Abstract
Based on measurements and theoretical analyses, we identified deletion of pyruvate kinase (PYK) activity as a possible route for elimination of acid formation in Bacillus subtilis cultures grown on glucose minimal media. Evidence consistent with the attenuation of PYK flux has come from metabolic flux calculations, metabolic pool and enzymatic activity measurements, and a series of nuclear magnetic resonance experiments, all suggesting a nearly complete inhibition of PYK activity for glucose-citrate fed cultures in which the amount of acid formation was nearly zero. In this paper, we report the construction and characterization of a pyk mutant of B. subtilis. Our results demonstrate an almost complete elimination of acid production in cultures of the pyk mutant in glucose minimal medium. The substantial reduction in acid production is accompanied by increased CO2 production and a reduced rate of growth. Metabolic analysis indicated a dramatic increase in intracellular pools of phosphoenolpyruvate (PEP) and glucose-6-P in the pyk mutant. The high concentrations of PEP and glucose-6-P could explain the decreased growth rate of the mutant. The substantial accumulation of PEP does not occur in Escherichia coli pyk mutants. The very high concentration of PEP which accumulates in the B. subtilis pyk mutant could be exploited for production of various aromatics.
Acid production is among the important factors that limit process stability and cell concentration and thus cell-based biotechnological processes (e.g., see references 14, 23, and 25). Numerous approaches have been used in an attempt to reduce acid formation. One mechanism involves maintaining low levels of glucose in fed batch reactors (24, 27). While this can lead to increased cell mass and product formation, it is a capital-intensive method. Manipulation of the growth media might also be used to reduce acid formation relative to the amount of glucose consumed (13, 24).
Majewski and Domach (17) used a constrained network analysis of the main metabolic pathways in conjunction with reported measurements of enzymatic activity levels to explain acid production by bacterial cells. This analysis suggested that Escherichia coli and Bacillus subtilis have excess glycolytic capacity relative to the Krebs cycle. This idea is consistent with the notion that given all the anabolic and catabolic tasks that metabolic networks must perform, stoichiometric conflicts and other conflicts arise, leading to imperfect coordination of all tasks. It is thus an overflow or “spilling” of excess carbon that leads to acid production.
In experiments using B. subtilis to test the overflow hypothesis, it was found that a small amount of citrate added to glucose minimal medium (0.1 mol of citrate/1 mol of glucose) caused the rate of glucose (or total carbon) use per cell to decline several-fold, while the maximal growth rate was not diminished (12). Further, acid production was undetectable in the glucose-citrate cultures. Subsequent work showed that productivity of recombinant protein (units of protein/vol of culture-time) in glucose-citrate medium was 5- to 10-fold higher (26), further emphasizing the utility of reducing acid production.
Although there have been several efforts to reduce acid formation in E. coli by metabolic engineering (6, 8), there has been no report on metabolic engineering of B. subtilis. Based on measurements and theoretical analyses, we identified pyruvate kinase (PYK) as the activity most likely affected by growth in glucose-citrate medium (12, 15). At the pH of the medium, citrate is transported by a symport with divalent metal ions (especially Ca2+) to maintain electroneutrality. Ca2+ is known to be a strong inhibitor of the enzyme PYK in procaryotes (3). Intracellular concentrations of Ca2+ are increased by cotransport with citrate to a level where PYK inhibition occurs. PYK inhibition would consequently elevate the phosphoenolpyruvate (PEP) concentration, which is known to inhibit the enzyme phosphofructokinase (PFK). The glucose flux through the terminal reactions of glycolysis is reduced, thus eliminating acid overflow. Measurements of intracellular metabolites were consistent with significant attenuation of PYK flux (13). Pyruvate levels were significantly decreased, and PEP and glucose-6-phosphate levels were elevated, for cells grown in glucose and citrate, compared to results for those grown with glucose alone (13). Thus there is biochemical evidence that is consistent with the inhibition of PYK suggested by the flux balances (12) and linear programming-generated flux scenarios (15).
