Abstract
Multiple Sclerosis (MS) is a debilitating degenerative disease characterized by an immunological attack on the myelin sheath leading to demyelination and axon degeneration. Mesenchymal stem/stromal cells (MSCs) and secreted extracellular vesicles (EVs) have become attractive targets as therapies to treat neurodegenerative diseases such as MS due to their potent immunomodulatory and regenerative properties. The placenta is a unique source of MSCs (PMSCs), demonstrates ‘fetomaternal’ tolerance during pregnancy, and serves as a novel source of MSCs for the treatment of neurodegenerative diseases. PMSCs and PMSC-EVs have been shown to promote remyelination in animal models of MS, however the molecular mechanisms by which modulation of autoimmunity and promotion of myelination occurs has not been well elucidated. The current review will address the molecular mechanisms by which PMSC-EVs can promote remyelination in MS.
Keywords: multiple sclerosis, mesenchymal stromal cell, placenta, extracellular vesicle, myelin regeneration, autoimmunity
1. Introduction
Multiple sclerosis (MS) is a central nervous system (CNS) disorder that gives rise to chronic neurological deficits. MS is caused by immunological attack on the myelin sheath leading to demyelination, axonopathy and neurodegeneration. MS is an extremely heterogenous disease and typically manifests as a progressive loss of motor function, with most cases involving relapsing flare-ups throughout disease progression. An estimated 2.5 million individuals are affected by MS, with the disease more commonly presenting in young adults and females.[1] The etiology of MS is not fully understood, but several factors may contribute to disease onset including genetics, vitamin D, and Epstein-Barr virus infection.[2,3] Current treatments for MS typically involve long-term use of disease-modifying drugs (DMDs), with most aiming to suppress or modulate the inflammatory component of the disease.[4] Long-term administration of anti-inflammatory medications can have detrimental effects to patients including risk of infection and malignancy from suppression of anti-microbial and anti-tumor immunity.[5] While these therapies can reduce the incidence of flare-ups, they do not prevent progressive axonal and neurological degeneration associated with MS pathology. Therefore cell-based therapies using mesenchymal stem/stromal cells (MSCs) have recently been under investigation in clinical trials for the treatment of neurogenerative diseases including MS.[6,7]
Therapeutically administered MSCs can migrate to sites of injury and interact with the inflammatory niche through cell-cell contact and/or paracrine signaling mechanisms.[8] Early gestational MSCs, including placenta-derived MSCs (PMSCs), preserve features from primitive embryonic layers and have been characterized by immunophenotyping and multipotency assays.[9] This unique cell subset has the potential to differentiate into various tissue types, has greater proliferative and immunomodulatory properties, and causes less immunogenicity than adult derived MSCs.[9] However, cell-based therapies are limited by several safety concerns including, teratoma formation and the possibility of eliciting immune responses or rejection of donor cells.[10] Increasingly, studies have shown that MSC survival and integration within the host after transplantation are usually poor, and that MSCs exert their therapeutic functions mainly via paracrine signaling mechanisms.[11] MSCs can release extracellular vesicles including exosomes, which are small nanovesicles that can carry protein, mRNA and microRNA and have been shown to play a key role in CNS inflammation.[12] Additionally, MSC-derived extracellular vesicles (EVs) are stable under long term storage conditions compared to freely secreted proteins and may serve as a superior source for cell-free therapy.[13]
MSC-derived EVs readily cross the BBB and deliver therapeutic cargo to reduce the effects of neuropathologic diseases such as MS.[14] It has been suggested that MSCs secrete multiple categories of EVs, which are involved in differing cellular processes.[15] A recent report demonstrated that MSC-derived EVs exhibit systemic immunomodulatory effects and can facilitate neurological recovery in vitro.[16] PMSC-derived EVs (PMSC-EVs) contain numerous proangiogenic, immunomodulatory and neuroprotective proteins.[17,18] In an experimental autoimmune encephalomyelitis (EAE) model of MS, it was demonstrated that PMSCs and PMSC-EVs could mitigate motor deficits in treated animals, likely by promoting oligodendrocyte precursor cells (OPC) to a mature myelinating phenotype.[19] While this pre-liminary data is promising, the molecular mechanisms by which PMSC and PMSC-EVs elicit these effects on central nervous system damage is not fully characterized. The current review addresses the molecular mechanisms by which PMSC and PMSC-EVs can promote remyelination in MS.
2. Multiple Sclerosis Pathology
The primary characteristic of MS pathology is autoreactive lymphocytes crossing the BBB into the CNS. Activation of peripheral myelin-specific T cells home to the CNS, where they can become reactivated by antigen presenting cells (APCs). Within the CNS, resident immune cells will become activated and secrete pro-inflammatory mediators that lead to the degeneration of myelinated axons. It has been suggested that human leukocyte antigen (HLA)-DR15 modulates homing of autoreactive CD4+ T cells, proliferation and infiltration of pro-inflammatory T helper cell (Th)-1 subsets into the CNS.[20] Integrin α4β1, also known as very late activation antigen (VLA)-4, has been implicated in mediating adhesion and migration of immune cells into the CNS through the interaction with integrin specific ligand vascular cell adhesion molecule (VCAM)-1 which in turn has been shown to be critical in mediating Th-1 cell migration in MS.[21][22] Activated peripheral T cells will enter circulating cerebral spinal fluid (CSF) through the subarachnoid space, which is mediated by constitutively expressed selectins and adhesion molecules.[22]
Evaluation of CSF obtained from MS patients showed presence of stimulated CD4+ Th-1 cells that secrete cytokines including interferon gamma (IFNγ), interleukin (IL)-2 and tumor necrosis factor alpha (TNFα).[23] These pro-inflammatory cytokines create a feedback loop signaling pathway which will in turn activate microglia and further drive a pro-inflammatory M1-like phenotype leading to upregulation of major histocompatibility complex (MHC)-II, driving reactivation of Th-1 cells.[24]
Th-17 cells can also mediate autoimmunity through expression of chemokine receptor (CCR)-6 which binds to chemokine ligand (CCL)-20 on vascular endothelium, allowing migration into the CNS.[25] Th-17 cells secrete IL-17A, which has been shown to inhibit proteins associated with tight junctions of the BBB, leading to increased permeability and entry of inflammatory cells and mediators into the CNS.[26] Th-1 and Th-17 cell responses will result in production of the inflammatory mediators IL-17 and IFNγ which can directly contribute to disease pathogenesis.[27] In the EAE model of MS, Th-17 cells producing IFNγ were shown to be enriched in myelin oligodendrocyte glycoprotein (MOG)-specific T cells, and can drive inflammatory responses in the CNS independent of Th-1 responses and have been shown to be effective in regulating astrocytic responses.[28] Astrocytes exist between the BBB and neurons, regulate interactions of the periphery with the CNS, and are responsible for neurogenesis and tissue repair. Astrocytes express an IL-17A receptor and can upregulate inflammatory cytokines and chemokines.[29] Therefore, reducing IL-17 signaling by astrocytes has been shown to ameliorate symptoms in the EAE model of MS.[29,30] Th-17 cells producing IL-17 have been shown to inhibit maturation and survival of oligodendrocyte lineage cells (OLs)[31] and cause apoptosis of myelinating cells.[32] Neurodegeneration and apoptosis of OLs is caused through direct cytotoxicity from antigen-specific T cells, autoantibodies, and T cell -mediated pro-inflammatory cytokines that activate resident microglia populations.[33] Overall, products of Th-17 cells, such as IFNγ and IL-17, can exacerbate inflammatory responses in astrocytes, oligodendrocytes and microglia. Regulating these inflammatory products that produce detrimental effects in CNS cells provides direction for future therapies.
