OBJECTIVES:
Few studies have explored the effect of frailty on the long-term survival of COVID-19 patients after ICU admission. Furthermore, the Clinical Frailty Scale (CFS) validity in critical care patients remains debated. We investigated the association between frailty and 6-month survival in critically ill COVID-19 patients. We also explored whether ICU resource utilization varied according to frailty status and examined the concurrent validity of the CFS in this setting.
DESIGN:
Ancillary study of a longitudinal prospective cohort.
SETTING:
University hospital in São Paulo.
PATIENTS:
Patients with severe COVID-19 admitted to ICU.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We assessed baseline frailty using the CFS (1–9; frail ≥ 5) and used validated procedures to compute a Frailty Index (0–1; frail > 0.25). We used Cox models to estimate associations of frailty status with 6-month survival after ICU admission and area under the receiver operating characteristic curves (AUCs) to estimate CFS’s accuracy in identifying frailty according to Frailty Index. We included 1,028 patients (mean age, 66 yr; male, 61%). Overall, 224 (22%) patients were frail (CFS ≥ 5), and 608 (59%) died over the 6-month follow-up. Frailty was independently associated with lower 6-month survival and further stratified mortality in patients with similar age and Sequential Organ Failure Assessment scores. We additionally verified that the CFS was highly accurate in identifying frailty as defined by the Frailty Index (AUC, 0.91; 95% CI, 0.89–0.93). Although treatment modalities did not diverge according to frailty status, higher CFS scores were associated with withholding organ support due to refractory organ failure.
CONCLUSIONS:
One in five COVID-19 patients admitted to the ICU was frail. CFS scores greater than or equal to 5 were associated with lower long-term survival and decisions on withholding further escalation of invasive support for multiple organ failure in the ICU. Clinicians should consider frailty alongside sociodemographic and clinical measures to have a fuller picture of COVID-19 prognosis in critical care.
Keywords: COVID-19, frailty, intensive care, mortality, prognosis
Frailty is an age-related syndrome marked by decreased physical, physiologic, and cognitive reserves. Frail patients are more vulnerable to adverse outcomes (e.g., mortality, disability), particularly in critical illness (1). Recently, frailty has been proposed as a significant risk factor for short-term mortality in critically ill patients with the COVID-19 (1–3).
However, some uncertainties persist concerning the relationship between frailty and COVID-19 prognosis. For instance, the association between frailty and mortality has been inconsistent across studies (4, 5). Also, we have little information about the effect of frailty on the long-term survival (i.e., follow-up period longer than 90 d) of COVID-19 patients admitted to ICUs. Additionally, data from developing countries and younger patients are underrepresented in the context of patients admitted to the ICU with COVID-19 (2). Furthermore, the association between frailty and ICU resource use is discordant between recent reports (2, 3, 6, 7). Finally, while the Clinical Frailty Scale (CFS) is the most commonly used frailty tool in acute care, its accuracy compared with other validated multidimensional frailty measures remains uncertain in the ICU context (8).
Therefore, we aimed 1) to investigate the association between frailty and 30-day and 6-month survival following ICU admission for COVID-19; 2) to explore whether ICU resource use varied according to frailty status; and 3) to compare the concurrent validity of the CFS with a validated multidimensional frailty measurement (the Frailty Index) of the same ICU population.
MATERIALS AND METHODS
Study Design and Patient Population
The datasets of two longitudinal studies conducted at Hospital das Clinicas, University of São Paulo Medical School (HCFMUSP), the CO-FRAIL [COVID-19 and Frailty] Study (1) and the EPICCoV [EPIdemiology of Critical COVID-19] Study (9) were assembled to build this cohort study (only patients common to both databases were included). HCFMUSP is a university hospital complex that converted 900 of its 2,400 beds to treat COVID-19 patients during the pandemic. The COVID-19 unit comprised 200 ICU beds and received severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients from 278 secondary hospitals located in 85 cities, mainly from the São Paulo metropolitan area (1, 10).
The CO-FRAIL Study comprised confirmed COVID-19 patients greater than or equal to 50 years old consecutively admitted to hospital between March 30, 2020, and July 7, 2020 (1). The EPICCoV Study included COVID-19 patients greater than or equal to 14 years old consecutively admitted to the ICU between March 30, 2020, and June 30, 2020 (9). Both CO-FRAIL and EPPICoV studies were reviewed and approved by HCFMUSP’s Research Ethics Committee (approval number 32037120.6.0000.0068 and 31382620.0.0000.0068) and registered in public databases (CO-FRAIL Study [Brazilian Clinical Trials Registry = RBR-7w5zhr] and EPICCoV Study [ClinicalTrials.gov = NCT04378582]). Recent analyses combining these two datasets have demonstrated the impact of COVID-19 on short-term patient-centered outcomes (e.g., frailty, functional disability) (11).
