PURPOSE
We annually treat more than 800 new patients with cervical cancer, where the majority (approximately 60%) have locally advanced disease and approximately 40% of them are infected with HIV. To optimally care for this large number of patients in low-income settings is difficult. From July 2011, we started using 45.0 Gy/15# hypofractionated radiotherapy (HFRT) as a substitute to 50.0 Gy/25# conventional fractionated radiotherapy (CFRT), for the treatment of locally advanced cervical cancer (LACC). This study aims at comparing the 5-year treatment outcomes between patients with LACC, known HIV serostatus, and treated with either CFRT or HFRT.
METHODS
A retrospective study was conducted according to demographic/clinical data, radiotherapy fractionations, and outcomes. Factors considered were FIGO stages IIB-IIIB, known HIV serostatus, and had completed external-beam radiotherapy and intracavitary brachytherapy. The primary end point was overall survival; the secondary end points were toxicity and compliance.
RESULTS
The study included 221 patients. Squamous cell carcinomas were 95.1% and adenocarcinomas 2.3%. The median age was 45.0 (interquartile range, 38.0-52.0) years. Stages IIB, IIIA, and IIIB were 38.9%, 6.3%, and 54.8%, respectively. HIV-positive and HIV-negative were 87 (39.4%) and 134 (60.6%), respectively. Chemoradiation was administered in 100 (45.2%), and 52 (52.0%) completed chemotherapy. CFRT/HFRT were 116 (52.5%)/105 (47.5%). At 24 months, the overall response was 54.1% for HIV-negative compared with 45.0% for HIV-positive (P value .262). There was no significant differences in acute/late toxicity grades ≥ 2 for HIV-negative/positive treated with HFRT/CFRT. At 60 months, the survival probabilities were 45.7% and 27.7% for HIV-negative and HIV-positive treated with CFRT (P value = .006), whereas it was 44.2% and 30.7% for HIV-negative and HIV-positive treated with HFRT (P value = .048), respectively.
CONCLUSION
For the treatment of LACC with known HIV serology, there was no significant statistical difference in terms of response, toxicity, and compliance between CFRT and HFRT. However, the difference in overall survival between HIV-negative and HIV-positive was significant.
INTRODUCTION
Cervical cancer is the second commonest cancer and third leading cause of cancer mortality among women in developing countries. Cervical cancer is also more common in women infected with HIV than in HIV-uninfected women.1 Multiple studies have confirmed that HIV-positive women are significantly more likely to develop cervical cancer.2-4 In the sub-Saharan Africa, where cervical cancer is the leading cause of cancer death, HIV-infected women are 6 times more likely than HIV-negatives to develop cervical cancer.2,5 GLOBOCAN 2020 shows that the incidence and mortality of cervical cancer in Uganda is about 7,000 and 4,600, respectively, with a 5-year prevalence rate of 55.5 per 100,000.6 Annually, we treat more than 800 new patients with cervical cancer annually, translating to approximately 40% of the workload, which is difficult to optimally care for these huge numbers of patients in low-income centers. Locally advanced cervical cancer (LACC) is characterized by large tumor (≥ 4 cm) within the cervix, extending to para-cervical tissues, parametrium, and pelvic side wall, but the cancer that has not spread to distant organs. In our center, LACCs account for approximately 60% of all cervical cancer patients, and HIV-infected patients amount to nearly 40% of all cervical cancers. Concomitant chemoradiotherapy using cisplatin is the standard of care for the treatment of LACC.7-9
CONTEXT
Key Objective
There is limited literature describing 5-year treatment outcomes of patients who are HIV-negative and HIV-positive with locally advanced cervical cancer (LACC), treated using either conventional fractionated radiotherapy-50.0 Gy/25# or hypofractionated radiotherapy (HFRT)-45.0 Gy/15#.
Knowledge Generated
A retrospective review of 221 patients' files was done, and the primary end point was overall survival. Inclusion criteria were FIGO stages IIB–IIIB cervical cancers, known HIV serostatus, and had completed external-beam radiotherapy and brachytherapy. Nearly 40% of patients were HIV-positive at diagnosis and 116 (52.5%) were treated with CFRT. There were no significant differences in acute or late toxicity grades ≥ 2. At 60 months, the survival probabilities for patients who are HIV-negative treated with either CFRT or HFRT were higher compared with HIV-positives.
Relevance
In low-income settings with high numbers of patients with locally advanced cervical cancer, the HFRT regime can be beneficial to both patients (shorter machine times and better compliance because of shorter hospital stays) and treatment institutions (more patients treated in the same time period, thereby saving resources).
In radiotherapy practice, the biologically effective dose is used for comparison and quantification of treatment outcomes for normal tissues and tumors.10,11 The biologically effective dose normalized in 2.0 Gy fractions is given by , where n is the number of fractions, d is the daily dose, and is the therapeutic ratio. For early radiation effects (acute toxicity) and tumor response, = 10; the hypofractionated radiotherapy (HFRT)-45.0 Gy/15# and conventional fractionated radiotherapy (CFRT)-50.0 Gy/25# have EQD2 values of 48.8 and 50.0, respectively. For intracavitary brachytherapy (ICBT), a 30.0 Gy single fraction was administered using Cs-137 low-dose-rate source in this study. Continuous low-dose-rate irradiation is given by , where D is the single fractional dose, and g is a factor that depends on cell-repair half-time (T1/2) and treatment duration (T), which ranged from 10 to 12 hours in this study. The T1/2 ranges from 0.5 to 5.0 hours, corresponding to g-factor ranges of 0.113-0.616. Cervical cancer, with T1/2 ≈ 1.5 hours, has the corresponding g-factor = 0.112. Using the above information for a single continuous brachytherapy dose of 30 Gy to point A, EQD2 = 33.4 Gy. The total EQD2 (to point A) from external-beam radiotherapy (EBRT) plus ICBT are 83.4 Gy and 82.2 Gy for CFRT and HFRT, respectively.
