Introduction:
Patients with inflammatory bowel disease (IBD) receiving tumor necrosis factor alpha inhibitors (TNFai) may be at higher risk for hepatitis B virus (HBV) infection. We conducted a quality improvement (QI) initiative to improve HBV vaccination rates in seronegative children with IBD.
Methods:
This QI initiative implemented an HBV vaccination strategy from September 2018 to March 2020 in patients with newly diagnosed IBD with hepatitis B surface antibody (HBsAb) <10 mIU/mL. The project aimed to (1) increase HBV vaccination rates in seronegative patients and (2) document immunogenicity after completing a three-dose vaccine series. Outcome measures included the percentage of seronegative patients who received HBV vaccines (dose 1 and three-dose series). Interventions included applying a standardized vaccination protocol, and creating a vaccine workflow in two clinical areas, previsit planning and stakeholder engagement.
Results:
One hundred seventy-four children and adolescents with IBD were evaluated during the study period, and 132 (76%) were HBsAb negative. After plan-do-study-act (PDSA) 1, the proportion of eligible patients who received HBV vaccine dose 1 increased from a baseline of 7% to 100% and was sustained for over 12 months. During PDSA 2, the proportion of patients completing the three-dose vaccine series improved from a baseline of 0% to 82% (n = 100); among 93 children in this subgroup who had repeat serology performed, 86 (92%) demonstrated serologic evidence of HBV protection.
Conclusions:
A multidisciplinary approach applying QI methodology allowed for improved and sustained HBV vaccination rates in at-risk seronegative children and adolescents with IBD. A three-dose HBV vaccine series proved immunogenic in 92% of eligible patients.
INTRODUCTION
Tumor necrosis factor alpha inhibitors (TNFai) are effective for the induction and maintenance treatment of children with inflammatory bowel disease (IBD). However, TNFai may increase the risk of vaccine-preventable infections (VPIs), including those caused by the Hepatitis B virus (HBV).1,2 HBV nonimmune children receiving TNFai may be at risk for either primary infection or reactivation, the latter occurring more frequently in patients receiving immunosuppression.3 Immunocompromised patients who are hepatitis B surface antigen-positive are at the highest risk of severe and rapidly progressive HBV disease and chronic infection.4 As such, TNFai agents carry a warning regarding HBV reactivation during use.5–7 A hepatitis B surface antibody (HBsAb) ≥ 10 mIU/mL indicates immunity against HBV infection, defines vaccine-induced seroprotection, and is considered a surrogate of clinical protection. Due to the morbidity and mortality associated with HBV, it is imperative to appropriately screen and vaccinate HBV surface antibody seronegative children and adolescents with IBD.2,8
Despite evidence of HBV vaccine efficacy, seroprotection may wane over time.9,10 Revaccination rates among children with IBD remain suboptimal because of unawareness of HBV seronegative status or vaccine recommendations.11,12 In addition, many children and adolescents may have their medical care fragmented between primary and subspecialty care providers, leading to potentially missed opportunities for vaccination.13,14 Some primary care providers (PCPs) may not be comfortable vaccinating immunocompromised individuals or have all vaccine formulations readily available in their offices.15 Published studies regarding HBV vaccination rates among patients with IBD have focused on increasing serologic testing and providing one additional HBV vaccine dose to seronegative patients with variable seroconversion rates.16–20 To reduce potential patient harm, this quality improvement (QI) project aimed to establish a process to improve HBV screening and delivery of a three-dose HBV vaccine series among HBsAb seronegative children and adolescents with newly diagnosed IBD and assess vaccine immunogenicity of this approach.
METHODS
Practice Description
This study was conducted at Nationwide Children’s Hospital (NCH), a 570-bed pediatric quaternary care academic hospital in Columbus, Ohio. The Center for Pediatric and Adolescent Inflammatory Bowel Disease at NCH is a subspecialty, multidisciplinary ambulatory clinic focusing on the comprehensive care of 700 children with IBD.
