the procedure and remained significantly lower than before the procedure (p < 0.000) after 3 months. In six patients (11%) two minor complications were observed, and four major consisting of two complete femoral fractures after ablation and cementoplasty of proximal lytic lesions, and two transient neuropathies resulting from damage to adjacent nerves during ablation.
From July 2011 to January 2012, Pusceddu et al. [76] analyzed 18 patients with symptomatic skeletal metastatic lesions undergoing treatment with MWA. Seven patients had been previously treated with RT, three patients with RT and chemotherapy, and seven with chemotherapy alone. In all these patients, pain had proven refractory to conventional approaches. Technical success was achieved in 100% of the cases. Postprocedural CT scans demonstrated no major complications. The mean BPI (Brief Pain Inventory) score, 7 days after the procedure, was reduced by 77%. Four weeks after treatment, nine out of 18 patients (50%) were completely pain free, and the remaining patients reported a 64% reduction in BPI score. Twelve weeks after the MWA procedure, the mean BPI score was reduced by 92% (range, 41–100%). Thirteen out of 18 patients (72%) were symptom-free and did not resume any therapy.
Regarding embolization as a palliative and/or adjuvant treatment of bone metastases the literature is smaller compared with ablative procedures. One of the larger series of cases was collected by Rossi et al. [98] that retrospectively studied 107 patients with BM from renal cell carcinoma treated from December 2002 to January 2011 with 163 embolizations with N-2-butyl cyanoacrylate (NBCA). The mean tumor diameter before embolization was 8.8 cm. A clinical response (defined by a reduction of at least 50% in pain and a reduction of at least 50% reduction in analgesic doses) was achieved in 157 embolizations (96%) and no response in six embolizations of sacroiliac metastases. The median duration of clinical response was 10 (range 1–12) months. Areas of hypoattenuating signal on CT (tumor necrosis index) were observed in all patients. Variable ossification was observed in 41 patients. The mean maximum tumor diameter after embolization was 4.0 cm.
3.3. Palliative Treatment of Both Bone Fracture and Impending Fracture
Indications
Patients with BM and impending pathologic fractures may be selected for possible treatment with cementoplasty.
Osteolytic tumors (e.g., metastases, lymphoma, multiple myeloma) located in the vertebrae, acetabulum, or condyles, causing local pain, disability, or being at risk for compression fracture are the primary indications for cementoplasty.
It is critical to determine whether compressive or torsional forces are predominant in the involved bone.
In cases where compressive forces are involved (i.e., spine, acetabulum, femoral condyles, tibial extremities, talus, and calcaneus), percutaneous injection of Poly Methyl MethAcrylate (PMMA) cement allows for bone consolidation and prevention of pathologic fractures. Due to its properties, PMMA leads to pain relief. PMMA polymerization is an exothermic process with a transient increase in temperature. This exothermic reaction has a direct toxic effect on the nociceptors; furthermore, the cement stabilizes the intratumoral microfractures, which are sources of pain in metastatic fractures. PMMA is injected as a dental paste material. Once deposited into the bone defect, it solidifies within 20–30 min. The distribution of PMMA within the bone defect is unpredictable [183,184]. However, percutaneous cementoplasty alone does not provide any radical cytotossic effect. Therefore, percutaneous cementoplasty should always be preceded by ablative treatment if a cytotossic effect (palliative or curative) is the aim of the procedure.
In the bony districts where torsional forces are most likely to act (the diaphysis of long bones), percutaneous cementoplasty can be sufficient to achieve pain palliation; however, it does not guarantee a definitive bone consolidation. Therefore, other forms of consolidation (intramedullary nailing or external fixation performed percutaneously or surgically) may be necessary along with cementoplasty. Even for long bone injuries, percutaneous cementoplasty alone (with or without other percutaneous or surgical consolidation strategies) has no curative effect.
Complications reported following cementoplasty are cement leakage from vertebrae, reported in 38% to 72.5% of cases [185]; pulmonary embolism, infection, and fracture in less than 1% of cases; allergic reactions, bleeding from the puncture site, pain in other areas, described in 14% of patients [186].
Available evidence
Wanget al. [16] retrospectively analyzed 35 patients with neoplastic vertebral lesions who received RFA combined with vertebroplasty (group A, 15 patients with 17 lesions) or single vertebroplasty (group B, 20 patients with 24 lesions) from March 2016 to June 2019.
VAS scores in group A decreased more rapidly 1 week after treatments and remained more stable at 6 months than in group B (p < 0.05).
Twenty-five patients with refractory painful vertebral metastases were included in the study by Kastler et al. [90] consecutively between 2012 and 2019. The decision to perform vertebroplasty was made based on the location, type, and extent of the lesion, and the Kostuik score was used to predict fracture risk.
The mean VAS before the procedure was 8.4/10. Significant pain reduction was achieved in 24/29 (83%) procedures at 1 month.
Therapeutic failures on pain palliation were observed in three procedures with advanced stage of disease. In each case, the lesions were large with significant prevertebral invasion of soft tissues and foramina. In five other patients, a decrease in pain of less than 50% was observed: at 1 month (one patient), at 3 months (two patients), at 6 months (two patients), and at 1 year (two patients). Significant growth of tumor lesions or new lesions was observed in these patients.
Approximately 82% of patients had a satisfactory analgesic outcome at 1 month with significant long-term pain palliation. Pain relief was obtained immediately after the procedure and provided significantly lasting pain relief. Therefore, end-of-life quality was improved in these patients suffering from intractable pain.
Madani et al. [89] between June 2016 and January 2021 retrospectively examined treatment complications, pain palliation, and skeletal complications after combined local treatments (CLT) for vertebral metastases with limited epidural extension (VMLEE).
Eighteen consecutive patients underwent CLT for 24 VMLEE, between June 2016 and January 2021. No major post-treatment complications were reported. Only one vertebra showed an increase in a preexisting vertebral fracture. Nine VMLEE had evidence of residual disease, including two that resulted in spinal cord compression (2, 11 months).
Pusceddu et al. [91] examined 35 patients with 41 spinal vertebral metastases undergoing targeted percutaneous radiofrequency ablation with a navigable radiofrequency ablation device associated with vertebral augmentation. Twenty-one patients (60%) had one or two metastatic lesions (Group A) and fourteen (40%) patients had multiple (>2) vertebral lesions (Group B).
The procedure was technically successful in all treated vertebrae. The VAS decrease over time between 1 week and 1 year after radiofrequency ablation was similar, suggesting that pain relief was immediate and durable.
From 1 October 2017, to 1 October 2019, Jiao et al. [18] analyzed 30 patients with 42 metastatic osteolytic or mixed osteolytic tumors, treated with percutaneous microwave ablation and percutaneous cementoplasty simultaneously under CT and scopic guidance to control local lesions and severe pain.
Opioids were discontinued in nine cases (30%) at 12 weeks. In five cases (16.7%), opioids were replaced with nonsteroidal anti-inflammatory drugs. QoL assessments at 4 weeks showed significant improvements in physical function, body pain, general health. Vitality (p < 0.01), social function (p = 0.002), role emotion (p = 0.02), and mental health (p = 0.007) showed significance at 12 weeks compared with pre-treatment values.
None of the patients showed serious complications. Asymptomatic bone cement loss occurred in two cases with extraspinal metastases and in one case with spinal metastases.
Yang et al. [93] conducted a retrospective study of 63 patients with osteolytic vertebral fractures treated by vesselplasty (a type of minimally invasive stabilization system for vertebral fractures) from September 2014 to January 2018. The rate of pain relief considered as VAS and ODI scores was 97.4% in 37 out of 38 patients at 1 year after surgery. The rate of bone cement loss was 16.2%, of which 3.8% (4/105) of the segments leaked into the spinal canal but without nerve symptoms. No cases of infection occurred.
A total of 274 patients with 367 bone metastases undergoing percutaneous RFA and CA between January 2008 and April 2018 were reviewed by De Marini et al. [94]. Metastases treated with RFA were more frequently treated in combination with cementoplasty during the same session 20/66 (30.3%) versus 64/301 (21.3%) of cases treated with CA (p < 0.001). Complications occurred in 49/367 (13.4%) of the bone metastases (major, 2.5% of cases, minor complications in 10.9%). Complications were more likely with RFA than with CA (23/66 [34.8%] versus 26/301 [8.6%], respectively; p < 0.001); minor complications (immediate post-procedural pain) occurred more frequently with RFA (22/66 (33.3%)) compared with CA (18/301 (6.0%); p < 0.001). Major complication rates were similar between RFA and CA (RFA 1/66 (1.5%) vs. CA 8/301 (2.7%); p = 1). RFA and CA were comparable for treatment efficacy (curative/palliative ratio 14/52 (26.9%) vs. 47/254 (18.5%), respectively; p = 0.27.
Deib et al. [95] performed a retrospective review that included 65 patients with 77 tumors who underwent microwave ablation and cementoplasty for 18 months. Complete ablation was achieved in all 77 tumors.
A mean decrease of 17 (SD, 12.9) ODI points was observed in the interval between before the procedure and 20–24 weeks after the procedure. A mean decrease of 4.30 (SD, 3.32) VAS points was observed between before and 20–24 weeks after the procedure.
3.4. Role of Radiation Therapy and Surgery
The gold standard treatment for painful BM is radiation therapy (RT) [187,188], a well-accepted local treatment modality. The aim of palliative radiation therapy is pain control, prevention of pathological fractures, and spinal cord compression to improve quality of life, reduce the need of analgesics, and, in some cases, improve the survival rate. In presence of no response to RT, partial response, or recurrence of pain, re-irradiation of the same area may be considered [189].
Re-irradiation is usually required in a substantial group, considering that 40% of patients achieve no pain relief after initial RT. Moreover, pain comes back in approximately 50% of patients within one year of RT.
Ablative approaches have been proposed as a therapeutic option to control painful BM [127,190], even in combination with RT. Life expectancy is crucial in the choice of the best surgical treatment of BM. Short life expectancy, due to histology, staging and conditions of the patient, requires palliative treatments. Good life expectancy may require more aggressive treatments of metastases, performed to last over time (excisional surgery) with radical intent [191,192].