Because of the close proximity of EQD2 for CFRT and HFRT, and the limited treatment facilities, our department commenced the use of 45.0 Gy/15# as an alternative to 50 Gy/25# from July 2011. This retrospective study compares 5-year clinical outcomes of known HIV serology patients with LACC, FIGO stages IIB-IIIB, who received concurrent chemoradiation with either HFRT or CFRT, followed by ICBT. There is limited literature describing treatment outcomes, responses, and toxicities between patients who are HIV-negative and HIV-positive treated with either CFRT or HFRT.
METHODS
Patients and Methods
This was a nonrandomized retrospective study of 221 patients with LACC, with FIGO stages IIB-IIIB,12 and with known HIV serostatus who had radiotherapy treatment at our center between January 2011 and December 2012. Inclusion criteria were patients with histologically confirmed LACC, with known HIV serostatus, and who had completed EBRT and intracavitary (ICBT) treatments. The review done in April 2021 was based on demographic and clinical data, HIV serology, waiting time (time between patient's first registration date in department and first EBRT session date), fractionations schedule (CFRT or HFRT), toxicities, responses, and 5-year survival probabilities. Phone calls were made for updated patients status.
Chemoradiation
The patient workup included clinical history, bimanual pelvic examination, chest radiograph, transabdominal/pelvic ultrasound, digital rectal examination, CBC count, and liver and renal function tests. A standard patient simulation procedure was used; target demarcation and 2D treatment planning in the supine position.13 Field borders used were (1) inferior: 3 cm below the inferior extent of the vaginal involvement (often at the inferior obturator-foramina), (2) superior: L4-L5 interdisc space, and (3) lateral: 1-2 cm lateral to the pelvic brim. Patients were advised to drink 500 mL of water to lessen gastrointestinal side effects after ≈30 minutes, before simulation and daily treatments. All patients reviewed/presented in this study were treated with curative intent and were given radical doses. Patients were treated with two parallel-opposed anterior-posterior/posterior-anterior portals using Cobalt-60 EBRT. The pelvic EBRT included dosages of 50 Gy/25# (CFRT) and 45 Gy/15# (HFRT). The 45 Gy/15# had been used for the treatment of other cancers, eg, non–small-cell lung cancers14 and glioblastoma.15 All patients who were fit for concurrent chemoradiation received cisplatin of 40 mg/m2 once a week, for 3-5 weeks,8,9,13 regardless of their serostatus. Blood samples (CBC, RFT, and LFT) were checked before weekly chemotherapy cycle. The CD4 threshold was 200 cells/mm3 for HIV-seropositives to receive chemotherapy. A 30.0 Gy single fraction of low-dose-rate Cs-137 ICBT to point A was delivered after EBRT. A departmental follow-up protocol of first review at 6 weeks, then every 3 months for the first 6 months, 6 months upto 1 year, and 12 months thereafter upto 60 months was used.
Evaluation of Treatment Outcomes
The evaluation was done according to age, histology, degree of differentiation, Eastern Cooperative Oncology Group (ECOG) performance status, HIV status, radiation dose, with or without chemotherapy (cisplatin), ICBT, treatment duration, response and toxicities during EBRT/follow-up, retreatments in the 5-year period, and survival probabilities. The retreatments were palliative, eg, 10.0 Gy/single fraction, 20.0 Gy/5 fractions to a reduced field size (true pelvis), or 20 Gy single fraction of ICBT to point A. The responses and toxicities to treatment were evaluated using the clinical notes in the patient's files while on treatment, at ICBT, and on successive follow-ups. At ICBT, visual inspection (tumor size, presence of discharge, or bleeding) and documentation was done by the radiation oncologist. These data were used to score response as per the RECIST guidelines16 as SD = stable disease, PR = partial response, CR = complete response, and DP = disease progression at ICBT. The scoring was CR for no tumor seen, PR for tumor < 1.5 cm diameter, SD for tumor > 1.5 cm, and DP for necrotic/bleeding tumor filling the cervix. The patient's data during the successive visits, eg, asymptomatic, pain, discharge, bleeding, and visual speculum examination, were used to grade as SD, PR, CR, and DP. The overall response rate was defined as the proportion of patients who had PR or CR to the treatment. The follow-up duration was defined as the time between the first registration date to date of death or follow-up end point (60 months).
Treatment-related toxicities were evaluated using RTOG criteria,17 on the basis of skin reactions, and gut and bladder toxicities as recorded in the patient's file. The toxicities were graded as grade 0, asymptomatic; grade 1 (mild toxicity), eg, increased urinary/bowel frequency, anorexia, nausea, vomiting, mild abdominal and rectal pains, and dry desquamation; grade 2 (moderate toxicity), moderate diarrhea, moderate abdominal and rectal pains, and intermittent bleeding; grade 3, skin ulceration, bloody stool and GI bleeding, fibrosis, and obstruction; and grade 4 (severe toxicity), eg, severe abdominal pains, wet desquamation, necrosis, and fistula.
Statistical Analysis
All statistical analyses were performed using STATA version 12. Quantitative data were presented by numbers, percentages, median, and interquartile range (IQR). Survival rates were computed using the Kaplan-Meier method. A P value < .05 was considered statistically significant.
Ethics Approval
The use of patients' data without obtaining consent in this retrospective study was approved by the Uganda Cancer Institute Research and Ethics Committee.
