Abstract
Simple Summary
This study was conducted to evaluate the efficacy of six ultraviolet light-emitting diodes (UV-LED) traps and a fluorescent light trap for sampling urban nocturnal mosquitoes. Results demonstrated that the fluorescent light trap outperformed all the UV-LED traps throughout the 72 sampling nights and between wet and dry seasons. Among the UV-LED traps, the LED375 trapped the highest number of mosquitoes. Additional field trials are needed to validate these findings in different ecological settings.
Abstract
Well-designed surveillance systems are required to facilitate a control program for vector-borne diseases. Light traps have long been used to sample large numbers of insect species and are regarded as one of the standard choices for baseline insect surveys. The objective of this study was to evaluate the efficacy of six ultraviolet light-emitting diodes and one fluorescent light for trapping urban nocturnal mosquito species within the Kasetsart University (KU), Bangkok. Ultraviolet light-emitting diodes (UV-LEDs), (LED365, LED375, LED385, LED395, and LED405) and a fluorescent light were randomly assigned to six different locations around the campus in a Latin square design. The traps were operated continuously from 18:00 h to 06:00 h throughout the night. The traps were rotated between six locations for 72 collection-nights during the dry and wet seasons. In total, 6929 adult mosquitoes were caught, with the most predominant genus being Culex, followed by Aedes, Anopheles, Armigeres and Mansonia. Among the Culex species, Culex quinquefasciatus (n = 5121: 73.9%) was the most abundant followed by Culex gelidus (n = 1134: 16.4%) and Culex vishnui (n = 21: 0.3%). Small numbers of Aedes, Armigeres, and Anopheles mosquitoes were trapped [Aedes albopictus (n = 219: 3.2%), Aedes pocilius (n = 137: 2.0%), Armigeres subalbatus (n = 97: 1.4%), Anopheles vagus (n = 70: 1.0%), Aedes aegypti (n = 23: 0.3%)]. There were 2582 specimens (37.2%) captured in fluorescent light traps, whereas 942 (13.6%), 934 (13.5%), 854 (12.3%), 820 (11.8%), and 797 (11.5%) were captured in the LED375, LED405, LED395, LED365, and LED385 traps, respectively. None of the UV-LED light traps were as efficacious for sampling nocturnal mosquito species as the fluorescent light trap. Among the five UV-LED light sources, LED375 trapped the greatest number of mosquitoes. Additional field trials are needed to validate these findings in different settings in order to substantially assess the potential of the LEDs to trap outdoor nocturnal mosquitoes.
Keywords: light traps, fluorescent, Culex, Aedes, Thailand
1. Introduction
Vector control is a key component of disease control and elimination. Several tools are under development to determine distribution, abundance, and infection rate of the mosquito, among which various traps are of interest for mosquito sampling and surveillance [1]. Light traps have been used for a long time as a common basic surveying equipment device for insects, and there have been many variations in the design of light traps over the decades. Although its use in urban environments is facilitated by the availability of nearby power sources, field applications are often limited by the requirement for electricity to power traditional lights. Traditional fluorescent bulbs usually do not run for more than 12 h from a conventional 12-volt power source, such as a car battery [2]. Light traps have been used to trap insects for over 100 years [3,4] with many different types of designs, with some being very complicated, involving both the lights and fans, while others have remained uncomplicated [5].
Most current traps use incandescent bulbs or actinic fluorescent traps as the light source because the spectrum of light emitted by these bulbs is effective in attracting insects [6,7]. However, maintaining the necessary power to illuminate these light sources is always an issue [8]. Typically, small bulbs of around 6–9 watts will require either a fixed mains-connected power source or a large portable power supply to provide illumination for the entire night. A typical power source is a 12-volt battery, which will provide approximately 6–8 h, depending on the type of battery. Due to the flight period of various insects varying from dusk to dawn, standard light sources might not be able to attract some of the existing insect population.
Nocturnal insects are attracted by artificial light sources. Recently, light sources that produce large amounts of ultraviolet light-emitting diode (UV-LED) light trap radiation have revolutionized light trap sampling. Light traps are generally expensive, but some are very effective for the collection of insects [9,10]. Various light sources have been used for sampling purposes, such as mercury vapor lamps, gas lamps, and fluorescent UV light traps [11]. The UV light trap (LT trap) was designed using black plastic material equipped with an electric fan and an artificial light source supplied by 220 V electricity [12,13]. However, there are three types of UV radiation: UVA, UVB, and UVC with wavelengths in the ranges of 315–400 nm, 280–315 nm, and 100–280 nm, respectively. These three types of UV radiation are grouped according to wavelength, which varies according to insect biological activity [14,15]. UVA (315–400 nm) consists of long-wave ultraviolet or black light and is not absorbed by the ozone layer, hence it is a safe wavelength for users [16]. Numerous studies have used and evaluated LED for trapping mosquitoes [17]. However, no study has been published that compared the different UVA wavelengths in trapping nocturnal biting mosquitoes in Thailand. To address these shortcomings, different spectral ranges of UVA (365, 375, 385, 395, and 405 nm) were evaluated by comparison with a fluorescent light trap source for collecting mosquitoes in Bangkok, Thailand.
2. Materials and Methods
2.1. Study Sites
This study was conducted at the Kasetsart University (KU) (13°50′32.96″ N, 100°34′2.98″ E), Bangkok, Thailand. The campus covers 135.7 hectares and is categorized as an urban area. The campus consists of either buildings or natural sites that provide an ideal breeding habitat for mosquitoes. In total, six square grids (approximately 1.3 km2/grid) were overlaid on a map of the campus and used as six study locations: Chobprachoom Building, Faculty of Fisheries (Loc. 1), Faculty of Agriculture (Loc. 2), Ngamwongwan 1 Parking Garage (Loc. 3), KU Dormitory for male students (Loc. 4), the Office of Agricultural Museum and Culture (Loc. 5) and the Entomology Research Building (Loc. 6), as shown in (Figure 1). Each grid was subdivided into six smaller squares in which trap locations were randomly selected.
