Abstract
The activity of nitrogenase in the nitrogen-fixing bacterium Azotobacter vinelandii grown diazotrophically under aerobic conditions is generally considered to be protected against O2 by a high respiration rate. In this work, we have shown that a high rate of respiration is not the prevailing mechanism for nitrogenase protection in A. vinelandii grown in phosphate-limited nitrogen-free chemostat culture. Instead, the formation of alginate appeared to play a decisive role in protecting the nitrogenase that is required for cell growth in this culture. Depending on the O2 tension and cell growth rate, the formation rate and composition of alginate released into the culture broth varied significantly. Furthermore, transmission electron microscopic analysis of cell morphology and the cell surface revealed the existence of an alginate capsule on the surface of A. vinelandii. The composition, thickness, and compactness of this alginate capsule also varied significantly. In general, increasing O2 tension led to the formation of alginate with a higher molecular weight and a greater l-guluronic acid content. The alginate capsule was accordingly thicker and more compact. In addition, the formation of the alginate capsule was found to be strongly affected by the shear rate in a bioreactor. Based on these experimental results, it is suggested that the production of alginate, especially the formation of an alginate capsule on the cell surface, forms an effective barrier for O2 transfer into the cell. It is obviously the quality, not the quantity, of alginate that is decisive for the protection of nitrogenase.
Azotobacter vinelandii is an N2-fixing bacterium commonly found in soil. The biological fixation of dinitrogen depends on the activity of the highly oxygen-sensitive nitrogenase enzyme complex (25). Despite this sensitivity, species of the diazotrophic azotobacters are able to grow under fully aerobic conditions (9, 26, 33). For the survival of these bacteria under aerated conditions, one of the priorities of their entire metabolism is to protect the active nitrogenase from being damaged by oxygen. Protection of this enzyme from oxygen has been proposed to occur in azotobacters mainly through two mechanisms: (i) high respiratory activity that removes oxygen already at the cell surface and (ii) reversible conversion of the enzyme into a protected inactivated state (24, 26, 29). The first mechanism is believed to explain the function of nitrogenase when cells grow diazotrophically in the presence of O2. The second mechanism is considered to be used to protect the reversibly inactivated enzyme from O2 damage when the respiratory protection becomes overburdened, such as with a sudden increase in the ambient O2 concentration (21, 25) or under conditions of phosphate limitation (43). In the latter case, the respiration rate of cells is limited due to shortage of phosphate for the oxidative phosphorylation. For growing cells which need an active nitrogenase system to provide their nitrogen requirement, the second protection mechanism can work only temporarily because it does not remove O2.
Although the respiratory-protection hypothesis is generally accepted, Post et al. (33) and Boiardi (3) have questioned it. Those authors found that at O2 concentrations ranging from 30 to 100% air saturation, A. vinelandii showed almost constant respiration rates and negligible decreases in nitrogenase activity. These results are incompatible with the concept of respiratory protection. Post et al. (32) observed a decrease in the cellular surface area per cell volume at elevated O2 levels and suggested that this decrease of cell surface may also provide some protection for the nitrogenase. In addition, it was postulated that the energy efficiency of respiration is more important than the respiration rate as a protective mechanism (18).
A. vinelandii is known to produce alginate under aerobic conditions (1, 2, 7, 8, 16, 19). The formation of alginate is strongly affected by oxygen tension, especially in nitrogen-free medium and with limited phosphate (17, 38). A possible link between alginate formation and protection of nitrogenase in this organism has not been examined so far in the literature. Studies of the nitrogenase protection mechanisms of Azotobacter have mostly been based on either the respiration rates or acetylene reduction measurements as indications of nitrogenase activity (25, 26, 29). In fact, the biological function of alginate formation in bacteria is not fully understood. Alginate is important for cyst formation in A. vinelandii as a coating protective polysaccharide material (30, 36, 39). This was evidenced by the fact that noncapsulate mutants of A. vinelandii 12837 were unable to form cysts (11). Such a coating protects the cells from desiccation and mechanical stress. Under favorable growth conditions, the coat swells and the cyst germinates, divides, and releases a vegetative cell. However, the formation of a cyst in A. vinelandii does not explain the formation of alginate by vegetative cells under conditions not favoring encystment (7, 41).
For the protection of nitrogenase in nitrogen-fixing microorganisms, a low intracellular oxygen concentration is essential (29, 33). For A. vinelandii the increase of viscosity of the culture broth during the course of a cultivation as a result of increasing biomass and alginate concentrations can reduce the oxygen transfer rate from the gas phase to the aqueous phase and from the bulk liquid to the cell surface. To avoid a high oxygen transfer rate into the cell, an effective oxygen barrier on the cell surface can be even more important. As a matter of fact, the present communication provides evidence for the importance of alginate capsule formation on the cell surface for the survival of diazotrophically growing A. vinelandii under aerobic conditions. Variations in the quantity and quality of the alginate produced are studied under different culture conditions. Based on the experimental results, a new protection mechanism for nitrogenase against oxygen is proposed.
MATERIALS AND METHODS
Microorganism and cultivation conditions.