Pathological fractures or injuries at risk of fracture located at meta-epiphyseal level and meta-epiphyseal lesions with poor response to non-surgical therapies are a good indication for resection surgery and replacement with prosthesis. Osteosynthesis is indicated in pathological fractures or injuries at risk of pathological diaphyseal fractures. In the literature, there are several attempts of standardization of the treatments of BM (Capanna and Campanacci, SIOT guidelines) in absence of univocal guidelines [193,194]. The feasibility study is the first step to determine the type of treatment to be performed. Patients who are not eligible for excisional surgery are evaluated by a multidisciplinary team formed by oncologists, radiotherapists, orthopedic surgeons, and interventional radiologists.
4. Indications in Primary Bone Tumors
Indications
Treatment of primary malignant bone tumors (osteosarcoma, Ewing’s sarcoma, and chondrosarcoma), which account for less than 1% of tumors diagnosed each year, is primarily surgical, with accompanying systemic therapy if necessary. An interdisciplinary approach may employ percutaneous ablation, as in the case of lesions located in anatomically unfavorable, inoperable, or recurrent sites. However, malignant tumors are too large at the time of patient presentation to allow effective local ablative control of the tumor. Therefore, it seems reasonable to use radiofrequency ablation to treat locally recurrent malignancy or when resection is not feasible. Percutaneous curative treatment of primary bone tumors is feasible for benign lesions such as OO, OB, and chondroblastoma. OO was the first tumor to be successfully treated with RFA at any site. RFA is curative in most patients, usually after a single treatment. If performed correctly, the frequency of complications is extremely low.
Available evidence
Nakatsuka et al. [179] enrolled 20 patients with unresectable malignant bone tumors. No adverse events occurred in 19 of the 20 patients (95%). VAS scores decreased by ≥2 points in 11 of 13 patients (84.6%). Tumor response (complete or partial response) was achieved in 16 of 18 patients (88.9%). The 1-year overall survival rate was 60.9%.
Yu et al. [180] performed a retrospective analysis of 27 patients who, from 2006 to 2010, had unresectable local recurrence of osteosarcoma. After HIFU treatment, the response rate was 51.8% and the local disease control rate was 85.2%. Local disease control rates at 1, 2, and 3 years were 59.2%, 40.7%, and 33.1%, respectively. The median time free from progression of local disease was 14 months, the median time free of progression was 13 months, and the median time to overall survival was 21 months.
Anselmetti et al. [181] analyzed 106 patients with multiple myeloma undergoing percutaneous vertebroplasty. The median pretreatment VAS score of 9 (range 4–10) decreased significantly (p < 0.001) to 1 (range 0–9) after vertebroplasty. Median pretreatment Oswestry Disability Index (ODI) values of 82% (range 36–89%) improved significantly to 7% (range 0–82%) (p < 0.001). Use of analgesic medications after treatment decreased significantly with a statistically significant difference (p < 0.001).
Using HIFU, Chen et al. treated 80 patients with bone tumors (osteosarcoma, chondrosarcoma, Ewing sarcoma, giant cell tumor) [21]. In combination with systemic chemotherapy, the survival of patients with stage 2b tumor disease was thereby significantly improved. In addition to individual case reports describing the treatment of aneurysmal bone cysts or giant cell tumors, when pain is in the forefront of symptoms, percutaneous ablation can additionally be employed in the palliation of primary bone tumors, analogously to the treatment of bone metastases [49]. Compared with traditional surgery for malignant bone tumors, HIFU has the advantage of being noninvasive and can be used to treat large malignant bone tumors that cannot be removed surgically. Limb shape and succession are not affected by HIFU, and treatment can be repeated on patients who have not fully responded or had recurrences. HIFU treatment provides pain relief and can be used in conjunction with chemotherapy. Li et al. [182] analyzed 25 patients with malignant bone tumors before and after HIFU treatment. After HIFU treatment, 21 (87.5%) patients were completely pain free, and 24 (100%) experienced significant relief. The response rate was 84.6%. The survival rates at 1, 2, 3, and 5 years were 100.0%, 84.6%, 69.2%, and38.5%, respectively.
Ablation with high-intensity focused ultrasound is also an option for palliative treatment. In the study by Chen et al. [21], the 5-year survival rate for patients with osteosarcoma stage III disease (Enneking staging system) was 15.8%, which is like the reported survival rate (15.7%) for a group of patients undergoing incomplete surgical excision (38). These results indicate that high-intensity focused ultrasound offers another choice for patients with advanced stages of bone neoplasia. However, patients with tumors larger than 10 cm, nerves involved by the tumors, or tumors in different locations, such as pelvic tumors, growing toward the pelvic cavity and partially covered by the bowel, should not be considered for curative treatment.
5. Conclusions
Skeletal metastases influence the quality of life of patients with metastatic diseases. In these patients, especially those with short survival expectancy, the indications for surgical treatment are limited, while immediate pain relief and improvement of the functional status are important, and complications of treatments are unwanted. Decision making regarding potential surgery for a metastatic disease necessarily requires availability of reliable information about patient survival and quality of life [195,196]. Currently, such novel mini-invasive surgical treatments as embolization, thermal ablation, and cementoplasty are valuable techniques in the management of patients with painful bone metastases both in terms of pain palliation and disease control. Many are the advantages of IR: immediate reduction of post-procedural pain, even in radio-resistant tumors, as a result of thermal destruction of fibers and pain receptors and reduction of the tumor volume (tumor debulking); low complication rate; low hospitalization costs; more comfort for the patient; possibility to repeat the procedure with the same technique without damaging the surrounding structures; possibility of repeated and multiple treatments, also in combination with other IR techniques such as cementoplasty or RT [8].
IR can also significantly help the management patients with primary bone tumors, even if the literature is still limited in this field. All these techniques, along with systemic treatment, surgery, and RT, offer a variety of treatment options for musculoskeletal oncologic patients.
Author Contributions
Conceptualization, F.A. and C.M.; methodology, F.B. and P.P.; formal analysis, P.P. and F.B.; investigation, F.B., F.S. and P.P.; data curation, F.S.; writing—original draft preparation, F.S.; writing—review and editing, F.A.; supervision, L.Z., N.S., C.Z., A.B. and C.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cazzato R.L., Arrigoni F., Boatta E., Bruno F., Chiang J.B., Garnon J., Zugaro L., Giordano A.V., Carducci S., Varrassi M., et al. Percutaneous management of bone metastases: State of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR) Radiol. Med. 2019;124:34–49. doi: 10.1007/s11547-018-0938-8. [DOI] [PubMed] [Google Scholar]
- 2.Shimohira M., Nagai K., Hashizume T., Nakagawa M., Ozawa Y., Sakurai K., Matsushita Y., Yamada S., Otsuka T., Shibamoto Y. Preoperative transarterial embolization using gelatin sponge for hypervascular bone and soft tissue tumors in the pelvis or extremities. Acta Radiol. 2016;57:457–462. doi: 10.1177/0284185115590435. [DOI] [PubMed] [Google Scholar]
- 3.Kickuth R., Waldherr C., Hoppe H., Bonel H.M., Ludwig K., Beck M., Triller J. Interventional Management of Hypervascular Osseous Metastasis: Role of Embolotherapy Before Orthopedic Tumor Resection and Bone Stabilization. AJR Am. J. Roentgenol. 2008;191:W240–W247. doi: 10.2214/AJR.07.4037. [DOI] [PubMed] [Google Scholar]
- 4.Kato S., Hozumi T., Takaki Y., Yamakawa K., Goto T., Kondo T. Optimal Schedule of Preoperative Embolization for Spinal Metastasis Surgery. Spine. 2013;38:1964–1969. doi: 10.1097/BRS.0b013e3182a46576. [DOI] [PubMed] [Google Scholar]
- 5.Rossi G., Mavrogenis A.F., Rimondi E., Braccaioli L., Calabrò T., Ruggieri P. Selective Embolization with N-butyl Cyanoacrylate for Metastatic Bone Disease. J. Vasc. Interv. Radiol. 2011;22:462–470. doi: 10.1016/j.jvir.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Chen-Xu S., Martel-Villagrán J., Bueno-Horcajadas Á. Percutaneous management of bone metastases: State of the art. Radiología. 2021;63:345–357. doi: 10.1016/j.rx.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Dalili D., Isaac A., Bazzocchi A., Åström G., Bergh J., Lalam R., Weber M.-A., Fritz J., Mansour R. Interventional Techniques for Bone and Musculoskeletal Soft Tissue Tumors: Current Practices and Future Directions—Part I. Ablation. Semin. Musculoskelet. Radiol. 2020;24:692–709. doi: 10.1055/s-0040-1719103. [DOI] [PubMed] [Google Scholar]