In the second quarter of 2018, a review of our institutional best practice guidelines measured adherence to screening practices for infectious diseases (ID) before starting TNFai therapies. It demonstrated that only 32% (111/351) of patients with IBD had serologic evidence of HBV immunity. Before starting this QI project, our process had been to refer HBV surface antibody-negative patients to their primary provider for HBV vaccination and request confirmation of vaccine receipt; when received, these data are reflected in the p-chart baseline rates. These data revealed an opportunity for improvement by vaccinating at-risk, HBsAb negative patients. Therefore, in July 2018, a QI team was formed consisting of gastroenterology (GI) and ID physicians, a certified pediatric nurse practitioner, GI IBD center nurses, infusion center nurses, and IBD nurse coordinators, a QI data specialist, and a GI clinical pharmacist.
Inclusion Criteria and Definition of Study Measures
The QI team evaluated all children and adolescents with newly diagnosed IBD receiving care at the NCH IBD center for inclusion from September 2018 to March 2020. The stop date of March 2020 was chosen because it was the start of COVID-19 pandemic measures at our hospital, which disrupted process performance, including in-person evaluations in clinics, and did not allow for vaccine delivery. The process measure was the percentage of patients with a new IBD diagnosis who underwent HBV serologic testing (hepatitis B surface antigen, core antibody, and quantitative surface antibody). Patients with a quantitative HBsAb less than 10 mIU/mL (by chemiluminescent microparticle immunoassay, NCH) were deemed seronegative and included in the study as eligible to receive the HBV vaccine (denominator).
The first outcome measure evaluated the proportion of seronegative patients who received the first dose of HBV vaccine within three months of initial serology results (numerator) divided by the total number of seronegative patients included. The second outcome measure evaluated the proportion of patients who completed the three-dose HBV vaccine series among all seronegative patients included in the study. In addition, a quantitative HBsAb was obtained >8 weeks after the third vaccine dose to assess immunogenicity to HBV vaccination.
Initial baseline data regarding vaccine delivery were collected by obtaining a report of all newly diagnosed patients with IBD with a confirmed HBsAb < 10 IU/mL between August 2017 and August 2018 from the electronic health record (EHR). In addition, before each HBV vaccine dose, we assessed immunization records in the EHR, a state-wide vaccine registry, and from primary medical providers. Unfortunately, we could not always reliably ascertain the number of previous HBV doses an individual had received; thus, we intended to vaccinate seronegative individuals with three doses of HBV vaccine.
Interventions to Improve Vaccination Rates
We created a key driver diagram (KDD) to focus the scope for each outcome measure in stages (Fig. 1). We followed the model for improvement, including repeated plan-do-study-act (PDSA) cycles to implement sequential interventions.21 For example, PDSA 1 focused on initial processes to reliably identify eligible patients and deliver the first HBV vaccine dose (outcome measure 1). PDSA 2 focused on interventions to provide the subsequent two vaccine doses and evaluate postvaccination antibody response (outcome measure 2).
Fig. 1.
Key driver diagram of factors impacting hepatitis B vaccination among nonimmune children with newly diagnosed IBD.
Reliably Identifying Eligible Patients
A priori, the QI team focused on identifying newly diagnosed patients with IBD to optimize vaccine delivery and immunogenicity at diagnosis and before any immunomodulatory therapy.2,22 At our IBD center, newly diagnosed patients undergo baseline laboratory evaluations ordered from within an EHR order set, including HBV serology. Upon identifying a patient as HBV seronegative, the QI team notified GI providers of patient eligibility and the need for HBV vaccination, initially via email to familiarize providers with the QI initiative, then by notification within the EHR. Following this notification, a multidisciplinary team within the clinic provided one-on-one teaching to newly diagnosed patients and their families. We used these visits to obtain updated vaccine records from the child’s PCP and administer the first dose of the HBV vaccine series.
Vaccine Delivery
The KDD involved the creation of a standardized vaccine protocol and algorithm that included vaccine availability and delivery. The QI team reviewed opportunities for optimizing HBV vaccine delivery during the regularly held QI meetings, and changes were considered to patient identification and vaccine delivery. This intervention resulted in formulating a vaccine delivery workflow in two distinct clinical areas: the GI clinic and the infusion center. Following evidence-based guidelines, the vaccine algorithm detailed age-appropriate HBV vaccine dosages and minimum intervals (Fig. 2).8 The vaccine algorithm was used to educate providers, nurses, and pharmacy staff and aid in ordering HBV vaccines. To increase accessibility to appropriate vaccines, pediatric and adult HBV vaccine formulations were stocked in the GI clinic and the infusion center at the start of this project. Revised workflows ensured both GI and infusion nurses could administer the vaccines. Staff received an SBAR (situation, background, assessment, recommendation) communication, and all nurses received vaccine education and completed a competency assessment related to HBV vaccine dosing, scheduling, and delivery. Individual competency was validated before vaccine administration. Throughout PDSA cycles, a missed or near-miss vaccine opportunity was reviewed and small adjustments made, including additional communication with stakeholders on opportunities for improvement and upcoming occasions to provide vaccines.