Further evidence consistent with the attenuation of PYK flux has come from a series of nuclear magnetic resonance experiments measuring the 13C enrichment at specific carbons of glutamate when [1-13C]glucose is used as a substrate (21). The relative enrichment at each carbon in glutamate can be used to predict the dominant metabolic route operating in the cell. Nuclear magnetic resonance analysis of extracts of B. subtilis cells growing in glucose-citrate media indicates a nearly complete inhibition of PYK activity.
Taken together, the data presented above suggest that mutation of the pyk gene would lead to a strain that would show reduced acid production when grown in glucose minimal media. Indeed, it is possible that reducing the PYK flux could be a generic strategy for eliminating acid production.
In this paper, we report the construction and characterization of a pyk mutant of B. subtilis. Our results demonstrate an almost complete elimination of acid production in cultures of the pyk mutant in glucose minimal medium. The substantial reduction in acid production is accompanied by increased CO2 production and a somewhat reduced growth rate. Metabolic analysis indicated a dramatic increase in intracellular pools of PEP and glucose-6-P in the pyk mutant. The high concentrations of PEP and glucose-6-P could explain the decreased growth rate of the mutant.
MATERIALS AND METHODS
Construction of a B. subtilis mutant.
The primers BspykF (5′ GGCAAGAACGTTGGAATTC 3′) and BspykR (5′ CGAGGGCAAGCTTTCTGG 3′) were designed using the sequence of the B. subtilis pyk gene obtained from GenBank and used to amplify a 1,050-bp fragment from B. subtilis genomic DNA. The fragment contained EcoRI and HindIII sites at the 5′ and 3′ ends, respectively. The fragment was digested with EcoRI and HindIII and cloned into the E. coli plasmid pUC19. Following isolation and characterization of the pUC/pyk plasmid, a chloramphenicol resistance gene (Cmr) was inserted in the middle of the cloned pyk DNA. The Cmr gene was obtained as a MspI-TaqI fragment from plasmid pUB110, and that fragment was cloned into a ClaI site in the middle of the cloned B. subtilis pyk DNA. This plasmid, pUC/BSPYK::CAM, was grown in E. coli, purified, linearized at the EcoRI site, and used to transform competent wild-type B. subtilis (ATCC 6051) as described previously (4). Transformants were selected on plates containing 3 μg of chloramphenicol/ml and then checked for the presence of plasmid DNA by agarose gel electrophoresis. Twelve candidate clones were identified and further characterized by PCR. Chromosomal DNA from the candidate colonies was isolated and subjected to PCR using the primers originally used to isolate the pyk fragment. Six of the colonies displayed an amplified band of about 2.7 kb (1 kb of pyk DNA and 1.7 kb for the Cmr gene), and wild-type B. subtilis DNA showed an approximately 1-kb band.
Growth medium.
One liter of media contained the following: 1 g of NH4Cl, 0.04 g of tryptophan, 1.0 g of KH2PO4, 2.72 g of K2HPO4, 0.284 g of Na2SO4, 0.17 g of NaNO3, 0.15 g of KCl, 25 mg of MgCl2 · 6H2O, 2.16 mg of FeCl3 · 6H2O, 15 mg of MnCl2 · 4H2O, 22 mg of CaCl2 · 6H2O, and 2.5 ml of 10% antifoam B. The concentrations of glucose and other carbon sources used in each experiment are noted in the text.
Cultivation and measurement of glucose and acids.
Fermentation vessels with a 2-liter capacity (Applikon, Austin, Tex.) were used. An online data acquisition system (1) collected data for the CO2 evolution rate from a Dycor mass spectrometer (Ametek, Pittsburgh, Pa.) using a standard gas mixture for calibration. The air flow rate was set at 2 liters/min, and the pH was constrained between 6.7 and 6.9. Optical density was measured offline using a Lambda 6 spectrophotometer (Perkin-Elmer, Norwalk, Conn.) from our calibrations (1 optical density at 660 nm [OD660] = 0.35 g of cell dry weight/liter). The glucose concentration was measured enzymatically (Sigma kit 16-UV; Sigma Chemicals, St. Louis, Mo.). To measure the concentrations of organic acids (lactate, acetate, acetoin, formate, fumarate, malate, pyruvate, succinate, and citrate), a high-pressure liquid chromatography gradient module (Bio-Rad, Richmond, Calif.) equipped with a UV-visible light monitor was employed (13).