- 8.Barile A., Arrigoni F., Bruno F., Palumbo P., Floridi C., Cazzato R.L., Reginelli A., Zappia M., Brunese L., Zugaro L., et al. Present role and future perspectives of interventional radiology in the treatment of painful bone lesions. Future Oncol. 2018;14:2945–2955. doi: 10.2217/fon-2017-0657. [DOI] [PubMed] [Google Scholar]
- 9.Callstrom M.R., Charboneau J.W. Image-Guided Palliation of Painful Metastases Using Percutaneous Ablation. Tech. Vasc. Interv. Radiol. 2007;10:120–131. doi: 10.1053/j.tvir.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal D., Callstrom M.R. Critical Review and State of the Art in Interventional Oncology: Benign and Metastatic Disease Involving Bone. Radiology. 2012;262:765–780. doi: 10.1148/radiol.11101384. [DOI] [PubMed] [Google Scholar]
- 11.Arrigoni F., De Cataldo C., Bruno F., Palumbo P., Zugaro L., Di Staso M., Gravina G.L., Barile A., Masciocchi C. Ablation, consolidation and radiotherapy for the management of metastatic lesions of the spine: Impact on the quality of life in a mid-term clinical and diagnostic follow-up in a pilot study. Med. Oncol. 2020;37:53. doi: 10.1007/s12032-020-01378-6. [DOI] [PubMed] [Google Scholar]
- 12.Di Staso M., Zugaro L., Gravina G.L., Bonfili P., Marampon F., Di Nicola L., Conchiglia A., Ventura L., Franzese P., Gallucci M., et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur. Radiol. 2011;21:2004–2010. doi: 10.1007/s00330-011-2133-3. [DOI] [PubMed] [Google Scholar]
- 13.Wallace A., Tomasian A., Vaswani D., Vyhmeister R., Chang R., Jennings J. Radiographic Local Control of Spinal Metastases with Percutaneous Radiofrequency Ablation and Vertebral Augmentation. Am. J. Neuroradiol. 2016;37:759–765. doi: 10.3174/ajnr.A4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy J., Hopkins T., Morris J., Tran N.D., David E., Massari F., Farid H., Vogel A., O’Connell W.G., Sunenshine P., et al. Radiofrequency Ablation for the Palliative Treatment of Bone Metastases: Outcomes from the Multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 Patients. J. Vasc. Interv. Radiol. 2020;31:1745–1752. doi: 10.1016/j.jvir.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Gardner C.S., Ensor J.E., Ahrar K., Huang S.Y., Sabir S.H., Tannir N.M., Lewis V.O., Tam A.L. Cryoablation of Bone Metastases from Renal Cell Carcinoma for Local Tumor Control. J. Bone Jt. Surg. Am. 2017;99:1916–1926. doi: 10.2106/JBJS.16.01182. [DOI] [PubMed] [Google Scholar]
- 16.Wang F., Gu J., Xu C., Li G., Lv P. The combination of radiofrequency ablation and vertebroplasty shows advantages over single vertebroplasty in treating vertebral neoplastic lesions. Skelet. Radiol. 2022;51:565–571. doi: 10.1007/s00256-021-03788-7. [DOI] [PubMed] [Google Scholar]
- 17.Pusceddu C., Dessì G., Melis L., Fancellu A., Ruggiu G., Sailis P., Congia S., Derudas D., Cau R., Senis I., et al. Combined Microwave Ablation and Osteosynthesis for Long Bone Metastases. Medicina. 2021;57:825. doi: 10.3390/medicina57080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao D., Yao Y., Li Z., Ren J., Han X. Simultaneous C-arm Computed Tomography-Guided Microwave Ablation and Cementoplasty in Patients with Painful Osteolytic Bone Metastases: A Single-center Experience. Acad. Radiol. 2022;29:42–50. doi: 10.1016/j.acra.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Caracciolo J.T., Letson G.D. Radiologic Approach to Bone and Soft Tissue Sarcomas. Surg. Clin. N. Am. 2016;96:963–976. doi: 10.1016/j.suc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Dea N., Gokaslan Z., Choi D., Fisher C. Spine Oncology–Primary Spine Tumors. Neurosurgery. 2017;80:S124–S130. doi: 10.1093/neuros/nyw064. [DOI] [PubMed] [Google Scholar]
- 21.Chen W., Zhu H., Zhang L., Li K., Su H., Jin C., Zhou K., Bai J., Wu F., Wang Z. Primary Bone Malignancy: Effective Treatment with High-Intensity Focused Ultrasound Ablation. Radiology. 2010;255:967–978. doi: 10.1148/radiol.10090374. [DOI] [PubMed] [Google Scholar]
- 22.Barton P.P., Waneck R.E., Karnel F.J., Ritschl P., Kramer J., Lechner G.L. Embolization of Bone Metastases. J. Vasc. Interv. Radiol. 1996;7:81–88. doi: 10.1016/S1051-0443(96)70738-8. [DOI] [PubMed] [Google Scholar]
- 23.Rybak L.D. Fire and Ice: Thermal Ablation of Musculoskeletal Tumors. Radiol. Clin. N. Am. 2009;47:455–469. doi: 10.1016/j.rcl.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Tomasian A., Wallace A., Jennings J. Benign Spine Lesions: Advances in Techniques for Minimally Invasive Percutaneous Treatment. AJNR Am. J. Neuroradiol. 2017;38:852–861. doi: 10.3174/ajnr.A5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupuy D.E., Liu D., Rn D.H., Hanna L., Blume J.D., Ahrar K., Lopez R.R., Safran H., A DiPetrillo T. Percutaneous radiofrequency ablation of painful osseous metastases: A multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao W., Wang H., Hu J.-H., Peng Z.-H., Chen J.-Z., Huang J.-Q., Jiang Y.-N., Luo G., Yi G.-F., Shen J., et al. Palliative pain relief and safety of percutaneous radiofrequency ablation combined with cement injection for bone metastasis. Jpn. J. Clin. Oncol. 2018;48:753–759. doi: 10.1093/jjco/hyy090. [DOI] [PubMed] [Google Scholar]
- 27.Madaelil T.P., Wallace A.N., Jennings J.W. Radiofrequency ablation alone or in combination with cementoplasty for local control and pain palliation of sacral metastases: Preliminary results in 11 patients. Skelet. Radiol. 2016;45:1213–1219. doi: 10.1007/s00256-016-2404-9. [DOI] [PubMed] [Google Scholar]
- 28.Cazzato R.L., Auloge P., De Marini P., Rousseau C., Chiang J.B., Koch G., Caudrelier J., Rao P., Garnon J., Gangi A. Percutaneous image-guided ablation of bone metastases: Local tumor control in oligometastatic patients. Int. J. Hyperth. 2018;35:493–499. doi: 10.1080/02656736.2018.1508760. [DOI] [PubMed] [Google Scholar]
- 29.Callstrom M.R., Charboneau J.W., Goetz M.P., Rubin J., Atwell T.D., Farrell M.A., Welch T.J., Maus T.P. Image-guided ablation of painful metastatic bone tumors: A new and effective approach to a difficult problem. Skelet. Radiol. 2006;35:1–15. doi: 10.1007/s00256-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 30.Barral M., Auperin A., Hakime A., Cartier V., Tacher V., Otmezguine Y., Tselikas L., de Baere T., Deschamps F. Percutaneous Thermal Ablation of Breast Cancer Metastases in Oligometastatic Patients. Cardiovasc. Interv. Radiol. 2016;39:885–893. doi: 10.1007/s00270-016-1301-x. [DOI] [PubMed] [Google Scholar]
- 31.Simon C.J., Dupuy D.E. Percutaneous Minimally Invasive Therapies in the Treatment of Bone Tumors: Thermal Ablation. Semin. Musculoskelet. Radiol. 2006;10:137–144. doi: 10.1055/s-2006-939031. [DOI] [PubMed] [Google Scholar]
- 32.Erickson J.K., Rosenthal D.I., Zaleske D.J., Gebhardt M.C., Cates J.M. Primary Treatment of Chondroblastoma with Percutaneous Radio-frequency Heat Ablation: Report of Three Cases. Radiology. 2001;221:463–468. doi: 10.1148/radiol.2212010262. [DOI] [PubMed] [Google Scholar]
- 33.Munk P.L., Malfair D., Rashid F., Torreggiani W.C. Radiofrequency Ablation of Solitary Eosinophilic Granuloma of Bone. Am. J. Roentgenol. 2008;191:W320. doi: 10.2214/AJR.08.1450. [DOI] [PubMed] [Google Scholar]
- 34.Kujak J.L., Liu P.T., Johnson G., Callstrom M.R. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skelet. Radiol. 2010;39:175–182. doi: 10.1007/s00256-009-0801-z. [DOI] [PubMed] [Google Scholar]
- 35.Janjan N.A., Payne R., Gillis T., Podoloff D., Libshitz H., Lenzi R., Theriault R., Martin C., Yasko A. Presenting Symptoms in Patients Referred to a Multidisciplinary Clinic for Bone Metastases. J. Pain Symptom Manag. 1998;16:171–178. doi: 10.1016/S0885-3924(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 36.Breckheimer A., Bruners P., Mahnken A. Interventional management of a rare complication in radiofrequency ablation of an osteosclerotic bone metastasis. Rofo. 2010;182:433. doi: 10.1055/s-0029-1245138. [DOI] [PubMed] [Google Scholar]