Data Collection and Definitions
Medical investigators collected the study data using case report forms after completing extensive examinations of electronic medical records and conducting structured telephone interviews with patients or their proxy. Data included demographics (e.g., age, sex), comorbidities as determined by the Charlson Comorbidity Index (12), duration of COVID-19 symptoms, ICU stay characteristics (e.g., Simplified Acute Physiology Score 3 [SAPS 3] [13, 14], presence and severity of organ dysfunction at admission according to the Sequential Organ Failure Assessment [SOFA] score [15], use of invasive organ support), and limitation of life-sustaining therapies (see Outcomes subsection below).
Frailty was measured using the CFS (16). Trained medical investigators followed existing guidelines to rate the CFS (17, 18), using their clinical judgment after assessing patients’ information on physical activity practice, reported symptoms (e.g., fatigue), level of independence in routine activities, and cognitive impairment, regarding the period 2–4 weeks before the infection. Similar to previous ICU studies (16, 19), CFS scores were classified according to four groups, 1–3 (“very fit” to “managing well”), 4 (“vulnerable”), 5 (“mildly frail”), and 6–9 (“moderately frail to terminally ill”). Frailty was defined as present by CFS scores greater than or equal to 5 (16). We also used the well-known concept of deficit accumulation to estimate a Frailty Index that could serve as a multidimensional frailty measure in our sample (20, 21). This Frailty Index (0–1; worse = 1) represented the proportion of impaired items across 40 age-related health conditions (Table S1, http://links.lww.com/CCX/B6), and scores greater than 0.25 defined frailty (1).
Outcomes
Our primary outcomes were time-to-death within 30 days and 6 months of ICU admission. Patients who were alive at the end of the 180-day follow-up period were right-censored. Secondary outcomes included resource utilization during the ICU stay (i.e., type of invasive organ support, such as invasive mechanical ventilation, vasoactive drugs, or renal replacement therapy) and decisions about treatment limitations. We assessed the following scenarios regarding decisions on treatment limitations: 1) lack of hospital resources during the patient stay (e.g., lack of ICU bed or equipment for organ support, such as mechanical ventilators); 2) avoiding initiation of invasive support for severe clinical condition (e.g., hypoxemic patient under supplementary oxygen with a decision for “do not intubate,” acute kidney injury with a decision for not starting renal replacement therapy); 3) withholding further escalation of invasive support already in place (e.g., patient already with a vasopressor in whom no further increase in infusions would occur); and 4) withdrawing invasive organ support (e.g., palliative extubation). During the pandemic, the clinical staff working at the COVID-19-only facility of our institution was trained to register information on treatment limitations (i.e., lack of resources and decisions on avoiding initiation of invasive support, withholding further escalation of invasive support, and withdrawing invasive organ support) in electronic hospital records. Medical investigators blinded to frailty data conducted detailed chart reviews to retrieve information on these treatment limitations. All cases identified as having decisions on treatment limitations were double-checked by a senior ICU physician blinded to the study protocol data. After confirming the outcome, this investigator classified the type of treatment limitation using the data available on electronic hospital records.
Analysis
Quantitative data were presented as mean and sds or median and interquartile ranges (IQRs) as applicable and the analysis of variance test was used to examine parametric data and Kruskal-Wallis test for nonparametric data. chi-square tests were used to assess categorical data, given as counts and percentages.
Kaplan-Meier curves were generated to estimate 6-month survival rates according to frailty status, stratifying the sample by age group and organ dysfunction severity. Cox proportional hazards models were fitted to examine the association between frailty and time-to-death within 30 days and 6 months of ICU admission. Conceptual diagrams were applied to select the variables for adjustment and test for multicollinearity (Fig. S1, http://links.lww.com/CCX/B6). Adjusted hazards ratios and 95% CIs for each variable of interest were reported. In our analyses, we chose to categorize SOFA and Charlson scores using quartiles since they do not have standard cutoff values. No imputation of missing data was performed.
Treatment limitations were compared across frailty categories using chi-square test. Use and type of invasive resource were also compared between those with and without any treatment limitation.