Measurements of intracellular glucose-6-phosphate, PEP, and pyruvate.
For metabolite assays, cell extracts were prepared using the formic acid extraction method (9). Flasks were immersed in liquid nitrogen and chilled to 4°C. A sorval Superspeed RC2-B Automatic refrigerated centrifuge was used for harvesting the cells. The cell pellet was resuspended in 1 N formic acid at 4°C and incubated at 4°C for 1 h. Cell debris was then removed by centrifugation. The supernatant was then lyophilized and resuspended in 0.2% of the original culture volume. A Perkin-Elmer fluorospectrometer was used for the assays in which NADPH or NADH served as the indicator, with the absorption set at 340 nm and emission measured at 460 nm. Glucose-6-phosphate, PEP, pyruvate, and citrate were measured according to the method of Lowry and Passonneau (16).
Assay of pyruvate kinase.
Pyruvate kinase assays were performed for both the wild-type and the pyk mutant as previously reported (18).
RESULTS
Pyruvate kinase activities.
The specific PYK activity (measured in units/milligram of protein) in the extracts of the mutant cells was 0.02 ± 0.002, significantly lower than the wild type level (0.09 ± 0.007). The low base level of activity for the mutant may reflect the contribution from other reactions in the cell extract. To the best of our knowledge, there is no second pyk gene in B. subtilis. This experiment has been repeated at least five times, and we always see the low level of activity. Moreover, while the specific PYK activity in the wild-type cell extract varies with changes in the PEP concentration (e.g., 0.036 with 2.5 mM PEP and 0.09 with 5 mM PEP), as expected (18), the specific activity for the mutant was at about 0.02 independent of the PEP concentration. The specific activity of 0.02 was also present when there was no PEP in the assay mixture. Thus, we are confident that this low level of activity represents the background reading of the cell extract mixture. Indeed, in our experiment with the E. coli pyk mutant, in which both PYK I and PYK II are inactivated, this low level is also observed.
Growth on glucose minimal media.
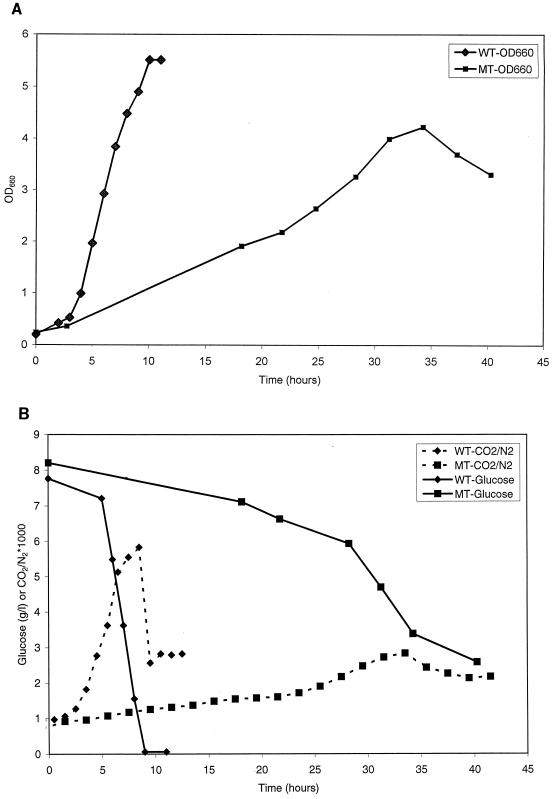
The results of growth in batch cultures with 8 g of glucose/liter are shown in Fig. 1. The mutant exhibited a considerably longer lag time and a lower growth rate than did the wild type (Fig. 1A). Although visually, on a linear scale, it appears that the growth rate of the mutant was not dramatically lower than that of the wild-type, displaying the data on a log scale will reveal that indeed the maximum growth rate of the mutant was only about 30% of that of the wild type. The longer lag phase and lower growth rate have also been observed consistently in our shake-flask experiments, which were repeated many times (data not shown). The final cell concentration was slightly lower than that for the wild type (Fig. 1A), but a significant portion of glucose remained unutilized (Fig. 1B). Thus, the cell yield on glucose was higher for the mutant. The same experiment was also repeated in the reactor experiment with 4.4 g of glucose/liter in medium (data not shown). The results displayed the same pattern of a significantly longer lag time and a slower maximum growth rate. Despite the longer lag period and slower growth rate in the glucose (4.4 g/liter) culture, the mutant reached a slightly higher cell mass. Further, all of the glucose was consumed in both the wild-type and the mutant cultures. While there were some differences in growth characteristics between the mutant and the wild type, the major differences were in the production of acids and intracellular metabolite pools.