- 37.Goetz M.P., Callstrom M.R., Charboneau J.W., Farrell M.A., Maus T.P., Welch T.J., Wong G.Y., Sloan J.A., Novotny P.J., Petersen I.A., et al. Percutaneous Image-Guided Radiofrequency Ablation of Painful Metastases Involving Bone: A Multicenter Study. J. Clin. Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 38.Mohan H., Nicholson P., Winter D.C., O’Shea D., O’Toole D., Geoghegan J., Maguire D., Hoti E., Traynor O., Cantwell C.P. Radiofrequency Ablation for Neuroendocrine Liver Metastases: A Systematic Review. J. Vasc. Interv. Radiol. 2015;26:935–942.e1. doi: 10.1016/j.jvir.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Connors J.C., Boike A.M., Rao N., Kingsley J.D. Radiofrequency Ablation for the Treatment of Painful Neuroma. J. Foot Ankle Surg. 2020;59:457–461. doi: 10.1053/j.jfas.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Brace C.L., Laeseke P.F., Sampson L.A., Frey T.M., van der Weide D.W., Lee F.T. Microwave Ablation with Multiple Simultaneously Powered Small-gauge Triaxial Antennas: Results from an in Vivo Swine Liver Model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 41.Wright A.S., Sampson L.A., Warner T.F., Mahvi D.M., Lee J.F.T. Radiofrequency versus Microwave Ablation in a Hepatic Porcine Model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 42.Ringe K.I., Lutat C., Rieder C., Schenk A., Wacker F., Raatschen H.-J. Experimental Evaluation of the Heat Sink Effect in Hepatic Microwave Ablation. PLoS ONE. 2015;10:e0134301. doi: 10.1371/journal.pone.0134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ringe K.I., Panzica M., von Falck C. Thermoablation of Bone Tumors. Rofo. 2016;188:539–550. doi: 10.1055/s-0042-100477. [DOI] [PubMed] [Google Scholar]
- 44.Hinshaw J.L., Lubner M.G., Ziemlewicz T.J., Lee F.T., Jr., Brace C.L. Percutaneous Tumor Ablation Tools: Microwave, Radiofrequency, or Cryoablation—What Should You Use and Why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrafiello G., Laganà D., Mangini M., Fontana F., Dionigi G., Boni L., Rovera F., Cuffari S., Fugazzola C. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int. J. Surg. 2008;6((Suppl. S1)):S65–S69. doi: 10.1016/j.ijsu.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Thacker P.G., Callstrom M.R., Curry T.B., Mandrekar J.N., Atwell T.D., Goetz M.P., Rubin J. Palliation of Painful Metastatic Disease Involving Bone with Imaging-Guided Treatment: Comparison of Patients’ Immediate Response to Radiofrequency Ablation and Cryoablation. AJR Am. J. Roentgenol. 2011;197:510–515. doi: 10.2214/AJR.10.6029. [DOI] [PubMed] [Google Scholar]
- 47.Susa M., Kikuta K., Nakayama R., Nishimoto K., Horiuchi K., Oguro S., Inoue M., Yashiro H., Nakatsuka S., Nakamura M., et al. CT guided cryoablation for locally recurrent or metastatic bone and soft tissue tumor: Initial experience. BMC Cancer. 2016;16:798. doi: 10.1186/s12885-016-2852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bang H.J., Littrup P.J., Currier B.P., Goodrich D.J., Aoun H.D., Klein L.C., Kuo J.C., Heilbrun L.K., Gadgeel S., Goodman A.C. Percutaneous Cryoablation of Metastatic Lesions from Non–Small-Cell Lung Carcinoma: Initial Survival, Local Control, and Cost Observations. J. Vasc. Interv. Radiol. 2012;23:761–769. doi: 10.1016/j.jvir.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cazzato R.L., Garnon J., Ramamurthy N., Koch G., Tsoumakidou G., Caudrelier J., Arrigoni F., Zugaro L., Barile A., Masciocchi C., et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016;33:140. doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 50.Gangi A., Buy X. Percutaneous Bone Tumor Management. Semin. Interv. Radiol. 2010;27:124–136. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bazzocchi A., Napoli A., Sacconi B., Battista G., Guglielmi G., Catalano C., Albisinni U. MRI-guided focused ultrasound surgery in musculoskeletal diseases: The hot topics. Br. J. Radiol. 2016;89:20150358. doi: 10.1259/bjr.20150358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Napoli A., Anzidei M., Marincola B.C., Brachetti G., Noce V., Boni F., Bertaccini L., Passariello R., Catalano C. MR Imaging–guided Focused Ultrasound for Treatment of Bone Metastasis. Radiographics. 2013;33:1555–1568. doi: 10.1148/rg.336125162. [DOI] [PubMed] [Google Scholar]
- 53.Masciocchi C., Conchiglia A., Gregori L.M., Arrigoni F., Zugaro L., Barile A. Critical role of HIFU in musculoskeletal interventions. Radiol. Med. 2014;119:470–475. doi: 10.1007/s11547-014-0414-z. [DOI] [PubMed] [Google Scholar]
- 54.Liberman B., Gianfelice D., Inbar Y., Beck A., Rabin T., Shabshin N., Chander G., Hengst S., Pfeffer R., Chechick A., et al. Pain Palliation in Patients with Bone Metastases Using MR-Guided Focused Ultrasound Surgery: A Multicenter Study. Ann. Surg. Oncol. 2009;16:140–146. doi: 10.1245/s10434-008-0011-2. [DOI] [PubMed] [Google Scholar]
- 55.Catane R., Beck A., Inbar Y., Rabin T., Shabshin N., Hengst S., Pfeffer R., Hanannel A., Dogadkin O., Liberman B., et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—Preliminary clinical experience. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007;18:163–167. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 56.Masciocchi C., Arrigoni F., La Marra A., Mariani S., Zugaro L., Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br. J. Radiol. 2016;89:20150356. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arrigoni F., Barile A., Zugaro L., Splendiani A., di Cesare E., Caranci F., Ierardi A.M., Floridi C., Angileri A.S., Reginelli A., et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): Imaging follow-up and clinical results. Med. Oncol. 2017;34:55. doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- 58.Mercadante S., Fulfaro F. Management of painful bone metastases. Curr. Opin. Oncol. 2007;19:308–314. doi: 10.1097/CCO.0b013e3281214400. [DOI] [PubMed] [Google Scholar]
- 59.Health Quality Ontario Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: A Systematic Review. [(accessed on 20 March 2022)];Ont. Health Technol. Assess Ser. 2016 16:1–34. Available online: https://pubmed.ncbi.nlm.nih.gov/27298655/ [PMC free article] [PubMed] [Google Scholar]
- 60.Masala S., Chiocchi M., Taglieri A., Bindi A., Nezzo M., De Vivo D., Simonetti G. Combined use of percutaneous cryoablation and vertebroplasty with 3D rotational angiograph in treatment of single vertebral metastasis: Comparison with vertebroplasty. Neuroradiology. 2013;55:193–200. doi: 10.1007/s00234-012-1096-7. [DOI] [PubMed] [Google Scholar]
- 61.van der Linden E., Kroft L.J., Dijkstra P.S. Treatment of Vertebral Tumor with Posterior Wall Defect Using Image-guided Radiofrequency Ablation Combined with Vertebroplasty: Preliminary Results in 12 Patients. J. Vasc. Interv. Radiol. 2007;18:741–747. doi: 10.1016/j.jvir.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Tsoumakidou G., Too C.W., Koch G., Caudrelier J., Cazzato R.L., Garnon J., Gangi A. CIRSE Guidelines on Percutaneous Vertebral Augmentation. Cardiovasc. Interv. Radiol. 2017;40:331–342. doi: 10.1007/s00270-017-1574-8. [DOI] [PubMed] [Google Scholar]
- 63.Saliou G., Kocheida E.M., Lehmann P., Depriester C., Paradot G., Le Gars D., Balut A., Deramond H. Percutaneous Vertebroplasty for Pain Management in Malignant Fractures of the Spine with Epidural Involvement. Radiology. 2010;254:882–890. doi: 10.1148/radiol.09081698. [DOI] [PubMed] [Google Scholar]
- 64.Cazzato R.L., Buy X., Eker O., Fabre T., Palussiere J. Percutaneous long bone cementoplasty of the limbs: Experience with fifty-one non-surgical patients. Eur. Radiol. 2014;24:3059–3068. doi: 10.1007/s00330-014-3357-9. [DOI] [PubMed] [Google Scholar]
- 65.Le Clair A., Gangi A., Lacaze F., Javier R.-M., Bonidan O., Kempf J.-F., Bonnomet F., Limbach F.-X., Kuntz J.-L., Dietmann J.-L., et al. Rapid chondrolysis after an intra-articular leak of bone cement in treatment of a benign acetabular subchondral cyst: An unusual complication of percutaneous injection of acrylic cement. Skelet. Radiol. 2000;29:275–278. doi: 10.1007/s002560050607. [DOI] [PubMed] [Google Scholar]
- 66.Kassamali R.H., Ganeshan A., Hoey E.T.D., Crowe P.M., Douis H., Henderson J. Pain management in spinal metastases: The role of percutaneous vertebral augmentation. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011;22:782–786. doi: 10.1093/annonc/mdq605. [DOI] [PubMed] [Google Scholar]
- 67.Halpin R.J., Bendok B.R., Sato K.T., Liu J.C., Patel J.D., Rosen S.T. Combination treatment of vertebral metastases using image-guided percutaneous radiofrequency ablation and vertebroplasty: A case report. Surg. Neurol. 2005;63:469–474. doi: 10.1016/j.surneu.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 68.Schaefer O., Lohrmann C., Markmiller M., Uhrmeister P., Langer M. Combined Treatment of a Spinal Metastasis with Radiofrequency Heat Ablation and Vertebroplasty. Am. J. Roentgenol. 2003;180:1075–1077. doi: 10.2214/ajr.180.4.1801075. [DOI] [PubMed] [Google Scholar]
- 69.Barragán-Campos H.M., Vallée J.-N., Lo D., Cormier E., Jean B., Rose M., Astagneau P., Chiras J. Percutaneous Vertebroplasty for Spinal Metastases: Complications. Radiology. 2006;238:354–362. doi: 10.1148/radiol.2381040841. [DOI] [PubMed] [Google Scholar]
- 70.Cazzato R.L., Koch G., Buy X., Ramamurthy N., Tsoumakidou G., Caudrelier J., Catena V., Garnon J., Palussiere J., Gangi A. Percutaneous Image-Guided Screw Fixation of Bone Lesions in Cancer Patients: Double-Centre Analysis of Outcomes including Local Evolution of the Treated Focus. Cardiovasc. Interv. Radiol. 2016;39:1455–1463. doi: 10.1007/s00270-016-1389-z. [DOI] [PubMed] [Google Scholar]
- 71.Mirels H. The Classic: Metastatic Disease in Long Bones A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 2003;415:S4–S13. doi: 10.1097/01.blo.0000093045.56370.dd. [DOI] [PubMed] [Google Scholar]