A. vinelandii (DSMZ 93-541b, a capsulated nonflagellated strain) was grown under conditions of dinitrogen fixation. The composition of the medium per liter of deionized water was as follows: sucrose, 20 g; MgSO4 · 7H2O, 0.4 g; NaCl, 0.4 g; CaCl2 · 2H2O, 84 mg; NaMoO4 · 2H2O, 2 mg; FeSO4 · 7H2O, 6 mg; H3BO4, 2.9 mg; CoCl2, 1.2 mg; CuSO4 · 5H2O, 0.1 mg; MnCl2 · 4H2O, 0.09 mg; ZnSO4 · 7H2O, 1.2 mg; KH2PO4, 20 mg/liter; and K2HPO4, 80 mg/liter. Sucrose, MgSO4, CaCl2, and the phosphate mixture were separated from the other medium components during sterilization. FeSO4 solution was sterilized by filtration using a 0.2-μm-pore-size Millipore filter.
Bioreactor and control.
Cultivations were carried out in a 5-liter stirred tank bioreactor (B. Braun Biotechnology, Melsungen, Germany) with a working volume of 2.5 liters. The control of the volume was realized by using a balance connected to a real-time operating computer system, UBICON (Universal Bioprocess Control System; GBF, Braunschweig, Germany). The reactor was equipped with pH, temperature, antifoam, and agitation speed controls.
The bioreactor was aerated with a fine-pore gas distributor. Thermal mass flow meters and controllers were used for the supply of gasses. The total aeration rate was controlled at a constant value (2 liters/min) with UBICON. The dissolved oxygen tension (partial O2 pressure [pO2]) was controlled in the range of 1% ± 1% to 20% ± 1% air saturation by mixing nitrogen and air in the inlet gas by a proportional-integral controller defined by the UBICON facilities. The agitation speed was 500 rpm if not otherwise stated.
The oxygen uptake rate, carbon dioxide production rate, and respiratory quotient (the carbon dioxide production rate divided by the oxygen uptake rate) were determined by online measurements of O2 and CO2 in the exit gas and compared with measurements taken at the inlet gas flow rate. Paramagnetic oxygen analyzers (OXYGOR 6N; Maihalk, Hamburg, Germany) and infrared carbon dioxide analyzers (UNOR 6N; Maihalk) were used for CO2 and O2 measurement.
Analytical methods.
Biomass and alginate dry weight were determined by gravimetrical methods as follows. One milliliter of 0.5 M EDTA–sodium salt and 0.5 ml of 5 M NaCl were added to a 25-ml sample of culture broth to separate the capsular alginate. After being stirred for 5 min, the sample was centrifuged at 38,000 × g and 20°C for 30 min to precipitate the cells. The cells were washed twice with distilled water, recentrifuged, and then dried at 80°C for 24 h. The supernatant was cooled, and alginate was then precipitated by the addition of 3 volumes of ice-cold isopropanol, which was then recovered by centrifugation at 38,000 × g at 4°C for 30 min. The precipitate was dissolved in water, precipitated again, centrifuged, and then finally dried at 80°C for 24 h. For each determination, at least two samples were used.
The poly-β-hydroxybutyrate content of cells was determined according to the method of Senior et al. (40). Sucrose was determined with the test kit combination of Boehringer (Mannheim, Germany). Phosphate was determined colorimetrically by the method of Boltz (4). The ratio of l-guluronic acid to d-mannuronic acid (G/M ratio) was estimated by colorimetric reaction of carbazole according to the method of Knutson and Jeanes (20).
The relative molecular weight of alginate was determined by gel permeation–high-pressure liquid chromatography as follows (15). The mobile phase used was 0.1 M phosphate buffer (pH 6.9), which was applied to two TSK gel columns (TSK G6000PWHR and then TSK G5000PWHR) arranged in rows. The signal was detected by a differential refractive index detector (Beckman model 156). The columns were calibrated with broad dextran standards. Before being applied to the columns, the alginate samples (0.5 g/liter) were prepared with purified alginate in the same elution buffer and filtered through a 1.2-μm-pore-size Millipore membrane to remove cellular debris.
The morphologies of cells and capsules were routinely examined by negatively staining the cells with 7% (wt/vol) aqueous nigrosin; the diameters of cells and capsules were then determined through visualization by dark-phase microscopy. Diameters of the cells were the means of those of at least 15 to 20 cells. The results agreed satisfactorily with those from later examination with a computer-aided image analyzing system from Zeiss.
Electron microscopy.
Transmission electron microscopy was used to investigate the surface view of alginate around the cells by negative staining. Samples were picked up with carbon-coated collodion grids (300 mesh, Cu). The grids were blotted with filter paper, and alginate was positively contrasted by incubation on freshly prepared 1% aqueous ruthenium red solution for 1 to 2 min at room temperature. The grids were washed three times with distilled water, with care being taken not to let the surface become dry. Finally, the cells were negatively stained with 1% uranyl acetate for 10 s, blotted, and air dried. Electron microscopy was done with a Zeiss CEM 902 microscope set at 80 kV with a magnification between ×16,000 and ×25,000.