T regulatory cells (Tregs) are another subset of T cells involved with MS pathology that can regulate Th-1, Th-17 and Th-2 responses. Tregs are involved in the maintenance of peripheral immunotolerance and modulate CD4+CD25+ T cell subsets and can suppress effector T cell responses to maintain self-tolerance. It has been reported that Treg populations are reduced in MS patients but are not functionally impaired.[34] It has also been demonstrated that severity of EAE can be mitigated by transfer of Treg cells.[35] Tregs can inhibit proliferation and function of inflammatory T cell subsets and can decrease migration into target organs. Tregs have been shown to facilitate remyelination through the secretion of cellular communication network factor 3 (CCN3), which promotes oligodendrocyte differentiation and myelination in mice.[36] Tregs are typically classified as CD8+/CD25+/FOXP3+ cells however, CD8+ Tregs have been presented as a novel subset of cells that can regulate self-reactive CD4+ T cells, and disruption in these processes may lead to autoimmune response induction.[37] [38,39]Effective Treg functions have been suggested to promote remyelination through direct mechanisms, but reduced Treg numbers in MS patients suggests the role of Tregs in the demyelinating pathology of MS.[40]
CD8+ effector T cells are typically referred to as cytotoxic T cells and target cells that present MHC I on their surface. Most resident CNS cells present MHC I in inflammatory conditions and thus can be targeted by CD8+ T cells. MHC I has been shown to be upregulated in macrophages in actively demyelinating lesions in early onset of MS, which suggests a direct role for activated macrophages and microglia in demyelination.[41] Additionally, the perivascular space around actively demyelinating MS lesions was found to contain CD8+ cells expressing IL-17.[42] [43]It is unclear whether CD8+ T cells play a pathogenic or regulatory role in MS because it has been suggested that CD8+ T cells can be both pro and anti-inflammatory immune mediators.[44] CD8+ T cells can suppress activity of myelin-specific CD4+ T cells, and MS relapses have been shown to correlate with reduced CD8+ T cell numbers.[45,46] However, it has also been shown that increases in CD8+ T cells can correlate with axon damage,[47] and polarize near demyelinated axons.[48] Functional Tregs have been suggested to promote remyelination through direct mechanisms, but reduced Tregs numbers in MS patients suggests the role of Tregs in disease pathology.[40] While the exact role of T cells is not fully understood, it appears that disruption of T cell subset expression and homeostasis leading to inflammatory responses is directly involved in the demyelinating component of MS pathology.[42] [43–48]
In MS, interactions between T lymphocytes and B lymphocytes are also likely disrupted. One study demonstrated that in 90% of patients, oligoclonal immunoglobulin (Ig) existed in CSF, suggesting a pathologic role of B cells in MS.[49] B cells can produce antibody responses that can target antigens including MOG and myelin basic protein (MBP), both of which are expressed in mature myelinating oligodendroglia cells.[50] Peripheral B cells may become autoreactive due to impaired or chronically exhausted Tregs, thus allowing B cells to interact with Th effector cells in lymphoid organs.[51] These interactions can lead to pathogenic cell infiltration through the BBB and allow activated CD8+ T cells to become reactivated in the CNS.[51]
Local inflammatory responses in the CNS will lead to disruptions in homeostasis and cause resident immune cell subsets to become activated. Microglia are APCs that can promote myelin regeneration by expressing anti-inflammatory mediators, removing debris, and facilitating tissue repair. Chronic T cell and microglia activation can lead to accompanying demyelination and neurodegeneration associated with MS.[52] Chronic inflammation in the CNS can result in several pathogenic molecular processes including oxidative stress, mitochondrial injury and ion channel dysfunction.[53] T cells and microglia have been shown to co-localize in demyelinating sites in MS patients,[54] thus suggesting interactions between these two cell types could occur through cell-cell contact or through the secretion of bioactive factors. Microglia express MHC molecules and can secrete several pro-inflammatory and anti-inflammatory mediators including TNFα, IL-10, and co-stimulatory molecules ICAM-1, B7–1 and B7–2.[55] Activation of microglia to an M1 inflammatory phenotype can support T lymphocyte homing and reactivation in the CNS. It was demonstrated that TNF secreted by microglia was induced by T cells, in part through interactions between VCAM-1 on microglia and α4β1 on T cells.[56] The interactions of encephalitogenic T cells with microglia can lead to further reactivation and inflammation, thus leading to cellular toxicity to neuronal cell populations leading to demyelination and degeneration. Clearly, interactions between activated microglia and T cells drive the immune component of MS pathology and require further mechanistic studies for understanding their role in molecular processes.
Micro-RNAs (miRNAs) have been proposed as key modulators of OPC maturation and differentiation. Mice lacking miRNA-processing enzyme Dicer demonstrated significant deficits in myelination.[57] Moreover, blocking studies demonstrated a critical role of miR-219 and miR-338 for oligodendrocyte differentiation and maturation.[58] In MS, deficits in OPC maturation have been observed.[59] OPCs express receptors for the pro-inflammatory mediators IFNγ,[60] IL-17[61] and TNFα,[62] all of which have been shown to be associated with MS pathology. Furthermore, IFNγ has been shown to induce a pro-inflammatory M1 microglia phenotype, and act on oligodendrocytes to cause endoplasmic reticulum stress, demyelination and degeneration.[60] It has been reported that OPC maturation can also be inhibited by effector T cell functions and overexpression of IFNγ.[63] In addition, IFNγ primed OPCs presented to CD8+ T cells resulted in OPC death, suggesting that inhibiting inflammatory process can directly result in remyelination.[63]
Understanding the interaction between inflammatory responses and accompanying demyelination and neurodegeneration associated with MS pathology is critical in developing intervention strategies that can promote remyelination. The process of remyelination has been described as beginning with proliferation of OPCs, then OPC migration to demyelinating axons, followed by OPC maturation and then myelination of premature oligodendrocytes with axons.[64] Cellular therapeutics that can protect and promote myelinating oligodendrocytes, axons and neurons, in addition to targeting the inflammatory component of MS, will provide a novel approach to treat the complex and heterogenous nature of MS.
3. Placenta-derived mesenchymal stem/stromal cells novels are a novel source of MSCs for the treatment of MS
Cell-based therapies using MSCs have been recently investigated in clinical trials for neurogenerative diseases including MS.[7,65] MSCs display potent immunomodulatory and regenerative capabilities through the secretion of bioactive factors, such as proteins, cytokines, chemokines as well as the release of EVs. It has been reported that MSCs reduce B cell proliferation and maturation,[66] regulate natural killer (NK) cell activities[67] and direct macrophage polarization.[68] Moreover, MSCs have the potential to suppress activated CD4+ and CD8+ T lymphocyte proliferation and promote the induction of Tregs, all of which are directly involved with MS pathology.[69–71] This unique T cell subset can reduce inflammatory immune response and plays an important role in peripheral immunity.[69] These functional properties of MSCs make them ideal candidates for treating degenerative and inflammatory diseases, including MS.[69–71]
The placenta is a unique source of MSCs that possesses robust immunomodulatory properties and has been reported to be beneficial in graft verse host diseases mouse models.[8,65] PMSCs have been suggested to have advantages in terms of proliferation and plasticity as compared to adult derived tissue sources.[72] It has been reported that PMSCs have the capability to differentiate towards neural lineages including oligodendrocytes and neurons.[73] Compared to adult sources of MSCs, PMSCs have been shown to have superior doubling times, easily expanded,[74] and are more homogenous.[75] Additionally, homing of PMSCs to sites of injury may be superior due to higher expression of VLA-4 which aids endothelium adherence.[76] PMSCs exert their therapeutic functions through cell-cell contact coupled with paracrine signaling factors. In addition, PMSCs have been shown to express programmed death ligand (PDL)-1 which can directly interact with PD-1 inhibitory molecule on T cells, inducing apoptosis and modulating Th-1, Th-17 and Th-2 responses.[77] Overall, PMSCs are an attractive therapeutic approach in comparison to other adult sources of MSCs.
PMSCs may be a more appropriate cell source for pediatric diseases given during pregnancy the placenta demonstrates “fetomaternal tolerance”, which is attributed to the expression of human leukocyte antigen-G (HLA-G), a non-classical MHC class I molecule that inhibits natural killer cell (NK) function.[78] Unlike bone marrow derived MSCs (BM-MSCs), PMSCs express HLA-G on their surface in response to IFNγ,[79] which is a key inflammatory mediator involved with the onset of MS.[80] Currently, a clinical trial is underway using term PMSCs for adult MS and no paradoxical worsening of MS lesion counts has been noted.[81]
Interestingly, pregnancy in MS patients has been known to effectively attenuate disease activity.[82] This suggests a possible unique role the placenta may play in modulating MS symptoms. A study by Vukusic et al. assessed the role of pregnancy on immunosuppression in MS patients and compared CD4+ and CD8+ T cell subsets in peripheral blood and the decidua. This study demonstrated elevated CD4+ T cells in peripheral blood of non-pregnant MS patients compared to non-pregnant healthy controls. CD4+ T cell populations increased in pregnant healthy controls in the second trimester of pregnancy, but in MS patients there was no increase or changes noted, thusCD4+ T cell levels were then comparable to controls. No changes in CD8+ T cell populations were noted in the peripheral blood. Tregs have been shown to be reduced and/or impaired in MS patients, and this study also showed decreased Tregs in peripheral blood of non-pregnant MS patients compared to non-pregnant controls. Once pregnant, Treg levels declined in healthy women, but in MS patients Treg levels were not altered through gestation. This data suggests that pregnancy can alter the function of Tregs and allow for more immunological tolerance. Furthermore, there is physiological immune regulation that occurs at the fetal-maternal interface that can act regardless of the pathological features of MS.[83] Another group demonstrated that pregnancy allows for the expansion of Treg clonotypes that can recognize EAE-associated antigen and can regulate autoreactive T cells.[84] These findings suggest that the placenta may have key features that innately modulate immune responses in MS, and suggest that the placenta is an advantageous source of MSCs for the treatment of MS.
