Abstract
Background
The systemic inflammatory response index (SIRI) is a novel inflammatory biomarker in many diseases.
Objectives
The aim of this study was to examine the association between SIRI and adverse events in patients with the acute coronary syndrome (ACS) undergoing percutaneous coronary intervention.
Methods
A total of 1724 patients with ACS enrolled from June 2016 to November 2017 at a single centre were included in this study, and SIRI was calculated for each patient. The primary endpoint was the composite of major adverse cardiovascular events (MACE), including overall death, non-fatal myocardial infarction, non-fatal stroke, and unplanned repeat revascularization.
Results
During a median follow-up of 927 days, 355 patients had MACE. Multivariate Cox analysis showed that SIRI was significantly associated with MACE (hazard ratio: 1.127, 95% confidence interval: 1.034–1.229 p = .007). The results were consistent in multiple sensitivity analyses. The addition of SIRI had an incremental effect on the predictive ability of the Global Registry of Acute Coronary Events risk score for MACE (integrated discrimination improvement: 0.007, p = .040; net reclassification improvement: 0.175, p = .020; likelihood ratio test: p < .001). The restricted cubic spline showed a monotonic increase with a greater SIRI value for MACE (p < .001).
Conclusion
SIRI was an independent risk factor for MACE and provided incremental prognostic information in patients with ACS undergoing percutaneous coronary intervention.
KEY MESSAGES
The SIRI is a strong and independent risk factor for adverse outcomes in patients with ACS undergoing percutaneous coronary intervention.
Higher SIRI is associated with a more severe disease status.
The SIRI could increase the prognostic value of the GRACE risk score.
Keywords: Systemic inflammatory response index, acute coronary syndrome, percutaneous coronary intervention, cardiovascular outcomes
Introduction
With a global prevalence of 154 million in 2016, coronary artery disease (CAD) represents 32.7% of the global burden of cardiovascular disease and is one of the leading causes of death [1]. Atherosclerosis, the main cause of CAD, is an inflammatory process wherein the immune response interacts with metabolic disorders to generate and activate coronary lesions. Much evidence has suggested that chronic inflammation plays a critical role in the pathogenesis of atherosclerosis [2,3]. Immune cells, including neutrophils, monocytes, lymphocytes, and mast cells, infiltrate the atherosclerotic lesions and initiate a cytokine cascade [4]. A larger number of white blood cells is associated with the onset and poor prognosis of CAD [5,6]. Inflammation also incites plaque destabilization and precipitates acute coronary syndrome (ACS) [7].
As a reflection of inflammation, peripheral blood inflammatory cell count and its derived indicators are now widely used in clinical practice. These indicators are considered to be inexpensive and easily accessible biomarkers that are associated with increased risk of CAD [8,9], stroke [10], and overall death [11]. For example, studies have found that neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), and monocyte to high-density lipoprotein cholesterol (HDL-C) ratio have strong predictive roles [12–14]. Recently, a novel indicator has emerged called the systemic inflammatory response index (SIRI). SIRI is a composite index based on the absolute count of three different inflammatory cells, namely, neutrophils, monocytes, and lymphocytes, and it is highly associated with cancer, hyperuricaemia, rheumatoid arthritis, and stroke [15–18]. Elevated SIRI values are related to an increased risk of myocardial infarction (MI) and overall death [19]. However, whether SIRI is an independent risk factor for adverse prognosis in patients with ACS is still unknown. Here, we investigated the prognostic value of SIRI in ACS patients undergoing percutaneous coronary intervention (PCI).
Method
This study was a single-centre, retrospective analysis derived from a prospective observational study (ChiCTR1800017417) of patients with ACS undergoing PCI conducted between June 2016 and November 2017 at Beijing Anzhen Hospital. A total of 1770 patients were consecutively enrolled and followed up after 1 month and every 6 months thereafter. The exclusion criteria of this study included patients with infection or rheumatic disease or a history of coronary artery bypass graft surgery. Eventually, 1724 patients were included in the final analysis, with a median follow-up of 927 days. This study was performed following the Helsinki Declaration of Human Rights and was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University. Written informed consent was obtained from all of the patients.
Data on demographics, medical history, angiographic information, and medicine intake were collected from the medical records system using a questionnaire. The SIRI was defined as neutrophils × monocytes/lymphocytes. Smoking status was defined as currently smoking or not smoking. ACS was classified into unstable angina, ST-segment elevation myocardial infarction (STEMI), and non-STEMI (NSTEMI). MI was defined as an elevated level of creatine kinase or cardiac troponin higher than the upper limit of the normal range accompanied by either ischaemic symptoms or electrocardiographic changes implicating ischaemia.