Fig. 2.
Algorithm for hepatitis B vaccination among nonimmune children with newly diagnosed IBD. *Minimum interval between doses 1 and 2 = 4 weeks, minimum interval between doses 2 and 3 = 8 weeks from dose 2 and 16 weeks from dose 1. **Risk for Hep A: clotting liver disease, clotting factor disorder, men who have sex with men, users of elicit drugs, close contact with international adoptee, travel to countries with intermediate/high endemnicity.
Team Engagement and Education
Multiple stakeholders were engaged to successfully coordinate the interventions needed to define the workflow and effectively complete the vaccine series. GI provider buy-in was necessary to educate patients and families and incorporate vaccination into their routine clinic visits. Nursing staff were educated on the purpose and timing of vaccine administration. In addition, we reviewed the EHR and followed up with providers and nurses to address missed vaccine opportunities, identify root causes, and inform subsequent efforts for timely vaccination. The ID physician and clinical pharmacist tracked all eligible patients, notified providers and nursing of results, provided vaccine recommendations, and performed weekly data reviews. Inpatient pharmacists also received HBV vaccine education and verified vaccines for administration.
Initially, some patients/families chose to return to their PCP to complete the vaccine series. This observation identified an opportunity to improve the interface between our subspecialty clinic and the PCP’s office for vaccine delivery. As a result, the PCP received a standardized letter from the IBD center explaining the serology results and patient-specific vaccine and testing recommendations.
Monthly meetings with the QI team and interested stakeholders allowed for real-time updates and data review, multidisciplinary assessment, and troubleshooting until the workflow and QI process stabilized.
Optimizing EHR and Tracking
The ID physician and clinical pharmacist organized the weekly previsit planning. All newly diagnosed children were added to a secure database to track vaccine completion and immunosuppressive medication administration, dates of serologies, administered and next HBV vaccines, and upcoming clinic or infusion center appointments. EHR smart-order sets were created to facilitate vaccine and serology ordering. To minimize missed vaccination opportunities, the clinical pharmacist preordered all vaccine-related orders for the GI providers to sign within the EHR before the scheduled visit in both infusion and outpatient clinic encounters (Figure 1, Supplemental Digital Content 1, http://links.lww.com/PQ9/A379). Providers were notified of the preordered HBV vaccines via EHR communications. In addition, the clinical pharmacist created a vaccine therapy plan within the EHR, allowing for HBV vaccine administration and postvaccination HBV serologies to be performed with a patient’s scheduled infusion. At the week’s conclusion, the team would confirm vaccine administration, serology results (if obtained), and update the database.
Analysis
We used statistical process control charts (p-charts) to graphically represent the percentage of eligible patients successfully vaccinated (received the first dose and completed three-dose vaccine series) from baseline, starting the vaccine interventions in September 2018 and continuing through March 2020. To assess for evidence of change over time, we followed special cause variation rules, as defined by the American Society for Quality.23
Ethical Considerations
Evidence-based clinical practice vaccine recommendations for immunocompromised patients served as the basis for this study’s interventions.8 Since the study was a QI initiative, NCH’s Institutional Review Board did not require review and approval.
RESULTS
During the study period, we identified 174 patients newly diagnosed with IBD: 172 (99%) had HBV serologies performed (process measure), of which 132 (77%) were HBV seronegative and eligible for vaccination (Fig. 3). Eligible patients were predominantly males, median age of 14 years (IQR 4–20 years), with underlying Crohn disease; the type of immunosuppression varied by vaccine timepoint per patient (Table 1). Eighty (66%) children received the pediatric formulation, and 42 (34%) adolescents received the adult formulation to complete the three-dose HBV vaccine series.