Embedding and ultrathin sectioning of A. vinelandii were done as follows. Cells were preincubated in 0.5% (wt/vol) ruthenium red for 30 min at ambient temperature in the growth medium. Fixation was done by addition of 25% (vol/vol) glutardialdehyde to a final concentration of 1.25% (vol/vol) for 72 h at ambient temperature, followed by fixation at 4°C. After centrifugation, the fixed cells were resuspended in 0.1 M cacodylate buffer (pH 7.2) and washed in three sedimentation-resuspension cycles for 10 min each at room temperature. Washed cells were immobilized in 0.1 M cacodylate (pH 7.2)-buffered 2% (wt/vol) agar and were finally fixed with 1% (wt/vol) OsO4–0.1 M cacodylate (pH 7.2) overnight at 4°C. Cells were dehydrated on ice with an acetone series and embedded in epoxy resin (42). Ultrathin sections (120-nm thickness) were poststained with lead citrate, according to the method of Reynolds (35), and were analyzed with a Zeiss model CEM 902 transmission electron microscope set at 80 kV in the primary magnification range of ×12,000 to ×20,000.
RESULTS
Specific rates of oxygen consumption and alginate formation and quality as functions of pO2 in phosphate-limited continuous culture.
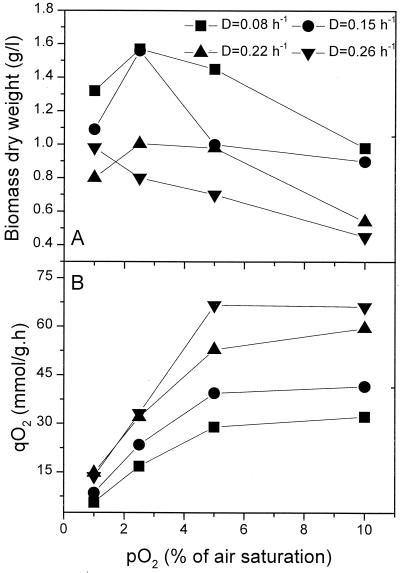
To gain better insight into the inhibitory effect of oxygen on nitrogenase activity and cell growth and to examine their relationships with alginate biosynthesis, the respiratory activities of cells were impaired through phosphate limitation in chemostat culture at different pO2 values over a dilution rate (growth rate) range of 0.08 to 0.26 h−1. The results of cell growth and the specific O2 consumption rate (i.e., respiratory rate) are shown in Fig. 1. Biomass concentrations showed maxima as a function of pO2 level except at the highest dilution rate (D = 0.26 h−1) studied. At high levels of pO2, biomass concentration declined, confirming an inhibitory effect of O2 on nitrogenase activity. Unlike cell growth, the specific rate of oxygen consumption (oxygen quotient [qO2]) increased as pO2 was elevated from 1 to 5% air saturation but remained essentially constant when pO2 was above about 5%. In general, qO2 was higher at higher growth rates. The initial increase of qO2 with pO2 is consistent with results expected from study of the respiratory protection mechanism. However, the behavior of the cells above a pO2 level of 5% cannot be explained by this mechanism. The leveling off of qO2 is not due to saturation of the respiratory capacity, as evidenced by the increase of qO2 with growth rate at both low and high pO2 levels. Consistent with the observations of Post et al. (33) and Boiardi (3), these results suggested that respiration is not the prevailing mechanism for protecting oxygen inhibition of nitrogen fixation and hence of cell growth under the experimental conditions.
FIG. 1.
Effect of O2 tension (percentage of air saturation) on the biomass production (A) and the specific respiration rate (qO2) (B) of A. vinelandii in phosphate-limited chemostat culture at different dilution rates (D).
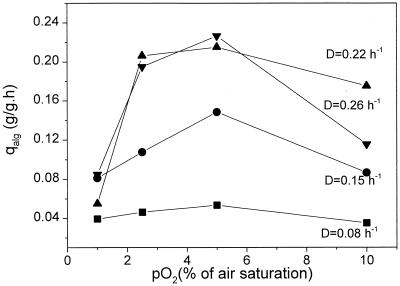
As mentioned in the introduction, the formation of alginate may be involved in the protection of nitrogenase. To test whether alginate production is up-regulated by increasing oxygen concentration, the specific formation rate of alginate (qalg) was examined (Fig. 2). Like biomass concentration, qalg showed a maximum as a function of pO2. At pO2 values above 5%, qalg decreased with increasing pO2. On the other hand, qalg increased with increasing growth rate. The effect of pO2 on the formation rate of alginate in continuous culture is in agreement with the results from pO2-controlled batch cultures with the same strain (38). Taken together, these results are useful for the optimization of the alginate production process. However, for understanding the role of alginate formation in the regulation of nitrogen fixation and cell growth, knowing the alginate concentration or formation rate alone is obviously not sufficient.
FIG. 2.
Specific alginate production rate (qalg) of A. vinelandii as a function of O2 tension in phosphate-limited continuous culture at different dilution rates (D).
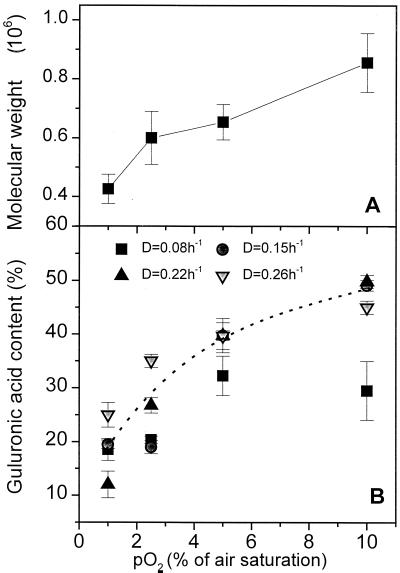
Therefore, the quality of alginate produced at different dissolved oxygen tensions was further examined in terms of molecular weight and alginate composition. The results are depicted in Fig. 3. It was interesting that both the molecular weight and the l-guluronic acid content monotonically increased with pO2. Unlike with the specific rates of oxygen consumption (Fig. 1) and alginate formation (Fig. 2), the effect of growth rate on the l-guluronic acid content of alginate was not significant. These results revealed that it is obviously the quality, not the quantity, of alginate that is the better determinant for the protection of nitrogenase in A. vinelandii.