Figure 1.
Locations for setting light traps (18:00 to 06:00 h) to capture adult mosquitoes at Kasetsart University, Bangkok, Thailand.
2.2. Trapping Method
Black Hole™ Mosquito trap units (Pan Science Co., Ltd., Bangkok, Thailand) were used as the reference trapping method. Briefly, the trap was made of durable black plastic material equipped with an electrical fan and used a fluorescent lamp as the ultraviolet light (UV) source in the range of 100–400 nm. As an alternative source of UV light to this fluorescent lamp, LED UV light traps purchased from a department store were investigated. The LED UV lights with titanium dioxide (TiO2) produced carbon dioxide (CO2) and had 5 spectral lines of UV light-emitting diodes (UV-LEDs) consisting of 365 nm (LED365), 375 nm (LED375), 385 nm (LED385), 395 nm (LED395) and 405 nm (LED405). These were examined for their effectiveness in sampling adult mosquitoes compared to the Black Hole™ Mosquito trap. This trap consisted of on O-ring hanging with standalone columns or beams in a box that operated without any spark or noise. Each trap was hung, approximately 1.5 m above the ground.
2.3. Experimental Design
A Latin square design was applied in this study to minimize the residual error in the experiment by eliminating variance due to any known and controllable disturbance variables [18]. The light traps for catching adult mosquitoes were rotated through the 6 sites based on a 6 × 6 Latin square design, where one replication comprised 6 consecutive nights of trapping. The experiments were performed for 6 replications (36 nights) each in the dry (February to April 2020) and wet (July to September 2020) seasons.
2.4. Mosquito Collection
A Nocturnal mosquito species were captured from the six light traps which were operated continuously from 18:00 to 06:00 h. All captured mosquitoes were removed from light traps every three hours at 21:00 h, 24:00 h, 03:00 h, and 06:00 h Morphological identification of mosquito species was performed following the standard illustrated keys to adult mosquitoes [19,20,21,22,23] the next morning. To confirm the mosquito species, larvae or pupae were sampled once nearby a trap position during a season and reared to adult mosquitoes in the insectary at the Department of Entomology, Faculty of Agriculture, Kasetsart University. The meteorological data (relative humidity, temperature, and rainfall) were recorded using climatological data for the period between 2020 and 2021 from Don Muang Airport Station, Bangkok.
2.5. Data Analysis
The total number of mosquitoes captured per light trap per night was transformed using the logarithm function (Log10(x + 1)) to normalize the distribution of the one-way analysis of variance (ANOVA), and the Tukey’s test for post hoc analysis was performed to evaluate the efficacy of the five UV-LEDs (LED365, LED375, LED385, LED395, and LED405) compared to the UV fluorescence. The percentage of mosquito species caught per trap per night was calculated by dividing the number of mosquito species from an individual light trap by the total number of mosquito species collected in the night and multiplying by 100. Mean percentages of mosquito species captured per trap per night were analyzed using the one-way ANOVA and Tukey’s test for post-hoc analysis.
A generalized linear model (GLM), consisting of a negative binomial model and a log link function, was used to analyze the main parameter of the light source and the co-parameter of the night collection that influenced the numbers of mosquitoes collected per trap per night in each season. Parameter coefficients were evaluated using the Wald chi-square test. Incident rate ratios (IRR) of the different light sources were calculated relative to the reference light trap of the UV fluorescence. Values of IRR greater or less than 1 indicated higher or lower trapping performance, respectively, relative to the reference to determine whether each of the alternative light sources (UV-LEDs) was correlated with the referenced light source (UV fluorescent). The Pearson’s correlation coefficient was used to investigate the relationship among the log-transformed catches for each mosquito species. All data were analyzed using the SPSS Statistics for Windows software, version 26.0 (IBM Corp, Armonk, NY, USA) with a significance level of 0.05.
3. Results
Five LED-UV traps with different spectral ranges (365–405 nm) were compared with one fluorescent light source for efficacy in trapping urban mosquito species. Traps were set for 72 nights collection in both the dry and wet seasons. In total, 6929 adult mosquitoes were recorded in the trap types: fluorescent UV (35.64%, 34.72%), LED405 (14.76%, 12.35%), LED375 (13.04%, 14.78%), LED395 (12.96%, 12.38%), LED385 (12.86%, 11.74%), and LED365 (11.15%, 14.52%), as shown in (Figure 2).
Figure 2.
Percentage of mosquitoes trapped using different light source traps between dry and wet seasons.
Species within five genera were morphologically identified as Ae. aegypti (n = 23: 0.3%), Ae. albopictus (n = 219: 3.2%), Ae. pocilius (n = 137: 2.0%), An. vagus (n = 70: 1.0%), Ar. subalbatus (n = 97: 1.4%), Cx. gelidus (n = 1134: 16.4%), Cx. quinquefaciatus (n = 5121: 73.9%), Cx. vishnui (n = 21: 0.3%), Mansonia uniformis (n = 28: 0.4%), and other species (n = 79: 1.1%). The highest number of mosquitoes was collected from fluorescent-UV light traps compared to the other five LED-UV light sources. In terms of abundance, the fluorescent light traps captured the greatest number of mosquitoes (n = 2582; 37.2%), followed by LED375 (n = 942; 13.6%), LED405 (n = 934; 13.5%), LED395 (n = 854; 12.3%), LED365 (n = 820; 11.8%), and LED385 (n = 797; 11.5%), respectively (Table 1). There were no significant differences in the mean numbers of collected mosquitoes from each light source between the two seasons during the collection period (Table 2).
Table 1.