Additionally, the Spearman rank correlation between the CFS and Frailty Index was calculated to investigate their concurrent validity. We still computed area under the receiver operating characteristic curves (AUCs) to measure the CFS’s accuracy in discriminating between frail and nonfrail patients as determined by the Frailty Index. Sensitivity, specificity, and likelihood ratios for each CFS score were estimated, and the Youden index (sensitivity + specificity–1) was applied to identify the cutoff with the best performance.
All hypotheses were two-tailed with a significance level of 0.05. To complete our analyses, either RStudio, Version 1.4.1717 (Boston, MA), or IBM SPSS Statistics, Version 21 (Armonk, NY) were used.
RESULTS
All 1,028 patients who took part in both studies, CO-FRAIL (n = 1,830) and EPICCoV (n = 1,503), were included in our sample. Patients had a mean age of 66 years, and 61% were male. The sample presented a median SAPS 3 score of 66 (Table 1), and 80% of patients (821/1,028) used invasive mechanical ventilation during ICU stay. Overall, 224 patients (22%) were classified as frail (CFS = 5–8), with no individuals rated as terminally ill (CFS = 9). The median Frailty Index of patients was 0.16 (IQR, 0.11–0.25).
TABLE 1.
Characteristics of the Study Participants According to Frailty Categories
| Characteristic | Total | CFS 1–3a | CFS 4a | CFS 5a | CFS 6–9a | p b |
|---|---|---|---|---|---|---|
| n | 1,028 | 629 | 175 | 108 | 116 | |
| Age (IQR), yr | 65 (59–73) | 64 (58–71) | 67 (60–73) | 71 (65–80) | 67 (61–76) | < 0.001 |
| Male, n (%) | 628 (61.1) | 417 (66.3) | 98 (56.0) | 53 (49.1) | 60 (51.7) | < 0.001 |
| Simplified Acute Physiology Score 3 (IQR) | 66 (55–77) | 65 (54–75) | 66 (54–79) | 72 (59–84) | 68 (56–80) | < 0.001 |
| Admission SOFA (IQR) | 8 (5–12) | 8 (5–12) | 9 (4–12) | 8 (5–12) | 9 (5–12) | 0.88 |
| Admission SOFA, respiratory component (IQR) | 3 (2–3) | 3 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.053 |
| Body mass index, kg/m2 (IQR) | 27.1 (24.1–31.2) | 27.4 (24.5–31.2) | 27.5 (24.0–32.7) | 26.3 (23.5–31.1) | 25.4 (22.6–30.7) | 0.04 |
| Duration of COVID-19 symptoms before ICU (IQR), d | 9 (6–13) | 9 (7–13) | 8 (5–12) | 8 (5–14) | 8 (5–13) | 0.002 |
| Comorbidities, n (%) | ||||||
| Hypertension | 764 (74.3) | 441 (70.1) | 140 (80.0) | 86 (79.6) | 97 (83.6) | 0.001 |
| Chronic obstructive pulmonary disease | 118 (11.5) | 61 (9.7) | 25 (14.3) | 14 (13.0) | 18 (15.5) | 0.14 |
| Heart failure | 145 (14.1) | 45 (7.2) | 42 (24.0) | 31 (28.7) | 27 (23.3) | < 0.001 |
| Coronary disease | 140 (13.6) | 58 (9.2) | 40 (22.9) | 21 (19.4) | 21 (18.1) | < 0.001 |
| Diabetes mellitus | 506 (49.2) | 276 (43.9) | 95 (54.3) | 63 (58.3) | 72 (62.1) | < 0.001 |
| Chronic kidney disease | 196 (19.1) | 77 (12.2) | 48 (27.4) | 34 (31.5) | 37 (31.9) | < 0.001 |
| Cerebrovascular disease | 107 (10.4) | 40 (6.4) | 18 (10.3) | 19 (17.6) | 30 (25.9) | < 0.001 |
| Dementia | 34 (3.3) | 8 (1.3) | 4 (2.3) | 6 (5.6) | 16 (13.8) | < 0.001 |
| Cancer | 155 (15.1) | 51 (8.1) | 39 (22.3) | 36 (33.3) | 29 (25.0) | < 0.001 |
| Charlson Comorbidity Index (IQR) | 2 (1–4) | 1 (0–2) | 2 (1–5) | 4 (2–6) | 3 (2–6) | < 0.001 |
| Frailty Index (0 to 1) | 0.16 (0.11–0.25) | 0.13 (0.10–0.16) | 0.20 (0.16–0.25) | 0.26 (0.23–0.31) | 0.41 (0.34–0.48) | < 0.001 |
CFS = Clinical Frailty Scale, IQR = interquartile range, SOFA = Sequential Organ Failure Assessment.
aPre-COVID-19 frailty was assessed based on characteristics from 2 to 4 wk preceding hospitalization.
bComparison between CFS categories.