FIG. 3.
Changes in the molecular weight (at a D of 0.08 h−1) (A) and the l-guluronic acid content (B) of alginate produced at different pO2 values. Molecular weight was based on a dextran standard.
Effect of shear rate on alginate capsule formation under a controlled pO2 value.
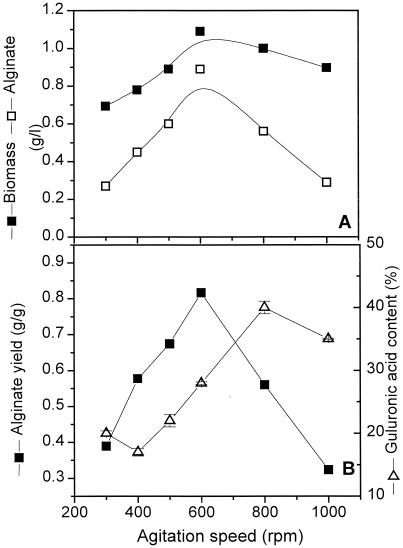
Based on observations of capsulated cells in a diazotrophically growing culture of A. vinelandii (38) and in view of the importance of alginate quality in coping with oxygen stress, as pointed out above, the formation of an alginate capsule and its quality were further studied with an elevated shear rate in a bioreactor. Figure 4 shows the results from a phosphate-limited continuous culture at different agitation speeds (300 to 1,000 rpm) with a controlled pO2 value (5% air saturation) and a fixed dilution rate of 0.15 h−1. Both alginate and biomass concentrations first increased with the agitation speed (up to 600 rpm) and then dropped. Alginate yield on biomass also reached a maximum at an agitation speed of 600 rpm. On the other hand, the l-guluronic acid content in alginate increased with increasing agitation speed and seemed to reach a saturation value of about 35 to 40%, which is typical for the pO2 value (see also Fig. 3).
FIG. 4.
Effect of agitation speed on the biomass production and the concentration, yield, and composition (expressed as weight percentages of l-guluronic acid content) of alginate in a phosphate-limited chemostat culture with a pO2 of 5% air saturation and a D of 0.15 h−1.
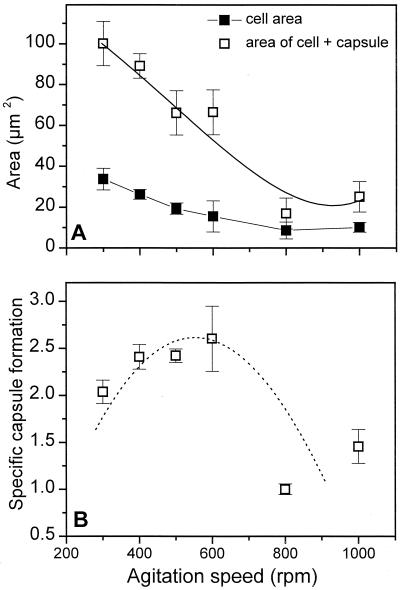
Microscopic examination of the cells revealed that the higher the shear rate, the smaller the average cell surface area (and also diameter), which reached a minimum of 8.9 μm2 at 800 rpm compared to the minimum of 33.8 μm2 reached at 300 rpm (Fig. 5). A decrease in the cellular surface area per cell volume, which was proposed by Post et al. (32) as a nitrogenase protection mechanism against oxygen, is clearly not involved in our culture system. In fact, the specific surface area per cell volume increased in this case.
FIG. 5.
Cell and capsule area (A) and specific capsule formation (B) as a function of the agitation speed (culture conditions were as described in the legend to Fig. 4). Specific capsule formation = capsule area/cell area; capsule area = area of capsule, including the cell − cell area.
Surprisingly, even in the presence of a high level of mixing intensity (800 to 1,000 rpm), capsules were always observed around the diazotrophically grown cells. The specific capsule area (capsule area/cell area) increased with agitation speed up to 600 rpm as shown in Fig. 5. The presence of a capsule around the cell, even during growth at a high shear rate in an agitated bioreactor, indicates how important the alginate capsules are for the survival of diazotrophically growing cells under phosphate limitation. Moreover, the results shown in Fig. 4 and 5 are consistent with those shown in Fig. 2 and 3 in the sense that they also suggest that not only the quantity but also the quality of the alginate (capsule) seems to be important for coping with the oxygen stress caused by high turbulence. In this connection, it is reasonable to assume that the effective pO2 value on the cell surface is higher at higher agitation speeds due to reduced O2 transfer resistance in the bulk liquid and a thinner liquid film and/or alginate capsule on the cell surface.
Morphological and ultrastructural variations of the alginate capsule.