Not only is the placenta a unique source of MSCs, but it has also been shown that placenta derived EVs are involved in biological processes during pregnancy. Placenta-EVs can interact with several cell types and can inhibit NK cell cytotoxicity,[85] inhibit T cell proliferation,[86] and drive monocyte and macrophage polarization[87]. Placenta-EVs can be detected in maternal circulation at 6 weeks of pregnancy.[88] Placenta-EVs have been shown to mediate immunosuppression via transfer of exosomal proteins to T cells, which lead to T cell apoptosis, inhibition of T cell proliferation, induction of Treg population and reduction of T cell cytotoxicity.[89] This data further demonstrates a unique role the placenta can play in mediating immune responses, and further shows a role for EVs in modulating inflammatory responses and demyelination associated with MS.
Cell-based therapies can be limited by potential immune rejection of donor cells and lack of cellular homing to sites of injury.[10] Increasingly, studies have shown that MSC survival and integration within the host after transplantation are usually poor and that MSCs exert their therapeutic functions mainly via paracrine signaling mechanisms.[11] Recently, conditioned media of BM-MSCs was shown to protect neurons from apoptosis, activate macrophages and be pro-angiogenic.[90] However, the use of MSC conditioned media is limited in that the secreted protein factors are unstable, which creates technical difficulties for “off-the-shelf” clinical use. MSC derived EVs alternatively, are stable under long term storage conditions compared to freely secreted proteins and may serve as a superior source for cell-free therapy.[13]
4. Potential mechanism of action by PMSC-EVs for myelin regeneration
Growing evidence suggests that MSCs exert their therapeutic functions through the secretion of EVs. EVs are small nanovesicles, which can play an important role in intercellular communication by transporting various functional molecules, including proteins, lipids, microRNAs, and mRNAs, all of which can regulate the behaviors of cellular targets.[91] MSC-derived EVs readily cross the BBB and deliver therapeutic cargo to reduce the effects of neuropathologic diseases, such as MS.[14] Several reports have demonstrated that MSC-derived EVs from adult tissue sources exhibit systemic immunomodulatory effects and can facilitate neurological recovery in vitro.[16,92] The functional properties of EVs are largely dependent on the parent cells from which they are produced. EVs can be derived from various tissues and cell types including but not limited to urine, plasma, saliva, tissues, cerebrospinal fluid and synovial fluid under physiological or pathological conditions.[93] EVs interact with target cells through internalization by endocytosis, direct membrane fusion or receptor-based induction of intracellular signaling pathways.[94] Target cell regulation can occur through the presence of certain surface proteins on EV membranes, or EV cargo such as miRNA which can modulate transcriptional activity of genes associated with biologic activity. Soluble factors packaged in EVs can play an important role in cell-cell interactions that lead to immune modulation and neuronal regeneration.[95] Moreover, EVs can also regulate mitochondrial transfer. Morrison et al. demonstrated that MSC-EVs promote an inflammatory phenotype in a lung injury model by mediating mitochondrial transfer to macrophages and promoting oxidative phosphorylation.[96] EVs are not only versatile in their composition but also immensely diverse in their mechanism of action.
The role of miRNA content in MSC-EVs has been suggested to be key modulators of myelin regeneration. miRNAs are small non-coding RNAs that can silence gene expression by inhibiting post-transcriptional activity or by inducing mRNA degradation.[97] Therefore, miRNAs can regulate cellular proliferation, differentiation and apoptosis in target cells, and have been shown to modulate inflammatory immune responses.[98] Dysregulation of miRNAs can lead to disrupted immune responses resulting in disease pathology. MSC-EV miRNAs taken up into target cells can regulate gene activity through gene silencing leading to downregulation of protein secretion by immune cell subsets. The miRNA content in MSC-EVs can also promote remyelination by modulating inflammatory responses or by directly promoting oligodendrocyte differentiation and maturation. Specifically, human BM-MSC-EVs stimulated by IFNγ have been shown to improve motor function scores in EAE animals, reduce demyelination and neuroinflammation, as well as upregulate Treg populations in the spinal cord of EAE mice. Priming MSCs with IFNγ led to the identification of miR-467f and miR-466q as modulators of inflammatory responses in microglia cultures as they downregulate TNF and IL-1β expression.[98] These were among several other differentially expressed miRNAs in MSC-derived EVs, indicating the role of MSC-EVs in affecting neuroinflammation in EAE mice. MSC-EVs enriched in the miR-17–92 cluster exhibited enhanced oligodendrogenesis, neurogenesis and neuron remodeling, indicating the role of native miR-17–92 in promoting these myelination-related processes.[99] MSC-EVs were also shown to transfer miRNA-133b, which lead to increased branch length and number to primary cultured astrocytes and neurons.[100] Additionally, miRNA-133b from human umbilical cord MSCs can augment trophoblast cell proliferation and migration, thus suggesting a critical role in trophoblast development.[101] Interestingly, the miRNA profile from MSCs derived from differing tissue sources including umbilical cord,[102] bone marrow and adipose tissues is altered.[103] These findings suggest that the source of MSC-EVs and miRNA transfer will have unique interactions on target cells and potentially different clinical impacts.[104] These MSC-EVs were found to reduce inflammatory responses by changing gene expression in activated macrophages. PMSCs-EVs also contain miRNA-138,[17] which has been suggested to play a key role in maturation of OPCs to OLs.[105] However, many of these findings were made in models of stroke and diabetes, and the role of miRNA content in MSC-EVs has not yet been explored in the context of MS. Altogether, these findings attribute the immunomodulatory and neurotrophic effects of MSC-EVs at least in part to their native miRNA content. Identification and subsequent enrichment of these miRNA represent a viable research direction for treating MS through the reduction of demyelination and promotion of remyelination.
PMSCs also contain numerous proangiogenic, immunomodulatory and neuroprotective proteins, that can play a critical role in promoting myelination in CNS disorders. Hepatocyte growth factor (HGF) is a pleiotropic factor shown to have neuronal and oligodendrocyte protective properties.[17,18,106] In the EAE rodent model of MS, overexpression of HGF by neurons conferred neuroprotection by reducing inflammation in the CNS and activation of Tregs.[106] HGF is secreted both in soluble form from MSCs and is also contained in exosomes; however, the effects of each form could lead to different cellular responses. It has been suggested that MSCs secrete multiple categories of exosomes, which are involved in differing cellular processes.[15] BM-MSC-EVs have been shown to modulate inflammatory responses, by increasing IL-10, TGFβ, PGE2 and IL-6 secretions and driving a Treg phenotype and thus switching to a Th-2 responses in a murine model of diabetes.[107] Engineered Tregs in the EAE model of MS effectively localized to brain tissue and were able to reduce IL-12 and IFNγ mRNAs in the brain and reduce inflammatory markers resulting in diminished symptoms in treated animals.[108] Furthermore, MSC-EVs have been shown to inhibit immunoglobulin production of B cells,[109] and co-cultures with various immune cell subsets demonstrated that B lymphocytes preferentially take up MSC-EVs.[110]
Galectin-1 (gal-1) is another soluble protein expressed in PMSC-EVs that may play a critical role in modulating inflammation and neurodegeneration.[17] Recent reports have suggested a critical role for gal-1 in chronic inflammatory diseases.[111] Gal-1 is a multifunctional ~14 kDa monomeric protein, which can dimerize and is involved in many cellular functions, such as cell growth and migration regulation, adhesion, angiogenesis and embryonic and adult tissue development.[112] Interestingly, this protein also plays many key functions in the immune system,[113,114] and has been shown to facilitate anti-inflammatory processes and modulate the adaptive immune system.[113] Gal-1 has the potential to activate T lymphocyte apoptosis and induce the differentiation Tregs.[113,114] Moreover, studies have also reported that gal-1 can promote phenotypic and functional changes after binding to microglia in vitro.[115] The mammalian protein can downregulate M1 microglia activation via the regulation of p38-, CREB- and NF-κB-dependent pathways and induce differentiation toward an M2 phenotype.[114] Macrophages are also involved in MS pathology as they contribute to tissue damage via the production of pro-inflammatory cytokines (TNF-α, IL-6, and IL-23).[116] Although no studies have assessed the effect of gal-1 on macrophage polarization, many publications have shown that those leukocytes respond to the same signals as microglia since they also undergo polarization toward an M2 or M1 phenotype in the presence of IL-4/13 or IFNγ/ lipopolysaccharide (LPS) respectively.[115] Overall, gal-1 expression on MSCs is a mediator of inflammation as it binds to microglia and downregulates M1 microglia activation. Further studies are needed, namely as it pertains to secreted MSC-EVs that share the same surface protein expression profile as their parent cells.