- 72.Cazzato R.L., Garnon J., Tsoumakidou G., Koch G., Palussière J., Gangi A., Buy X. Percutaneous image-guided screws meditated osteosynthesis of impeding and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur. J. Radiol. 2017;90:1–5. doi: 10.1016/j.ejrad.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Garnon J., Koch G., Ramamurthy N., Caudrelier J., Rao P., Tsoumakidou G., Cazzato R.L., Gangi A. Percutaneous CT and Fluoroscopy-Guided Screw Fixation of Pathological Fractures in the Shoulder Girdle: Technical Report of 3 Cases. Cardiovasc. Interv. Radiol. 2016;39:1332–1338. doi: 10.1007/s00270-016-1333-2. [DOI] [PubMed] [Google Scholar]
- 74.Deschamps F., de Baere T., Hakime A., Pearson E., Farouil G., Teriitehau C., Tselikas L. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur. Radiol. 2016;26:1631–1639. doi: 10.1007/s00330-015-3971-1. [DOI] [PubMed] [Google Scholar]
- 75.Gangi A., Tsoumakidou G., Buy X., Quoix E. Quality Improvement Guidelines for Bone Tumour Management. Cardiovasc. Interv. Radiol. 2010;33:706–713. doi: 10.1007/s00270-009-9738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pusceddu C., Sotgia B., Fele R.M., Melis L. Treatment of Bone Metastases with Microwave Thermal Ablation. J. Vasc. Interv. Radiol. 2013;24:229–233. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Cazzato R.L., De Marini P., Leonard-Lorant I., Dalili D., Koch G., Autrusseau P.A., Mayer T., Weiss J., Auloge P., Garnon J., et al. Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control. Diagn. Interv. Imaging. 2021;102:355–361. doi: 10.1016/j.diii.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Ma Y., Wallace A.N., Waqar S.N., Morgensztern D., Madaelil T.P., Tomasian A., Jennings J.W. Percutaneous Image-Guided Ablation in the Treatment of Osseous Metastases from Non-small Cell Lung Cancer. Cardiovasc. Interv. Radiol. 2018;41:726–733. doi: 10.1007/s00270-017-1843-6. [DOI] [PubMed] [Google Scholar]
- 79.Vaswani D., Wallace A.N., Eiswirth P.S., Madaelil T.P., Chang R.O., Tomasian A., Jennings J.W. Radiographic Local Tumor Control and Pain Palliation of Sarcoma Metastases within the Musculoskeletal System with Percutaneous Thermal Ablation. Cardiovasc. Interv. Radiol. 2018;41:1223–1232. doi: 10.1007/s00270-018-1932-1. [DOI] [PubMed] [Google Scholar]
- 80.Erie A.J., Morris J.M., Welch B.T., Kurup A.N., Weisbrod A.J., Atwell T.D., Schmit G.D., Kwon E.D., Callstrom M.R. Retrospective Review of Percutaneous Image-Guided Ablation of Oligometastatic Prostate Cancer: A Single-Institution Experience. J. Vasc. Interv. Radiol. 2017;28:987–992. doi: 10.1016/j.jvir.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Aubry S., Dubut J., Nueffer J.-P., Chaigneau L., Vidal C., Kastler B. Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur. Radiol. 2016;27:1477–1485. doi: 10.1007/s00330-016-4528-7. [DOI] [PubMed] [Google Scholar]
- 82.Tomasian A., Wallace A., Northrup B., Hillen T., Jennings J. Spine Cryoablation: Pain Palliation and Local Tumor Control for Vertebral Metastases. AJNR Am. J. Neuroradiol. 2015;37:189–195. doi: 10.3174/ajnr.A4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deschamps F., Farouil G., Ternes N., Gaudin A., Hakime A., Tselikas L., Teriitehau C., Baudin E., Auperin A., De Baere T. Thermal ablation techniques: A curative treatment of bone metastases in selected patients? Eur. Radiol. 2014;24:1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 84.McMenomy B.P., Kurup A.N., Johnson G., Carter R.E., McWilliams R.R., Markovic S.N., Atwell T.D., Schmit G.D., Morris J.M., Woodrum D.A., et al. Percutaneous Cryoablation of Musculoskeletal Oligometastatic Disease for Complete Remission. J. Vasc. Interv. Radiol. 2013;24:207–213. doi: 10.1016/j.jvir.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Littrup P.J., Bang H.J., Currier B.P., Goodrich D.J., Aoun H.D., Heilbrun L.K., Adam B.A. Soft-Tissue Cryoablation in Diffuse Locations: Feasibility and Intermediate Term Outcomes. J. Vasc. Interv. Radiol. 2013;24:1817–1825. doi: 10.1016/j.jvir.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bang H.J., Littrup P.J., Goodrich D.J., Currier B.P., Aoun H.D., Heilbrun L.K., Vaishampayan U., Adam B., Goodman A.C. Percutaneous Cryoablation of Metastatic Renal Cell Carcinoma for Local Tumor Control: Feasibility, Outcomes, and Estimated Cost-effectiveness for Palliation. J. Vasc. Interv. Radiol. 2012;23:770–777. doi: 10.1016/j.jvir.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arrigoni F., Bianchi G., Formiconi F., Palumbo P., Zugaro L., Gravina G.L., Barile A., Masciocchi C. CT-guided cryoablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022;127:199–205. doi: 10.1007/s11547-021-01437-6. [DOI] [PubMed] [Google Scholar]
- 88.Jennings J.W., Prologo J.D., Garnon J., Gangi A., Buy X., Palussière J., Kurup A.N., Callstrom M., Genshaft S., Abtin F., et al. Cryoablation for Palliation of Painful Bone Metastases: The Motion Multicenter Study. Radiol. Imaging Cancer. 2021;3:16–18. doi: 10.1148/rycan.2021200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madani K., Najafi A., Boticella A., Roux C., Tselikas L., Delpla A., Al Ahmar M., de Baere T., Deschamps F. Combined local treatments for vertebral metastases with limited epidural extension. Support. Care Cancer. 2022;30:337–345. doi: 10.1007/s00520-021-06443-y. [DOI] [PubMed] [Google Scholar]
- 90.Kastler A., Barbé D.-A., Alemann G., Hadjidekov G., Cornelis F.H., Kastler B. Bipolar Radiofrequency Ablation of Painful Spinal Bone Metastases Performed under Local Anesthesia: Feasibility Regarding Patient’s Experience and Pain Outcome. Medicina. 2021;57:966. doi: 10.3390/medicina57090966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pusceddu C., De Francesco D., Melis L., Ballicu N., Fancellu A. The Role of a Navigational Radiofrequency Ablation Device and Concurrent Vertebral Augmentation for Treatment of Difficult-to-Reach Spinal Metastases. Curr. Oncol. 2021;28:4004–4015. doi: 10.3390/curroncol28050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y., Li Y., Wu Y., Qiu S., Liu C., Wang Q., Hong Y., Lyu J., Zhang Y., Du D. Retrospective analysis of CT-guided percutaneous cryoablation for treatment of painful osteolytic bone metastasis. Cryobiology. 2020;92:203–207. doi: 10.1016/j.cryobiol.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Yang X.-G., Wu G., Sun Y.-Y., Pang H.-R., Huang X.-Q., Xu G.-H. Vesselplasty using the Mesh-Hold™ bone-filling container for the treatment of pathological vertebral fractures due to osteolytic metastases: A retrospective study. Eur. J. Radiol. 2020;126:108962. doi: 10.1016/j.ejrad.2020.108962. [DOI] [PubMed] [Google Scholar]
- 94.De Marini P., Cazzato R.L., Auloge P., Koch G., Dalili D., Garnon J., Gangi A. Percutaneous image-guided thermal ablation of bone metastases: A retrospective propensity study comparing the safety profile of radio-frequency ablation and cryo-ablation. Int. J. Hyperth. 2020;37:1386–1394. doi: 10.1080/02656736.2020.1859628. [DOI] [PubMed] [Google Scholar]
- 95.Deib G., Deldar B., Hui F., Barr J.S., Khan M.A. Percutaneous Microwave Ablation and Cementoplasty: Clinical Utility in the Treatment of Painful Extraspinal Osseous Metastatic Disease and Myeloma. AJR Am. J. Roentgenol. 2019;212:1377–1384. doi: 10.2214/AJR.18.20386. [DOI] [PubMed] [Google Scholar]
- 96.Tanigawa N., Arai Y., Yamakado K., Aramaki T., Inaba Y., Kanazawa S., Matsui O., Miyazaki M., Kodama Y., Anai H., et al. Phase I/II Study of Radiofrequency Ablation for Painful Bone Metastases: Japan Interventional Radiology in Oncology Study Group. Cardiovasc. Interv. Radiol. 2018;41:1043–1048. doi: 10.1007/s00270-018-1944-x. [DOI] [PubMed] [Google Scholar]
- 97.Prologo J.D., Passalacqua M., Patel I., Bohnert N., Corn D. Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: A single-center experience. Skelet. Radiol. 2014;43:1551–1559. doi: 10.1007/s00256-014-1939-x. [DOI] [PubMed] [Google Scholar]
- 98.Rossi G., Mavrogenis A.F., Casadei R., Bianchi G., Romagnoli C., Rimondi E., Ruggieri P. Embolisation of bone metastases from renal cancer. Radiol. Med. 2013;118:291–302. doi: 10.1007/s11547-012-0802-4. [DOI] [PubMed] [Google Scholar]
- 99.Campanacci L., Bianchi G., Cevolani L., Errani C., Ciani G., Facchini G., Spinnato P., Tognù A., Massari L., Cornelis F.H., et al. Operating procedures for electrochemotherapy in bone metastases: Results from a multicenter prospective study on 102 patients. Eur. J. Surg. Oncol. (EJSO) 2021;47:2609–2617. doi: 10.1016/j.ejso.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Koirala N., McLennan G. Percutaneous reinforced osteoplasty for long bone metastases: A feasibility study. Skelet. Radiol. 2019;49:375–382. doi: 10.1007/s00256-019-03288-9. [DOI] [PubMed] [Google Scholar]