FIG. 1.
Changes in the OD660 (A) and glucose and CO2 levels (B) in the batch cultures of the wild type (WT) and the pyk mutant (MT) of B. subtilis with an initial glucose concentration of about 8 g/liter. CO2/N2 is the molar ratio of CO2 to N2 in the effluent gas stream. Since the amount of N2 does not change, the variation in the CO2/N2 ratio represents the changes in the CO2 concentration.
Acid production by wild-type and pyk mutant strains.
The concentrations of acidic metabolic by-products found in the culture and the carbon balances are given in Table 1. The data in Table 1 correspond to the end of exponential phase of growth. The data show a somewhat higher output of carbon than the carbon input as glucose. This difference might be attributed, at least partly, to an overestimation of the amount of CO2. The percentage of carbon in biomass was calculated based on the formula of 1 OD660 = 0.35 g of cell dry weight/liter and a cell's carbon content of 45%. These values are fairly conservative estimates, and thus the cell's carbon content should not be overestimated. Moreover, the assay kit used for measuring the glucose concentration is reliable and has an error margin of less than 5%.
TABLE 1.
Yield and acid production in cultures of the B. subtilis pyk mutant (pyk MT) and the wild type (WT)a
| Trial | Cell type | Amt (g/liter) of:
|
% Carbon in:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Glucose in medium | Residual glucose | Acetate | Pyruvate | Lactate | Cells | Acids | CO2 | ||
| 1 | pyk MT | 4.4 ± 0.2 | 0 | 0 | 0 | 0 | 55 | 0 | 64 |
| WT | 4.4 ± 0.2 | 0 | 0.68 ± 0.11 | 0 | 0 | 47 | 15 | 43 | |
| 2 | pyk MT | 8.2 ± 0.3 | 3.4 | 0 | 0 | 0 | 44 | 0 | 74 |
| WT | 7.8 ± 0.3 | 0 | 1.16 ± 0.18 | 0.66 ± 0.08 | 0.93 ± 0.12 | 36 | 37 | 36 | |
Percent carbon in cells is defined as (grams of carbon in cells/total grams of carbon consumed) × 100. The amount of carbon consumed is calculated based on the initial and final glucose concentrations. Percent carbon in acids and that in CO2 are based on grams of carbon in either acids or CO2/total grams of carbon consumed. The acids and glucose concentrations were measured three times, and the standard deviations are relatively small, as shown.
The data reveal that in contrast to results for the wild-type cultures, there was no acid formed by the mutant, but the amount of CO2 formation was substantially higher in the mutant cultures. The mutant also converted a higher percentage of glucose carbon to cell mass. The low amount of acid formation in the pyk mutant is consistent with our hypothesis that levels of acid formation will be low in the mutant. Since there was very little or no acid formed in the mutant cultures, the lower growth rate of the mutant cannot be attributed to the inhibitory role of acidic by-products, such as acetate.
Intracellular metabolite pools.