In the EAE model of MS, it was shown that murine BM-MSC-EVs express gal-1, PD-L1, and TGFβ which lead to reduced lymphocyte proliferation and induced secretion of IL-10 and TGFβ.99 Using the BV-2 microglia cell line, it has been shown that BM-MSC-EVs modulate microglia activation through the MAPK signaling pathway, which will lead to reduction in pro-inflammatory gene expression, thus modulating CNS inflammation.[109,117] It was also demonstrated that MSC-EVs can polarize microglia to an M2 phenotype and upregulate modulatory proteins including IL-10 and TGFβ in the EAE model, which lead to resolution of clinical scores.[118] Additionally, priming of MSCs can result in altered cargo loading or expression to EVs, and is a valuable tool to evaluate the translational applications of PMSC-EVs in a diseased environment. Priming MSCs with IFNγ led to the identification of miR-467f and miR-466q as modulators of pro-inflammatory responses in microglia cultures by downregulating TNF and Il-1β expression.[98] Lipopolysaccharide stimulated umbilical cord MSC-EVs immunomodulatory properties have been shown to be mediated by miRNA let-7b.[119] Human BM-MSC-EVs stimulated by IFNγ have been shown to improve motor function scores in EAE animals, reduce demyelination and neuroinflammation, upregulated Treg cells in the spinal cord of EAE mice.[120] Additionally, this group showed that BM-MSC-EVs reduced activated peripheral blood monocular cell proliferation and reduced Th-1 and Th-17 cytokines in vitro.[120] These studies are critical for mimicking the disease environment and can provide insights into alterations of molecular signaling mechanisms by MSC-EVs when used for disease intervention.
MSC-EV mediated anti-inflammatory properties are largely mediated by immunoregulatory miRNAs and immunomodulatory protein delivery in inflammatory immune cells (M1 macrophages, dendritic cells (DCs), CD4+Th-1 and Th-17 cells), enabling their phenotypic conversion into immunosuppressive M2 macrophages, tolerogenic DCs, and Tregs, respectively.[5] The placenta represents a novel source of MSCs that have unique properties for clinical applications to MS. PMSC-EVs have been shown to be neuroprotective, immunomodulatory and pro-angiogenic.[17,18,121] It was recently demonstrated that PMSCs and PMSCs-EVs could lead to symptom improvements in the EAE model of MS, which was due to the promotion of remyelination by the maturation of OPCs to mature OLs.[19] The promotion of remyelination by PMSC-EVs is due to interactions with multiple cellular targets that can modulate inflammatory responses preventing further reactivation of immune cell subsets and damage to myelin, as well as interactions with myelinating cells promote differentiation into mature phenotypes. While the multifunctional properties of PMSC-EVs make them a promising therapeutic agent to promote remyelination in MS, limitations exist on the use of native EV sources. Namely, in vivo EV biodistribution studies revealed that after systemic administration, a significant amount of EVs accumulated in the spleen and liver, while very few were detected in the CNS.[122] Therefore, while PMSC-EVs are a promising new approach to address the inflammatory and neurodegenerative component of MS, additional, new strategies can be employed for superior targeting and delivery of EV cargo to pathological target cells to promote remyelination.
5. Bioengineering Approaches for EVs to promote remyelination for MS
Recent work has been done to apply engineering principles to the design of EVs for increased therapeutic potential. These techniques can be categorized broadly into three approaches: parent cell engineering, miRNA loading, and surface targeting modifications. The role for these strategies and current practices of engineering MSC-EVs as they apply to MS and CNS disorders, as well as highlighted potential avenues for future studies are discussed below.
5.1. Parent cell engineering approaches
A variety of methods have been applied to alter the state of parent cells to induce specific EV features through canonical EV biogenesis pathways. One such method involves gene editing of parent cells by transfection. A range of cell types (BV2 microglia, HEK293T) have been infected with lentiviral plasmids to overexpress either surface proteins or miRNAs internally, which are similarly expressed on EV surfaces as well internally.[123] This method of cell engineering takes advantage of natural EV biogenesis pathways to generate engineered EVs for various applications. For example, miR-219a-5p has been overexpressed in HEK293T cells to obtain EVs that induced OPC differentiation for remyelination applications.[123]
Another method to engineer EVs through modifications to their parent cells is preconditioning. Parent cells are subjected to modified culture conditions to promote or suppress the expression of certain proteins in the subsequently produced EVs. This was done in the context of ischemic stroke in which BV2 microglia were polarized using IL-4 to differentially express angiogenin and therefore promote angiogenesis in C57BL/J mice.[124] Currently, no studies have been performed to precondition cells to obtain EVs for treating other CNS disorders including MS and Alzheimer’s. Microglia have been preconditioned with stimulants such as LPS for the study and treatment of CNS disorders through their secreted EVs, which are implicated to regulate many of their neuroinflammatory functions.[125] Hypoxia preconditioning has been used MSCs to stimulate secretion of EVs promoting bone fracture healing, indicating the feasibility of such an approach.[126] Additionally, hypoxic preconditioning of MSCs has shown to improve MSC-EV immunomodulatory properties, which can promote an anti-inflammatory phenotype and in turn preserve or promote myelination.[123] This represents a potential direction for future work, where preconditioned cells can secrete EVs with increased myelinating or neuromodulatory capabilities.
5.2. EV surface targeting modification approaches
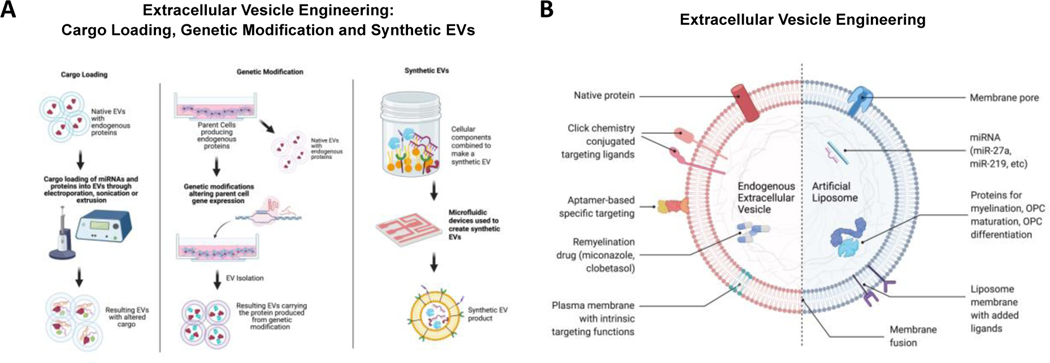
EV surface engineering has also been heavily studied to develop targeted drug delivery vehicles for the treatment of several diseases. For example, many groups have successfully used Click chemistry to conjugate functional ligands of interest onto EVs. Click chemistry refers to a class of covalent reactions used in bioconjugation that is both procedurally simple and high yield. It has previously been shown that conjugation of the integrin ligand LLP2A to an electrospun scaffold for the recruitment of PMSC-EVs improves vascularization.[127] In the context of CNS disorders, Click chemistry has been used to conjugate peptides onto MSC-derived EVs to treat cerebral ischemic stroke.[128] This type of surface modification generally allows for improved targeting and increased uptake by cells of interest. It has already been established that the autoimmune component of MS pathology involves several cell types including T cells, B cells, astrocytes, oligodendrocytes and microglia. Pathogenetic cell types all represent viable targets for engineering of targeting ligands to EV surfaces.