Total number and percentage of adult mosquito species captured in traps with 6 different light sources over 6 replications (6 nights/replication) in dry and wet seasons at Kasetsart University, Bangkok.
| Trap Light Source | Collection Nights | Total Number of Mosquito Species (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | Ae. pocilius | An. vagus | Ar. subalbatus | Cx. quinquefasciatus | Cx. gelidus | Cx. vishnui | Ma. uniformis | Others * | Total | ||
| LED365 | 72 | 3 | 29 | 36 | 16 | 5 | 519 | 183 | 6 | 5 | 18 | 820 (11.8) |
| LED375 | 72 | 3 | 35 | 11 | 7 | 6 | 743 | 127 | 0 | 3 | 7 | 942 (13.6) |
| LED385 | 72 | 8 | 22 | 17 | 12 | 36 | 576 | 102 | 6 | 5 | 13 | 797 (11.5) |
| LED395 | 72 | 2 | 25 | 18 | 14 | 13 | 639 | 132 | 0 | 1 | 10 | 854 (12.3) |
| LED405 | 72 | 3 | 29 | 21 | 8 | 5 | 754 | 100 | 3 | 4 | 7 | 934 (13.5) |
| Fluorescent | 72 | 4 | 79 | 34 | 13 | 32 | 1890 | 490 | 6 | 10 | 24 | 2582 (37.3) |
| Total | 23(0.3) | 219(3.2) | 137(2.0) | 70(1.0) | 97(1.4) | 5121(73.9) | 1134(16.4) | 21(0.3) | 28(0.4) | 79(1.1) | 6929(100) | |
* These specimens could not be identified to the species level due to damage and insufficient numbers to make comparisons.
Table 2.
Mean number of mosquitoes obtained from 6 light sources over 6 replications (6 nights/replication) at Kasetsart University, Bangkok between dry and wet seasons.
| Trap Light Source | Collection Nights Dry/Wet |
Total (%) Dry/Wet |
Mean ± SD * | 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|---|---|
| Dry ** | Wet ** | Dry | Wet | |||||
| Lower | Upper | Lower | Upper | |||||
| LED365 | 36/36 | 365 (10.1)/ 832 (22.5) |
0.85 ± 0.07 a | 0.95 ± 0.07 a | 0.70 | 1.01 | 0.81 | 1.09 |
| LED375 | 36/36 | 480 (13.2)/ 462 (12.5) |
0.09 ± 0.07 a | 0.97 ± 0.06 a | 0.74 | 1.06 | 0.83 | 1.10 |
| LED385 | 36/36 | 449 (12.3)/ 348 (9.4) |
0.91 ± 0.07 a | 0.88 ± 0.06 a | 0.76 | 1.06 | 0.76 | 1.00 |
| LED395 | 36/36 | 441 (12.1)/ 440 (11.9) |
0.85 ± 0.08 a | 0.92 ± 0.06 a | 0.68 | 1.02 | 0.78 | 1.06 |
| LED405 | 36/36 | 562 (15.5)/ 372 (10.1) |
0.97 ± 0.08 a | 0.96 ± 0.04 a | 0.80 | 1.13 | 0.87 | 1.06 |
| Fluorescent | 36/36 | 1339 (36.8)/ 1243 (33.6) |
1.40 ± 0.06 a | 1.41 ± 0.05 a | 1.27 | 1.53 | 1.29 | 1.53 |
* Mean number of log-transformed data ± SD of all adult mosquitoes captured by each light source trap carried out during 36 sampling nights in dry and wet seasons. ** Values with the same lowercase superscripts in a column for each season are not significantly different using one-way ANOVA with a multiple Tukey’s test comparison at the 0.05 level.
The efficacy of the light traps at catching the two Culex species is provided in Table 3. Overall, the greatest number of mosquitoes were collected from the UV fluorescence light traps (38.4% in dry and 37.7% in wet), regardless of the season. Specifically, a higher number of Cx. quinquefasciatus was caught during the dry season compared to the wet season, whereas there was a higher number of Cx. gelidus caught in the wet season. There was no significant difference in the mean number of mosquitoes caught from each trap source between the two seasons for both Culex species, except those collected from UV fluorescence light trap, where there was a significant difference between seasons.
Table 3.
Mean numbers of Cx. quinquefasciatus and Cx. gelidus collected during 36 trapping nights in dry and wet seasons using 6 different light source traps.
| Trap Light Source | Night Dry/Wet |
Total (%) Dry/Wet |
Mean ± SD ** | |||
|---|---|---|---|---|---|---|
| Cx. quinquefasciatus | Cx. gelidus | |||||
| Dry * | Wet * | Dry * | Wet * | |||
| LED365 | 36/36 | 296 (9.0)/ 406 (13.6) |
7.89 ± 1.44 a | 6.53 ± 1.15 a | 0.33 ± 0.15 a | 4.74 ± 1.05 a |
| LED375 | 36/36 | 453 (13.8)/ 417 (14.0) |
12.31 ± 2.82 a | 8.33 ± 1.55 a | 0.28 ± 0.13 a | 3.25 ± 0.65 a |
| LED385 | 36/36 | 367 (11.2)/ 311 (10.4) |
9.69 ± 1.98 a | 6.31 ± 1.30 a | 0.50 ± 0.22 a | 2.33 ± 0.40 a |
| LED395 | 36/36 | 381 (11.6)/ 390 (13.1) |
10.44 ± 2.07 a | 7.31 ± 1.54 a | 0.14 ± 0.58 a | 3.53 ± 1.05 a |
| LED405 | 36/36 | 522 (15.9)/ 332 (11.1) |
14.17 ± 2.93 a | 6.78 ± 0.74 a | 0.33 ± 0.13 a | 2.44 ± 0.57 a |
| Fluorescent | 36/36 | 1258 (38.4)/ 1122 (37.7) |
33.92 ± 6.11 b | 18.58 ± 1.77 a | 1.03 ± 0.33 a | 12.58 ± 3.42 b |
** Mean numbers (± SD) of all adult mosquitoes captured in each light source trap carried out during 36 sampling nights in dry and wet seasons. * Values in each column with different lowercase superscripts are significantly different using one-way ANOVA with a multiple Tukey’s test comparison at the 0.05 level.