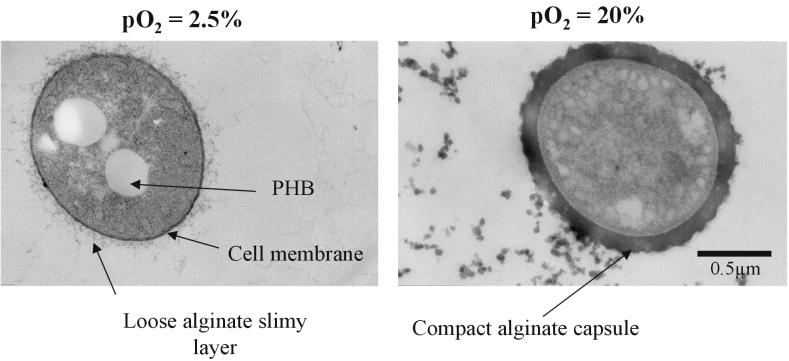
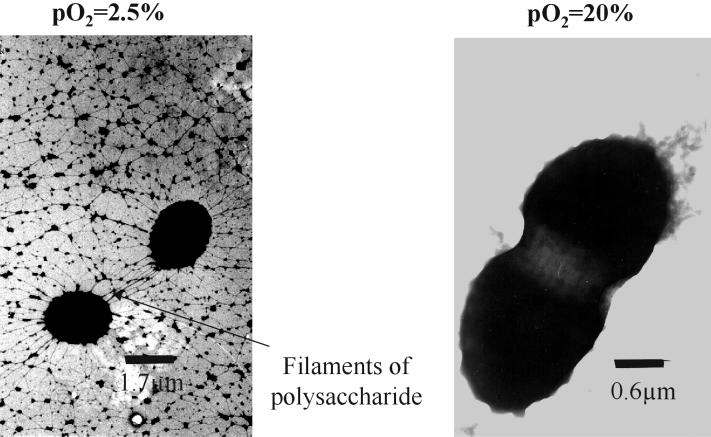
To gain more information on the development and quality of the alginate capsule, an O2-controlled chemostat culture of A. vinelandii was studied at a low pO2 value (2.5% air saturation) and at a relatively high pO2 value (20% air saturation). Transmission electron microscopy was used to investigate the appearance of the alginate layer around the negatively stained vegetative cell in a surface view as well as in thin sections. Surface view micrographs of vegetative cells at both pO2 values showed a net-structured polysaccharide capsule around the cell (Fig. 6). However, the polysaccharide structure differed distinctly by forming filamentous structures radiating from the bacterial surface at the low pO2 value (2.5% air saturation), while a compact dense layer of capsular polysaccharide was generated at a pO2 of 20%, which totally covered the cell surface.
FIG. 6.
Surface view electron micrographs of A. vinelandii cells grown at 2.5 and 20% air saturation in a phosphate-limited chemostat. PHB, poly-β-hydroxybutyrate.
Electron micrographs of negatively stained A. vinelandii cells seen in ultrathin sections are shown in Fig. 7. A. vinelandii cells grown diazotrophically at pO2 values of 2.5 and 20% formed capsules with significant differences in the thickness and compactness of the polysaccharide materials. These differences in the polysaccharide structure are also reflected by large variations in the G/M ratio of alginate around the cells. The G/M ratios of alginate formed were determined to be 45 and 88% for the pO2 values of 2.5 and 20%, respectively.
FIG. 7.
Thin section of negatively stained A. vinelandii cells grown at low and high pO2 values in a phosphate-limited chemostat.
DISCUSSION
The results presented in this work clearly showed that the respiratory protection of nitrogenase against oxygen stress, a widely accepted conjecture in the literature for nitrogenase protection in nitrogen-fixing bacteria, is not the prevailing mechanism involved in diazotrophically growing A. vinelandii in phosphate-limited culture (Fig. 1). This conclusion is in agreement with the results of Post et al. (33) and Boiardi (3). Other possible mechanisms such as reversible conversion of nitrogenase into a protected inactivated state and reduction of cell-specific surface area cannot explain our experimental results either. These facts and the observations of alginate formation, its variation in quality, and especially the varied morphology of the alginate capsule on the cell surface strongly suggested that the formation of alginate (capsule) plays an important role in overcoming oxygen stress and regulating the nitrogenase activity and hence the growth of A. vinelandii, particularly under phosphate-limited conditions. This conclusion is also supported by several other previous observations: (i) the production of alginate takes place mainly in the phosphate-limited phase in batch culture (38), (ii) growth in the presence of ammonium (the final product of nitrogen fixation) inhibits alginate formation in A. vinelandii (5, 37), and (iii) alginate production is stimulated under conditions of limited synthesis of nitrogenase caused by limitation of either molybdate or iron (1, 10; N. F. Ferrala, P. Westervelt, G. A. Mabbott, and F. A. Fekete, Abstr. Annu. Meet. Am. Soc. Microbiol. 1986, abstr. K-143, 1986). This increased formation of alginate can better protect the activity of the synthesized nitrogenase and thus compensate for the limitation of molybdate and/or iron to a certain extent.