Glycosylation of EV surface represents another active area of study, especially in the context of CNS disorders. Glycoproteins naturally present on cell and EV membranes and facilitate a variety of functions, such as cell-cell interactions and the immune response. By altering the composition of glycoproteins on the cell or EV surface, biodistribution and targeting behaviors can be changed. For instance, modification of surface glycosylation through neuraminidase treatment resulted in increased accumulation of EVs in the brain.[129] This allows EVs to more efficiently perform their naturally functions maintaining homeostasis, or to promote remyelination through their engineered material.[129,130] While parent cell engineering represents a top-down approach for generating EVs, taking advantage of natural EV biogenesis pathways to promote remyelination, synthetic EV production involves a bottom-up method of creating EVs. Essentially, components are combined to create a well-defined synthetic EV, allowing for control over membrane protein expression. Microfluidic platforms have previously been used for characterization and isolation of EVs, but now have been shown to be viable platforms for the generation of synthetic EVs. PDMS microfluidic chips specifically have successfully created synthetic surface engineered EVs expressing tumor peptides as candidates for cancer immunotherapy.[131] These EVs lack the compositional complexity of native EVs, but can be decorated with specific targeting moieties and cargo to induce OPC differentiation or promote remyelination.
An alternate method for synthetically engineering EV surfaces is through fusion of distinct membranes. Combining two separate membranes provides the benefit of increased cellular uptake due to the unique expression of surface proteins on the fused membrane. Membrane fusion has been done via extrusion to create synthetic EVs with a hybrid lipid membrane structure. For example, a library of lipids was fused with EVs using a membrane extrusion technique to generate synthetic EVs with significantly increased cellular uptake to lung cancer cells with subsequent gene silencing.[132] This represents a novel approach in which multiple combinations of lipid and cellular membranes can be performed to produce EVs with increased targeting potential. This platform technology has the potential to promote downstream myelination processes for MS through improved EV biodistribution. A summary of these approaches is shown in Figure 1.
Figure 1.
(A) Summary of bioengineering strategies to modify PMSC-EVs. One popular method of EV engineering is loading EVs with miRNA or protein cargo using techniques like electroporation, sonication or extrusion. Another approach is to genetically modify the parent cells to release customized EVs. Creating synthetic EVs using microfluidic devices is a more recent technique for engineering and customizing EVs. (B) EV modification approaches to promote remyelination. EV surfaces can be functionalized with biomolecules through membrane fusion or chemical modifications for targeting applications. Exogenous cargo, including remyelination drugs, miRNA and proteins can be loaded for remyelination applications.
5.3. Cargo loading into EVs
In addition to and often alongside surface modification of EVs, loading of exogenous cargo represents an increasingly studied method for engineering EVs to improve therapeutic outcomes. Of the several types of cargo used for loading EVs, miRNAs stand out as a commonly researched approach with the potential to promote various molecular processes to modulate inflammation and remyelination.
Loading miRNA in EVs represents a promising approach for the treatment of neurodegenerative diseases such as MS. As previously mentioned, neuroinflammation is a prominent component of MS pathology, and is mediated in part by microglia. Therefore, regulating the immune response via microglia modulation can potentially restore the immune cell homeostasis in the CNS. In one study, dendritic cell EVs were loaded with miR-124 and were able to reduce the expression of inflammatory markers by microglia.[133] miRNA was loaded into the EVs through incubation and lead to decreased microglial activation in mice.[133] Due to the role of neuroinflammation in driving MS disease progression, the neuromodulatory behavior of miRNA-loaded EVs demonstrates the potential of miRNA loading for treatment of MS.
To date, loading miRNA into EVs to treat MS has not been performed; however, studies have shown the effects of miRNA delivery in promoting myelination and other pathological processes. EVs loaded with miR-210 successfully promoted angiogenesis and increased mice survival rate in a model of cerebral ischemia.[58] Parent MSCs have been transfected with the miR-17–92 cluster to produce EVs that promote oligodendrogenesis and neurogenesis.[99] These EVs were shown in a rat model to promote neurite remodeling, neural plasticity, and functional recovery. Many other miRNAs have been identified to promote remyelination, representing future research avenues loading them into EVs for efficacy studies. These include miR-219 and miR-338, which have been found to promote both myelin repair and myelination in the CNS.[134] This avenue of research has not been investigated in the context of myelination and MS but can allow for an alternative technique for EV engineering.
As a result, current engineering techniques allow for modification of EV surfaces and the contents inside EVs. This results in a cell-free drug delivery platform that can specifically target certain cells or tissues. In the context of MS, PMSC-EVs can be modified to exhibit increased targeting and uptake by cell types of interest, such as activated T cells, microglia, and oligodendrocytes.
6. Conclusion
In summary, MS is an autoimmune disorder of the CNS, largely characterized by an attack on the myelin sheath and subsequent loss of motor function. Recent studies have elucidated many of the processes contributing to and driving MS disease progression. As an autoimmune disease, lymphocytes have been heavily implicated in MS, specifically with increased pro-inflammatory T cell behavior, reduced T regulatory cell behavior, and disrupted T cell and B cell interactions. Leukocyte invasion and resident immune cell activation leads to reduced oligodendrocyte viability and ultimately the loss of protective myelin sheaths. Innate immune responses were found to be primarily driven by activated microglia, which in turn induce endoplasmic reticulum stress on oligodendrocytes to promote demyelination and degeneration.
In this immune-dominated environment, PMSCs represent a promising novel therapeutic agent because of their intrinsic immunomodulatory functions during fetal development. Furthermore, secreted PMSC-EVs have been shown to play a significant role in neuroinflammation and myelin regeneration processes in the CNS. The mechanism of action has been proposed to involve specific components of secreted EVs, namely their surface protein expression and interior miRNA content. Not only have MSC-EVs been found to directly induce remyelinating processes such as neurogenesis and oligodendrogenesis, but both miRNA and proteins have been shown to be critical mediators of MSC-EVs inflammatory, neuroprotective and neuroregenerative properties. This involves driving an immuno-regulatory phenotype in immune cell subsets as well as promoting the secretion of anti-inflammatory cytokines. Recent studies have also shown strong potential for PMSC-EVs to be applied to MS research. Understanding the role of PMSC and native PMSC-EVs on CNS cell populations and the interface of oligodendroglia-axonal interactions and accompanying neuronal degeneration will be warranted in future studies. These studies demonstrate the potential of PMSC-EVs in both modulating upstream immune responses as well as promoting remyelinating processes.
Currently, many MSC and MSC-EV studies employ the intravenous administration strategy to treat MS. While optimization of intervention strategies is warranted, engineering approaches can serve as advantageous tools for superior targeting to pathologic cell types and lesions associated with neurodegenerative disease. Multiple engineering techniques previously established for EV engineering in different disease applications represent promising avenues of future research applying engineered PMSC-EVs for MS treatment. In tandem with the therapeutic properties of PMSCs and the native content of PMSC-EVs, bioengineered PMSC-EVs could allow for the development of novel MS treatments affecting a significant and therapy-lacking population.
Acknowledgements
Financial support for this study was provided by the Shriners Hospitals for Children research grant (85108-NCA-19), National Institute on Aging of the National Institutes of Health (NIH), No. P30AG010129, through the UC Davis Alzheimer’s Disease Center Pilot program, and NIH grants (1R01NS100761-01A1, 1R01NS115860-01A1). Kaitlin Clark was supported by the Willis W. and Ethel M. Clark Foundation Investment in Community Fellowship, the Lodric Maddox Graduate Fellowship, and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Biographies

Kaitlin Clark is a PhD candidate in Biochemistry, Molecular, Cellular and Developmental Biology at the University of California, Davis emphasizing in translational research. She received her master’s degree in Stem Cell biology from CSU Sacramento. Kaitlin’s main research focus is on the immunoregulatory properties of mesenchymal stem/stromal cells and is also investigating the use of MSC derived extracellular vesicles for the treatment of neurodegenerative diseases. She has additionally worked in veterinary regenerative medicine and is interested in utilizing naturally occurring large animal disease models to evaluate biomedical engineering approaches and cellular therapies using MSCs and MSC derived extracellular vesicles.