Hypertension was diagnosed as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg and/or receiving anti-hypertension treatment. Fasting plasma glucose (FPG) ≥7.0 mmol/L and/or casual plasma glucose ≥11.1 mmol/L, and/or 2-h blood glucose after an oral glucose tolerance test ≥11.1 mmol/L, and/or using antidiabetic medicine were considered to indicate diabetes. Dyslipidemia was defined as a fasting serum total cholesterol >5.17 mmol/L, low-density lipoprotein cholesterol (LDL-C) >3.36 mmol/L, triglyceride >1.69 mmol/L, HDL-C <1.03 mmol/L, and/or use of lipid-lowering drugs. Hyperuricaemia was defined when uric acid was >416 μmol/L in men and >357 μmol/L in women. Patients with previous ischaemic strokes or transient ischaemic attacks were defined as having a history of cerebrovascular disease. Chronic kidney disease (CKD) was defined as the estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Patients with congestive heart failure signs/symptoms, ongoing heart failure therapy, or left ventricular ejection fraction (LVEF) <40% were defined as having heart failure. The Synergy between PCI with TAXUS and Cardiac Surgery (SYNTAX) score and the Global Registry of Acute Coronary Events (GRACE) risk score were calculated for each patient.
Information on adverse events was obtained through telephone contact or the medical records system. The primary endpoint was the composite of major adverse cardiovascular events (MACE) including overall death, non-fatal MI, non-fatal stroke, and unplanned repeat revascularization (URR). Non-fatal MI was defined as an elevated level of cardiac creatine kinase or cardiac troponin accompanied by either ischaemic symptoms or electrocardiographic changes. Non-fatal MI was also diagnosed when patients had new pathological Q waves in ≥2 contiguous electrocardiogram leads. Patients with an acute ischaemic cerebral vascular event underlying a neurological event were diagnosed with stroke. Only Q-wave MI was considered MI within 1 week after the index PCI. Any non-staged revascularization after the index PCI was considered URR. If the same event occurred more than twice, the first event was used in the analysis. The most severe endpoint event was selected for the analysis if >1 event occurred during follow-up (death > stroke > MI > URR).
Continuous variables are summarised as mean ± standard deviation or median (interquartile range). Comparisons were performed using the t-test or Mann–Whitney U-test according to the distribution. Categorical variables are expressed as the number (percentage) for which the chi-square test or Fisher’s exact test was used accordingly. Receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off value of SIRI for MACE, and the area under the ROC curve was also calculated. SIRI was analyzed as continuous and categorical (tertiles) variables. Cumulative incidence curves were visualized using the Kaplan–Meier analyses, and the log-rank test was performed. Cox proportional hazard analyses with six models were performed to detect independent risk factors by computing the hazard ratio with a 95% confidence interval. Subgroup analyses according to age, sex, hypertension, diabetes, dyslipidemia, and ACS type were carried out. Restricted cubic spline (RCS) with 4 knots based on model 6 in the multivariate analysis was used to evaluate the association between the SIRI and MACE. To analyze the incremental value of SIRI, we introduced the SIRI into the GRACE risk score and compared their performance, for which the C-statistic, net reclassification improvement (NRI) index, and integrated discrimination improvement (IDI) index were used to evaluate the discrimination properties. The likelihood ratio (LR) test, Akaike information criterion (AIC), and Bayesian information criterion (BIC) were calculated to assess calibration properties. The statistical analyses were performed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA), R version 3.6.1 (The R Project for Statistical Computing, Vienna, Austria), and MedCalc version 20.0.1 (MedCalc Software, Ostend, Belgium).
Results
Among the 1724 patients with a median follow-up of 927 days, the mean age was 60 ± 10 years, and 1323 (76.74%) patients were men. The baseline characteristics of the participants are summarised in Table 1. Patients with higher tertiles of SIRI were older and more likely to be male, and these patients had higher percentages of smoking, dyslipidemia, previous MI, heart failure, and the presence of STEMI. These patients also had higher neutrophil and monocyte counts, high-sensitivity C-reactive protein (hs-CRP), FPG, and SYNTAX scores but lower DBP, LVEF, lymphocyte count, total cholesterol, HDL-C, and eGFR. In angiography, patients with higher SIRI tertiles had a higher SYNTAX score and more frequent left anterior descending coronary artery intervention and a lower percentage of complete revascularization. As for medicine intake, these patients had a lower percentage of aspirin intake and higher percentages of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and β-blocker prescriptions at discharge.