The protective role of alginate has also been demonstrated for Pseudomonas aeruginosa with respect to growth stresses caused by antibiotics, virulent phages, and metal toxicity (13, 23). However, for A. vinelandii and P. aeruginosa, the mechanisms by which alginate exerts its protective role are so far not well understood. We have shown in this work that alginate production does not linearly increase with increasing pO2 (Fig. 2). A similar trend was observed for A. vinelandii growing at an increased shear rate (Fig. 4). These results suggested that alginate formation alone cannot explain the experimental observations. We therefore looked at the quality of alginate by measuring the molecular weight and l-guluronic acid content of alginate released into the culture and the morphology, thickness, and compactness of the alginate capsule on the cell surface. The molecular weight of alginate increased monotonically with increased pO2 (Fig. 3). It is understood that the increase of molecular weight increases the viscosity of the culture broth and hence reduces oxygen transport. The high molecular weight may also increase the density of the alginate capsule on the cell surface. For the barrier effect of alginate against O2, its guluronic acid content may play an even more important role. As shown in Fig. 3 and 4, the guluronic acid content of alginate increased significantly under both oxidative and shear force stresses. It is known that increasing the l-guluronic acid fraction of the alginate tends to lead to the formation of dense gels through a characteristic interaction between alginate chains, according to the so-called egg-box model (44), but that alginate with a high d-mannuronic acid content forms only soft gels with a lower affinity for chain binding (28). These findings may well explain the formation of filamentous and loosely structured alginate at a low pO2 (2.5% air saturation), in contrast to the dense and compact alginate that forms at a high pO2 (20% air saturation) (Fig. 6 and 7). Alginate formed at a pO2 of 2.5% had a much lower l-guluronic acid content (45%) than alginate formed at a pO2 of 20% (88% of l-guluronic acid). In this connection, it is interesting that the calcium content of the medium may also affect the quality of the alginate capsule. It is the calcium alginate gel that is postulated to have the egg-box-like structure.
Taken together, the results presented in this work allow us to draw the conclusion that it is the quality, not the quantity, of alginate that is decisive for the protection of nitrogenase against oxygen for diazotrophically growing A. vinelandii in phosphate-limited culture. The formation of alginate and, above all, the variation of its quality and structure seem to help maintain the intracellular oxygen concentration at a level low enough for an effective nitrogenase system. With the determinant role of alginate quality in mind, we may now also understand the occurrence of an optimal formation rate of alginate at pO2 levels between 2.5 and 5% (Fig. 2) and at an intermediate agitation speed (Fig. 4). Obviously, at high levels of oxygen stress, cells tend to produce an alginate as the capsule on the cell surface that is more compact and has a greater guluronic acid content at the expense of release of alginate into culture broth. In this connection, it is worth mentioning that the formation of a compact slime layer or capsule on the cell surface is not common for other polysaccharide-producing bacterial strains under highly turbulent conditions in a bioreactor, as was discussed by Peters et al. (31) for the formation of xanthan by Xanthomonas campestris and by Lobas et al. (27) for the production of gellan by Sphingomonas paucimobilis. Both X. campestris and S. paucimobilis are non-O2-sensitive strains and form no slime layer at high shear rates. These findings underline again the importance of the alginate capsule for the survival of A. vinelandii under particular growth conditions.
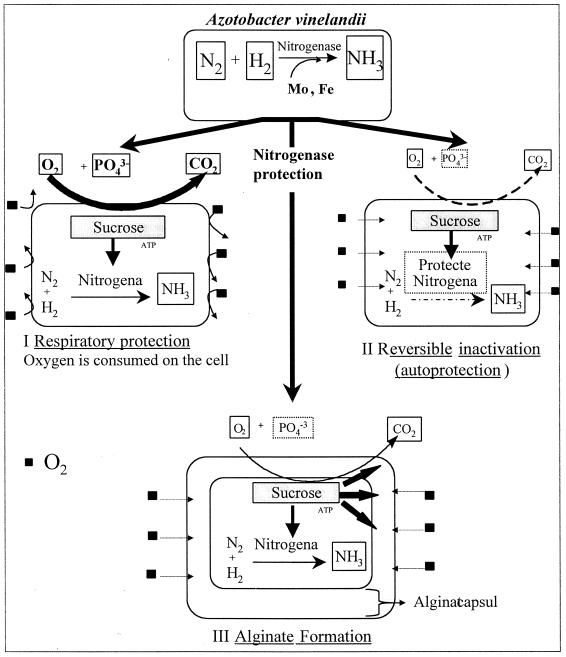
Of course, the respiratory protection and enzyme conversion mechanisms may still be involved in A. vinelandii, especially in nonslimy variants (6), but do not play a decisive role under the experimental conditions considered in this work. The overall mechanisms for the protection of nitrogenase against O2 in A. vinelandii are schematically shown in Fig. 8. Depending on the growth conditions, the relative importance of the different protection mechanisms may vary.
FIG. 8.
Summation of protection mechanisms for nitrogenase against O2 in A. vinelandii. The formation of a compact alginate capsule is proposed in this work as the decisive protection mechanism for A. vinelandii grown under phosphate-limited conditions.
It should be mentioned that several questions related to the formation of alginate and its role in A. vinelandii remain to be answered. It is not known, for example, if the alginate capsule represents also a strong barrier for the transport of other essential nutrients necessary for growth. Such a barrier effect may explain the observed decline of biomass concentration in our culture (Fig. 1). An earlier study showed that free alginate in medium limits the diffusion of O2 in culture of P. aeruginosa, thereby restricting its growth (14). It is, however, also possible that the protection of nitrogenase by the formation of alginate has its limitation at relatively high pO2 levels and that the activity of nitrogenase will still be impaired, leading to a decrease in cell growth. To address this issue, a measurement of intracellular oxygen concentration and a quantitative knowledge of the sensitivity of nitrogenase toward oxygen are necessary. From fundamental and application points of view, it is also important to ask the question of how the quantity and quality of alginate at different O2 concentration are regulated at physiological and genetic levels. Oxygen concentration can regulate several alginate biosynthetic enzymes and genes (22). From our results, an oxygen-dependent up-regulation of epimerase enzymes and especially of the gene algE2, which is responsible for introducing guluronic acid blocks into alginate, may be inferred since compact alginate with a high guluronic acid content was characteristic at high pO2 values (Fig. 6). Answers to these questions and the possibility of physiologically and genetically altering alginate synthesis and its quality will certainly help to improve the microbial production of alginate, which is of increasing industrial interest (12, 34).