David Wang is a PhD candidate in Biomedical Engineering at the University of California, Davis. His research focuses on engineering extracellular vesicles to target neuroinflammation and neurodegenerative diseases, notably Alzheimer's Disease. David aspires to be a leading scientist in industry working in the research and development of novel therapeutics.

Priyadarsini Kumar is a senior scientist with over 25 years of research experience. She obtained her PhD from University of California, Davis, and Master’s/Bachelor’s degrees from University of Madras, India. Her research focus is stem cell and stem cell derived extracellular vesicles for treatment of neurodegenerative diseases. She is currently leading the cell manufacturing team for the Phase1/2a spina bifida clinical trial. Her other research focus is in utilizing placenta-derived extracellular vesicles and developing a serum-free cell-free product for clinical applications like adult acquired spinal cord injury.

Sabrina Valentina Lazar is a first-year medical student at Albany Medical College in New York. She received her Bachelor’s of Science in Cell Biology from the University of California, Davis, where she also minored in Professional Writing. Sabrina is pursuing a career in academic medicine and cutting-edge biomedical research to improve patient health outcomes.

Edwin Kulubya is a Neurological Surgery resident at UC Davis Medical Center in Sacramento, CA. He is a graduate of Columbia University and earned a joint MD/MBA degree from the USC Keck School of Medicine and Marshall School of Business. He plans to complete a fellowship in Pediatric Neurosurgery in order to help children and their families deal with neurological diseases. His research interests include cellular therapy for the fetal treatment of spina bifida and spina cold injury.

Sirjan Mor is a second-year medical student at the UC Davis School of Medicine. She received her M.S. in Stem Cells and Regenerative Medicine, and B.S. in Biological Sciences at the University of Southern California (USC). Her interests include investigating the role of neuroinflammation in neurodegenerative disorders and the use of placental mesenchymal stem cells (PMSCs) in attenuating neuroinflammation driven by astrocytes and microglia.

Aijun Wang is a Chancellor’s Fellow, Professor of Surgery and of Biomedical Engineering at the University of California, Davis (UC Davis). He is the vice chair for Translational Research, Innovation and Entrepreneurship, and codirector of the Center for Surgical Bioengineering at the Department of Surgery, UC Davis School of Medicine. Dr. Wang’s research focuses on developing tools and technologies that combine molecular, cellular, tissue, and biomaterial engineering to promote regeneration and restore function. The Wang Group engineers and develops stem cells, extracellular vesicles/nanomedicine and extracellular matrix/biomaterial scaffolds for the treatment of surgical conditions and diseases.
References
- [1].Dilokthornsakul P. et al. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 86, 1014–1021, doi: 10.1212/wnl.0000000000002469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guan Y, Jakimovski D, Ramanathan M, Weinstock-Guttman B. & Zivadinov R. The role of Epstein-Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen Res 14, 373–386, doi: 10.4103/1673-5374.245462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scazzone C, Agnello L, Bivona G, Lo Sasso B. & Ciaccio M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem Genet 59, 1–30, doi: 10.1007/s10528-020-10010-1 (2021). [DOI] [PubMed] [Google Scholar]
- [4].Chisari CG et al. An update on the safety of treating relapsing-remitting multiple sclerosis. Expert Opin Drug Saf 18, 925–948, doi: 10.1080/14740338.2019.1658741 (2019). [DOI] [PubMed] [Google Scholar]
- [5].Harrell CR, Jovicic N, Djonov V, Arsenijevic N. & Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 8, doi: 10.3390/cells8121605 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen JA et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England), 1352458517703802, doi: 10.1177/1352458517703802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamout B. et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. Journal of neuroimmunology 227, 185–189, doi: 10.1016/j.jneuroim.2010.07.013 (2010). [DOI] [PubMed] [Google Scholar]
- [8].Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS & Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta, doi: 10.1016/j.placenta.2017.04.003 (2017). [DOI] [PubMed] [Google Scholar]
- [9].Lee JM et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol 13, 219–224, doi: 10.1016/j.intimp.2012.03.024 (2012). [DOI] [PubMed] [Google Scholar]
- [10].Munir H. & McGettrick HM Mesenchymal Stem Cell Therapy for Autoimmune Disease: Risks and Rewards. Stem Cells Dev 24, 2091–2100, doi: 10.1089/scd.2015.0008 (2015). [DOI] [PubMed] [Google Scholar]
- [11].Kim HJ, Lee JH & Kim SH Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. Journal of neurotrauma 27, 131–138, doi:10.1089/neu.2008-0818 10.1089/neu.2008.0818 (2010). [DOI] [PubMed] [Google Scholar]
- [12].Selmaj I, Mycko MP, Raine CS & Selmaj KW The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. Journal of neuroimmunology 306, 1–10, doi: 10.1016/j.jneuroim.2017.02.002 (2017). [DOI] [PubMed] [Google Scholar]
- [13].Maumus M, Rozier P, Boulestreau J, Jorgensen C. & Noel D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front Bioeng Biotechnol 8, 997, doi: 10.3389/fbioe.2020.00997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andras IE & Toborek M. Extracellular vesicles of the blood-brain barrier. Tissue Barriers 4, e1131804, doi: 10.1080/21688370.2015.1131804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beretti F. et al. Amniotic fluid stem cell exosomes: Therapeutic perspective. Biofactors 44, 158–167, doi: 10.1002/biof.1407 (2018). [DOI] [PubMed] [Google Scholar]
- [16].Bonafede R. et al. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res 340, 150–158, doi: 10.1016/j.yexcr.2015.12.009 (2016). [DOI] [PubMed] [Google Scholar]
- [17].Kumar P. et al. Neuroprotective effect of placenta-derived mesenchymal stromal cells: role of exosomes. FASEB J 33, 5836–5849, doi: 10.1096/fj.201800972R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Komaki M. et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther 8, 219, doi: 10.1186/s13287-017-0660-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clark K. et al. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells 8, doi: 10.3390/cells8121497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jelcic I. et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 175, 85–100 e123, doi: 10.1016/j.cell.2018.08.011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baiula M, Spampinato S, Gentilucci L. & Tolomelli A. Novel Ligands Targeting alpha4beta1 Integrin: Therapeutic Applications and Perspectives. Front Chem 7, 489, doi: 10.3389/fchem.2019.00489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ransohoff RM, Kivisakk P. & Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3, 569–581, doi: 10.1038/nri1130 (2003). [DOI] [PubMed] [Google Scholar]
- [23].Gutcher I. & Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest 117, 1119–1127, doi: 10.1172/JCI31720 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prajeeth CK et al. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain Behav Immun 37, 248–259, doi: 10.1016/j.bbi.2014.01.001 (2014). [DOI] [PubMed] [Google Scholar]
- [25].Reboldi A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10, 514–523, doi: 10.1038/ni.1716 (2009). [DOI] [PubMed] [Google Scholar]
- [26].Kebir H. et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13, 1173–1175, doi: 10.1038/nm1651 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Annunziato F. et al. Phenotypic and functional features of human Th17 cells. J Exp Med 204, 1849–1861, doi: 10.1084/jem.20070663 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Duhen R. et al. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol 190, 4478–4482, doi: 10.4049/jimmunol.1203172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Elain G, Jeanneau K, Rutkowska A, Mir AK & Dev KK The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 62, 725–735, doi: 10.1002/glia.22637 (2014). [DOI] [PubMed] [Google Scholar]
- [30].Kang Z. et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32, 414–425, doi: 10.1016/j.immuni.2010.03.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kang Z. et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nature neuroscience 16, 1401–1408, doi: 10.1038/nn.3505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paintlia MK, Paintlia AS, Singh AK & Singh I. Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J Neurochem 116, 508–521, doi: 10.1111/j.1471-4159.2010.07136.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dulamea AO Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis. Adv Exp Med Biol 958, 91–127, doi: 10.1007/978-3-319-47861-6_7 (2017). [DOI] [PubMed] [Google Scholar]
- [34].Venken K. et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res 83, 1432–1446, doi: 10.1002/jnr.20852 (2006). [DOI] [PubMed] [Google Scholar]
- [35].Stephens LA, Malpass KH & Anderton SM Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur J Immunol 39, 1108–1117, doi: 10.1002/eji.200839073 (2009). [DOI] [PubMed] [Google Scholar]