Fig. 3.
Process flow diagram of HBV nonimmune children with newly diagnosed IBD who underwent HBV vaccination.
Table 1.
Demographic and Clinical Characteristics of Children and Adolescents with Newly Diagnosed IBD and HBV Nonimmune, who Received any HBV Vaccination
| Patient Characteristics | N = 122 | |||
|---|---|---|---|---|
| Male, n (%) | 71 (58) | |||
| Age in years, median (range) | 14 (4–20) | |||
| IBD phenotype, n (%) Crohn disease Ulcerative colitis Indeterminate colitis |
90 (74) 27 (22) 5 (4) |
|||
| Locations of vaccine administration Gastroenterology clinic Infusion clinic Primary care provider Inpatient Hospital Other |
N = 336 189 (56) 80 (24) 45 (13) 14 (4) 8 (2) |
|||
| Immunosuppression being received at each vaccine dosing interval (N = patients) | Dose 1 (N = 122) | Dose 2 (N = 114) | Dose 3 (N = 100) | |
| None | No immunosuppression | 20 | 2 | 1 |
| TNFai | Monotherapy | 44 | 51 | 44 |
| Combination with prednisone | 6 | 2 | 1 | |
| Combination with other* | 17 | 28 | 30 | |
| No TNFai | Prednisone-containing regimen | 10 | 5 | 1 |
| Nonprednisone regimen† | 25 | 26 | 23 | |
TNFai, tumor necrosis factor alpha inhibitor.
*Combination with methotrexate (N = 46), 6-mercaptopurine (N = 29).
†Methotrexate (N = 12), 6-mercaptopurine (N = 6), mesalamine (N = 41), vedolizumab (N = 11), and budesonide (N = 4).
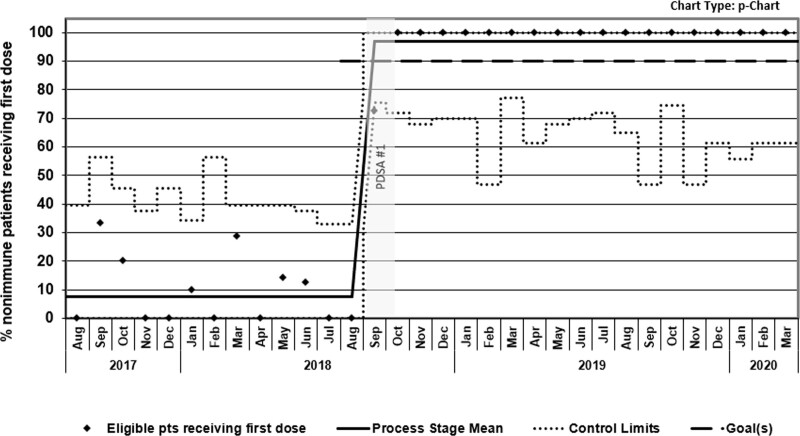
After 2 months of active interventions in PDSA 1, the percentage of HBV seronegative children receiving the first dose of age-appropriate HBV vaccine increased from a historical baseline of 7% to 100% of patients, meeting the KDD aim; this improvement was sustained for over one year (Fig. 4). In total, 122 (92%) eligible patients received at least one dose of HBV vaccine during the study period, with 67 (55%) patients receiving it during their new diagnosis teaching day in the IBD clinic. As a balancing measure for potential process burden from the new vaccine workflow, we measured the amount of time a patient with newly diagnosed IBD spent in the clinic for the project’s first 3 months. These data were reviewed with nursing monthly, noting no significant differences in time spent in the clinic for patients who required and received HBV vaccination compared with patients who did not receive vaccines (data not shown).
Fig. 4.
Shewhart P-chart of proportion of HBV nonimmune children and adolescents with newly diagnosed IBD receiving the first dose of HBV vaccine.