A negative binomial regression GLM was performed to determine whether two key factors (light source, season) influenced the efficacy of light traps in capturing nocturnal mosquito species. From the goodness-of-fit test, the deviance (1.033) and Pearson chi-square (443.689) demonstrated that the negative binomial regression was perfectly suitable (Omnibus test; p = 0.000). Based on results from Table 4, only the light source parameter was a significant predictor that influenced the number of mosquitoes captured per trap, while seasons and nights were not significant variables in the prediction model (p = 0.45). For the fluorescent UV set as the reference (IRR = 1), the IRR values for LED375, LED405, LED395, LED365, and LED385 were 0.364, 0.362, 0.331, 0.327, and 0.307, respectively. These results indicated that the fluorescent UV was the most efficient light source to capture mosquitoes compared to the other LED light sources (Table 4).
Table 4.
Incidence rate ratios of factors influencing efficacy of light traps to capture nocturnally active mosquitoes.
| Parameter Estimate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | B | Std. Error | 95% Wald Confidence Interval | Hypothesis Test | IRR | 95% Wald Confidence Interval for Exp(B) | ||||
| Lower | Upper | Wald Chi-Square | df | Sig. | Lower | Upper | ||||
| (Intercept) | 3.611 | 0.1589 | 3.300 | 3.922 | 516.572 | 1 | 0.000 | 37.003 | 27.102 | 50.521 |
| LED365 | −1.119 | 0.1720 | −1.456 | −0.782 | 42.309 | 1 | 0.000 | 0.327 | 0.233 | 0.458 |
| LED375 | −1.010 | 0.1710 | −1.345 | −0.675 | 34.910 | 1 | 0.000 | 0.364 | 0.260 | 0.509 |
| LED385 | −1.181 | 0.1716 | −1.518 | −0.845 | 47.387 | 1 | 0.000 | 0.307 | 0.219 | 0.430 |
| LED395 | −1.105 | 0.1714 | −1.441 | −0.769 | 41.598 | 1 | 0.000 | 0.331 | 0.237 | 0.463 |
| LED405 | −1.017 | 0.1716 | −1.353 | −0.681 | 35.138 | 1 | 0.000 | 0.362 | 0.258 | 0.506 |
| Fluorescent UV | 0 a | 1 | ||||||||
| Dry season | 0.076 | 0.1003 | −0.121 | 0.272 | 0.572 | 1 | 0.449 | 1.079 | 0.886 | 1.313 |
| Wet season | 0 a | 1 | ||||||||
| Night | −0.021 | 0.0283 | −0.077 | 0.034 | 0.570 | 1 | 0.450 | 0.979 | 0.926 | 1.035 |
| (Scale) | 1 b | |||||||||
| (Negative binomial) | 1 b | |||||||||
a Set to zero because this parameter is redundant. b Fixed at displayed value.
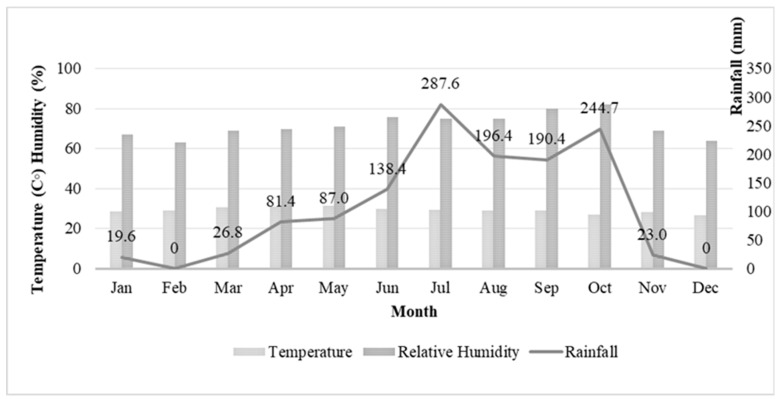
The experiment was conducted during two seasons: dry (February–April) and wet (July–September). The meteorological data indicated that there was higher rainfall between July and October than for the other months of the year. The highest rainfall was in July (287.6 mm), whereas no rainfall (0 mm) was recorded in February and December. The mean relative humidity and temperature were also recorded (Figure 3).
Figure 3.
Monthly environmental parameters (mean temperature, relative humidity, and total rainfall) for study area.
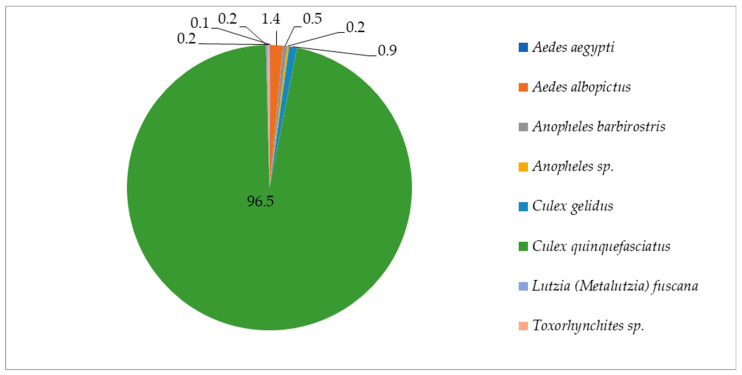
Larval collections made in the vicinity of trap placements identified six species belonging to four genera (Culex, Aedes, Anopheles, Toxorhynchites), the highest number of Cx. quinquefasciatus (n = 815: 96.5%), Cx. gelidus (n = 7: 0.8%), Lutzia (Metalutzia) fuscana (n = 2: 0.2%), Ae. albopictus (n = 12: 1.4%), Ae. aegypti (n = 1: 0.1%) and Anopheles barbrirostris (n = 4: 0.5%).
Two specimens of Anopheles (n = 2: 0.2%) and two from Toxorhynchites (n = 2: 0.2%) could not be identified due to specimen damage (Figure 4).
Figure 4.