Table 1.
Baseline characteristics of the study population according to the SIRI tertiles.
| Variables | T1: <0.63 | T2: 0.63–1.02 | T3: >1.02 | p Value |
|---|---|---|---|---|
| SIRI | 0.46 ± 0.12 | 0.81 ± 0.11 | 1.74 ± 1.06 | <.001 |
| Age (years) | 59 ± 10 | 60 ± 11 | 61 ± 11 | .019 |
| Male sex, n (%) | 380 (66.2) | 458 (79.5) | 485 (84.5) | <.001 |
| BMI (kg/m2) | 25.66 ± 2.99 | 25.84 ± 3.03 | 25.56 ± 3.22 | .297 |
| SBP (mmHg) | 130 ± 15 | 130 ± 16 | 130 ± 18 | .926 |
| DBP (mmHg) | 77 ± 9 | 76 ± 11 | 75 ± 12 | .022 |
| Risk factors | ||||
| Smoking, n (%) | 218 (38.0) | 271 (47.0) | 272 (47.4) | .001 |
| Hypertension, n (%) | 353 (61.5) | 370 (64.2) | 376 (65.6) | .353 |
| Diabetes, n (%) | 269 (46.9) | 263 (45.7) | 260 (45.3) | .856 |
| Dyslipidemia, n (%) | 439 (76.5) | 470 (81.6) | 469 (81.7) | .041 |
| Hyperuricaemia, n (%) | 108 (18.8) | 133 (23.1) | 130 (22.6) | .153 |
| Cancer, n (%) | 2 (0.3) | 6 (1.0) | 5 (0.9) | .367 |
| Previous MI, n (%) | 88 (15.3) | 119 (20.7) | 123 (21.4) | .017 |
| Previous PCI, n (%) | 104 (18.1) | 120 (20.8) | 117 (20.4) | .465 |
| Previous CVD, n (%) | 28 (4.9) | 29 (5.0) | 43 (7.5) | .105 |
| CKD, n (%) | 13 (2.3) | 15 (2.6) | 25 (4.4) | .088 |
| Heart failure, n (%) | 31(5.4) | 32 (5.6) | 59 (10.3) | .001 |
| LVEF (%) | 65 (61–68) | 65 (60–68) | 63 (58–68) | <.001 |
| Clinical presentation | ||||
| UA, n (%) | 484 (84.3) | 417 (72.4) | 378 (65.9) | <.001 |
| NSTEMI, n (%) | 57 (9.9) | 86 (14.9) | 77 (13.4) | .034 |
| STEMI, n (%) | 33 (5.7) | 73 (12.7) | 119 (20.7) | <.001 |
| GRACE risk score | 79 (65–91) | 82 (66–100) | 87 (73–107) | <.001 |
| Laboratory results | ||||
| Neutrophil (×106/μL) | 3.07 (2.61–3.61) | 3.94 (3.42–4.55) | 5.18 (4.40–6.06) | <.001 |
| Monocyte count (×106/μL) | 0.29 (0.22–0.33) | 0.35 (0.30–0.42) | 0.48 (0.40–0.58) | <.001 |
| Lymphocyte (×106/μL) | 1.89 (1.58–2.34) | 1.73 (1.42–2.14) | 1.57 (1.31–2.00) | <.001 |
| hs-CRP (mg/L) | 0.93 (0.45–2.05) | 1.32 (0.67–2.86) | 2.43 (0.92–6.68) | <.001 |
| Total cholesterol (mmol/L) | 4.23 ± 1.00 | 4.11 ± 0.96 | 4.10 ± 1.00 | .045 |
| LDL-C (mmol/L) | 2.48 ± 0.83 | 2.41 ± 0.79 | 2.43 ± 0.80 | .274 |
| HDL-C (mmol/L) | 1.08 ± 0.24 | 1.01 ± 0.22 | 1.00 ± 0.24 | <.001 |
| Triglycerides (mmol/L) | 1.43 (0.99–1.99) | 1.49 (1.04–2.10) | 1.45 (1.01–2.12) | <.001 |
| FPG (mmol/L) | 6.22 ± 1.64 | 6.32 ± 1.72 | 6.49 ± 1.79 | .026 |
| Glycosylated haemoglobin (%) | 6.10 (5.60–7.03) | 6.05 (5.60–7.10) | 6.10 (5.60–7.20) | .300 |
| eGFR (mL/min/1.73 m2) | 93.19 ± 14.04 | 93.87 ± 13.50 | 90.55 ± 15.70 | <.001 |