- [36].Dombrowski Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nature neuroscience 20, 674–680, doi: 10.1038/nn.4528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Correale J. & Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol 67, 625–638, doi: 10.1002/ana.21944 (2010). [DOI] [PubMed] [Google Scholar]
- [38].Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani SH & Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. Journal of neuroimmunology 262, 106–112, doi: 10.1016/j.jneuroim.2013.06.007 (2013). [DOI] [PubMed] [Google Scholar]
- [39].Kimura K. et al. Disrupted balance of T cells under natalizumab treatment in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 3, e210, doi: 10.1212/NXI.0000000000000210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Astier AL, Meiffren G, Freeman S. & Hafler DA Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest 116, 3252–3257, doi: 10.1172/JCI29251 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gobin SJ et al. Upregulation of transcription factors controlling MHC expression in multiple sclerosis lesions. Glia 36, 68–77, doi: 10.1002/glia.1096 (2001). [DOI] [PubMed] [Google Scholar]
- [42].Tzartos JS et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172, 146–155, doi: 10.2353/ajpath.2008.070690 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Elong Ngono A. et al. Frequency of circulating autoreactive T cells committed to myelin determinants in relapsing-remitting multiple sclerosis patients. Clin Immunol 144, 117–126, doi: 10.1016/j.clim.2012.05.009 (2012). [DOI] [PubMed] [Google Scholar]
- [44].Crawford MP et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood 103, 4222–4231, doi: 10.1182/blood-2003-11-4025 (2004). [DOI] [PubMed] [Google Scholar]
- [45].Baughman EJ et al. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun 36, 115–124, doi: 10.1016/j.jaut.2010.12.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cunnusamy K. et al. Disease exacerbation of multiple sclerosis is characterized by loss of terminally differentiated autoregulatory CD8+ T cells. Clin Immunol 152, 115–126, doi: 10.1016/j.clim.2014.03.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T. & Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123 ( Pt 6), 1174–1183, doi: 10.1093/brain/123.6.1174 (2000). [DOI] [PubMed] [Google Scholar]
- [48].Neumann H, Medana IM, Bauer J. & Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci 25, 313–319, doi: 10.1016/s0166-2236(02)02154-9 (2002). [DOI] [PubMed] [Google Scholar]
- [49].Disanto G, Morahan JM, Barnett MH, Giovannoni G. & Ramagopalan SV The evidence for a role of B cells in multiple sclerosis. Neurology 78, 823–832, doi: 10.1212/WNL.0b013e318249f6f0 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kothur K. et al. B Cell, Th17, and Neutrophil Related Cerebrospinal Fluid Cytokine/Chemokines Are Elevated in MOG Antibody Associated Demyelination. PLoS One 11, e0149411, doi: 10.1371/journal.pone.0149411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].van Langelaar J, Rijvers L, Smolders J. & van Luijn MMB and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front Immunol 11, 760, doi: 10.3389/fimmu.2020.00760 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ransohoff RM How neuroinflammation contributes to neurodegeneration. Science 353, 777–783, doi: 10.1126/science.aag2590 (2016). [DOI] [PubMed] [Google Scholar]
- [53].Friese MA, Schattling B. & Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 10, 225–238, doi: 10.1038/nrneurol.2014.37 (2014). [DOI] [PubMed] [Google Scholar]
- [54].Lucchinetti CF et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365, 2188–2197, doi: 10.1056/NEJMoa1100648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ponomarev ED, Shriver LP, Maresz K. & Dittel BN Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res 81, 374–389, doi: 10.1002/jnr.20488 (2005). [DOI] [PubMed] [Google Scholar]
- [56].Chabot S, Williams G. & Yong VW Microglial production of TNF-alpha is induced by activated T lymphocytes. Involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest 100, 604–612, doi: 10.1172/JCI119571 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhao X. et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626, doi: 10.1016/j.neuron.2010.02.018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang H. et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology 17, 29, doi: 10.1186/s12951-019-0461-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kuhlmann T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758, doi: 10.1093/brain/awn096 (2008). [DOI] [PubMed] [Google Scholar]
- [60].Tirotta E, Kirby LA, Hatch MN & Lane TE IFN-gamma-induced apoptosis of human embryonic stem cell derived oligodendrocyte progenitor cells is restricted by CXCR2 signaling. Stem Cell Res 9, 208–217, doi: 10.1016/j.scr.2012.06.005 (2012). [DOI] [PubMed] [Google Scholar]
- [61].Wang C. et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun 8, 15508, doi: 10.1038/ncomms15508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bonora M. et al. Tumor necrosis factor-alpha impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ 21, 1198–1208, doi: 10.1038/cdd.2014.35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kirby L. et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10, 3887, doi: 10.1038/s41467-019-11638-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Prineas JW & Connell F. Remyelination in multiple sclerosis. Ann Neurol 5, 22–31, doi: 10.1002/ana.410050105 (1979). [DOI] [PubMed] [Google Scholar]
- [65].Jang MJ et al. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta haematologica 129, 197–206, doi: 10.1159/000345267 (2013). [DOI] [PubMed] [Google Scholar]
- [66].Fan L. et al. Interaction between Mesenchymal Stem Cells and B-Cells. International journal of molecular sciences 17, doi: 10.3390/ijms17050650 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Petri RM et al. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem cell reports 9, 985–998, doi: 10.1016/j.stemcr.2017.06.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shin TH et al. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell death & disease 7, e2524, doi: 10.1038/cddis.2016.442 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marti LC, Ribeiro AA & Hamerschlak N. Immunomodulatory effect of mesenchymal stem cells. Einstein (Sao Paulo, Brazil) 9, 224–228, doi: 10.1590/s1679-45082011rw1843 (2011). [DOI] [PubMed] [Google Scholar]
- [70].Kovach TK, Dighe AS, Lobo PI & Cui Q. Interactions between MSCs and immune cells: implications for bone healing. Journal of immunology research 2015, 752510, doi: 10.1155/2015/752510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang H. et al. Umbilical cord-derived mesenchymal stem cells reversed the suppressive deficiency of T regulatory cells from peripheral blood of patients with multiple sclerosis in a co-culture - a preliminary study. Oncotarget 7, 72537–72545, doi: 10.18632/oncotarget.12345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Parolini O. et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26, 300–311, doi: 10.1634/stemcells.2007-0594 (2008). [DOI] [PubMed] [Google Scholar]
- [73].Portmann-Lanz CB et al. Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol 202, 294 e291–294 e211, doi: 10.1016/j.ajog.2009.10.893 (2010). [DOI] [PubMed] [Google Scholar]
- [74].Heo JS, Choi Y, Kim HS & Kim HO Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med 37, 115–125, doi: 10.3892/ijmm.2015.2413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Barlow S. et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev 17, 1095–1107, doi: 10.1089/scd.2007.0154 (2008). [DOI] [PubMed] [Google Scholar]
- [76].Karlsson H. et al. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol 167, 543–555, doi: 10.1111/j.1365-2249.2011.04540.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].D’Addio F. et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol 187, 4530–4541, doi: 10.4049/jimmunol.1002031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Alegre E. & Rizzo R. Some basic aspects of HLA-G biology. 2014, 657625, doi: 10.1155/2014/657625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu KJ et al. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell transplantation 20, 1721–1730, doi: (2011). [DOI] [PubMed] [Google Scholar]
- [80].Martino G. & Hartung HP Immunopathogenesis of multiple sclerosis: the role of T cells. Current opinion in neurology 12, 309–321 (1999). [DOI] [PubMed] [Google Scholar]
- [81].Lublin FD et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: a randomized, placebo-controlled, multiple-dose study. Mult Scler Relat Disord 3, 696–704, doi: 10.1016/j.msard.2014.08.002 (2014). [DOI] [PubMed] [Google Scholar]
- [82].Vukusic S. et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127, 1353–1360, doi: 10.1093/brain/awh152 (2004). [DOI] [PubMed] [Google Scholar]
- [83].Spadaro M. et al. Immunomodulatory Effect of Pregnancy on Leukocyte Populations in Patients With Multiple Sclerosis: A Comparison of Peripheral Blood and Decidual Placental Tissue. Front Immunol 10, 1935, doi: 10.3389/fimmu.2019.01935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Engler JB, Heckmann NF, Jager J, Gold SM & Friese MA Pregnancy Enables Expansion of Disease-Specific Regulatory T Cells in an Animal Model of Multiple Sclerosis. J Immunol 203, 1743–1752, doi: 10.4049/jimmunol.1900611 (2019). [DOI] [PubMed] [Google Scholar]
- [85].Mincheva-Nilsson L. et al. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol 176, 3585–3592, doi: 10.4049/jimmunol.176.6.3585 (2006). [DOI] [PubMed] [Google Scholar]