RESULTS
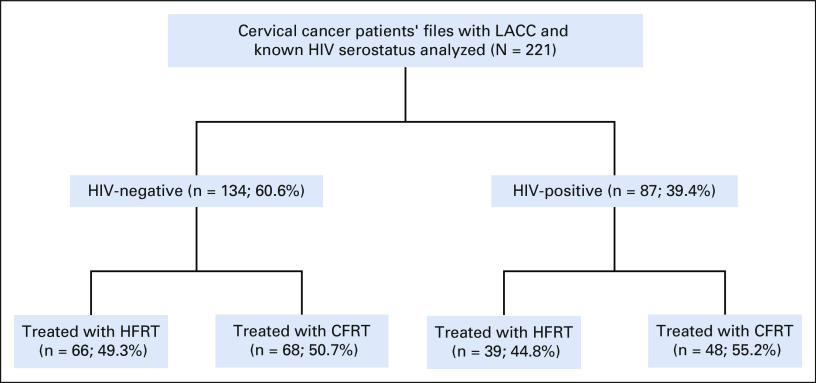
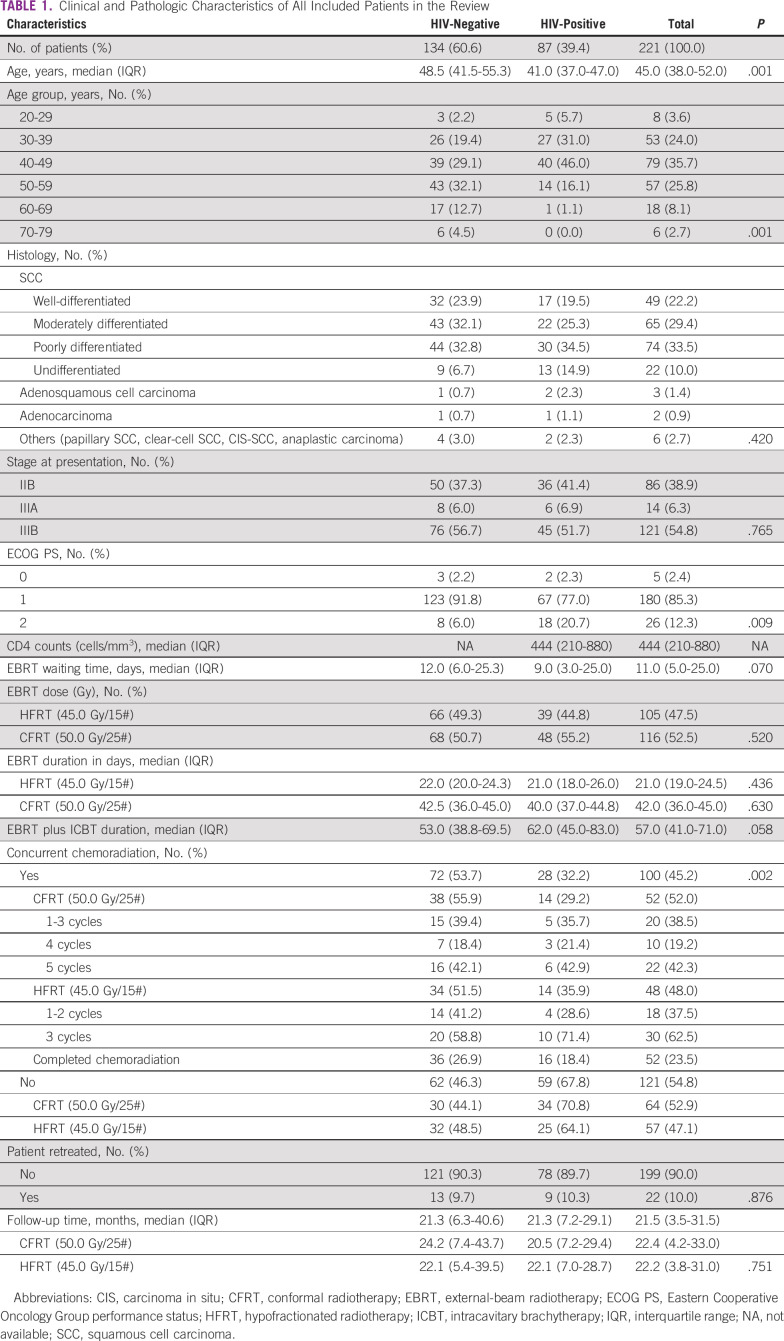
Two hundred twenty-one patients with LACC and known HIV serostatus received pelvic radiotherapy and/or chemoradiation. Nearly 40% (87) of patients were HIV-positive at diagnosis, of whom 48 (55.2%) received CFRT while 39 (44.8%) received HFRT. Figure 1 summarizes the enrollment of patients included in the analysis. Table 1 shows the demographic, clinical, and pathologic characteristics of the patients in the study. Squamous cell carcinoma was the primary histologic subtype (95.1%), followed by adenocarcinoma (2.3%) and others (2.7%). The age ranged from 24 to 78 years, with a median of 45.0 (IQR 38.0-52.0) years and peak age group of 40-49 years. Stages IIB, IIIA, and IIIB were 38.9%, 6.3%, and 54.8%, respectively. The ECOG performance status was categorized as 0, 1, and 2, contributing 2.4%, 85.3%, and 12.3%, respectively. The ECOG performance status for patients who are HIV-positive were statistically lower than for patients who are HIV-negative (P value = .009). The median CD4 counts (cells/mm3) was 444 (IQR = 210-880). The median waiting time to start EBRT was 11.0 (IQR = 5.0-25.0) days. Concomitant chemoradiation was administered to 100 (45.2%), of whom only 52 (52.0%) completed the chemotherapy. Logistical problems (80.2%) and clinical factors (19.8%) were the major reasons of not completing the chemotherapy cycles. CFRT and HFRT were 116 (53.4%) and 105 (47.5%), respectively. The median overall treatment time (time duration from start of EBRT to completion of brachytherapy) was 53.0 (IQR = 38.8-69.5) days for HIV-negative and 62.0 (IQR = 45.0-83.0) days for HIV-positive. The median follow-up duration was 23.2 (IQR = 6.4-41.6) months for HIV-negative and 21.3 (IQR = 7.1-29.2) months for HIV-positive. Retreatments were 9.7% and 10.3% for HIV-negative and HIV-positive (P value = .862), respectively. At 60 months, the survival probabilities were 45.7% and 27.7% for HIV-negative and HIV-positive treated with CFRT (P value = .006), whereas it was 44.2% and 30.7% for HIV-negative and HIV-positive treated with HFRT (P value = .048), respectively. The median external radiation duration was 22.0 and 21.0 days for HIV-negative and HIV-positive treated with HFRT, whereas it was 42.5 and 40.0 days for HIV-negative and HIV-positive treated with CFRT, respectively. Retreatments during the 5 years of follow-up were 9.7% and 10.3% for HIV-negative and HIV-positive, respectively (P value = .862). There were no statistical differences in distribution of histology types, FIGO stage, EBRT/ICBT treatment prescriptions, toxicities, and retreatments between patients who are HIV-positive and HIV-negative.