- 101.Giles S.L., Brown M.R., Rivens I., Deppe M., Huisman M., Kim Y.-S., Farquhar-Smith P., Williams J.E., ter Haar G.R., Desouza N.M. Comparison of Imaging Changes and Pain Responses in Patients with Intra- or Extraosseous Bone Metastases Treated Palliatively with Magnetic Resonance-Guided High-Intensity–Focused Ultrasound. J. Vasc. Interv. Radiol. 2019;30:1351–1360.e1. doi: 10.1016/j.jvir.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sundararajan S.H., Calamita S., Girgis P., Ngo G., Ranganathan S., Giglio M., Gendel V., Goyal S., Nosher J., Roychowdhury S. Sequential Interventional Management of Osseous Neoplasms via Embolization, Cryoablation, and Osteoplasty. J. Oncol. 2019;2019:5247837. doi: 10.1155/2019/5247837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian Q.-H., Liu H.-F., Wang T., Cheng Y.-S., Wu C.-G. Percutaneous Sacroplasty for Painful Sacral Metastases Involving Multiple Sacral Vertebral Bodies: Initial Experience with an Interpedicular Approach. Korean J. Radiol. 2019;20:939–946. doi: 10.3348/kjr.2018.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H.-F., Wu C.-G., Tian Q.-H., Wang T., Yi F. Application of Percutaneous Osteoplasty in Treating Pelvic Bone Metastases: Efficacy and Safety. Cardiovasc. Interv. Radiol. 2019;42:1738–1744. doi: 10.1007/s00270-019-02320-8. [DOI] [PubMed] [Google Scholar]
- 105.Cazzato R.L., Garnon J., Caudrelier J., Rao P.P., Koch G., Gangi A. Low-power bipolar radiofrequency ablation and vertebral augmentation for the palliative treatment of spinal malignancies. Int. J. Hyperth. 2018;34:1282–1288. doi: 10.1080/02656736.2017.1422557. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z.-Q., Wang C.-R., Ma X.-J., Sun W., Shen J.-K., Sun M.-X., Fu Z.-Z., Hua Y.-Q., Cai Z.-D. Evaluation of Quality of Life Using EORTC QLQ-BM22 in Patients with Bone Metastases after Treatment with Magnetic Resonance Guided Focused Ultrasound. Orthop. Surg. 2018;10:264–271. doi: 10.1111/os.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan M., Deib G., Deldar B., Patel A., Barr J. Efficacy and Safety of Percutaneous Microwave Ablation and Cementoplasty in the Treatment of Painful Spinal Metastases and Myeloma. AJNR Am. J. Neuroradiol. 2018;39:1376–1383. doi: 10.3174/ajnr.A5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Couraud G., Gaston A.-P., Thuillier T., Eymard F., Hourdille A., Chevalier X.-J., Boussion H., Guignard S. Evaluation of short-term efficacy of extraspinal cementoplasty for bone metastasis: A monocenter study of 31 patients. J. Bone Oncol. 2018;13:136–142. doi: 10.1016/j.jbo.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fares A., Shaaban M.H., Reyad R.M., Ragab A.S., Sami M.A. Combined percutaneous radiofrequency ablation and cementoplasty for the treatment of extraspinal painful bone metastases: A prospective study. J. Egypt. Natl. Cancer Inst. 2018;30:117–122. doi: 10.1016/j.jnci.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Bertrand A.-S., Iannessi A., Natale R., Beaumont H., Patriti S., Xiong-Ying J., Baudin G., Thyss A. Focused ultrasound for the treatment of bone metastases: Effectiveness and feasibility. J. Ther. Ultrasound. 2018;6:8. doi: 10.1186/s40349-018-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coupal T.M., Pennycooke K., I Mallinson P., A Ouellette H., Clarkson P.W., Hawley P., Munk P.L. The Hopeless Case? Palliative Cryoablation and Cementoplasty Procedures for Palliation of Large Pelvic Bone Metastases. Pain Physician. 2017;20:E1053–E1061. doi: 10.36076/ppj/2017.7.E1053. [DOI] [PubMed] [Google Scholar]
- 112.Pusceddu C., Fancellu A., Ballicu N., Fele R.M., Sotgia B., Melis L. CT-guided percutaneous screw fixation plus cementoplasty in the treatment of painful bone metastases with fractures or a high risk of pathological fracture. Skelet. Radiol. 2017;46:539–545. doi: 10.1007/s00256-017-2584-y. [DOI] [PubMed] [Google Scholar]
- 113.Reyad R., Ghobrial H., Hakim S., Hashem R., Elsaman A., Shaaban M. Thick cement usage in percutaneous vertebroplasty for malignant vertebral fractures at high risk for cement leakage. Diagn. Interv. Imaging. 2017;98:721–728. doi: 10.1016/j.diii.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Motta A., Caltabiano G., Palmucci S., Failla G., Basile A. Feasibility of percutaneous cryoablation of vertebral metastases under local anaesthesia in ASAIII patients. Eur. J. Radiol. 2017;95:13–17. doi: 10.1016/j.ejrad.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 115.McArthur T.A., Narducci C.A., Lander P.H., Lopez-Ben R. Percutane Image-Guided Cryoablation of Painful Osseous Metastases: A Retrospective Single-Center Review. Curr. Probl. Diagn. Radiol. 2017;46:282–287. doi: 10.1067/j.cpradiol.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 116.Bagla S., Sayed D., Smirniotopoulos J., Brower J., Rutledge J.N., Dick B., Carlisle J., Lekht I., Georgy B. Multicenter Prospective Clinical Series Evaluating Radiofrequency Ablation in the Treatment of Painful Spine Metastases. Cardiovasc. Interv. Radiol. 2016;39:1289–1297. doi: 10.1007/s00270-016-1400-8. [DOI] [PubMed] [Google Scholar]
- 117.Facchini G., Di Tullio P., Battaglia M., Bartalena T., Tetta C., Errani C., Mavrogenis A.F., Rossi G. Palliative embolization for metastases of the spine. Eur. J. Orthop. Surg. Traumatol. 2015;26:247–252. doi: 10.1007/s00590-015-1726-y. [DOI] [PubMed] [Google Scholar]
- 118.Pusceddu C., Sotgia B., Fele R.M., Ballicu N., Melis L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc. Interv. Radiol. 2015;39:74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 119.Anzidei M., Napoli A., Sacconi B., Boni F., Noce V., Di Martino M., Saba L., Catalano C. Magnetic resonance-guided focused ultrasound for the treatment of painful bone metastases: Role of apparent diffusion coefficient (ADC) and dynamic contrast enhanced (DCE) MRI in the assessment of clinical outcome. Radiol. Med. 2016;121:905–915. doi: 10.1007/s11547-016-0675-9. [DOI] [PubMed] [Google Scholar]
- 120.Wang F.A., He S.C., Xiao E.H., Wang S.X., Sun L., Lv P.H., Huang W.N. Sequential Transarterial Embolization Followed by Percu-taneous Vertebroplasty Is Safe and Effective in Pain Management in Vertebral Metastases. Pain Physician. 2016;19:E559–E567. [PubMed] [Google Scholar]
- 121.Chen F., Xia Y.-H., Cao W.-Z., Shan W., Gao Y., Feng C., Wang D. Percutaneous kyphoplasty for the treatment of spinal metastases. Oncol. Lett. 2016;11:1799–1806. doi: 10.3892/ol.2016.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bianchi G., Campanacci L., Ronchetti M., Donati D.M. Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial. World J. Surg. 2016;40:3088–3094. doi: 10.1007/s00268-016-3627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiao D., Wu G., Ren J., Han X. Radiofrequency ablation versus 125I-seed brachytherapy for painful metastases involving the bone. Oncotarget. 2016;7:87523–87531. doi: 10.18632/oncotarget.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joo B., Park M.-S., Lee S.H., Choi H.J., Lim S.T., Rha S.Y., Rachmilevitch I., Lee Y.H., Suh J.-S. Pain Palliation in Patients with Bone Metastases Using Magnetic Resonance-Guided Focused Ultrasound with Conformal Bone System: A Preliminary Report. Yonsei Med. J. 2015;56:503–509. doi: 10.3349/ymj.2015.56.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wallace A.N., Greenwood T.J., Jennings J.W. Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J. Neuro-Oncol. 2015;124:111–118. doi: 10.1007/s11060-015-1813-2. [DOI] [PubMed] [Google Scholar]
- 126.Wei Z., Zhang K., Ye X., Yang X., Zheng A., Huang G., Wang J. Computed tomography-guided percutaneous microwave ablation combined with osteoplasty for palliative treatment of painful extraspinal bone metastases from lung cancer. Skelet. Radiol. 2015;44:1485–1490. doi: 10.1007/s00256-015-2195-4. [DOI] [PubMed] [Google Scholar]
- 127.Di Staso M., Gravina G.L., Zugaro L., Bonfili P., Gregori L., Franzese P., Marampon F., Vittorini F., Moro R., Tombolini V., et al. Treatment of Solitary Painful Osseous Metastases with Radiotherapy, Cryoablation or Combined Therapy: Propensity Matching Analysis in 175 Patients. PLoS ONE. 2015;10:e0129021. doi: 10.1371/journal.pone.0129021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cazzato R., Bonichon F., Buy X., Godbert Y., de Figuereido B., Pointillart V., Palussière J. Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur. J. Surg. Oncol. (EJSO) 2015;41:1247–1255. doi: 10.1016/j.ejso.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 129.Tian Q.-H., Wu C.-G., Gu Y.-F., He C.-J., Li M.-H., Cheng Y.-D. Combination Radiofrequency Ablation and Percutaneous Osteoplasty for Palliative Treatment of Painful Extraspinal Bone Metastasis: A Single-Center Experience. J. Vasc. Interv. Radiol. 2014;25:1094–1100. doi: 10.1016/j.jvir.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 130.Kastler A., Alnassan H., Aubry S., Kastler B. Microwave Thermal Ablation of Spinal Metastatic Bone Tumors. J. Vasc. Interv. Radiol. 2014;25:1470–1475. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Hurwitz M.D., Ghanouni P., Kanaev S.V., Iozeffi D., Gianfelice D., Fennessy F.M., Kuten A., Meyer J.E., LeBlang S.D., Roberts A., et al. Magnetic Resonance–Guided Focused Ultrasound for Patients with Painful Bone Metastases: Phase III Trial Results. JNCI J. Natl. Cancer Inst. 2014;106:dju082. doi: 10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alemann G., Kastler A., Barbé D.-A., Aubry S., Kastler B. Treatment of Painful Extraspinal Bone Metastases with Percutaneous Bipolar Radiofrequency Under Local Anesthesia: Feasibility and Efficacy in Twenty-Eight Cases. J. Palliat. Med. 2014;17:947–952. doi: 10.1089/jpm.2013.0531. [DOI] [PubMed] [Google Scholar]