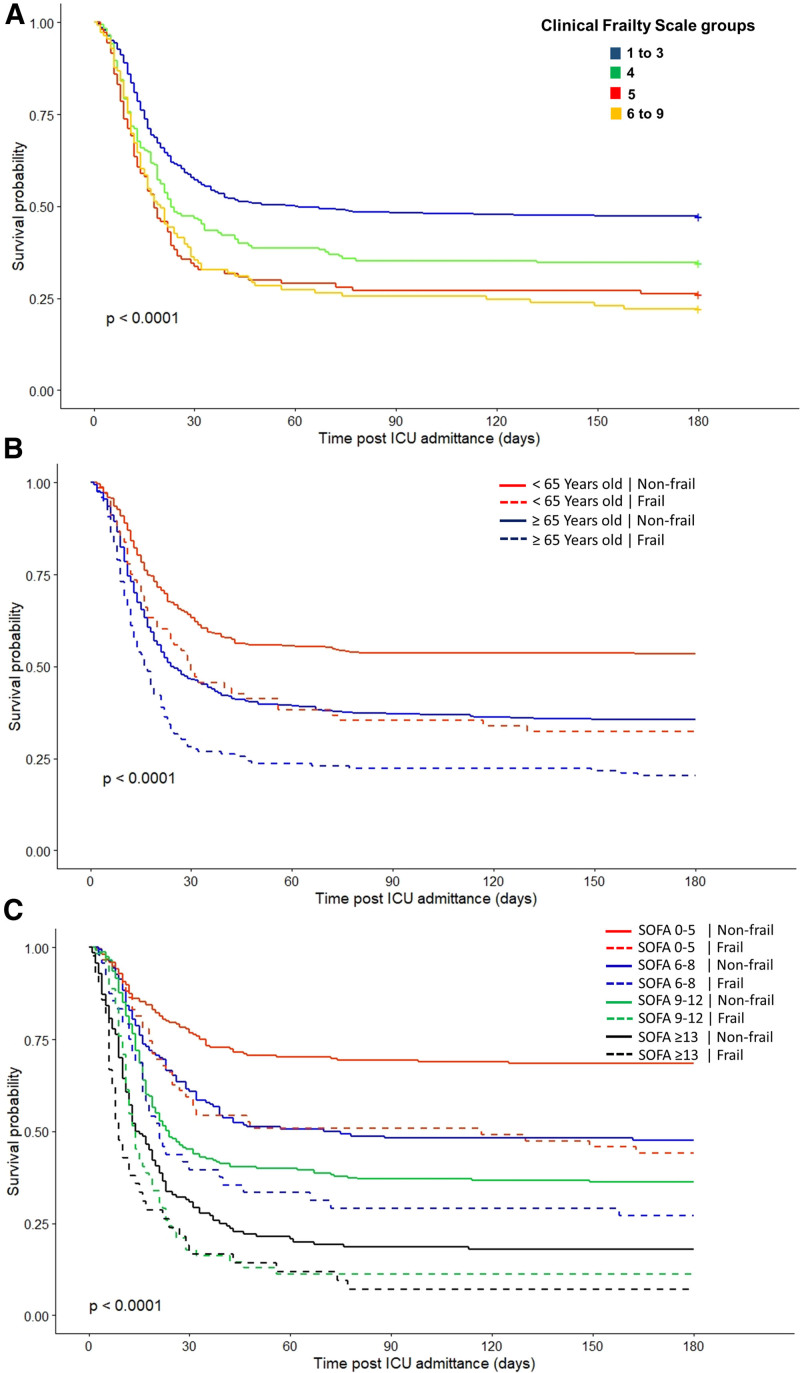
During the 6-month follow-up, 608 patients died (59%), 585 in the hospital, and 23 after discharge. Thirteen patients (1%) were lost to follow-up. Six-month survival varied significantly between CFS groups (Fig. 1A). We also observed that frailty was associated with higher rates of mortality within the same age groups (Fig. 1B) and SOFA score strata (Fig. 1C). We further demonstrated that higher CFS scores were independently associated with 30-day and 6-month mortality (Table 2).
Figure 1.