FIG 1.
The study summary of 221 patients with LACC, known HIV serology, and treated with either CFRT or HFRT. CFRT, conventional fractionated radiotherapy; HFRT, hypofractionated radiotherapy; LACC, locally advanced cervical cancer.
TABLE 1.
Clinical and Pathologic Characteristics of All Included Patients in the Review
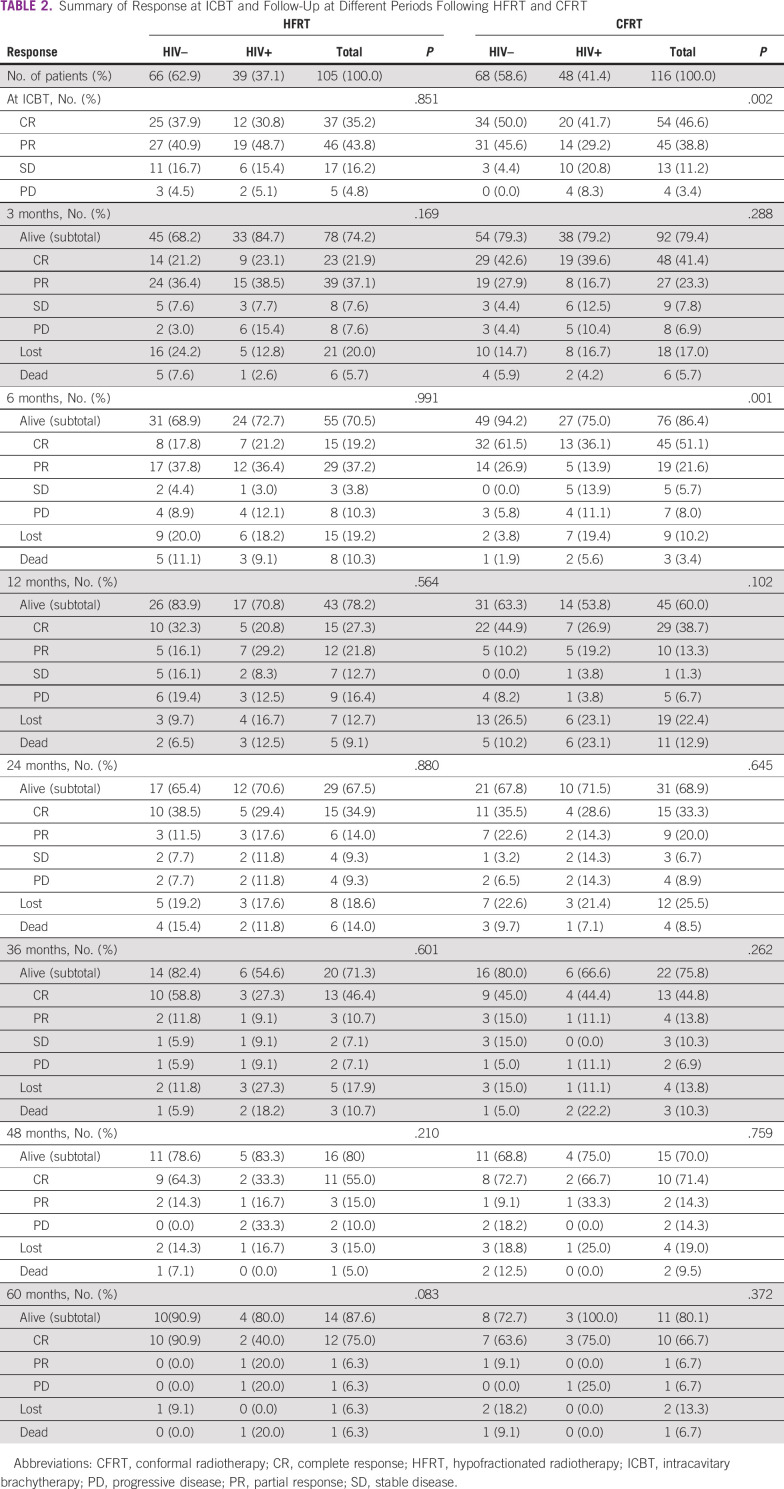
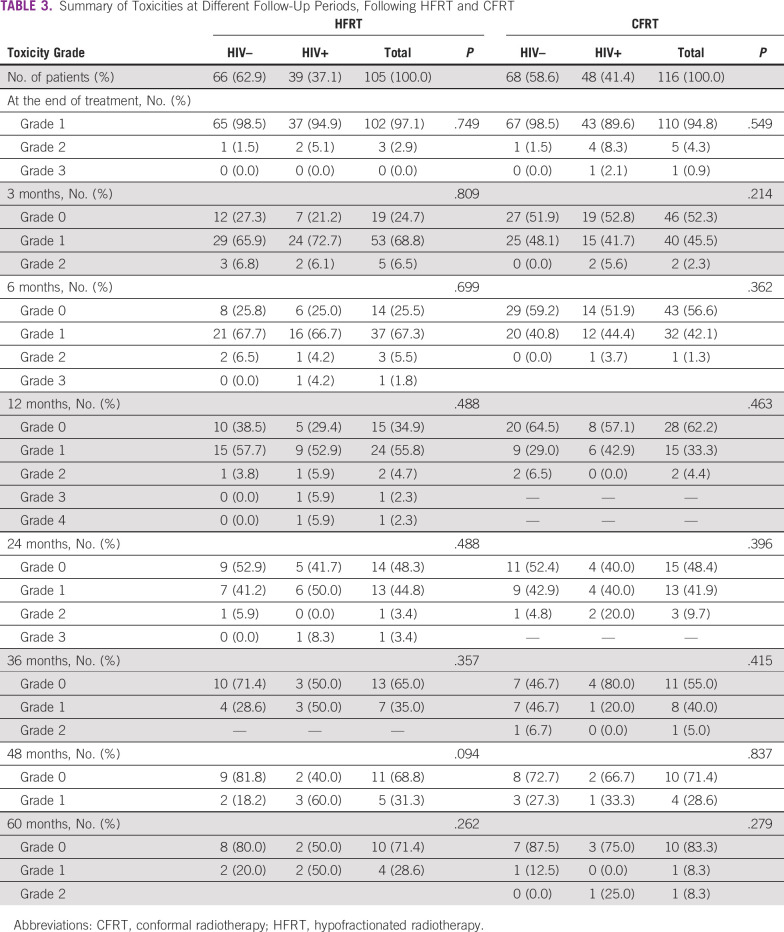
Table 2 summarizes the response and follow-up at different intervals treated with HFRT and CFRT. At 24 months, the overall response rate was 50.0% for HIV-negative compared with 47.0% for HIV-positive, treated with HFRT, whereas it was 58.1% for HIV-negative compared with 42.9% for HIV-positive treated with CFRT (P value .262). Table 3 summarizes the toxicities at different periods treated with HFRT and CFRT. Acute toxicity grades ≥ 2 were 4.2% and 5.6% for HIV-negative and HIV-positive treated with HFRT, whereas they were 4.5% and 8.0% for HIV-negative and HIV-positive treated with CFRT. Late toxicity grades ≥ 2 occurred mainly between 3 and 24 months, and they were 5.4% and 11.4% for HIV-negative and HIV-positive treated with HFRT, whereas they were 3.8% and 2.0% for HIV-negative and HIV-positive treated with CFRT, respectively. For all grade ≥ 2 toxicities, dermatologic, gastrointestinal, and genital-urinary were 25.3%, 20.4%, and 15.5%, respectively. The grade 4 toxicity was due to gastrointestinal complications, 4 months after completing treatment.
TABLE 2.
Summary of Response at ICBT and Follow-Up at Different Periods Following HFRT and CFRT
TABLE 3.
Summary of Toxicities at Different Follow-Up Periods, Following HFRT and CFRT
The study evaluated the 5-year overall survival probabilities for patients with LACC and known HIV status (HIV-positive v HIV-negative), treated with either HFRT or CFRT. Figures 2A‐2F show their corresponding Kaplan-Meier plots. The 5-year overall survival rates were 45.7% and 27.7% for HIV-negative and HIV-positive treated with CFRT, P value = .006 (Fig 2A), whereas the rates were 44.2% and 30.7% for HIV-negative and HIV-positive treated with HFRT, P value = .030 (Fig 2B), respectively. Comparison of 5-year overall survival for CFRT versus HFRT on HIV-negative (Fig 2C) and on HIV-positive (Fig 2D) shows that there was no significant statistical differences in both groups. The 5-year overall survival probabilities were 40.2% for all patients treated with CFRT compared with 43.0% for HFRT, P value = .245 (Fig 2E), and for all HIV-positive and HIV-negative, it was 30.0% and 44.9%, P values = .012 (Fig 2F), respectively.
FIG 2.