Proportion of mosquito species obtained from larval collections of nearby trap locations.
4. Discussion
Mosquito traps are generally used in surveillance to monitor the distribution and abundance of mosquito populations [24]. Recently, several traps have been developed not only for surveillance or monitoring but also for vector control. A light trap is one type of trap that is popular for trapping mosquitoes [25]. The several types of light sources of LED lights have demonstrated efficacy in attracting various insects and pests [26,27,28,29,30]. Although some studies using light traps have been reported in Thailand [31,32], no known study has been published on the use of different wavelengths of LED lights for collecting nocturnally active urban mosquitoes in Thailand. Therefore, we compared the efficacy of five different wavelengths of UV-LED light sources to catch nocturnal mosquitoes in an urban environment of Bangkok, Thailand.
In this study, fluorescent lights outperformed all five different wavelengths of LED lights in catching urban mosquito species. Overall, nine nocturnal mosquito species were collected, with the most predominant species being Cx. quinquefasciatus (73.9%) across all collections, regardless of the light configuration, trap location, or collection date. The potential of Cx. quinquefasciatus as a vector in urban areas is further indicated by its typical breeding underground in sewers and drains [33]. This species is a very common, cosmopolitan urban nighttime biting mosquito and is generally active during the entire evening, depending on the availability of vertebrate hosts [34,35,36,37]. Culex quinquefasciatus is a primary vector of lymphatic filariasis disease, which has caused serious negative social, economic, and health impacts in tropical and subtropical countries [38]. A report in Thailand revealed that this mosquito species is able to transmit several other medically important pathogens, such as Japanese encephalitis, West Nile virus, Zika virus, and Tembusu virus [39].
Kasetsart University is located in a heavily urbanized area of the Bangkok metropolis. The university grounds provide many suitable larval habitats covering large bodies of open stagnant/slow moving water, numerous rainwater drainage lines, and various artificial containers infused with varying degrees of organic matter and higher levels of pollution that provide suitable breeding areas in different environments. Culex gelidus was the second-most common species (12.6%) captured in all traps. This species is of particular interest, as it is a natural vector of Japanese encephalitis virus (JEV) between host birds and humans [40]. The campus is also home for both migratory and resident wild bird species (Family Ardeidae: egrets, herons and bitterns), that are potential reservoirs of JEV. In addition, the campus is normally congested with human activity during the day and at nighttime; thus, virus transmission to humans is a possibility.
The results demonstrated that the traps may not be species-specific, perhaps suggesting that other factors, such as mosquito density, could influence the trapping efficacy for a particular species. However, abundance estimates of mosquito species and their relative composition provided by different trapping devices could provide beneficial information for guiding surveillance methods and control efforts.
Differences between the two seasons in indices, such as ambient temperature, relative humidity, and rainfall, could be the factors that greatly influence adult mosquito activity and behavior [41]. Furthermore, the moon phase (waxing and waning) may influence the numbers of mosquitoes trapped due to the effect of the moonlight intensity and duration of illumination. The effect of the moonlight and lunar periodicity on light trap catches of mosquito species has been described by several authors [42,43]. Moonlight appears to reduce the number of mosquito collected from light traps under certain circumstances; for example, possibly by providing competitive illumination between the brightness (intensity) of the trap light source against background illumination resulting from the moon and thereby decreasing the contrast (attractiveness) by reducing the area in which mosquitoes are drawn to the trap [44]. Although the moon phase was recorded for each collection night throughout the study, several other factors influencing catch size and the study design itself precluded quantifying the possible effects of moonlight on catch size [45,46,47].
The first mosquito survey trap developed in the 1930s (the New Jersey light trap) remains among the most productive and efficient traps available for mosquito surveillance [5,48,49]. Several types of mosquito trapping devices have been developed and utilized over the years for mosquito surveillance [50,51,52]. Centers for Disease Control and Prevention (CDC) miniature light traps of different designs have been the standard used in at least one other study to conduct mosquito surveillance in Thailand [17]. Various devices, such as CDC traps baited with CO2 (or other semiochemicals) and Biogents (BG) lures with a combination of olfactory attractants, are available for adult mosquito sampling [51,53]. Recently, new traps using different light sources, modified designs, and attractors have been developed and evaluated against CDC light traps. However, there are no known studies published that have evaluated the attractive efficacy of LED illumination compared to fluorescent UV light. Additionally, the collection of outdoor active mosquitoes is limited in terms of the effectiveness of trapping devices. The present study in KU found that a fluorescent UV light trap achieved the greatest yield of attracted mosquitoes. Previous studies have demonstrated that among the five LEDs in the current study, the fluorescent UV light wavelength was an effective attractant for capturing mosquitoes [54,55,56,57]. Operationally, the Black Hole™ Mosquito trap was an acceptable device for mosquito collection. However, there are limits to its application for placement and position due to the need to for direct current electricity (from the main electricity distribution system) compared to other traps powered by batteries. Consequently, LED traps are increasingly used in mosquito traps since they have several advantages, including energy efficiency, durability, long lifetime, and good temporal stability.
This study was conducted to evaluate the attractiveness of different specific UVA wavelengths in trapping nocturnal mosquito species. As previously reported, mosquito species were attracted by different specific wavelengths of LED lights [27]. Wild Culex. pipiens has been reported as a species closely related to Cx. quinquefasciatus [58], which responded differently depending on the wavelengths of the light source. The most effective wavelengths to attract this species were between 333 nm and 405 nm in near UV wavelengths [59]. UV light can be categorized into three groups based on wavelength, UVA (315–400 nm), UVB (280–315 nm), and UVC (100–280 nm) [60]. Other publications have reported that UVA displayed high relative efficacy in attracting and trapping nocturnal mosquitoes [61,62]. Observations based on an electroretinogram have shown the highest responses of Cx. pipiens to 335 nm, corresponding to the UVA range (315–400 nm) [63].