ACKNOWLEDGMENTS
We thank G. Skjak-Braek for supplying alginate samples with different G/M ratios. We also thank T. Gäbel for the excellent assistance with the computer control system UBICON.
W. A. Sabra gratefully acknowledges financial support by DAAD.
REFERENCES
- 1.Annison G, Couperwhite I. Effect of limiting substrate concentration, growth rate and aeration on alginate composition and production by Azotobacter vinelandii in continuous culture. Food Hydrocoll. 1986;1:101–111. [Google Scholar]
- 2.Annison G, Couperwhite I. Influence of calcium on alginate production and composition in continuous cultures of Azotobacter vinelandii. Appl Microbiol Biotechnol. 1986;25:55–61. [Google Scholar]
- 3.Boiardi J L. Metabolic cost of nitrogen incorporation by N2-fixing Azotobacter vinelandii is affected by the culture pH. Biotechnol Lett. 1994;16:1195–1198. [Google Scholar]
- 4.Boltz D F. Total phosphorus. In: Holmann M H, editor. Analytical chemistry of phosphorus compound. H. New York, N.Y: Wiley Interscience; 1972. pp. 1–31. [Google Scholar]
- 5.Brivonese A, Sutherland W I. Polymer production by a mucoid strain of Azotobacter vinelandii in batch culture. Appl Microbiol Biotechnol. 1989;30:97–102. [Google Scholar]
- 6.Bush J A, Wilson P W. A non-gummy chromogenic strain of Azotobacter vinelandii. Nature. 1959;184:381. [Google Scholar]
- 7.Clementi F. Alginate production by Azotobacter vinelandii. Crit Rev Biotechnol. 1997;17:327–361. doi: 10.3109/07388559709146618. [DOI] [PubMed] [Google Scholar]
- 8.Deavin L, Jarman T R, Lawson C J, Richelato R C, Slocombe S. The production of alginic acid by Azotobacter vinelandii in batch and continuous culture. In: Sanford P A, Laskin A, editors. Extracellular microbial polysaccharides. Washington, D.C.: American Chemical Society; 1977. pp. 14–26. [Google Scholar]
- 9.Drozd J, Postgate J R. Effect of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970;63:63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- 10.Duyvis M, Wassink H, Haaker H. Nitrogenase of Azotobacter vinelandii: kinetic analysis of the Fe protein redox cycle. Biochemistry. 1998;37:17345–17354. doi: 10.1021/bi981509y. [DOI] [PubMed] [Google Scholar]
- 11.Fyfe J A M, Govan J R W. Synthesis, regulation and biological function of bacterial alginate. Prog Ind Microbiol. 1983;18:45–83. [Google Scholar]
- 12.Gacesa P. Bacterial alginate biosynthesis—recent progress and future prospects. Microbiology. 1998;144:1122–1143. doi: 10.1099/00221287-144-5-1133. [DOI] [PubMed] [Google Scholar]
- 13.Govan J R W, Fyfe J A M, Jarman T R. Isolation of alginate producing mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. J Gen Microbiol. 1981;125:217–220. doi: 10.1099/00221287-125-1-217. [DOI] [PubMed] [Google Scholar]
- 14.Hassett D J. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J Bacteriol. 1996;178:7322–7325. doi: 10.1128/jb.178.24.7322-7325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst H, Peters U H, Suh S I, Schumpe A, Deckwer W-D. Monitoring xanthan quality during fermentation by size exclusion chromatography. Biotechnol Tech. 1988;2:101–104. [Google Scholar]
- 16.Horan N J, Jarman T R, Dawes E A J. Effect of carbon source and inorganic phosphate concentration on the production of alginic acid by a mutant of Azotobacter vinelandii and on the enzyme involved in its biosynthesis. J Gen Microbiol. 1981;127:185–191. [Google Scholar]
- 17.Horan N J, Jarman T R, Dawes A J. Studies on some enzymes of alginic acid biosynthesis in Azotobacter vinelandii grown in continuous culture. J Gen Microbiol. 1983;129:2985–2990. [Google Scholar]
- 18.Iwahashi H, Someya J. Oxygen sensitivity of nitrogenase is not always a limiting factor of growth under nitrogen fixing conditions in Azotobacter vinelandii. Biotechnol Lett. 1992;14:227–232. [Google Scholar]
- 19.Jarman T R. Bacterial alginate synthesis. In: Barkeley U S, editor. Microbial polysaccharides and polysaccharases. London, United Kingdom: Academic Press; 1979. pp. 35–50. [Google Scholar]
- 20.Knutson C A, Jeanes A. Determination of the composition of uronic acid mixtures. Anal Biochem. 1968;24:482–490. doi: 10.1016/0003-2697(68)90155-3. [DOI] [PubMed] [Google Scholar]
- 21.Kuhla J, Oelze J. Dependence of nitrogenase switch-off upon oxygen stress on the nitrogenase activity in Azotobacter vinelandii. J Bacteriol. 1988;170:5325–5329. doi: 10.1128/jb.170.11.5325-5329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitäo J H, Correia I S. Oxygen dependent upregulation of transcription of alginate genes algA, algC and algD in Pseudomonas aeruginosa. Res Microbiol. 1997;148:37–43. doi: 10.1016/S0923-2508(97)81898-0. [DOI] [PubMed] [Google Scholar]