- [86].Stenqvist AC, Nagaeva O, Baranov V. & Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol 191, 5515–5523, doi: 10.4049/jimmunol.1301885 (2013). [DOI] [PubMed] [Google Scholar]
- [87].Atay S, Gercel-Taylor C, Suttles J, Mor G. & Taylor DD Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol 65, 65–77, doi: 10.1111/j.1600-0897.2010.00880.x (2011). [DOI] [PubMed] [Google Scholar]
- [88].Sarker S. et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12, 204, doi: 10.1186/1479-5876-12-204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bai L. et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nature neuroscience 15, 862–870, doi: 10.1038/nn.3109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cantinieaux D. et al. Conditioned Medium from Bone Marrow-Derived Mesenchymal Stem Cells Improves Recovery after Spinal Cord Injury in Rats: An Original Strategy to Avoid Cell Transplantation. PLOS ONE 8, e69515, doi: 10.1371/journal.pone.0069515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Phan J. et al. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles 7, 1522236, doi: 10.1080/20013078.2018.1522236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fathollahi A. et al. Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int Immunopharmacol 90, 107207, doi: 10.1016/j.intimp.2020.107207 (2021). [DOI] [PubMed] [Google Scholar]
- [93].Street JM et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10, 5, doi: 10.1186/1479-5876-10-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kurian NK & Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. Journal of assisted reproduction and genetics 36, 189–198, doi: 10.1007/s10815-018-1343-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fayazi N, Sheykhhasan M, Soleimani Asl S. & Najafi R. Stem Cell-Derived Exosomes: a New Strategy of Neurodegenerative Disease Treatment. Mol Neurobiol, doi: 10.1007/s12035-021-02324-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Morrison TJ et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med 196, 1275–1286, doi: 10.1164/rccm.201701-0170OC (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bartel DP MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233, doi: 10.1016/j.cell.2009.01.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Giunti D. et al. Role of miRNAs shuttled by mesenchymal stem cell-derived small extracellular vesicles in modulating neuroinflammation. Sci Rep 11, 1740, doi: 10.1038/s41598-021-81039-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Xin H. et al. MicroRNA cluster miR-17–92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 48, 747–753, doi: 10.1161/STROKEAHA.116.015204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Qiu G. et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 9, 320, doi: 10.1186/s13287-018-1069-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wang D, Na Q, Song GY & Wang L. Human umbilical cord mesenchymal stem cell-derived exosome-mediated transfer of microRNA-133b boosts trophoblast cell proliferation, migration and invasion in preeclampsia by restricting SGK1. Cell Cycle 19, 1869–1883, doi: 10.1080/15384101.2020.1769394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fang S. et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med 5, 1425–1439, doi: 10.5966/sctm.2015-0367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baglio SR et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 6, 127, doi: 10.1186/s13287-015-0116-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xu HK, Chen LJ, Zhou SN, Li YF & Xiang C. Multifunctional role of microRNAs in mesenchymal stem cell-derived exosomes in treatment of diseases. World J Stem Cells 12, 1276–1294, doi: 10.4252/wjsc.v12.i11.1276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fitzpatrick JM, Anderson RC & McDermott KW MicroRNA: Key regulators of oligodendrocyte development and pathobiology. Int J Biochem Cell Biol 65, 134–138, doi: 10.1016/j.biocel.2015.05.021 (2015). [DOI] [PubMed] [Google Scholar]
- [106].Benkhoucha M. et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 107, 6424–6429, doi: 10.1073/pnas.0912437107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Favaro E. et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 59, 325–333, doi: 10.1007/s00125-015-3808-0 (2016). [DOI] [PubMed] [Google Scholar]
- [108].Fransson M. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation 9, 112, doi: 10.1186/1742-2094-9-112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chen W. et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res 64, 831–840, doi: 10.1007/s12026-016-8798-6 (2016). [DOI] [PubMed] [Google Scholar]
- [110].Di Trapani M. et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep 6, 24120, doi: 10.1038/srep24120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sundblad V, Morosi LG, Geffner JR & Rabinovich GA Galectin-1: A Jack-of-All-Trades in the Resolution of Acute and Chronic Inflammation. J Immunol 199, 3721–3730, doi: 10.4049/jimmunol.1701172 (2017). [DOI] [PubMed] [Google Scholar]
- [112].Cedeno-Laurent F. & Dimitroff CJ Galectin-1 research in T cell immunity: past, present and future. Clin Immunol 142, 107–116, doi: 10.1016/j.clim.2011.09.011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Camby I, Le Mercier M, Lefranc F. & Kiss R. Galectin-1: a small protein with major functions. Glycobiology 16, 137r–157r, doi: 10.1093/glycob/cwl025 (2006). [DOI] [PubMed] [Google Scholar]
- [114].Starossom SC et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37, 249–263, doi: 10.1016/j.immuni.2012.05.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nakagawa Y. & Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel, Switzerland) 7, 1028–1048, doi: 10.3390/ph7121028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Vogel DY et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 10, 35, doi: 10.1186/1742-2094-10-35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Jaimes Y, Naaldijk Y, Wenk K, Leovsky C. & Emmrich F. Mesenchymal Stem Cell-Derived Microvesicles Modulate Lipopolysaccharides-Induced Inflammatory Responses to Microglia Cells. Stem Cells 35, 812–823, doi: 10.1002/stem.2541 (2017). [DOI] [PubMed] [Google Scholar]
- [118].Li Z. et al. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol 67, 268–280, doi: 10.1016/j.intimp.2018.12.001 (2019). [DOI] [PubMed] [Google Scholar]
- [119].Ti D. et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med 13, 308, doi: 10.1186/s12967-015-0642-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Riazifar M. et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 13, 6670–6688, doi: 10.1021/acsnano.9b01004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS & Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 59, 87–95, doi: 10.1016/j.placenta.2017.04.003 (2017). [DOI] [PubMed] [Google Scholar]
- [122].Wiklander OP et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4, 26316, doi: 10.3402/jev.v4.26316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Andrews S, Maughon T, Marklein R. & Stice S. Priming of MSCs with inflammation-relevant signals affects extracellular vesicle biogenesis, surface markers, and modulation of T cell subsets. Journal of Immunology and Regenerative Medicine 13, 100036, doi: 10.1016/j.regen.2020.100036 (2021). [DOI] [Google Scholar]
- [124].Tian Y. et al. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Advances in clinical and experimental medicine : official organ Wroclaw Medical University 28, 421–430, doi: 10.17219/acem/91826 (2019). [DOI] [PubMed] [Google Scholar]
- [125].Chen Z. et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 11706–11715, doi: 10.1523/JNEUROSCI.0730-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Liu W. et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater 103, 196–212, doi: 10.1016/j.actbio.2019.12.020 (2020). [DOI] [PubMed] [Google Scholar]
- [127].Hao D. et al. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front Bioeng Biotechnol 8, 633, doi: 10.3389/fbioe.2020.00633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tian T. et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 11, 6507–6521, doi: 10.7150/thno.56367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Royo F, Cossío U, Ruiz de Angulo A, Llop J. & Falcon-Perez JM Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 11, 1531–1537, doi: 10.1039/c8nr03900c (2019). [DOI] [PubMed] [Google Scholar]
- [130].Dusoswa SA et al. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles 8, 1648995, doi: 10.1080/20013078.2019.1648995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao Z, McGill J, Gamero-Kubota P. & He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab on a chip 19, 1877–1886, doi: 10.1039/c8lc01279b (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jhan YY et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. International journal of pharmaceutics 573, 118802, doi: 10.1016/j.ijpharm.2019.118802 (2020). [DOI] [PubMed] [Google Scholar]
- [133].Chivero ET et al. Engineered Extracellular Vesicles Loaded With miR-124 Attenuate Cocaine-Mediated Activation of Microglia. Front Cell Dev Biol 8, 573, doi: 10.3389/fcell.2020.00573 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tripathi A. et al. Oligodendrocyte Intrinsic miR-27a Controls Myelination and Remyelination. Cell Rep 29, 904–919 e909, doi: 10.1016/j.celrep.2019.09.020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]