| Angiographic findings | ||||
| Left-main and/or multivessel disease, n (%) | 477 (83.1) | 486 (84.4) | 498 (86.8) | .216 |
| Chronic total occlusion, n (%) | 123 (21.4) | 122 (21.2) | 120 (20.9) | .977 |
| Lesions with length >20 mm, n (%) | 299 (52.1) | 284 (49.3) | 322 (56.1) | .068 |
| Bifurcation or trifurcation lesions, n (%) | 428 (74.6) | 440 (76.4) | 431 (75.1) | .761 |
| SYNTAX score | 18 (12–26) | 20 (12–28) | 22 (14–32) | <.001 |
| Procedural results | ||||
| Target vessel-LM, n (%) | 17 (6.3) | 21 (8.0) | 18 (6.9) | .751 |
| Target vessel-LAD, n (%) | 117 (43.5) | 143 (54.4) | 137 (52.7) | .026 |
| Target vessel-LCX, n (%) | 71 (26.4) | 75 (28.5) | 78 (30.0) | .651 |
| Target vessel-RCA, n (%) | 119 (44.2) | 102 (38.8) | 102 (39.2) | .363 |
| Complete revascularization, n (%) | 380 (66.2) | 358 (62.2) | 320 (55.7) | .001 |
| Periprocedural medications | ||||
| Low molecular weight heparin, n (%) | 368 (64.1) | 380 (66.0) | 389 (67.8) | .425 |
| Bivalirudin, n (%) | 69 (12.0) | 64 (11.1) | 63 (11.0) | .832 |
| GP IIb/IIIa receptor antagonist, n (%) (17.0) 42 (22.3) | 111 (19.3) | 129 (22.4) | 129 (22.5) | .336 |
| Prescription at discharge | ||||
| Aspirin, n (%) | 573 (99.8) | 575 (99.8) | 560 (97.6) | <.001 |
| Clopidogrel, n (%) | 519 (90.4) | 534 (92.7) | 530 (92.3) | .315 |
| Ticagrelor, n (%) | 55 (9.6) | 42 (7.3) | 44 (7.7) | .321 |
| Statins, n (%) | 574 (100.0) | 576 (100.0) | 574 (100.0) | .135 |
| ACEI/ARBs, n (%) | 256 (44.6) | 254 (44.1) | 320 (55.7) | <.001 |
| β-blockers, n (%) | 384 (66.9) | 403 (70.0) | 426 (74.2) | .024 |
| Oral anticoagulants, n (%) | 4 (0.7) | 4 (0.7) | 0 (0.0) | .135 |
| Nitroglycerin, n (%) | 412 (71.8) | 436 (75.7) | 444 (77.4) | .082 |
| Calcium channel blockers, n (%) | 131 (22.8) | 156 (27.1) | 145 (25.3) | .247 |
SIRI: systemic inflammatory response index; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MI: myocardial infarction; PCI: percutaneous coronary intervention; CVD: cerebrovascular disease; CKD: chronic kidney disease; LVEF: left ventricular ejection fraction; UA: unstable angina; NSTEMI: non ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; GRACE: Global registry of acute coronary events; hs-CRP: high sensitive C-reactive protein; LDL-C: low-density lipoprotein-cholesterol; HDL-C: high-density lipoprotein-cholesterol; FPG: fasting plasma glucose; eGFR: estimated glomerular filtration rate; SYNTAX: Synergy between PCI with TAXUS and Cardiac Surgery; LM: left main artery; LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker.