We speculated that high levels of PEP might be accumulating in the pyk mutant of B. subtilis. High levels of PEP could potentially be responsible for the lower growth rate of the mutant. High PEP levels would inhibit phosphofructokinase (2, 13) and lead to accumulation of glucose 6-P. High glucose 6-P levels in turn would lead to a lower rate of glucose consumption. For the mutant, the concentration of PEP is very low and often undetectable in bacteria. Experiments were conducted to measure the PEP and glucose-6-P levels for the wild type and the mutant. We found a dramatic increase in PEP and glucose 6-P concentrations in the pyk mutant of B. subtilis. The PEP concentration for the wild type was undetectable (below 0.02 mM), and the concentration for the mutant was 1.30 mM (with a standard deviation of ±0.05 in two independent experiments). Furthermore, the concentration of glucose-6-P was about 1.36 mM (standard deviation, ±0.12 in two independent experiments) for the mutant but below 0.10 mM for the wild type. The high PEP levels in the mutant open the possibility of using this strain as a starting point in the construction of strains with the capacity to produce products that use PEP as a primary substrate (e.g., phenylalanine).
Growth in glucose-pyruvate medium.
The hypothesis that PEP accumulation and the resultant pyruvate deficit were responsible for the lower growth rate of the mutant was further confirmed by performing an experiment in which a small amount of pyruvate (0.2 mol of pyruvate/mol of glucose) was included in the growth medium. The pyruvate addition restored the growth rate of the mutant to the wild-type level and resulted in about a 15% higher final cell density than that for the wild-type cultures. The addition of pyruvate (0.2 mol of pyruvate/mol of glucose) had very little effect on either the growth rate or the final cell density for the wild-type culture.
DISCUSSION
The lower acetic acid production for the pyk mutant of B. subtilis is encouraging. However, as indicated by the pyruvate addition experiment, the B. subtilis mutant may be experiencing a pyruvate limitation when PYK activity is completely eliminated. This limitation could potentially be alleviated by controlling the expression of the pyk gene at a low but finite level, thereby reducing the high intracellular concentration of PEP.
The high intracellular concentration of PEP found in the pyk mutant of B. subtilis may be exploited for the production of various aromatic compounds. Successful commercial production of aromatic compounds in bacteria requires high-yield conversion of glucose to such products. Some of the commercially important aromatic products are tryptophan, tyrosine, phenylalanine, quinic acid, catechol, and adipic acids. The first dedicated step of the pathways leading to formation of various aromatics is the condensation of E4P and PEP catalyzed by DAHP synthase. Several attempts have been made to increase the in vivo pools of PEP in E. coli. They include the deletion of the PEP-consuming reaction PEP carboxylase (19) or pyruvate kinase (22) and use of either an alternative transport system utilizing ATP as the phosphate donor rather than PEP for the phosphotransferase (PTS) system (10) or an alternative carbon source, such as xylose, which does not rely on the PTS system (20). Although these strategies appeared to increase the level of PEP, the increase was not substantial (still remaining below the detection limit) or was not measured (10). Recently, Chen et al. (5) evaluated pts mutations for effects on production of phenylalanine from E. coli. This strategy did not increase the PEP pool or the production of the phenylalanine.
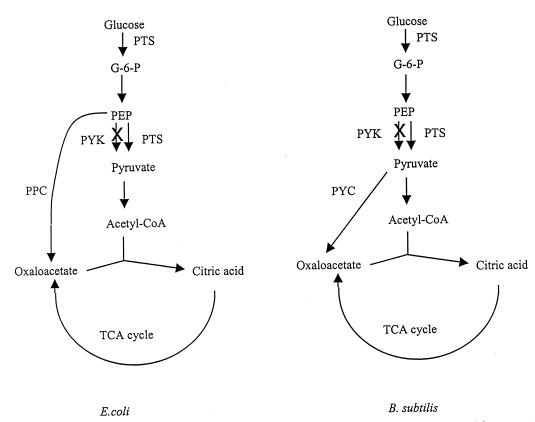
Our recent work with an E. coli pyk mutant (strain PB25 [22]) lacking both PYK I and PYK II activities indicated that the PEP pool in the E. coli pyk mutant was not substantially greater than in the wild type (T. Zhu et al., unpublished data). Unlike the B. subtilis pyk mutant, the E. coli pyk mutant had a growth rate similar to that of the wild type. The difference between the B. subtilis and E. coli networks is primarily at the level of oxaloacetate formation (see Fig. 2 for a schematic). In B. subtilis, oxaloacetate is formed from pyruvate, but in E. coli, PEP is the precursor. Thus, while the E. coli pyk mutant cannot convert PEP to pyruvate via PYK activity, PEP can enter the trichloroacetic acid cycle via PEP carboxylase activity (PEP to oxaloacetate). PEP may even be converted to pyruvate via the combination of PEP carboxylase and malic enzyme activities. In contrast, in the B. subtilis mutant PEP can only be converted to pyruvate via the PTS system; there are no other reactions that can convert PEP to trichloroacetic acid cycle metabolites for further processing. Consequently, substantial accumulation of PEP and reduction of glucose uptake are likely.