Kaplan-Meier survival curves over 180 d after ICU admission. Survival curves were stratified according to frailty status (A), according to the frailty status within the same age group (B) and stratum of Sequential Organ Failure Assessment (SOFA) score (C). Frailty was assessed using the Clinical Frailty Scale. Comparison between survival curves was performed using the log-rank test.
TABLE 2.
Association Between Frailty and Mortality in Critically Ill Patients With COVID-19
| Variable | 30-d Mortality | 6-mo Mortalityb |
|---|---|---|
| Adjusted Hazard ratio (95% CI) | Adjusted Hazard ratio (95% CI) | |
| Age (yr) | 1.03 (1.02–1.04) | 1.03 (1.02–1.04) |
| Male sex | 1.35 (1.12–1.63) | 1.46 (1.22–1.73) |
| Charlson score | ||
| 0 point | (Reference) | (Reference) |
| 1 point | 1.03 (0.78–1.36) | 1.07 (0.83–1.38) |
| 2–3 points | 1.36 (1.03–1.80) | 1.31 (1.02–1.69) |
| ≥ 4 points | 1.78 (1.36–2.33) | 1.70 (1.33–2.18) |
| Sequential Organ Failure Assessment score | ||
| 0–5 points | (Reference) | (Reference) |
| 6–8 points | 1.61 (1.20–2.17) | 2.14 (1.64–2.79) |
| 9–12 points | 2.06 (1.58–2.68) | 2.98 (2.34–3.78) |
| ≥ 13 points | 2.89 (2.19–3.82) | 4.78 (3.71–6.16) |
| Clinical Frailty Scalea | ||
| 1–3 | (Reference) | (Reference) |
| 4 | 1.17 (0.91–1.50) | 1.28 (1.02–1.60) |
| 5 | 1.55 (1.17–2.06) | 1.51 (1.15–1.99) |
| 6–9 | 1.49 (1.21–1.97) | 1.58 (1.22–2.05) |
aFrailty status was assessed based on characteristics from 2 to 4 wk preceding hospitalization.
bThirteen patients (1.2%) were lost to follow-up.
Estimates were calculated using Cox proportional hazards models. The adjusted model included age, sex, Charlson Comorbidity scores, Sequential Organ Failure Assessment (SOFA) scores, and the Clinical Frailty Scale. Quartiles defined the categories of Charlson and SOFA scores.
Severity of organ dysfunction measured by the SOFA scores on ICU admission did not vary significantly across frailty groups (Table 1). Most patients required invasive mechanical ventilation and vasoactive drugs during their ICU stay, regardless of frailty status. We found no significant differences in the utilization of organ support, including dialysis, across frailty groups (Table 3).
TABLE 3.
Resource Use, Treatment Limitations, and Outcomes of the Study Participants According to Frailty Categories
| Resource/Outcome | Total | CFS 1–3a | CFS 4a | CFS 5a | CFS 6–9a | p b |
|---|---|---|---|---|---|---|
| n | 1,028 | 629 | 175 | 108 | 116 | |
| Resource use, n (%) | ||||||
| Vasoactive drugs | 790 (76.8) | 479 (76.2) | 132 (75.4) | 87 (80.6) | 92 (79.3) | 0.66 |
| Invasive mechanical ventilation | 821 (79.9) | 511 (81.2) | 140 (80.0) | 84 (77.8) | 86 (74.1) | 0.33 |
| Dialysis | 389 (37.8) | 244 (38.8) | 68 (38.9) | 39 (36.1) | 38 (32.8) | 0.63 |
| Life-supporting limitations | 227 (22.1) | 103 (16.4) | 40 (22.9) | 38 (35.2) | 46 (39.7) | < 0.001 |
| Outcomes, n (%) | ||||||
| Hospital mortality | 585 (56.9) | 321 (51.0) | 107 (61.1) | 78 (72.2) | 79 (68.1) | < 0.001 |
| 6-mo mortalityc | 608/1,015 (59.9) | 328/622 (52.7) | 113/173 (65.3) | 79/107 (73.8) | 88/113 (77.9) | < 0.001 |
CFS = Clinical Frailty Scale.
aPre-COVID-19 frailty was assessed based on characteristics from 2 to 4 wk preceding hospitalization.
bComparison between CFS categories.
cThirteen patients were lost during 6-mo follow-up (seven in CFS 1–3 group, two in CFS 4 group, one in CFS 5 group, and three in CFS 6–9 group). Percentages are taking into account the missing patients.