Interventions during PDSA 2 measured compliance with subsequent HBV vaccine doses completed within 4–6 months among the 122 patients who received dose 1. At baseline before the QI initiative, no patients completed the HBV vaccine series at our center. After implementing PDSA 2, completing the three-dose vaccine series administered at any location increased to 82% though it did not meet our original goal of >90%. In total, 114 (93%) children completed dose-2, and 100 (82%) patients completed the full three-dose HBV vaccine series (Fig. 5). Among the 100 patients who received three HBV vaccine doses, 93 (93%) had repeat HBsAb performed and 86 (92%) seroconverted, confirming immunogenicity to a three-dose HBV vaccination. The median HBsAb concentration was 993 mIU/mL (range 38.42–1001), collected a median of 77 days (range 8–357) after the third HBV vaccine dose. Seven children (8%) did not seroconvert after completing the three-dose vaccine series; in six of these patients, we had documentation confirming prior receipt of the primary HBV vaccine series. Their median age was 15 years (range 13–19), and all were receiving immunosuppressive therapy throughout the entire vaccine series, including TNFai monotherapy (N = 4), combination TNFai plus immunomodulator (N = 2), and monotherapy with 6-mercaptopurine until adalimumab was added before vaccine dose 3. Two of these patients did not complete the three-dose series within 6 months.
Fig. 5.
Shewhart P-chart of proportion of HBV nonimmune children and adolescents with newly diagnosed IBD completing the three-dose HBV series. A, Start of COVID-19 pandemic.
HBV vaccines were administered predominantly in GI (56% of patients), then infusion (24%) clinics (Table 1). All patients tolerated the vaccine series with no adverse events reported. Of the 22 patients who did not receive the three doses, eight were followed at off-site GI clinics without HBV vaccine stock available, one patient was lost to follow-up, five patients had serology drawn before completing all three vaccine doses and were found to be HBV seropositive, and eight were seen via telehealth during the start of the COVID-19 pandemic and were unable to complete the series within 6 months of the first vaccine dose.
DISCUSSION
The number of children and adolescents diagnosed with IBD is increasing, as is the number of patients prescribed TNFai treatments.24 TNF-alpha is involved in controlling HBV; thus, inhibiting TNF-alpha could allow for HBV reactivation in susceptible individuals.1,2 TNFai can reactivate HBV in patients with IBD, leading societies to recommend HBV vaccination in patients lacking serologic evidence of HBV immunity.7,25–27 Although the current HBV infection rate in the US children is low, children and adolescents not previously vaccinated or HepBsAb seronegative individuals at risk for HBV infection (by sexual or percutaneous exposure) should be vaccinated to protect them from this VPI.9,28
In our cohort of 174 patients, only 24% of patients demonstrated serologic evidence of HBV immunity at the time of IBD diagnosis. This rate is similar to other published studies demonstrating low rates of HBV seroprotection (28%–36%) among children with IBD.17,18,20,29 A recent systematic review reported the pooled response rate to HBV vaccination among 1,688 children and adults with IBD to be approximately 61% (95% CI, 53–69); younger age during IBD remission and receipt of no immunosuppressive therapies were predictive of vaccine immunogenicity.30 Most pediatric studies evaluate the benefit of one booster HBV vaccine dose, resulting in seroconversion in 50%–79% of previously seronegative children with IBD.16,18,20,29,31 However, current guidelines recommend that seronegative individuals receive a three-dose HBV vaccine series.22 A pediatric study evaluating revaccination with a three-dose HBV series reported that 98% of 59 study participants seroconverted.29 This seroconversion rate aligns with our cohort data, where 92% (86/93 tested) had serologic evidence of protection after receiving the three-dose vaccine series. These data suggest that receiving a three-dose HBV vaccine series may be more immunogenic than a single HBV booster dose, even among patients receiving TNFai, a previously described risk factor for poor seroresponse.32,33
Compliance with vaccination is tracked as a quality metric, and applying QI processes can improve vaccination rates against other VPI (eg, influenza) in children with underlying IBD.34,35 We chose to leverage QI methodology to ensure delivery of a three-dose HBV vaccine series in a pediatric cohort of seronegative patients with IBD. Since many patients with IBD receive therapy infusions every 4–8 weeks, aligning the vaccine therapy plan with infusion clinic appointments proved to be advantageous to project success. Although this was a workflow change for the infusion clinic and required additional staff education, it allowed timely HBV vaccination in 24% of patients.
The collaboration between ID, pharmacy, and GI provided a comprehensive team approach that allowed rapid identification of challenges and problem-solving. The clinical pharmacist played a crucial role in the project’s success, from previsit planning, maintaining up-to-date vaccine records, and preordering vaccines and serology at appropriate intervals. This involvement limited the burden on providers to track and order the proper vaccine.