One of the primary goals for understanding mosquito biology and ecology is measuring mosquito populations and species dynamics to facilitate the design and implementation of appropriate prevention and control strategies. An in-depth evaluation and analysis of a mechanical light trapping system for attracting nocturnally active mosquitoes can provide important information for conducting mosquito surveillance. The current study was the first attempt to assess various light sources as mosquito attractants in a densely populated urban area of Bangkok, while also obtaining information on mosquito species present at the Kasetsart University. Studies are continuing at the same location to evaluate the attractive responses to different UV wavelengths and trapping systems.
5. Conclusions
None of the tested UV-LED light traps were as efficacious for sampling nocturnal mosquito species as the fluorescent light trap. There were no significant differences in the numbers of collected mosquitoes from each LED light source. Among the five UV-LEDs, LED375 trapped the greatest number of mosquitoes. The comparative trapping efficacy of all light sources did not vary with season. However, owing to the efficacy and advantages of the LED light traps, they could have potential for mosquito surveillance as well as vector control. Additional field trials are needed to validate these findings in different settings and to assess the effectiveness of different wavelengths for LED light sources in trapping mosquito species.
Acknowledgments
The late Michael J. Bangs’ helpful advice on trapping methodology and Alex Ahebwa provided useful information in preparing this manuscript. This research was supported by the Medical Entomology Laboratory, Department of Entomology, Faculty of Agriculture, Kasetsart University for assistance with this research.
Author Contributions
All authors have contributed significantly to this study. S.P. and T.C. conceived of and designed the experiment; S.P. and T.C. performed the experiment; S.P. and R.N.-K. analyzed the data; S.P. and T.C. wrote the manuscript; T.C., A.S. and R.N.-K. consulted, read, corrected, and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the article.
Conflicts of Interest
There are no conflict of interest to declare.
Funding Statement
This research is supported in part by the Graduate Program Scholarship from The Graduate School, Kasetsart University and Kasetsart University Research and Development Institute (KURDI) (Grant No. FF (KU) 14.64).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanou A., Moussa Guelbéogo W., Nelli L., Hyacinth Toé K., Zongo S., Ouédraogo P., Cissé F., Mirzai N., Matthiopoulos J., Sagnon N.F., et al. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar. J. 2019;18:386. doi: 10.1186/s12936-019-3030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green K.W., Zelbst P.J., Meacham J., Bhadauria V.S. Green supply chain management practices: Impact on performance. Int. J. Supply Chain Manag. 2012;17:290–305. doi: 10.1108/13598541211227126. [DOI] [Google Scholar]
- 3.Balamurugan R., Kandasamy P. Effectiveness of portable solar-powered light-emitting diode insect trap: Experimental investigation in a groundnut field. J. Asia-Pac. Entomol. 2021;24:1024–1032. doi: 10.1016/j.aspen.2021.09.013. [DOI] [Google Scholar]
- 4.Green D., Mackay D., Whalen M. Next generation insect light traps: The use of LED light technology in sampling emerging aquatic macroinvertebrates. Aust. Entomol. 2012;39:189–194. [Google Scholar]
- 5.Muirhead-Thompson R. Trap Responses of Flying Insects: The Influence of Trap Design on Capture Efficiency. Academic Press; Cambridge, MA, USA: 2012. p. 304. [Google Scholar]
- 6.Marchioro M., Rassati D., Faccoli M., Van Rooyen K., Kostanowicz C., Webster V., Mayo P., Sweeney J. Maximizing bark and ambrosia beetle (Coleoptera: Curculionidae) catches in trapping surveys for longhorn and jewel beetles. J. Econ. Entomol. 2020;113:2745–2757. doi: 10.1093/jee/toaa181. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann P., Ammunét T., Barton M., Battisti A., Eigenbrode S.D., Jepsen J.U., Kalinkat G., Neuvonen S., Niemelä P., Terblanche J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020;18:141–150. doi: 10.1002/fee.2160. [DOI] [Google Scholar]
- 8.O’hagan J., Khazova M., Price L. Low-energy light bulbs, computers, tablets and the blue light hazard. Eye. 2016;30:230–233. doi: 10.1038/eye.2015.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimoda M., Honda K.-I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013;48:413–421. doi: 10.1007/s13355-013-0219-x. [DOI] [Google Scholar]
- 10.Truxa C., Fiedler K. Attraction to light-from how far do moths (Lepidoptera) return to weak artificial sources of light? Eur. J. Entomol. 2012;109:77–84. doi: 10.14411/eje.2012.010. [DOI] [Google Scholar]
- 11.Tamuri A., Muhamad A., Akmal S., Lani M., Kundwal M., Daud Y. Ultravoilet (UV) Light Spectrum of Flourescent Lamps; Proceedings of the 8th SEATUC Symposium; Johor Bahru, Malaysia. 4–5 March 2014. [Google Scholar]
- 12.Cohnstaedt L., Gillen J.I., Munstermann L.E. Light-emitting diode technology improves insect trapping. J. Am. Mosq. Control Assoc. 2008;24:331. doi: 10.2987/5619.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh A.H., Thomas M., Bhandari R., Meshram H. Malaise trap and insect sampling: Mini Review. Bio Bull. 2016;2:35–40. [Google Scholar]
- 14.Hoel M., de Zeeuw A. Can a focus on breakthrough technologies improve the performance of international environmental agreements? Environ. Resour. Econ. 2010;47:395–406. doi: 10.1007/s10640-010-9384-3. [DOI] [Google Scholar]