- 133.Botsa E., Mylona S., Koutsogiannis I., Koundouraki A., Thanos L. CT image guided thermal ablation techniques for palliation of painful bone metastases. Ann. Palliat. Med. 2014;3:47–53. doi: 10.3978/j.issn.2224-5820.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 134.Li Z., Ni C., Chen L., Sun Z., Yang C., Zhao X., Wang Y. Kyphoplasty versus vertebroplasty for the treatment of malignant ver-tebral compression fractures caused by metastases: A retrospective study. Chin. Med. J. 2014;127:1493–1496. [PubMed] [Google Scholar]
- 135.Li F., Wang W., Li L., Chang Y., Su D., Guo G., He X., Li M. An Effective Therapy to Painful Bone Metastases: Cryoablation Combined with Zoledronic Acid. Pathol. Oncol. Res. 2014;20:885–891. doi: 10.1007/s12253-014-9769-7. [DOI] [PubMed] [Google Scholar]
- 136.Sun G., Jin P., Liu X.-W., Li M., Li L. Cementoplasty for managing painful bone metastases outside the spine. Eur. Radiol. 2014;24:731–737. doi: 10.1007/s00330-013-3071-z. [DOI] [PubMed] [Google Scholar]
- 137.Callstrom M.R., Dupuy D., Solomon S.B., Beres R.A., Littrup P.J., Davis K.W., Paz-Fumagalli R., Hoffman C., Atwell T.D., Charboneau J.W., et al. Percutaneous image-guided cryoablation of painful metastases involving bone. Cancer. 2012;119:1033–1041. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Napoli A., Anzidei M., Marincola B.C., Brachetti G., Ciolina F., Cartocci G., Marsecano C., Zaccagna F., Marchetti L., Cortesi E., et al. Primary Pain Palliation and Local Tumor Control in Bone Metastases Treated with Magnetic Resonance-Guided Focused Ultrasound. Investig. Radiol. 2013;48:351–358. doi: 10.1097/RLI.0b013e318285bbab. [DOI] [PubMed] [Google Scholar]
- 139.Anselmetti G.C., Manca A., Tutton S., Chiara G., Kelekis A., Facchini F.R., Russo F., Regge D., Montemurro F. Percutaneous verte-bral augmentation assisted by PEEK implant in painful osteolytic vertebral metastasis involving the vertebral wall: Experience on 40 patients. Pain Physician. 2013;16:E397–E404. doi: 10.36076/ppj.2013/16/E397. [DOI] [PubMed] [Google Scholar]
- 140.Trumm C.G., Jakobs T.F., Stahl R., Sandner T.A., Paprottka P.M., Reiser M.F., Zech C.J., Hoffmann R.-T. CT fluoroscopy-guided vertebral augmentation with a radiofrequency-induced, high-viscosity bone cement (StabiliT®): Technical results and polymethylmethacrylate leakages in 25 patients. Skelet. Radiol. 2012;42:113–120. doi: 10.1007/s00256-012-1386-5. [DOI] [PubMed] [Google Scholar]
- 141.Kastler A., Alnassan H., Pereira P.L., Alemann G., Barbé D.-A., Aubry S., Tiberghien F., Kastler B. Analgesic Effects of Microwave Ablation of Bone and Soft Tissue Tumors under Local Anesthesia. Pain Med. 2013;14:1873–1881. doi: 10.1111/pme.12242. [DOI] [PubMed] [Google Scholar]
- 142.Iannessi A., Amoretti N., Marcy P.-Y., Sedat J. Percutaneous cementoplasty for the treatment of extraspinal painful bone lesion, a prospective study. Diagn. Interv. Imaging. 2012;93:859–870. doi: 10.1016/j.diii.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Rossi G., Mavrogenis A.F., Rimondi E., Ciccarese F., Tranfaglia C., Angelelli B., Fiorentini G., Bartalena T., Errani C., Ruggieri P., et al. Selective arterial embolisation for bone tumours: Experience of 454 cases. Radiol. Med. 2011;116:793–808. doi: 10.1007/s11547-011-0670-0. [DOI] [PubMed] [Google Scholar]
- 144.Masala S.A., Guglielmi G., Petrella M.C., Mastrangeli R., Meschini A., Anselmetti G.C., A Bartolucci D., Mammucari M., Manenti G., Simonetti G. Percutaneous ablative treatment of metastatic bone tumours: Visual analogue scale scores in a short-term series. Singap. Med. J. 2011;52:182–189. [PubMed] [Google Scholar]
- 145.Masala S., Schillaci O., Bartolucci A.D., Calabria F., Mammucari M., Simonetti G. Metabolic and clinical assessment of efficacy of cryoablation therapy on skeletal masses by 18F-FDG positron emission tomography/computed tomography (PET/CT) and visual analogue scale (VAS): Initial experience. Skelet. Radiol. 2010;40:159–165. doi: 10.1007/s00256-010-0960-y. [DOI] [PubMed] [Google Scholar]
- 146.Masala S.A., Volpi T., Fucci F.P.M., Cantonetti M., Postorino M., Simonetti G. Percutaneus osteoplasty in the treatment of extraspinal painful multiple myeloma lesions. Support. Care Cancer. 2010;19:957–962. doi: 10.1007/s00520-010-0910-1. [DOI] [PubMed] [Google Scholar]
- 147.Kashima M., Yamakado K., Takaki H., Kaminou T., Tanigawa N., Nakatsuka A., Takeda K. Radiofrequency Ablation for the Treatment of Bone Metastases from Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2010;194:536–541. doi: 10.2214/AJR.09.2975. [DOI] [PubMed] [Google Scholar]
- 148.Carrafiello G., Laganà D., Pellegrino C., Fontana F., Mangini M., Nicotera P., Petullà M., Bracchi E., Genovese E., Cuffari S., et al. Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: Preliminary experience. Radiol. Med. 2009;114:608–625. doi: 10.1007/s11547-009-0395-5. [DOI] [PubMed] [Google Scholar]
- 149.Delpla A., Tselikas L., De Baere T., Laurent S., Mezaib K., Barat M., Nguimbous O., Prudhomme C., Al-Hamar M., Moulin B., et al. Preventive Vertebroplasty for Long-Term Consolidation of Vertebral Metastases. Cardiovasc. Interv. Radiol. 2019;42:1726–1737. doi: 10.1007/s00270-019-02314-6. [DOI] [PubMed] [Google Scholar]
- 150.Thanos L., Mylona S., Galani P., Tzavoulis D., Kalioras V., Tanteles S., Pomoni M. Radiofrequency ablation of osseous metastases for the palliation of pain. Skelet. Radiol. 2008;37:189–194. doi: 10.1007/s00256-007-0404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gianfelice D., Gupta C., Kucharczyk W., Bret P., Havill D., Clemons M. Palliative Treatment of Painful Bone Metastases with MR Imaging–guided Focused Ultrasound. Radiology. 2008;249:355–363. doi: 10.1148/radiol.2491071523. [DOI] [PubMed] [Google Scholar]
- 152.Basile A., Giuliano G., Scuderi V., Motta S., Crisafi R., Coppolino F., Mundo E., Banna G.L., Di Raimondo F., Patti M.T., et al. Cementoplasty in the management of painful extraspinal bone metastases: Our experience. Radiol. Med. 2008;113:1018–1028. doi: 10.1007/s11547-008-0314-1. [DOI] [PubMed] [Google Scholar]
- 153.Anselmetti G.C., Manca A., Ortega C., Grignani G., DeBernardi F., Regge D. Treatment of Extraspinal Painful Bone Metastases with Percutaneous Cementoplasty: A Prospective Study of 50 Patients. Cardiovasc. Interv. Radiol. 2008;31:1165–1173. doi: 10.1007/s00270-008-9396-3. [DOI] [PubMed] [Google Scholar]
- 154.Trumm C.G., Jakobs T.F., Zech C.J., Helmberger T.K., Reiser M.F., Hoffmann R.-T. CT Fluoroscopy–guided Percutaneous Vertebroplasty for the Treatment of Osteolytic Breast Cancer Metastases: Results in 62 Sessions with 86 Vertebrae Treated. J. Vasc. Interv. Radiol. 2008;19:1596–1606. doi: 10.1016/j.jvir.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 155.Tuncali K., Morrison P.R., Winalski C.S., Carrino J.A., Shankar S., Ready J.E., Vansonnenberg E., Silverman S.G. MRI-Guided Percutaneous Cryotherapy for Soft-Tissue and Bone Metastases: Initial Experience. AJR Am. J. Roentgenol. 2007;189:232–239. doi: 10.2214/AJR.06.0588. [DOI] [PubMed] [Google Scholar]
- 156.Forauer A.R., Kent E., Cwikiel W., Esper P., Redman B. Selective palliative transcatheter embolization of bony metastases from renal cell carcinoma. Acta Oncol. 2007;46:1012–1018. doi: 10.1080/02841860701280725. [DOI] [PubMed] [Google Scholar]
- 157.Callstrom M.R., Atwell T.D., Charboneau J.W., Farrell M.A., Goetz M.P., Rubin J., Sloan J.A., Novotny P.J., Welch T.J., Maus T.P., et al. Painful Metastases Involving Bone: Percutaneous Image-guided Cryoablation—Prospective Trial Interim Analysis. Radiology. 2006;241:572–580. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]
- 158.Weber C.H., Krötz M., Hoffmann R.-T., Euler E., Heining S., Pfeifer K.-J., Reiser M., Linsenmaier U. CT-gesteuerte Vertebro- und Kyphoplastie: Vergleichende Untersuchung zu technischem Erfolg und Komplikationen bei 101 Eingriffen [CT-guided vertebroplasty and kyphoplasty: Comparing technical success rate and complications in 101 cases] Rofo. 2006;178:610–617. doi: 10.1055/s-2006-926726. [DOI] [PubMed] [Google Scholar]
- 159.Callstrom M.R., Charboneau J.W. Percutaneous ablation: Safe, effective treatment of bone tumors. Oncology. 2005;19((Suppl. S4)):22–26. [PubMed] [Google Scholar]
- 160.Cai H.-Y., Liu X.-D., Cao H.-P., Wang X.-Q., Zhang Z.-Y., Dong X.-C. Treatment effect of percutaneous vertebroplasty combined with interventional chemotherapy on vertebral metastases. Ai Zheng. 2005;24:488–493. [PubMed] [Google Scholar]