We were unable to confirm the exact number of HBV vaccine doses a patient had previously received at the time of initial IBD diagnosis; it is possible that previously vaccinated and unvaccinated patients were included. HBV vaccination could proceed for this study despite this limitation since serologic testing confirmed a HBsAb < 10 mIU/mL. We also did not assess for seroconversion after each vaccine dose, which could have provided additional data for or against the need for three vaccine doses. The amount of time and manual work required to perform previsit planning may limit the generalizability of this process at other centers. This QI study underscored the importance of a dedicated, multidisciplinary team to ensure that one aspect of medical care—providing an age-appropriate HBV vaccine—was performed optimally in a complex patient population. The clinical pharmacist spent approximately 1–1.5 hours weekly reviewing vaccines administered and preordering vaccine doses and serologies for the upcoming week. In addition, confirming vaccine delivery when issued outside of the organization and not documented within the EHR or state-wide vaccine registry was problematic. Although we encouraged vaccination, we rarely received requested vaccination records from families and PCPs, which may have underestimated the percentage of patients who received and completed the HBV vaccines series.
Logistical barriers related to the timing of outpatient follow-up and off-site clinic locations also prevented timely and complete vaccination in all patients. For example, patients in clinical remission not receiving TNFai would follow every 4–6 months, rendering it challenging to administer vaccines within the recommended 6-month time frame from the initial vaccine dose. In addition, a subset of patients with IBD received care at one of the five off-site clinics not equipped with HBV vaccines.
Given the success of this initiative at the main hospital clinic and infusion center, immediate future directions include obtaining HBV vaccine stock and establishing a vaccine workflow at the off-site clinic locations. Additionally, we plan to expand vaccination efforts to established patients with IBD who are HBV seronegative. However, this project’s sustainability and widespread implementation in other patient populations will depend upon improvements within and leveraging the EHR, including processes to facilitate automated integration of vaccine registry data in real-time. Moreover, building clinical decision-making tools into the EHR to address health and vaccine maintenance for immunocompromised children is essential to identify eligible patients, automate tracking, monitor missed vaccination opportunities, and prevent errors successfully and sustainably.
CONCLUSIONS
The risk of HBV infection among patients with IBD is highest among those who are HBsAb seronegative and receiving immunosuppressive therapies. In our cohort, 76% of patients were HBV seronegative at IBD diagnosis and, thus, at potential risk for a VPI. Utilizing QI methodology, we successfully implemented and sustained an HBV vaccine algorithm and workflow in patients receiving medical care at our ambulatory and infusion clinics. Implementing sequential PDSA cycle interventions met the study aim. In addition, they demonstrated that delivering a three-dose HBV vaccine series was possible, sustainable, and resulted in successful HBV seroconversion among 92% of at-risk children and adolescents with IBD. A multidisciplinary approach and well-established protocol with previsit planning were essential to project success.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
We want to thank the NCH GI providers, pharmacy personnel, and nursing staff for their crucial role in the implementation and success of this project.
Supplementary Material
Footnotes
Published online June 23, 2022
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Mcnicol M, Donegan A, Hawa K, Boutzoukas AE, Drobnic B, Oates M, Orraca-tettah M, Michel HK, Maltz RM, Dotson JL, Buckingham D, Boyle B, Ardura MI. Improving Hepatitis B Vaccination Rates among At-risk Children and Adolescents with Inflammatory Bowel Disease. Pediatr Qual Saf 2022;7:e570.