- 15.Maverakis E., Miyamura Y., Bowen M.P., Correa G., Ono Y., Goodarzi H. Light, including ultraviolet. J. Autoimmun. 2010;34:J247–J257. doi: 10.1016/j.jaut.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 17.Ponlawat A., Khongtak P., Jaichapor B., Pongsiri A., Evans B.P. Field evaluation of two commercial mosquito traps baited with different attractants and colored lights for malaria vector surveillance in Thailand. Parasites Vectors. 2017;10:378. doi: 10.1186/s13071-017-2315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirikasemsuk K. AIP Conference Proceedings. AIP Publishing LLC; Long Island, NY, USA: 2016. A review on incomplete latin square design of any order; p. 030022. [Google Scholar]
- 19.Rattanarithikul R., Harrison B.A., Panthusiri P., Coleman R.E. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J. Trop. Med. Public Health. 2005;36:1–80. [PubMed] [Google Scholar]
- 20.Rattanarithikul R., Harbach R.E., Harrison B.A., Panthusiri P., Jones J., Coleman R.E. Illustrated key to the mosquito of Thailand II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health. 2005;36:1–97. [PubMed] [Google Scholar]
- 21.Rattanarithikul R., Harrison B.A., Panthusiri P., Peyton E.L., Coleman R.E. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J. Trop. Med. Public Health. 2006;37:1–85. [PubMed] [Google Scholar]
- 22.Rattanarithikul R., Harrison B.A., Harbach R.E., Panthusiri P., Coleman R.E. Illustrated Keys to the mosquitoes of Thailand IV. Anopheles. Southeast Asian J. Trop. Med. Public Health. 2006;37:1–128. [PubMed] [Google Scholar]
- 23.Rattanarithikul R., Harbach R.E., Harrison B.A., Panthusiri P., Coleman R.E. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. Public Health. 2007;38:1–65. [PubMed] [Google Scholar]
- 24.McDermott E.G., Mullens B.A. The Dark Side of Light Traps. J. Med. Entomol. 2017;55:251–261. doi: 10.1093/jme/tjx207. [DOI] [PubMed] [Google Scholar]
- 25.Abong’o B., Gimnig J.E., Longman B., Odongo T., Wekesa C., Webwile A., Oloo B., Nduta M., Muchoki M., Omoke D., et al. Comparison of four outdoor mosquito trapping methods as potential replacements for human landing catches in western Kenya. Parasites Vectors. 2021;14:320. doi: 10.1186/s13071-021-04794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop A.L., Bellis G.A., McKenzie H.J., Spohr L.J., Worrall R.J., Harris A.M., Melville L. Light trapping of biting midges Culicoides spp.(Diptera: Ceratopogonidae) with green light-emitting diodes. Aust. J. Entomol. 2006;45:202–205. doi: 10.1111/j.1440-6055.2006.00538.x. [DOI] [Google Scholar]
- 27.Bentley M.T., Kaufman P.E., Kline D.L., Hogsette J.A. Response of adult mosquitoes to light-emitting diodes placed in resting boxes and in the field. J. Am. Mosq. Control Assoc. 2009;25:285–291. doi: 10.2987/08-5815.1. [DOI] [PubMed] [Google Scholar]
- 28.Wakefield A., Broyles M., Stone E.L., Jones G., Harris S. Experimentally comparing the attractiveness of domestic lights to insects: Do LED s attract fewer insects than conventional light types? Ecol. Evol. 2016;6:8028–8036. doi: 10.1002/ece3.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng L., Zheng Y., Wu W., Fu Y. Field evaluation of different wavelengths light-emitting diodes as attractants for adult Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) Neotrop. Entomol. 2014;43:409–414. doi: 10.1007/s13744-014-0228-7. [DOI] [PubMed] [Google Scholar]
- 30.González M., Alarcón-Elbal P.M., Valle-Mora J., Goldarazena A. Comparison of different light sources for trapping Culicoides biting midges, mosquitoes and other dipterans. Vet. Parasitol. 2016;226:44–49. doi: 10.1016/j.vetpar.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Saeung M., Jhaiaun P., Bangs M.J., Ngoen-Klan R., Chareonviriyaphap T. Transmitted light as attractant with mechanical traps for collecting nocturnal mosquitoes in urban Bangkok, Thailand. J. Am. Mosq. Control Assoc. 2021;37:132–142. doi: 10.2987/20-6984.1. [DOI] [PubMed] [Google Scholar]
- 32.Jhaiaun P., Panthawong A., Saeung M., Sumarnrote A., Kongmee M., Ngoen-Klan R., Chareonviriyaphap T. Comparing Light—Emitting—Diodes light traps for catching anopheles mosquitoes in a forest setting, Western Thailand. Insects. 2021;12:1076. doi: 10.3390/insects12121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lillibridge K.M., Parsons R., Randle Y., Da Rosa A.P.T., Guzman H., Siirin M., Wuithiranyagool T., Hailey C., Higgs S., Bala A.A. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am. J. Trop. Med. Hyg. 2004;70:676–681. doi: 10.4269/ajtmh.2004.70.676. [DOI] [PubMed] [Google Scholar]
- 34.Pipitgool V., Waree P., Sithithaworn P., Limviroj W. Studies on biting density and biting cycle of Culex quinquefasciatus, say in Khon Kaen City, Thailand. Southeast Asian J. Trop. Med. Public Health. 1998;29:333–336. [PubMed] [Google Scholar]
- 35.Uttah E.C., Wokem G.N., Okonofua C. The abundance and biting patterns of Culex quinquefasciatus Say (Culicidae) in the coastal region of Nigeria. Int. Sch. Res. Not. 2013;2013:640691. doi: 10.1155/2013/640691. [DOI] [Google Scholar]
- 36.Bhattacharya S., Basu P., Sajal Bhattacharya C. The southern house mosquito, Culex quinquefasciatus: Profile of a smart vector. J. Entomol. Zool. Stud. 2016;4:73–81. [Google Scholar]