In our cohort, withholding further escalation of organ support accounted for the only form of treatment limitation and occurred a median 12 days (IQR, 5–22 d) after ICU admission. No case of lack of ICU resources occurred, and we also did not verify situations of withdrawing invasive organ support. Comparing ICU resource use between patients with versus without decisions on withholding further escalation of organ support, we observed that invasive mechanical ventilation (89% vs 77%; p < 0.001), vasoactive drugs (86% vs 73%; p < 0.001), and dialysis (43% vs 36%; p = 0.06) tended to be more frequent in those who ended up having decisions on treatment limitations. Of note, decisions on withholding further escalation of organ support were more common in patients with higher CFS scores (Table 3). Finally, 223 of 227 patients (98%) who ended up having decisions on withholding further escalation of organ support died a median of 2 days (IQR, 1–4 d) after those decisions due to refractory multiple organ dysfunctions.
We verified that CFS scores were highly correlated with the Frailty Index (Spearman coefficient = 0.68; p < 0.001) and had an excellent accuracy in discriminating frail and nonfrail patients as defined by Frailty Index scores (AUC, 0.91; 95% CI, 0.89–0.93). We found that CFS scores greater than or equal to 4 had the best performance to detect frailty in our sample (sensitivity = 91%, specificity = 76%, positive likelihood ratio = 3.71). Sensitivity, specificity, and likelihood ratios for each CFS score are detailed in Table S2 (http://links.lww.com/CCX/B6).
DISCUSSION
In a large sample of COVID-19 patients admitted to the ICU, we verified that one in four participants were frail and that frailty was independently associated with lower short- and long-term survival despite similar rates of invasive organ support. Frailty assessment further identified individuals within the same age groups and SOFA score strata with differing survival levels over 6 months. We also observed that assessing frailty with the CFS had an excellent accuracy in identifying critically ill patients who are frail. Our results suggest that frailty has a relevant prognostic value in COVID-19 critical care and, therefore, should be included in the clinical triage routines alongside other ICU measures (e.g., age, sex, comorbidities, severity of illness).
Other studies have investigated the value of assessing frailty in patients with COVID-19 (4, 5). However, few analyses have focused on the critical care scenario (2). While some variation exists in the reported frailty prevalence (22), our study found a frequency similar to a recent multicenter cohort study on critically ill patients with COVID-19 (2). However, our study advances prior research by demonstrating, in addition to its predictive usefulness, the concurrent validity of the CFS in comparison to the Frailty Index, a well-validated multidimensional frailty measure. Since the CFS is a feasible and well-known frailty tool in critical care (2, 22, 23), our results encourage its incorporation into the ICU practice. It is also worth mentioning that differently from previous studies (3, 23), we determined the prognostic effect of frailty on 6-month mortality, incorporating aspects related to long-COVID complications. Exploring long-term survival is relevant as recent work has indicated that frailty increases the risk of patient-centered adverse outcomes (e.g., functional disability) in COVID-19 ICU survivors after hospital discharge (11).
Our results support intensive care providers who wish to combine the prognostic information captured by their patients’ frailty status with other elements of risk, thereby contributing to better informed and evidence-based conversations about therapeutic choices and expectations in severe COVID-19 (1, 23). For example, despite a patient’s chronological age, a low burden of chronic diseases (Charlson scores 0–1) and no evidence of baseline frailty (CFS scores < 4) may support decisions favoring the highest degree of care and invasive organ support. On the other hand, evidence of multiple chronic conditions (Charlson scores ≥ 2) and greater levels of baseline frailty (CFS scores ≥ 4) might be essential in conversations about goals of care, including advance care planning and therapeutic limitations, in the context of COVID-19 in the ICU.
The use of invasive organ support was similar across the different levels of frailty in our sample. Thus, the increased cumulative occurrence rate of death among those with higher levels of frailty occurred despite comparable levels of intensive physiologic support. This finding illustrates the concept of frailty: the same stressor (i.e., severe COVID-19), causing similar acute physiologic dysfunctions (as measured by admission SOFA scores) and receiving equivalent therapeutic measures (i.e., invasive organ support) still resulted in worse outcomes in frail patients. Although frail patients were associated with decisions on withholding further escalation of organ support, these decisions happened after nearly 2 weeks of aggressive organ support, in a context of persistent multiple organ failure and virtually irreversible process of death.