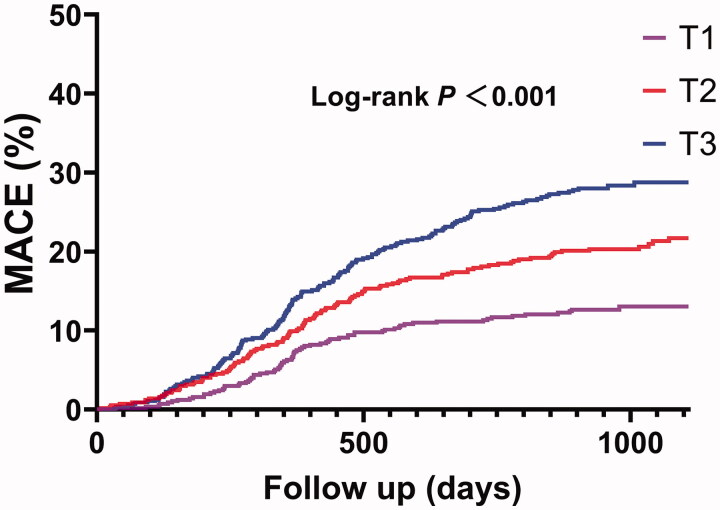
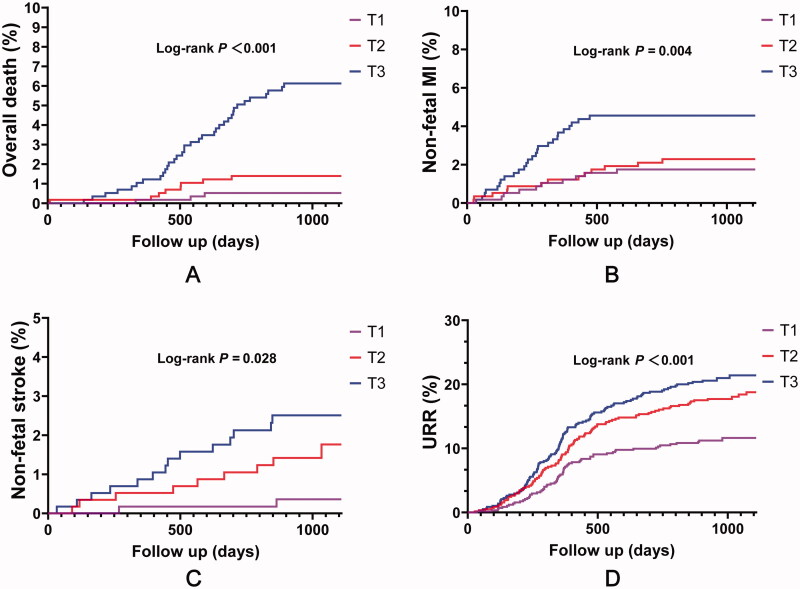
At follow-up, patients with higher SIRI tertiles had a higher percentage of MACE (Table 2). These patients had higher percentages of overall death, non-fatal MI, non-fatal stroke, and URR (p < .05 for all). Kaplan–Meier survival analysis showed that the cumulative incidence of MACE increased with higher SIRI tertiles (log-rank test, p < .001) (Figure 1). Considering each component of MACE, patients with higher SIRI tertiles had a higher cumulative incidence of overall death (p < .001), non-fatal MI (p = .004), non-fatal stroke (p = .028), and URR (p < .001) (Figure 2). When considering SIRI as a continuous variable, SIRI was significantly associated with MACE in both univariate and multivariate Cox regression analyses (p < .05 for all models) (Table 3). Additionally, when considering SIRI as a categorical variable, this association was also significant (p < .05 for all models) (Table 3).
Table 2.
Adverse cardiovascular events according to SIRI tertiles during follow-up.
| Adverse cardiovascular events | T1: <0.63 | T2: 0.63–1.02 | T3: >1.02 | p Value |
|---|---|---|---|---|
| MACE, n (%) | 73 (12.7) | 120 (20.8) | 162 (28.2) | <.001 |
| Overall death, n (%) | 3 (0.5) | 8 (1.4) | 35 (6.1) | <.001 |
| Non-fatal MI, n (%) | 10 (1.7) | 13 (2.3) | 26 (4.5) | .010 |
| Non-fatal stroke, n (%) | 2 (0.3) | 9 (1.6) | 14 (2.4) | .012 |
| URR, n (%) | 65 (11.3) | 104 (18.1) | 118 (20.6) | <.001 |
The MACE was defined as the composite of overall death, non-fatal stroke, non-fatal MI, and URR.
MACE: major adverse cardiovascular events; MI: myocardial infarction; URR: unplanned repeat revascularization.
Figure 1.
Cumulative incidence plot of the MACE stratified by SIRI tertiles. MACE included overall death, non-fatal MI, non-fatal stroke, and URR. MI: myocardial infarction; URR: unplanned repeat revascularization.
Figure 2.
Cumulative incidence plots of each component of MACE stratified by SIRI tertiles. (A) overall death; (B) non-fatal stroke; (C) non-fatal MI; (D) URR. MI: myocardial infarction; URR: unplanned repeat revascularization.
Table 3.