- 161.Masala S., Schillaci O., Massari F., Danieli R., Ursone A., Fiori R., Simonetti G. MRI and bone scan imaging in the preoperative evaluation of painful vertebral fractures treated with ver-tebroplasty and kyphoplasty. In Vivo. 2005;19:1055–1060. [PubMed] [Google Scholar]
- 162.Guzman R., Dubach-Schwizer S., Heini P., Lovblad K.-O., Kalbermatten D.F., Schroth G., Remonda L. Preoperative transarterial embolization of vertebral metastases. Eur. Spine J. 2004;14:263–268. doi: 10.1007/s00586-004-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Masala S., Lunardi P., Fiori R., Liccardo G., Massari F., Ursone A., Simonetti G. Vertebroplasty and Kyphoplasty in the Treatment of Malignant Vertebral Fractures. J. Chemother. 2004;16:30–33. doi: 10.1080/1120009X.2004.11782379. [DOI] [PubMed] [Google Scholar]
- 164.Poggi G., Gatti C., Melazzini M.G., Bernardo G., Strada M., Teragni C., Delmonte A., Tagliaferri C., Bonezzi C., Barbieri M., et al. Percutaneous ultrasound-guided radiofrequency thermal ablation of malignant osteolyses. Anticancer Res. 2004;23:4977–4983. [PubMed] [Google Scholar]
- 165.Eustatia-Rutten C.F.A., Romijn J.A., Guijt M.J., Vielvoye G.J., Berg R.V.D., Corssmit E.P.M., Pereira A.M., Smit J.W.A. Outcome of Palliative Embolization of Bone Metastases in Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2003;88:3184–3189. doi: 10.1210/jc.2003-030231. [DOI] [PubMed] [Google Scholar]
- 166.Fourney D.R., Schomer D.F., Nader R., Chlan-Fourney J., Suki D., Ahrar K., Rhines L.D., Gokaslan Z.L. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J. Neurosurg. Spine. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 167.Winking M., Stahl J.P., Oertel M., Schnettler R., Böker D.K. PMMA vertebroplasty in patients with malignant vertebral destruc-tion of the thoracic and lumbar spine. Ger. Med. Sci. 2003;1:Doc08. [PMC free article] [PubMed] [Google Scholar]
- 168.Alvarez L., Calvo E., Rossi R.E. Vertebroplasty in the treatment of vertebral tumors: Postprocedural outcome and quality of life. Eur. Spine J. 2003;12:356–360. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hierholzer J., Anselmetti G., Fuchs H., Depriester C., Koch K., Pappert D. Percutaneous Osteoplasty as a Treatment for Painful Malignant Bone Lesions of the Pelvis and Femur. J. Vasc. Interv. Radiol. 2003;14:773–777. doi: 10.1097/01.RVI.0000079987.80153.85. [DOI] [PubMed] [Google Scholar]
- 170.Grönemeyer D.H., Schirp S., Gevargez A. Image-Guided Radiofrequency Ablation of Spinal Tumors: Preliminary experience with an expandable array electrode. Cancer J. 2002;8:33–39. doi: 10.1097/00130404-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 171.Chatziioannou A.N., Johnson M.E., Pneumaticos S.G., Lawrence D.D., Carrasco C.H. Preoperative embolization of bone metastases from renal cell carcinoma. Eur. Radiol. 2000;10:593–596. doi: 10.1007/s003300050969. [DOI] [PubMed] [Google Scholar]
- 172.Barr J.D., Barr M.S., Lemley T.J., McCann R.M. Percutaneous Vertebroplasty for Pain Relief and Spinal Stabilization. Spine. 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 173.Martin J., Jean B., Sugiu K., Ruíz D.S.M., Piotin M., Murphy K., Rüfenacht B., Muster M., Rüfenacht D. Vertebroplasty: Clinical experience and follow-up results. Bone. 1999;25((Suppl. S2)):11S–15S. doi: 10.1016/S8756-3282(99)00126-X. [DOI] [PubMed] [Google Scholar]
- 174.Sun S., Lang E.V. Bone Metastases from Renal Cell Carcinoma: Preoperative Embolization. J. Vasc. Interv. Radiol. 1998;9:263–269. doi: 10.1016/S1051-0443(98)70267-2. [DOI] [PubMed] [Google Scholar]
- 175.Weill A., Chiras J., Simon J.M., Rose M., Sola-Martinez T., Enkaoua E. Spinal metastases: Indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 176.Cotten A., Dewatre F., Cortet B., Assaker R., Leblond D., Duquesnoy B., Chastanet P., Clarisse J. Percutaneous vertebroplasty for osteolytic metastases and myeloma: Effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 177.Breslau J., Eskridge J.M. Preoperative Embolization of Spinal Tumors. J. Vasc. Interv. Radiol. 1995;6:871–875. doi: 10.1016/S1051-0443(95)71205-2. [DOI] [PubMed] [Google Scholar]
- 178.Corcos G., Dbjay J., Mastier C., Leon S., Auperin A., De Baere T., Deschamps F. Cement Leakage in Percutaneous Vertebroplasty for Spinal Metastases: A retrospective evaluation of incidence and risk factors. Spine. 2014;39:E332–E338. doi: 10.1097/BRS.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 179.Nakatsuka A., Yamakado K., Uraki J., Takaki H., Yamanaka T., Fujimori M., Hasegawa T., Sakuma H. Safety and Clinical Outcomes of Percutaneous Radiofrequency Ablation for Intermediate and Large Bone Tumors Using a Multiple-Electrode Switching System: A Phase II Clinical Study. J. Vasc. Interv. Radiol. 2016;27:388–394. doi: 10.1016/j.jvir.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 180.Yu W., Tang L., Lin F., Yao Y., Shen Z., Zhou X. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 2015;24:9–15. doi: 10.1016/j.suronc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 181.Anselmetti G.C., Manca A., Montemurro F., Hirsch J., Chiara G., Grignani G., Schianca F.C., Capaldi A., Scalabrini D.R., Sardo E., et al. Percutaneous Vertebroplasty in Multiple Myeloma: Prospective Long-Term Follow-Up in 106 Consecutive Patients. Cardiovasc. Interv. Radiol. 2012;35:139–145. doi: 10.1007/s00270-011-0111-4. [DOI] [PubMed] [Google Scholar]
- 182.Li C., Zhang W., Fan W., Huang J., Zhang F., Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010;116:3934–3942. doi: 10.1002/cncr.25192. [DOI] [PubMed] [Google Scholar]
- 183.Kim N.J., Kim T.W., Park K.H., Chi M.P., O Kim J. The Proper Volume and Distribution of Cement Augmentation on Percutaneous Vertebroplasty. J. Korean Neurosurg. Soc. 2010;48:125–128. doi: 10.3340/jkns.2010.48.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chevalier Y., Pahr D., Charlebois M., Heini P., Schneider E., Zysset P. Cement Distribution, Volume, and Compliance in Vertebroplasty: Some answers from an anatomy-based nonlinear finite element study. Spine. 2008;33:1722–1730. doi: 10.1097/BRS.0b013e31817c750b. [DOI] [PubMed] [Google Scholar]
- 185.Laredo J.-D., Hamze B. Complications of percutaneous vertebroplasty and their prevention. Semin. Ultrasound CT MRI. 2005;26:65–80. doi: 10.1053/j.sult.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 186.Shimony J.S., Gilula L.A., Zeller A.J., Brown D.B. Percutaneous Vertebroplasty for Malignant Compression Fractures with Epidural Involvement. Radiology. 2004;232:846–853. doi: 10.1148/radiol.2323030353. [DOI] [PubMed] [Google Scholar]
- 187.Johnstone C., Lutz S.T. External beam radiotherapy and bone metastases. Ann. Palliat. Med. 2014;3:175–185. doi: 10.3978/j.issn.2224-5820.2014.04.06. [DOI] [PubMed] [Google Scholar]
- 188.Steenland E., Leer J., van Houwelingen H.C., Post W.J., Hout W.B.V.D., Kievit J., de Haes H., Martijn H., Oei B., Vonk E., et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999;52:101–109. doi: 10.1016/S0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 189.De Felice F., Piccioli A., Musio D., Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8:25691–25699. doi: 10.18632/oncotarget.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huisman M., Bosch M.A.V.D., Wijlemans J.W., van Vulpen M., van der Linden Y., Verkooijen H.M. Effectiveness of Reirradiation for Painful Bone Metastases: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:8–14. doi: 10.1016/j.ijrobp.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 191.Harvey N., Ahlmann E.R., Allison D.C., Wang L., Menendez L.R. Endoprostheses Last Longer Than Intramedullary Devices in Proximal Femur Metastases. Clin. Orthop. Relat. Res. 2012;470:684–691. doi: 10.1007/s11999-011-2038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Potter B.K., Chow V.E., Adams S.C., Letson G.D., Temple H.T. Endoprosthetic proximal femur replacement: Metastatic versus primary tumors. Surg. Oncol. 2009;18:343–349. doi: 10.1016/j.suronc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 193.Capanna R., De Biase P., Sensi L. Minimally invasive techniques for treatment of metastatic cancer. Orthopade. 2009;38:343–347. doi: 10.1007/s00132-008-1378-2. [DOI] [PubMed] [Google Scholar]
- 194.Carrafiello G., Laganà D., Pellegrino C., Mangini M., Fontana F., Piacentino F., Recaldini C., Rovera F., Dionigi G., Boni L., et al. Ablation of painful metastatic bone tumors: A systematic review. Int. J. Surg. 2008;6((Suppl. S1)):S47–S52. doi: 10.1016/j.ijsu.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 195.Kurup A.N., Callstrom M.R. Ablation of Musculoskeletal Metastases: Pain Palliation, Fracture Risk Reduction, and Oligometastatic Disease. Tech. Vasc. Interv. Radiol. 2013;16:253–261. doi: 10.1053/j.tvir.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 196.Kurup A.N., Morris J.M., Callstrom M.R. Ablation of Musculoskeletal Metastases. AJR Am. J. Roentgenol. 2017;209:713–721. doi: 10.2214/AJR.17.18527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.