A recent international multicenter COVID-19 cohort of older ICU patients reported that frailty was independently associated with a higher rate of treatment limitations, notably a lesser use of invasive mechanical ventilation (2). In fact, during the first wave of the pandemics (when most of the publications were performed, as recently reviewed in an individual patient data meta-analysis [3]), frail patients were less likely to receive mechanical ventilation (3). These studies contrast with our observation of similar rate of invasive organ support across levels of frailty. The reasons for the differences between our data and these publications might be speculated. We did not experience a shortage of resources during the pandemic. Therefore, policies restricting the use of critical care or ICU admissions were not implemented as previously recommended (24). Furthermore, end-of-life practices during critical illness vary worldwide, and in Latin America, such care restrictions often take longer to implement and occur when the patient is near death (25). This practice might differ from what is commonly reported in Europe and North America in the pre-COVID-19 period (25) and might explain our distinct findings from previous reports (2, 3).
Our study has notable strengths. First, we included patients consecutively admitted to the ICU and had minimal missing data, even after a 6-month follow-up. Second, data were collected in conjunction with admissions by medical investigators trained in geriatrics and critical care, providing high-quality information. Finally, we used different validated instruments to assess baseline frailty and severity of illness in the ICU.
The study also had limitations. First, although our data came from Latin America’s largest university hospital, our findings represent the treatment provided in a single institution. Second, cultural and religious affiliations significantly impact support-limitation decisions, which might have affected some of our results. In addition, even though the clinical staff was trained to register decisions on treatment limitations, we recognize that data from electronic hospital records might be subject to information bias, especially in work overload due to the pandemic. Third, investigators trained in geriatric medicine ranked the CFS, improving our internal validity but limiting the generalizability of the study. Nonetheless, the CFS has been proved to be a valid measure regardless of medical specialties (2, 26). Finally, our database concerns the first wave period, which was characterized by the lack of evidence-based specific therapies (e.g., antivirals, corticosteroids, vaccines) and early SARS-CoV-2 variants. As the pandemic advances, other investigations on the associations of frailty, organ support, and long-term survival in critically ill patients with COVID-19 might provide new insights. However, recent systematic reviews studying frailty in hospitalized COVID-19 patients demonstrated that all reports concern databases from 2020, similar to ours (3, 27). And the association of the concept of frailty with ICU outcomes has been demonstrated before COVID-19 (23), highlighting that it is not specific to this infectious disease. Taking the example of the COVID-19 pandemic, clinicians should consider frailty alongside sociodemographic and clinical measures before estimating the prognosis of older patients in the ICU.
CONCLUSIONS
In conclusion, frailty was common and a strong predictor of short- and long-term survival in COVID-19 patients admitted to the ICU. While invasive organ support did not vary across frailty levels, decisions on withholding further escalation of organ support were more frequent in frailer patients, particularly in the context of multiple organ failure. Intensive care providers should be aware that assessing frailty can aid valuable prognostic information during decision-making processes in COVID-19 care.
ACKNOWLEDGMENTS
We would like to acknowledge the dedicated work performed by healthcare workers and staff in our hospital during the COVID-19 crisis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This work was supported by Hospital das Clinicas, University of São Paulo Medical School (HCFMUSP), Faculdade de Medicina, Universidade de São Paulo, Brazil, from donations to the #HCCOMVIDA campaign. The #HCCOMVIDA fundraising campaign was a joint initiative from ordinary citizens, healthcare professionals, and researchers appealing to the broader society to support HCFMUSP’s frontline work against the COVID-19 pandemic. Donations were directed to the institution’s emergency initiatives related to fighting COVID-19, including research projects.
Dr. Taniguchi was involved in conceptualization, data curation, formal analysis, methodology, and writing-original draft. Dr. Avelino-Silva was involved in conceptualization, data curation, investigation, resources, and writing-original draft. Dr. Dias was involved in conceptualization, investigation, and writing-original draft. Dr. Jacob-Filho was involved in conceptualization, funding acquisition, resources, supervision, and writing—review and editing. Dr. Aliberti was involved in conceptualization, data curation, formal analysis, investigation, methodology, project administration, and writing-original draft. All authors approved the final version before submission.
Dr. Aliberti is supported by a scholarship from Hospital das Clinicas, University of São Paulo Medical School, with funds donated by NUBANK under the #HCCOMVIDA scheme. The remaining authors have disclosed that they do not have any potential conflicts of interest.
A complete list of investigators in the CO-FRAIL Study Group, EPICCoV Study Group, and COVID HCFMUSP Study Group is provided in the Supplementary Materials (http://links.lww.com/CCX/B6).
Brazilian Clinical Trials Registry (ReBEC): RBR-7w5zhr and ClinicalTrials.gov: NCT04378582.