- 23.Leitäo J H, Correia I S. Effect of growth inhibitory concentrations of copper on alginate biosynthesis in highly mucoid Pseudomonas aeruginosa. Microbiology. 1997;143:481–488. doi: 10.1099/00221287-143-2-481. [DOI] [PubMed] [Google Scholar]
- 24.Linkerhägner K, Oelze J. Cellular ATP level and nitrogenase switchoff upon oxygen stress in chemostat cultures of Azotobacter vinelandii. J Bacteriol. 1995;177:5289–5293. doi: 10.1128/jb.177.18.5289-5293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkerhägner K, Oelze J. Nitrogenase activity and regeneration of the cellular ATP pool in Azotobacter vinelandii adapted to different oxygen concentrations. J Bacteriol. 1997;179:1362–1367. doi: 10.1128/jb.179.4.1362-1367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J K, Lee F T, Lin C S, Yao X T, Davenport J W, Wong T Y. Alternative function of the electron transport system in Azotobacter vinelandii: removal of excess reductant by the cytochrome d pathway. Appl Environ Microbiol. 1995;61:3998–4003. doi: 10.1128/aem.61.11.3998-4003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobas D, Schumpe S, Deckwer W-D. The production of gellan polysaccharide with Sphingomonas paucimobilis E2 (DSM 6314) Appl Microbiol Biotechnol. 1992;37:411–415. [Google Scholar]
- 28.Matsumoto T, Kawal M, Masuda T. Influence of concentration and mannuronate/guluronate ratio on steady flow properties of alginate aqueous systems. Biorheology. 1992;29:411–417. doi: 10.3233/bir-1992-29404. [DOI] [PubMed] [Google Scholar]
- 29.Moshiri F, Crouse B R, Johnson M K, Maier R J. The 'nitrogenase protective' FeSII protein of Azotobacter vinelandii: overexpression, characterisation and crystallization. Biochemistry. 1995;34:12973–12982. doi: 10.1021/bi00040a007. [DOI] [PubMed] [Google Scholar]
- 30.Nunez C, Moreno S, Soberon-Chavez G, Espin G. The Azotobacter vinelandii response regulator AlgR is essential for cyst formation. J Bacteriol. 1999;181:141–148. doi: 10.1128/jb.181.1.141-148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters H-U, Herbst H, Hesselink P G M, Lünsdorf H, Schumpe A, Deckwer W-D. The influence of agitation rate on xanthan production by Xanthomonas campestris. Biotechnol Bioeng. 1989;34:1393–1397. doi: 10.1002/bit.260341108. [DOI] [PubMed] [Google Scholar]
- 32.Post E, Golecki J R, Oelze J. Morphological and ultrastructural variations in Azotobacter vinelandii growing in oxygen-controlled continuous culture. Arch Microbiol. 1982;133:75–82. [Google Scholar]
- 33.Post E, Kleiner D, Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983;134:68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- 34.Rehm H A, Valla S. Bacterial alginate: biosynthesis and applications. Appl Microbiol Biotechnol. 1997;48:281–288. doi: 10.1007/s002530051051. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds E S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruppen M, Garner G, Sadoff L H. Protein turnover in Azotobacter vinelandii during encystment and germination. J Bacteriol. 1983;156:1243–1248. doi: 10.1128/jb.156.3.1243-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabra W A. Microaerophilic production of alginate by Azotobacter vinelandii. Ph.D. dissertation. Braunschweig, Germany: Technische Universität Braunschweig; 1999. [Google Scholar]
- 38.Sabra W A, Zeng A-P, Sabry S, Omar S, Deckwer W-D. Effect of phosphate and oxygen concentrations on alginate production and stoichiometry of metabolism of Azotobacter vinelandii under microaerobic conditions. Appl Microbiol Biotechnol. 1999;52:773–780. [Google Scholar]
- 39.Sadoff H L. Encystment and germination in Azotobacter vinelandii. Bacteriol Rev. 1975;39:516–539. doi: 10.1128/br.39.4.516-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senior P J, Beech G A, Ritchie G A F, Dawes E A. The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J. 1972;128:1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skjak-Braek G. Alginate: biosynthesis and some structure function relationships relevant to biomedical and biotechnological applications. Biochem Soc Trans. 1992;20:27–33. doi: 10.1042/bst0200027. [DOI] [PubMed] [Google Scholar]
- 42.Spurr A R. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 43.Tsai J C, Aladegbami S L, Vela G R. Phosphate-limited culture of Azotobacter vinelandii. J Bacteriol. 1979;139:639–645. doi: 10.1128/jb.139.2.639-645.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valla S, Skjak-Braek G. Food Ingred. Eur. 1996(September):38–41. 1996. Alginate: a target molecule for genetic engineers and a versatile material for the biotechnologist. [Google Scholar]