- 37.Chou C.-H., Chen F.-C. Plasmonic nanostructures for light trapping in organic photovoltaic devices. Nanoscale. 2014;6:8444–8458. doi: 10.1039/C4NR02191F. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization . Good Health Adds Life to Years: Global Brief for World Health Day 2012. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 39.Raksakoon C., Potiwat R. Current arboviral threats and their potential vectors in Thailand. Pathogens. 2021;10:80. doi: 10.3390/pathogens10010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Q., Li C., Liu Y., Zhang J., Wang X.-J., Liu F. Manipulating trap filling of persistent phosphors upon illumination by using a blue light-emitting diode. J. Mater. Chem. C. 2020;8:6988–6992. doi: 10.1039/D0TC01427C. [DOI] [Google Scholar]
- 41.Reinhold J.M., Lazzari C.R., Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects. 2018;9:158. doi: 10.3390/insects9040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upham N.S. Los Angeles, California. Occidental College; Los Angeles, CA, USA: 2008. [Google Scholar]
- 43.Nirmal A., Sidar Y.K., Gajbhiye R., Laxmi J. The effects of moonlight phases on light-trap catches of insects. J. Entomol. Zool. Stud. 2017;5:1209–1210. [Google Scholar]
- 44.Yela J.L., Holyoak M. Effects of moonlight and meteorological factors on light and bait trap catches of noctuid moths (Lepidoptera: Noctuidae) Environ. Entomol. 1997;26:1283–1290. doi: 10.1093/ee/26.6.1283. [DOI] [Google Scholar]
- 45.Provost M.W. The influence of moonlight on light-trap catches of mosquitoes. Ann. Entomol. Soc. Am. 1959;52:261–271. doi: 10.1093/aesa/52.3.261. [DOI] [Google Scholar]
- 46.Barr R.A., Smith T.A., Boreham M.M., White K.E. Evaluation of some factors affecting the efficiency of light traps in collecting mosquitoes. J. Econ. Entomol. 1963;56:123–127. doi: 10.1093/jee/56.2.123. [DOI] [Google Scholar]
- 47.Bidlingmayer W. The effect of moonlight on the flight activity of mosquitoes. Ecology. 1964;45:87–94. doi: 10.2307/1937110. [DOI] [Google Scholar]
- 48.Mulhern T.D. A new development in mosquito traps. N. J. Mosq. Extermin. Assoc. Proc. 21. 1934;137:140. [Google Scholar]
- 49.Reinert W.C. Proceedings of the Seventy-Sixth Annual Meeting of the New Jersey Mosquito Control Association. Atlantic County Mosquito Unit; Trenton, NJ, USA: 1989. The New Jersey light trap: An old standard for most mosquito control programs; pp. 17–25. [Google Scholar]
- 50.Kline D.L. Traps and trapping techniques for adult mosquito control. J. Am. Mosq. Control Assoc. 2006;22:490–496. doi: 10.2987/8756-971X(2006)22[490:TATTFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 51.Silver J.B. Mosquito Ecology: Field Sampling Methods. Springer; Berlin/Heidelberg, Germany: 2008. pp. 845–946. [Google Scholar]
- 52.Ritchie S.A., Cortis G., Paton C., Townsend M., Shroyer D., Zborowski P., Hall-Mendelin S., Van Den Hurk A.F. A simple non-powered passive trap for the collection of mosquitoes for arbovirus surveillance. J. Med. Entomol. 2013;50:185–194. doi: 10.1603/ME12112. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Su X., Zhou G., Zhang H., Puthiyakunnon S., Shuai S., Cai S., Gu J., Zhou X., Yan G. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasites Vectors. 2016;9:446. doi: 10.1186/s13071-016-1724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Service M. A battery-operated light-trap for sampling mosquito populations. Bull. World Health Organ. 1970;43:635. [PMC free article] [PubMed] [Google Scholar]
- 55.Wilton D., Fay R. Responses of adult Anopheles stephensi to light of various wavelengths. J. Med. Entomol. 1972;9:301–304. doi: 10.1093/jmedent/9.4.301. [DOI] [PubMed] [Google Scholar]
- 56.Lee H.I., Seo B.Y., Shin E.-H., Burkett D.A., Lee J.-K., Shin Y.H. Efficiency evaluation of Nozawa-style black light trap for control of anopheline mosquitoes. Korean J. Parasitol. 2009;47:159. doi: 10.3347/kjp.2009.47.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C.-X., Smith M.L., Fulcher A., Kaufman P.E., Zhao T.-Y., Xue R.-D. Field evaluation of three new mosquito light traps against two standard light traps to collect mosquitoes (Diptera: Culicidae) and non-target insects in northeast Florida. Fla. Entomol. 2015;98:114–117. doi: 10.1653/024.098.0118. [DOI] [Google Scholar]
- 58.Miller B., Crabtree M., Savage H. Phylogeny of fourteen Culex mosquito species, including the Culex pipiens complex, inferred from the internal transcribed spacers of ribosomal DNA. Insect Mol. Biol. 1996;5:93–107. doi: 10.1111/j.1365-2583.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen X., Wang C., Baker E., Sun C. Numerical and experimental investigation of light trapping effect of nanostructured diatom frustules. Sci. Rep. 2015;5:11977. doi: 10.1038/srep11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hori M., Shibuya K., Sato M., Saito Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 2014;4:7383. doi: 10.1038/srep07383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H.-C., Kim M.-S., Choi K.-S., Hwang D.-U., Johnson J.L., Klein T.A. Comparison of adult mosquito black-light and light-emitting diode traps at three cowsheds located in malaria-endemic areas of the Republic of Korea. J. Med. Entomol. 2017;54:221–228. doi: 10.1093/jme/tjw136. [DOI] [PubMed] [Google Scholar]
- 62.Mwanga E.P., Ngowo H.S., Mapua S.A., Mmbando A.S., Kaindoa E.W., Kifungo K., Okumu F.O. Evaluation of an ultraviolet LED trap for catching Anopheles and Culex mosquitoes in south-eastern Tanzania. Parasites Vectors. 2019;12:418. doi: 10.1186/s13071-019-3673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peach D.A.H., Ko E., Blake A.J., Gries G. Ultraviolet inflorescence cues enhance attractiveness of inflorescence odour to Culex pipiens mosquitoes. PLoS ONE. 2019;14:e0217484. doi: 10.1371/journal.pone.0217484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the article.