Univariate and multivariate Cox proportional hazard analyses for the MACE according to the SIRI value.
| SIRI as a continuous variable |
SIRI as a categorical variable |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95 CI% | p Value | ||
| Model 1 | 1.218 | 1.138–1.303 | <.001 | T1 | Reference | ||
| T2 | 1.700 | 1.271–2.274 | <.001 | ||||
| T3 | 2.402 | 1.824–3.169 | <.001 | ||||
| Model 2 | 1.213 | 1.133–1.299 | <.001 | T1 | Reference | ||
| T2 | 1.718 | 1.282–2.303 | <.001 | ||||
| T3 | 2.437 | 1.838–3.231 | <.001 | ||||
| Model 3 | 1.203 | 1.120–1.292 | <.001 | T1 | Reference | ||
| T2 | 1.654 | 1.231–2.223 | .001 | ||||
| T3 | 2.374 | 1.782–3.164 | <.001 | ||||
| Model 4 | 1.145 | 1.054–1.243 | .001 | T1 | Reference | ||
| T2 | 1.572 | 1.168–2.115 | .003 | ||||
| T3 | 2.132 | 1.585–2.866 | <.001 | ||||
| Model 5 | 1.114 | 1.022–1.214 | .014 | T1 | Reference | ||
| T2 | 1.565 | 1.162–2.107 | .003 | ||||
| T3 | 1.944 | 1.443–2.618 | <.001 | ||||
| Model 6 | 1.127 | 1.034–1.229 | .007 | T1 | Reference | ||
| T2 | 1.582 | 1.174–2.132 | .003 | ||||
| T3 | 1.999 | 1.482–2.696 | <.001 | ||||
Model 1: Unadjusted.
Model 2: Adjusted for age, sex.
Model 3: Model 2 + smoking, hypertension, diabetes, dyslipidemia, cancer, hyperuricaemia, previous MI, previous PCI, previous CVA, type of ACS.
Model 4: Model 3 + hs-CRP, HDL-C, LDL-C, total cholesterol, triglycerides.
Model 5: Model 4 + GRACE risk score, SYNTAX score, complete revascularization.
Model 6: Model 5 + aspirin at discharge + ACEI/ARBs at discharge + β blocker at discharge.
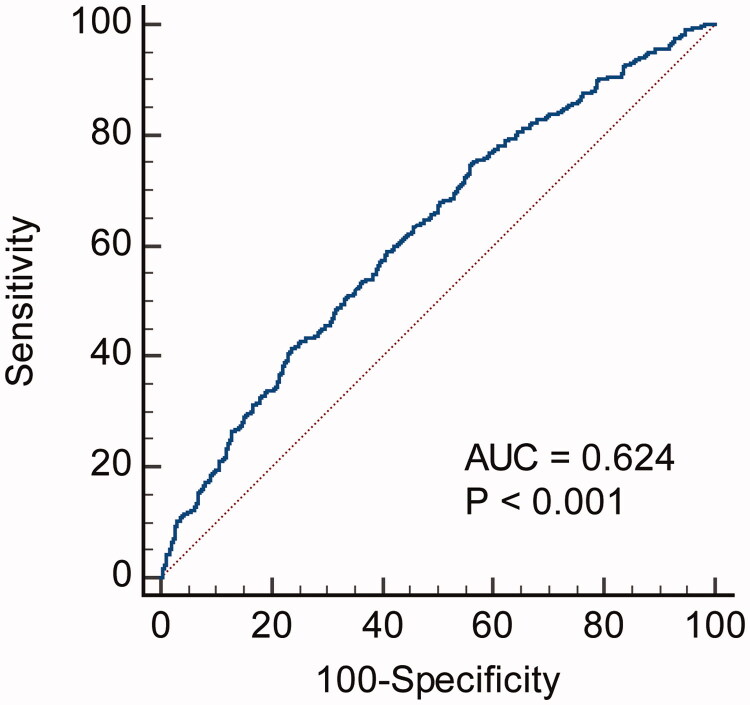
In view of each specific adverse event, SIRI as a continuous variable was associated with overall death, non-fatal MI, and URR in univariate Cox regression analyses (Table 4). In multivariate analyses, SIRI exhibited an association with non-fatal MI and URR. ROC curve analysis showed that the C-index of SIRI in predicting MACE was 0.624, with a sensitivity of 75.2% and specificity of 43.6% (Figure 3). Moreover, compared with each specific component of SIRI (neutrophils, monocytes, and lymphocytes), the SIRI has the highest C-index (Supplemental Table 1). As a categorical variable according to tertiles, SIRI was associated with overall death, non-fatal MI, non-fatal stroke, and URR in univariate analyses. In multivariate analyses, the SIRI was associated with overall death, non-fatal MI, and URR.