REFERENCES
- 1.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. [DOI] [PubMed] [Google Scholar]
- 2.Ardura MI, Toussi SS, Siegel JD, et al. NASPGHAN clinical report: surveillance, diagnosis, and prevention of infectious diseases in pediatric patients with inflammatory bowel disease receiving tumor necrosis factor-α inhibitors. J Pediatr Gastroenterol Nutr. 2016;63:130–155. [DOI] [PubMed] [Google Scholar]
- 3.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244.e3. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KR, Beavers KL, Hammond SP, et al.; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219; quiz e16. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–S165. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, et al. ; BIOGEAS Study Group. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore). 2011;90:359–371. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman V, Wise KA, Cotton W, et al. Previsit planning improves pneumococcal vaccination rates in childhood-onset SLE. Pediatrics. 2020;145:e20183141. [DOI] [PubMed] [Google Scholar]
- 8.Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–100. [DOI] [PubMed] [Google Scholar]
- 9.Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lao TT. Immune persistence after hepatitis B vaccination in infancy—Fact or fancy? Hum Vaccin Immunother. 2016;12:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasan SK, Calderwood AH, Long MD, et al. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 13.Holland KJ, Slaven JE, Ren CL, et al. A Retrospective cohort study of growth in the first 2 years of life in preterm infants with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2020;70:115–120. [DOI] [PubMed] [Google Scholar]
- 14.Michel HK, Siripong N, Noll RB, et al. Caregiver and adolescent patient perspectives on comprehensive care for inflammatory bowel diseases: building a family-centered care delivery model. Crohns Colitis 360. 2020;2:otaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd AAM, Wise K, Chavarin D, Crandall W, Boyle B, Sivaraman V. Barriers to Vaccination in Immunocompromised Children. Pediatric Academic Societies Meeting; 2019. [Google Scholar]
- 16.Jean MR, Weaver A, Mastin-Diebold T, et al. Improving a process to obtain hepatitis B serology among patients treated with infliximab at a large urban children’s hospital. BMJ Open Qual. 2017;6:e000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts A, Bennett WE, Molleston JP, et al. Incidence of low seroimmunity to Hepatitis B virus in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;65:551–554. [DOI] [PubMed] [Google Scholar]
- 18.Moses J, Alkhouri N, Shannon A, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol. 2012;107:133–138. [DOI] [PubMed] [Google Scholar]
- 19.Aljaberi N, Ghulam E, Smitherman EA, et al. Maintaining Hepatitis B protection in immunocompromised pediatric rheumatology and inflammatory bowel disease patients. J Rheumatol. 2021;48:1314–1321. [DOI] [PubMed] [Google Scholar]
- 20.Phatak UP, Rojas-Velasquez D, Pashankar DS. Seroimmunity to Hepatitis B virus in children with inflammatory bowel disease: effects of booster vaccination. J Pediatr Gastroenterol Nutr. 2018;66:e137. [DOI] [PubMed] [Google Scholar]
- 21.Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. John Wiley & Sons; 2009. [Google Scholar]
- 22.Lu Y, Bousvaros A. Immunizations in children with inflammatory bowel disease treated with immunosuppressive therapy. Gastroenterol Hepatol (N Y). 2014;10:355–363. [PMC free article] [PubMed] [Google Scholar]
- 23.American Society for Quality. Control chart. https://asq.org/quality-resources/control-chart. Accessed November 20, 2020.
- 24.Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteve M, Saro C, González-Huix F, et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. AAP, 2018: 401–428. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Hepatitis B Information: Hepatitis B Questions and Answers for Health Professionals. https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm. Accessed December 10, 2021.
- 28.Shapiro CN. Epidemiology of hepatitis B. Pediatr Infect Dis J. 1993;12:433–437. [DOI] [PubMed] [Google Scholar]
- 29.Brenner EJ, Jhaveri R, Kappelman MD, et al. Evaluating Hepatitis B seroprotection and revaccination for children with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:e108. [DOI] [PubMed] [Google Scholar]
- 30.Jiang HY, Wang SY, Deng M, et al. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine. 2017;35:2633–2641. [DOI] [PubMed] [Google Scholar]
- 31.Urganci N, Kalyoncu D. Immunogenecity of hepatitis A and B vaccination in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:412–415. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, et al. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1460–1466. [DOI] [PubMed] [Google Scholar]
- 33.Pratt PK, Jr, David N, Weber HC, et al. Antibody response to Hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. [DOI] [PubMed] [Google Scholar]
- 34.Parker S, Chambers White L, Spangler C, et al. A quality improvement project significantly increased the vaccination rate for immunosuppressed patients with IBD. Inflamm Bowel Dis. 2013;19:1809–1814. [DOI] [PubMed] [Google Scholar]
- 35.Egberg MD, Gulati AS, Gellad ZF, et al. Improving quality in the care of patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2018;24:1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.