FIG. 2.
Oxaloacetate formation in E. coli and B. subtilis.
In summary, we have constructed a B. subtilis pyk mutant and quantified its growth characteristics. There was very little or no acid formed in the mutant when it was grown on up to 8 g of glucose/liter, a condition under which the wild type produces as much as 2.75 g of acid by-products/liter (see Table 1). Furthermore, we have shown that in contrast to results for an E. coli pyk mutant, substantial accumulation of PEP occurs in our B. subtilis pyk mutant. This high accumulation of PEP should be further explored for production of various aromatics in B. subtilis.
REFERENCES
- 1.Beitle R R, Ataai M M. A unique cultivation monitoring and control system. Biotechnol Tech. 1991;5:77–82. [Google Scholar]
- 2.Blangy D, Buc H, Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968;31:13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- 3.Boiteux A, Markus M, Plesser T, Hess B, Malcovati M. Interaction of pyruvate kinase from Escherichia coli with fructose 1,6-biphosphate and calcium ions. Biochem J. 1983;211:631–640. doi: 10.1042/bj2110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bron S. Plasmids. In: Harwood C R, Cutting S M, editors. Molecular biological methods for bacillus. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 75–174. [Google Scholar]
- 5.Chen R Z, Hatzimanikatis V, Yap W M G J, Postma P W, Bailey J E. Metabolic consequences of phosphotransferase (PTS) mutation in a phenylalanine-producing recombinant Escherichia coli. Biotechnol Prog. 1997;13:768–775. doi: 10.1021/bp970060h. [DOI] [PubMed] [Google Scholar]
- 6.Chou C H, Bennett G N, San K Y. Effect of modifying glucose uptake using genetic engineering techniques on high level recombinant protein production in Escherichia coli dense culture. Biotechnol Bioeng. 1994;44:952–969. doi: 10.1002/bit.260440811. [DOI] [PubMed] [Google Scholar]
- 7.Evans P R, Hudson P J. Structure and control of phosphofructokinase from Bacillus stearothemophilus. Nature. 1979;279:500–504. doi: 10.1038/279500a0. [DOI] [PubMed] [Google Scholar]
- 8.Farmer W R, Liao J C. Reduction of aerobic acetate production by Escherichia coli. Appl Environ Microbiol. 1997;63:3205–3210. doi: 10.1128/aem.63.8.3205-3210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher S H, Magasanik B. Synthesis of oxaloacetate in Bacillus subtilis mutants lacking the a-ketoglutarate dehydrogenase enzyme complex. J Bacteriol. 1984;158:55–62. doi: 10.1128/jb.158.1.55-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores N, Xiao J, Berry A, Boliver F, Valle F. Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol. 1995;14:620–623. doi: 10.1038/nbt0596-620. [DOI] [PubMed] [Google Scholar]
- 11.Goel A, Ferrance J, Ataai M M. Analysis of metabolic fluxes in batch and continuous cultures of Bacillus subtilis. Biotechnol Bioeng. 1993;42:686–696. doi: 10.1002/bit.260420603. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, Lee J-W, Domach M M, Ataai M M. Suppressed acid formation by cofeeding glucose and citrate in Bacillus cultures: emergence of pyruvate kinase as a potential metabolic engineering site. Biotechnol Prog. 1995;11:380–386. doi: 10.1021/bp00034a003. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Domach M M, Ataai M M. Metabolite fluxes, pools and enzyme measurements suggest a tighter coupling of the energetic and biosynthetic reactions requires reduction in pyruvate kinase flux. Biotechnol Bioeng. 