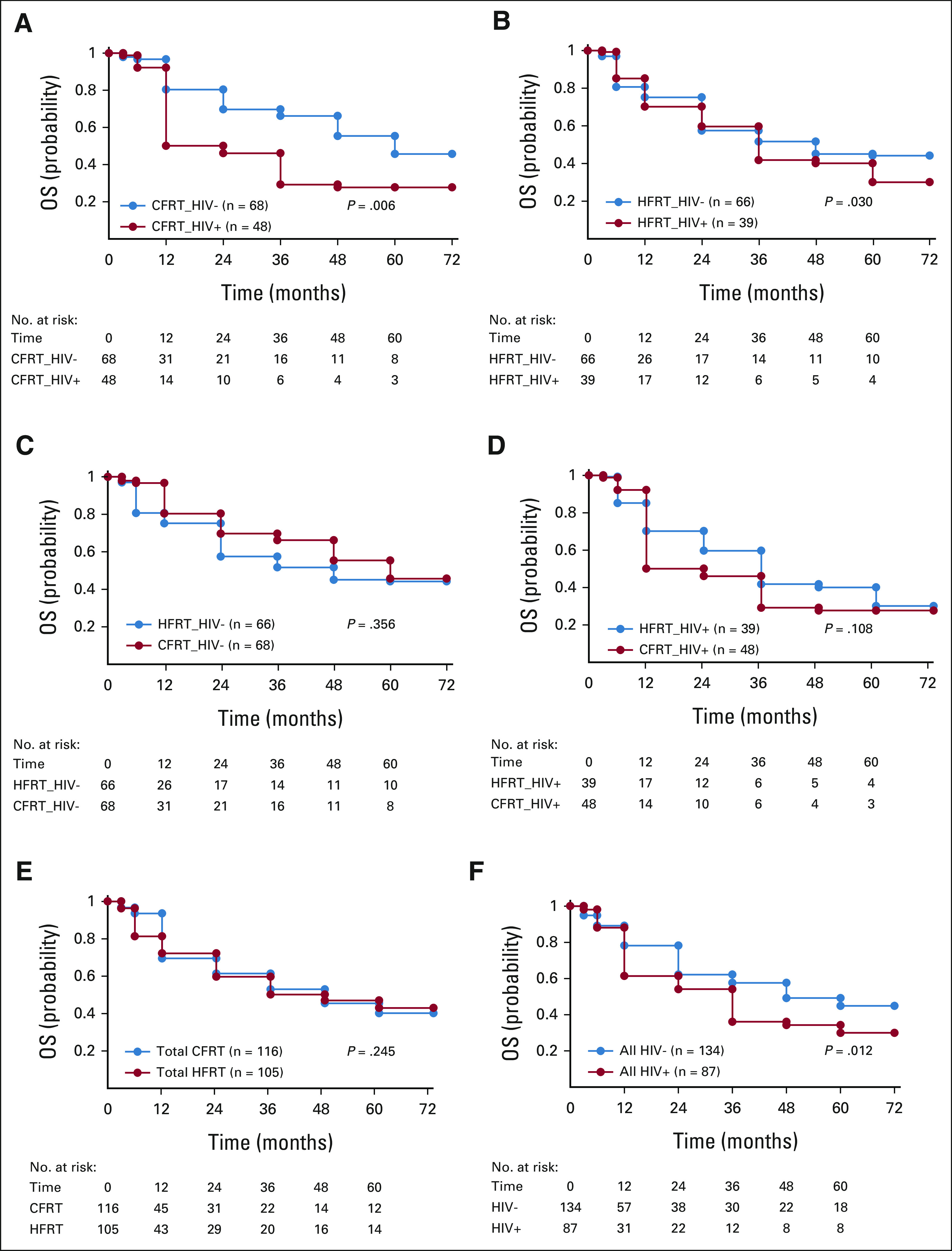
Kaplan-Meier plots and at-risk tables comparing OS probability for (A) CFRT/HIV– versus CFRT/HIV+, (B) HFRT/HIV– v HFRT/HIV+, (C) CFRT/HIV– v HFRT/HIV–, (D) CFRT/HIV+ v HFRT/HIV+, (E) total CFRT v total HFRT, and (F) all HIV– v all HIV+. CFRT, conventional radiotherapy; HFRT, hypofractionated radiotherapy; OS, overall survival.
DISCUSSION
In this analysis of patients with LACC and known HIV serostatus treated with either conventional or hypofractionated radiotherapy regimens, the tumor control rates and survival probabilities at 60 months were similar between CFRT and HFRT. The results in Table 2 show that for HFRT, there are evident differences in percentage overall responses between HIV-negative and HIV-positive at several evaluation periods (overall response for HIV-negative was better than that for HIV-positive); however, the differences are not statistically significant when compared with the HFRT regimen. For CFRT, there are noticeable statistically significant differences in percentage overall responses between HIV-negative and HIV-positive at several evaluation periods. These findings indicate that response is to a greater extent better in patients who are HIV-negative than in those who are HIV-positive, which is in agreement with other studies,18,19 which showed that women who are HIV-seropositive were three times likely to have residual disease, compared with HIV-negative. The results in Table 3 show that there were no significant statistical differences in toxicity between patients who are HIV-positive and HIV-negative treated with either HFRT or CFRT at all periods during the 5 years of follow-up. The complications ranged from no to mild complaints. On the contrary, Mangena et al20 reported that treatment toxicities occurred significantly more in patients who are HIV-positive than in those who are HIV-negative. The grade 3-4 toxicity profiles for both groups in our study were much lower compared with other studies21,22 that reported values in the range of 18.0%-21.6%.
The 3-year and 5-year overall survival rates of all HIV-positive women were significantly lower than those of their HIV-negative counterparts. The average 3-year survival rate in this study for patients with LACC and who are HIV-positive was 35.5%, which is comparable with the results by Dryden-Peterson et al.2 There was a significant 5-year survival difference between patients who are HIV-negative and HIV-positive (44.9% v 30.0%, P value = .012). A study by Einstein et al23 also showed that overall survival rates of HIV-positive women were significantly lower than those of their HIV-negative counterparts.
The 5-year survival in our study for patients who are HIV-negative of about 45%, which is lower compared with 58%-60% indicated in other studies,24,25 for LACC. Factors that may have contributed include:
1. Failure to complete the prescribed treatments (EBRT, ICBT, and chemotherapy) within the specified times; the overall treatment time should not exceed 56 days.7
2. Inadequate treatment resources and patient-associated logistical problems such as accommodation, transport, feeding, etc, resulted in some patients' failure to complete treatments on time;
3. Utilization of conventional two-dimensional radiotherapy treatment planning, which may contribute to poor dose conformity and higher risk for treatment-related toxicity.