Table 4.
Univariate and multivariate Cox proportional hazard analyses for adverse prognosis according to the SIRI.
| Univariate analyses |
Multivariate analyses |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | ||
| SIRI – categorical variable | |||||||
| Overall death | T1 | Reference | Reference | ||||
| T2 | 2.662 | 0.706–10.034 | .148 | 2.272 | 0.590–8.752 | .233 | |
| T3 | 11.912 | 3.664–38.730 | <.001 | 7.206 | 2.080–24.963 | .002 | |
| Non-fatal MI | T1 | Reference | Reference | ||||
| T2 | 1.300 | 0.570–2.964 | .533 | 1.339 | 0.580–3.092 | .494 | |
| T3 | 2.665 | 1.285–5.525 | .008 | 2.384 | 1.078–5.271 | .032 | |
| Non-fatal stroke | T1 | Reference | Reference | ||||
| T2 | 4.480 | 0.968–20.737 | .055 | 4.218 | 0.892–19.944 | .069 | |
| T3 | 7.177 | 1.631–31.580 | .009 | 4.659 | 0.960–22.610 | .056 | |
| URR | T1 | Reference | Reference | ||||
| T2 | 1.643 | 1.205–2.240 | .002 | 1.554 | 1.130–2.138 | .007 | |
| T3 | 1.940 | 1.433–2.627 | <.001 | 1.724 | 1.245–2.388 | .001 | |
| SIRI – continuous variable | |||||||
| Overall death | 1.364 | 1.207–1.541 | <.001 | 1.096 | 0.908–1.324 | .339 | |
| Non-fatal MI | 1.325 | 1.158–1.515 | <.001 | 1.235 | 1.030–1.480 | .022 | |
| Non-fatal stroke | 1.196 | 0.905–1.580 | .208 | 0.961 | 0.555–1.663 | .887 | |
| URR | 1.175 | 1.078–1.282 | <.001 | 1.129 | 1.015–1.255 | .025 | |
Multivariate analyses were based on model 6 in Table 3.
MACE: major adverse cardiovascular events; MI: myocardial infarction; URR: unplanned repeat revascularization.
Figure 3.
ROC analysis shows the predictive value of SIRI for MACE.
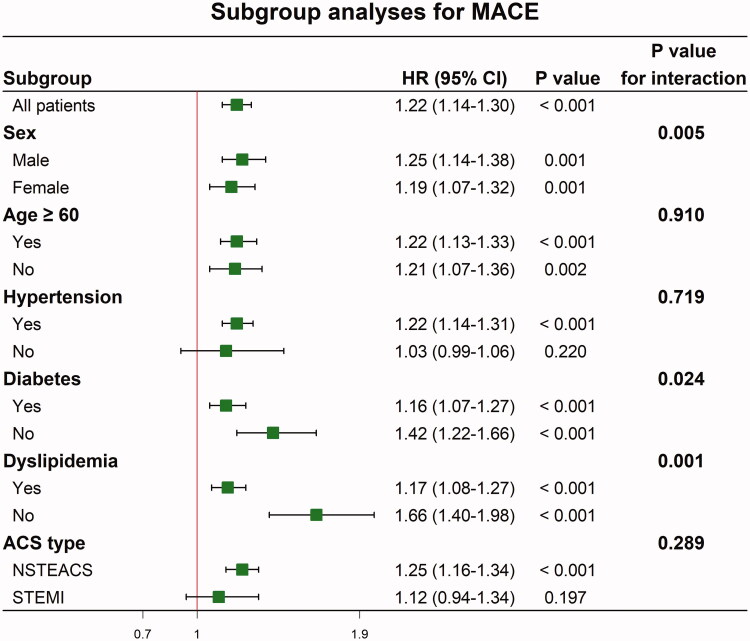
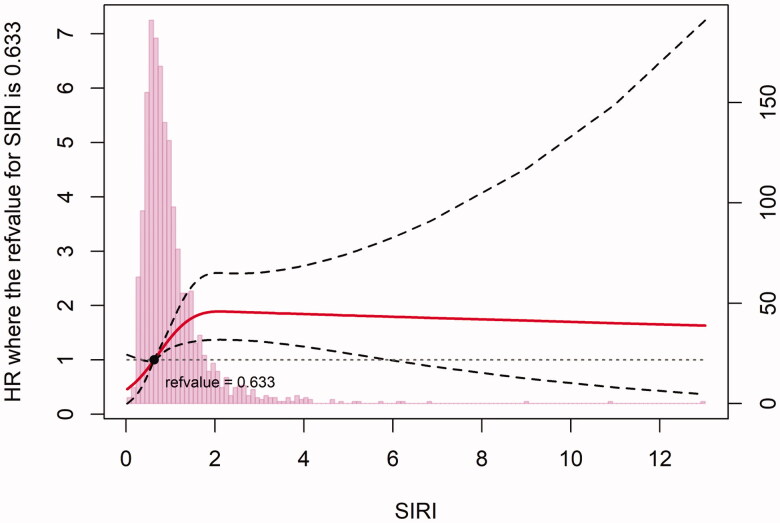
Table 5 shows that the inclusion of SIRI improved MACE prediction compared with the GRACE risk score. SIRI increased the C-statistic value and showed significant improvement in the NRI and IDI. SIRI also increased the calibration properties as indicated in the LR test (p < .001), AIC, and BIC. Subgroup analyses according to sex, age, hypertension, diabetes, dyslipidemia, and ACS type are shown in Figure 4. In patients presenting with STEMI or without hypertension, SIRI was not significantly associated with MACE. The restricted cubic spline with 4 knots based on multivariate analysis of model 6 showed that SIRI was positively associated with MACE (p < .001) (Figure 5).
Table 5.
Model performance after the addition of SIRI to the GRACE risk score for predicting clinical outcomes.
| MACE |
Death |
Death + MI |
||||
|---|---|---|---|---|---|---|
| GRACE | GRACE + SIRI | GRACE | GRACE + SIRI | GRACE | GRACE + SIRI | |