1999;64:129–134. doi: 10.1002/(sici)1097-0290(19990720)64:2<129::aid-bit1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Ko Y-F, Bentley W E, Weigand W A. An integrated modeling approach to describe the energy efficiency of E. coli fermentation under oxygen-limited conditions; cellular energetics, carbon flux, and acetate production. Biotechnol Bioeng. 1993;42:843–853. doi: 10.1002/bit.260420709. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Goel A, Ataai M M, Domach M M. Supply-side analysis of growth of Bacillus subtilis on glucose-citrate medium: feasible network alternatives and yield optimality. Appl Environ Microbiol. 1997;63:710–718. doi: 10.1128/aem.63.2.710-718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry O H, Passonneau O V. A flexible system of enzymatic analysis. New York, N.Y: Academic Press, Inc.; 1973. [Google Scholar]
- 17.Majewski R A, Domach M M. Simple constrained-optimization view of acetate overflow in E. coli. Biotechnol Bioeng. 1990;35:732–738. doi: 10.1002/bit.260350711. [DOI] [PubMed] [Google Scholar]
- 18.Malcovati M, Valentini G. AMP and fructose 6-phosphate activated pyruvate kinase from E. coli. Methods Enzymol. 1982;90:170–179. doi: 10.1016/s0076-6879(82)90123-9. [DOI] [PubMed] [Google Scholar]
- 19.Miller J E, Beckman K C, O'Connor M J, Hatch R T. Production of phenylalanine and organic acids by PEP carboxylase deficient mutants of Escherichia coli. J Ind Microbiol. 1987;2:143–149. [Google Scholar]
- 20.Patnaik R, Spitzer R G, Liao J C. Pathway engineering for production of aromatics in Escherichia coli: confirmation of stoichiometric analysis of independent modulation of AroG, TktA, and Pps activities. Biotechnol Bioeng. 1994;46:361–370. doi: 10.1002/bit.260460409. [DOI] [PubMed] [Google Scholar]
- 21.Phalakornkule C, Fry B, Zhu T, Koepsel R, Ataai M M, Domach M M. 13C NMR evidence for pyruvate kinase flux attenuation underlying suppressed acid formation in Bacillus subtilis. Biotechnol Prog. 2000;16:169–175. doi: 10.1021/bp000007k. [DOI] [PubMed] [Google Scholar]
- 22.Ponce E, Flores N, Martinez A, Bolivar F, Valle F. Simulation of glucose catabolism through the pentose pathway by the absence of the two pyruvate kinase isoenzymes in Escherichia coli. Biotechnol Bioeng. 1998;58:292–295. doi: 10.1002/(sici)1097-0290(19980420)58:2/3<292::aid-bit25>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Shiloach J, Kaufman J, Guillard A S, Fass R. Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (lambda DE3) and Escherichia coli JM109. Biotechnol Bioeng. 1996;49:421–428. doi: 10.1002/(SICI)1097-0290(19960220)49:4<421::AID-BIT9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Snay J, Jeong J, Ataai M M. Effect of growth conditions on carbon utilization and organic by-product formation. Biotechnol Prog. 1989;5:63–69. [Google Scholar]
- 25.Van de Walle M, Shiloach J. Proposed mechanism of acetate accumulation in two recombinant Escherichia coli strains during high density fermentation. Biotechnol Bioeng. 1998;57:71–78. doi: 10.1002/(sici)1097-0290(19980105)57:1<71::aid-bit9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Vierheller C, Goel A, Domach M M, Ataai M M. Sustained and constitutive high levels of protein production in continuous cultures of B. subtilis. Biotechnol Bioeng. 1995;47:520–525. doi: 10.1002/bit.260470503. [DOI] [PubMed] [Google Scholar]
- 27.Zabriskie D W, Arcuri E J. Factors influencing productivity of fermentations employing recombinant microorganisms. Enzyme Microb Technol. 1986;16:933–941. [Google Scholar]