Mapanga et al18 reported that poor response to treatment was significantly associated with advanced stages and receiving less-than-recommended radiation dose. Treatment toxicity, response, and outcome depend upon many variables, and many of these factors relate to tumor biology and patients' general condition, and are potentially influenced by HIV status.20
The median (IQR) age for patients who are HIV-positive was 41.0 (37.0-47.0) years compared with 48.5 (41.5-55.3) years for HIV-negatives (P value = .001). The results show that median age of patients who are HIV-positive and who have cervical cancer was nearly a decade lower than that of patients who are HIV-negative (41 years v 48.5 years). A comparable inclination is observed in age groups, where a bigger portion of patients who are HIV-positive presents at lower age group compared with patients who are HIV-negative, and the difference is statistically significant (P = .0001), in agreement with other studies.20,26 This has been attributed to the fact that the high virulence and hence the progression of HPV infection to cause invasive cervical cancer are faster in patients who are HIV-positive than in patients who are seronegative.27 There was no significant statistical difference (P value = .058) in the overall treatment time between patients who are HIV-positive and HIV-negative, in contrary to Gichangi et al,19 who reported that patients who are HIV-positive were twice as likely to have treatment interruptions. They further reported that those infected with HIV were younger and had advanced cervical cancer compared with those who were HIV-negative. Advanced cervical cancer stage, immunosuppressive level including those on HAART, and multisystem toxicities because of treatment are associated with inferior treatment completion, prognostic outcomes, and survival outcomes.
Nearly 40% of all patients with cervical cancer were HIV-positive, compared with 7.1%, which at that time was Uganda's HIV/AIDS incidence rate for women age 15 years and older.28 This suggests that HIV is one of the main contributors to the cervical cancer burden. Other studies even reported much higher prevalence rates. For example, Chambuso et al29 reported that the prevalence of cervical cancer lesions was 71.8% in the HIV-positive women compared with 27.3% in the HIV-negative.
Further prospective, randomized, conformal radiotherapy studies and are needed in the investigation of best care of LACC, many of whom are also infected with HIV. Exploration is needed to find out whether there are biological differences between tumors seen in young and older generation or if it is the HIV influence and whether these need to be addressed differently. Three recent systematic review studies of the optimal management of cervical cancer in patients who are HIV-positive have indicated that currently there are no standard guidelines and that there is limited literature regarding the management of patients who are HIV-positive diagnosed with cervical cancer and that these are managed like their HIV-negative counterparts.18,30
The median follow-up time in this study is lower compared with the 5-year results of 28 months reported by Cetina et al31 for LACC; our results are, however, comparable with other studies from limited-resource settings.32 Following are the limitations of this study:
-
1. The use of 2-fields (AP/PA) 2D treatment planning:
i. Failure to raise the dose to ≥ 85.0 Gy of both EBRT and ICT—required for most LACC.
ii. Anticipated increased toxicity.
2. Treatments were done on a Cobalt-60 unit that had a somewhat low-dose rate.
3. Radiation therapy treatment (EBRT plus ICBT) and chemotherapy were received inappropriately.
4. This was a retrospective study, mainly observational and descriptive; hence, comprehensive complication rates, particularly the major treatment-related side effects, could have been undetected.
Our department will commence on a prospective randomized study to assess a 3-week 45.0 Gy/15# HFRT schedule versus a 5-week 50.0 Gy/25# CFRT regimen for the treatment of LACC, taking into consideration the serostatus of the patients. Patients will be treated with IMRT/VMAT 6 MV or 10 MV photons, with weekly chemotherapy (cisplatin 40 mg/m2) followed by HDR brachytherapy (8.0 Gy × 3#) to point A, once a week. There are currently two NIH randomized studies, comparing concurrent chemoradiation CFRT with HFRT, followed by brachytherapy for the treatment of LACC. The first33 admits stages IIIA, IIIB, and IIIC, comparing 50 Gy/25# with 37.5 Gy/15#, plus ICBT 28 Gy (7 Gy/4#) to point A with weekly cisplatin. The second34 admits stages IB2, IB3, IIA, and IIB, comparing 45.0 Gy/25# with 40.0 Gy/15#, plus three or four fractions of ICBT with weekly cisplatin.
In conclusion, the 5-year overall survival rates of HIV-positive women was significantly lower than those of their HIV-negative counterparts treated with either HFRT or CFRT. There were no significant statistical differences in toxicity profiles between patients who are HIV-positive and HIV-negative treated with either HFRT or CFRT. No significant statistical differences were noted in survival rates for patients with LACC and known HIV serology treated with either HFRT or CFRT. In low-income centers with high cervical cancer burden, the shorter regimen of 45.0 Gy/15# can be advantageous to both patients and treatment centers because of (1) shorter overall machine time, resulting in reduced time patients take while waiting to commence EBRT, (2) shorter hospital stays, resulting in better patient compliance, and (3) many patients are treated within the same time frame, thereby saving resources.
ACKNOWLEDGMENT
The authors thank Mr Mulumba Yusuf, a biostatistician, for his exemplary role in data management and analysis, and Ms Bangidde Cissy Namutale for her determined role in data collection.
SUPPORT
Supported by the Varian Medical Systems Inc, Palo Alto, CA.