REFERENCES
- 1.Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. ; COVID HCFMUSP Study Group: COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriatr Soc 2021; 69:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung C, Flaatten H, Fjølner J, et al. ; COVIP study group: The impact of frailty on survival in elderly intensive care patients with COVID-19: The COVIP study. Crit Care 2021; 25:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramaniam A, Anstey C, Curtis JR, et al. : Characteristics and outcomes of patients with frailty admitted to ICU with coronavirus disease 2019: An individual patient data meta-analysis. Crit Care Explor 2022; 4:e0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolford SJ, D’Angelo S, Curtis EM, et al. : COVID-19 and associations with frailty and multimorbidity: A prospective analysis of UK Biobank participants. Aging Clin Exp Res 2020; 32:1897–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen RK, Conroy SP, Taub N, et al. : Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records. Age Ageing 2021; 50:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kundi H, Çetin EHÖ, Canpolat U, et al. : The role of frailty on adverse outcomes among older patients with COVID-19. J Infect 2020; 81:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sablerolles RSG, Lafeber M, van Kempen JAL, et al. ; COMET research team: Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): An international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev 2021; 2:e163–e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utino Taniguchi L, Ibrahim Q, Azevedo LCP, et al. : Comparison of two frailty identification tools for critically ill patients: A post-hoc analysis of a multicenter prospective cohort study. J Crit Care 2020; 59:143–148 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira JC, Ho YL, Besen BAMP, et al. ; EPICCoV Study Group: Protective ventilation and outcomes of critically ill patients with COVID-19: A cohort study. Ann Intensive Care 2021; 11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliberti MJR, Covinsky KE, Garcez FB, et al. : A fuller picture of COVID-19 prognosis: The added value of vulnerability measures to predict mortality in hospitalised older adults. Age Ageing 2021; 50:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi LU, Avelino-Silva TJ, Dias MB, et al. : Patient-centered outcomes following COVID-19: Frailty and disability transitions in critical care survivors. Crit Care Med 2022; In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 13.Metnitz PG, Moreno RP, Almeida E, et al. ; SAPS 3 Investigators: SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med 2005; 31:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno RP, Metnitz PG, Almeida E, et al. ; SAPS 3 Investigators: SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Stelfox HT, McDermid RC, et al. : Association between frailty and short- and long-term outcomes among critically ill patients: A multicentre prospective cohort study. CMAJ 2014; 186:E95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence: COVID- 19 Rapid Guideline: Critical Care in Adults. NICE Guideline [NG159]. 2020. Available at: https://www.nice.org.uk/guidance/ng159. Accessed March 2020. [PubMed]
- 18.Rockwood K, Theou O: Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J 2020; 23:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Stelfox HT, Johnson JA, et al. : Long-term association between frailty and health-related quality of life among survivors of critical illness: A prospective multicenter cohort study. Crit Care Med 2015; 43:973–982 [DOI] [PubMed] [Google Scholar]
- 20.Mitnitski AB, Mogilner AJ, Rockwood K: Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poco PCE, Aliberti MJR, Dias MB, et al. : Divergent: Age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci 2021; 76:e46–e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dres M, Hajage D, Lebbah S, et al. ; COVID-ICU investigators: Characteristics, management, and prognosis of elderly patients with COVID-19 admitted in the ICU during the first wave: Insights from the COVID-ICU study: Prognosis of COVID-19 elderly critically ill patients in the ICU. Ann Intensive Care 2021; 11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscedere J, Waters B, Varambally A, et al. : The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med 2017; 43:1105–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emanuel EJ, Persad G, Upshur R, et al. : Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; 382:2049–2055 [DOI] [PubMed] [Google Scholar]
- 25.Avidan A, Sprung CL, Schefold JC, et al. ; ETHICUS-2 Study Group: Variations in end-of-life practices in intensive care units worldwide (Ethicus-2): A prospective observational study. Lancet Respir Med 2021; 9:1101–1110 [DOI] [PubMed] [Google Scholar]
- 26.Surkan M, Rajabali N, Bagshaw SM, et al. : Interrater reliability of the Clinical Frailty Scale by geriatrician and intensivist in patients admitted to the intensive care unit. Can Geriatr J 2020; 23:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rottler M, Ocskay K, Sipos Z, et al. : Clinical Frailty Scale (CFS) indicated frailty is associated with increased in-hospital and 30-day mortality in COVID-19 patients: A systematic review and meta-analysis. Ann Intensive Care 2022; 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.