AUTHOR CONTRIBUTIONS
Conception and design: Awusi Kavuma, Israel Luutu, Daniel Kanyike
Financial support: Awusi Kavuma, Israel Luutu
Administrative support: Awusi Kavuma, Israel Luutu, Daniel Kanyike
Provision of study materials or patients: Awusi Kavuma, Daniel Kanyike
Collection and assembly of data: Awusi Kavuma, Israel Luutu, Solomon Kibudde
Data analysis and interpretation: Awusi Kavuma, Solomon Kibudde, Daniel Kanyike
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Einstein MH, Phaëton R: Issues in cervical cancer incidence and treatment in HIV. Curr Opin Oncol 22:449-455, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. : HIV infection and survival among women with cervical cancer J Clin Onco 34:3749-3757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palefsky JM: HPV-associated anal and cervical cancers in HIV-infected individuals: Incidence and prevention in the antiretroviral therapy era. Curr Opin HIV AIDS 12:26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghebre RG, Grover S, Xu MJ, et al. : Cervical cancer control in HIV-infected women: Past, present and future. Gynecol Oncol Rep 21:101-108, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grulich AE, Van Leeuwen MT, Falster MO, et al. : Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 370:59-67, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Keys H, Gibbons SK: Optimal management of locally advanced cervical carcinoma. J Natl Cancer Inst Monogr 21:89-92, 1996 [PubMed] [Google Scholar]
- 8.Chung Y-L, Jian JJ-M, Cheng SH, et al. : Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: A phase I/II study. Gynecol Oncol 97:126-135, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Rose PG, Ali S, Watkins E, et al. : Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group study. J Clin Oncol 25:2804-2810, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Nilsson P, Thames HD, Joiner MC: A generalized formulation of the “incomplete-repair” model for cell survival and tissue response to fractionated low dose-rate irradiation. Int J Radiat Biol 57:127-142, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Fowler JF: 21 years of biologically effective dose. Br J Radiol 83:554-568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haie-Meder C, Morice P, Castiglione M: Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v37-v40, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Gandhi AK, Sharma DN, Rath GK, et al. : Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys 87:542-548, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Amini A, Lin SH, Wei C, et al. : Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat Oncol 7:1-7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hingorani M, Colley WP, Dixit S, et al. : Hypofractionated radiotherapy for glioblastoma: Strategy for poor-risk patients or hope for the future? Br J Radiol 85:e770-e781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edeline J, Boucher E, Rolland Y, et al. : Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 118:147-156, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Cox JD, Stetz JA, Pajak TF: Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341-1346, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Mapanga W, Singh E, Feresu SA, et al. : Treatment of pre-and confirmed cervical cancer in HIV-seropositive women from developing countries: A systematic review. Syst Rev 9:1-16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gichangi P, Bwayo J, Estambale B, et al. : HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol Oncol 100:405-411, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mangena M, Snyman L, Dreyer G, et al. : The impact of HIV infection on women receiving radiation for cervical cancer. South Afr J Gynaecol Oncol 7:44-51, 2015 [Google Scholar]
- 21.Medina-Jiménez AK, Monroy-Torres R: Repurposing individualized nutritional intervention as a therapeutic component to prevent the adverse effects of radiotherapy in patients with cervical cancer. Front Oncol 10:2504, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan LT, Zahra M: Long-term survival and late toxicity after chemoradiotherapy for cervical cancer—The Addenbrooke’s experience. Clin Oncol 20:358-364, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Einstein MH, Ndlovu N, Lee J, et al. : Cisplatin and radiation therapy in HIV-positive women with locally advanced cervical cancer in sub-Saharan Africa: A phase II study of the AIDS malignancy consortium. Gynecol Oncol 153:20-25, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RA, Cokkinides V, Eyre HJ: American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin 53:27-43, 2003 [DOI] [PubMed] [Google Scholar]
- 25.American Society of Clinical Oncology : Cervical Cancer: Statistics. Cancer.net, 2018. https://www.cancer.net/cancer-types/cervical-cancer/statistics [Google Scholar]
- 26.Ntekim A, Campbell O, Rothenbacher D: Optimal management of cervical cancer in HIV‐positive patients: A systematic review. Cancer Med 4:1381-1393, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amit A, Edwards CL, Athey P, et al. : Extensive subcutaneous metastases from squamous cell carcinoma of the cervix in patient with HIV. Int J Gynecol Cancer 11:78-80, 2001 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization : Joint United Nations Programme on HIV/AIDS (UNAIDS)—WHO: Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec 72:81-87, 1997 [PubMed] [Google Scholar]
- 29.Chambuso RS, Shadrack S, Lidenge SJ, et al. : Influence of HIV/AIDS on cervical cancer: A retrospective study from Tanzania. JCO Glob Oncol 3:72-78, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohanty S, Gurram L, Chopra S, et al. : Cervical cancer treatment in HIV-positive patients: A survey of treatment practices in India. JCO Glob Oncol 7:843-848, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cetina L, Rivera L, Hinojosa J, et al. : Routine management of locally advanced cervical cancer with concurrent radiation and cisplatin. Five-year results. BMC Womens Health 6:1-7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George J, Tuli S, Monare B, et al. : Stage and outcomes of cervical cancer with or without HIV infection in Botswana 2013-2020. Int J Radiat Oncol Biol Phys 111:e612-e613, 2021 [Google Scholar]
- 33.NIH Clinical Trials : Chemotherapy and Pelvic Hypofractionated Radiation Followed by Brachytherapy for Cervical Cancer. https://clinicaltrials.gov/ct2/show/NCT04070976 [Google Scholar]
- 34.NIH Clinical Trials : Hypofractionated External Beam Radiotherapy for Intact Cervical Cancer (HEROICC). https://clinicaltrials.gov/ct2/show/NCT04583254 [Google Scholar]