| Discrimination | ||||||
| C-statistic | 0.527 | 0.601 | 0.681 | 0.742 | 0.613 | 0.688 |
| p-Value | <.001 | <.001 | <.001 | |||
| IDI (95% CI) | 0.007 (0.001 to 0.020) | −0.001 (−0.002 to 0.004) | 0.189 (0.074 to 0.292) | |||
| p-Value | .040 | .851 | <.001 | |||
| NRI (95% CI) | 0.175 (0.027 to 0.215) | −0.001 (−0.002 to 0.004) | −0.001 (−0.001 to 0.012) | |||
| p-Value | .020 | .059 | .119 | |||
| Calibration | ||||||
| LR test, p-value | <.001 | .018 | <.001 | |||
| AIC | 5195 | 5179 | 661 | 658 | 1307 | 1296 |
| BIC | 5199 | 5188 | 663 | 661 | 1309 | 1301 |
MACE: major adverse cardiovascular events; GRACE: Global registry of acute coronary events; MI: myocardial infarction; IDI: integrated discrimination improvement; NRI: net reclassification improvement; LR: likelihood ratio; AIC: Akaike information criterion; BIC: Bayesian information criterion.
Figure 4.
Subgroup analyses of continuous SIRI for MACE.
Figure 5.
Restricted cubic spline demonstrated the association between SIRI and MACE.
Discussion
This study was focussed on the association of SIRI with adverse prognosis in patients with ACS undergoing PCI, and the remarkable significance of SIRI was revealed. Specifically, we found a quantitative association between SIRI and MACE. Moreover, SIRI increased the prognostic role of the GRACE risk score and was independent of hs-CRP.
The inflammatory process in atherosclerosis is dominated by the innate immune response in the early stage of plaque formation. As a precursor of foam cells, monocytes transform into macrophages, proliferate, and phagocytose oxidized LDL-C, ultimately forming the lipid core of plaque [20]. Monocytes also promote destabilization of the fibrous cap, participating in plaque rupture through secreting lytic enzymes, such as matrix metalloproteinases [21]. Monocytes are also involved in thrombus propagation, leading to a coagulation cascade [22]. The number of monocytes is negatively associated with the extent of myocardial salvage and recovery of left ventricular function after MI [23]. Inhibition of monocyte infiltration and differentiation could attenuate early atherogenesis [24].
Secretion by neutrophils of reactive oxygen species and proteases results in the activation and dysregulation of the endothelial layer, resulting in LDL-C extravasation and promoting foam cell formation. Neutrophils localize near plaques, especially those with rupture-prone features. During the atherosclerosis process, neutrophils can also enhance monocyte adhesion and transmigration. Epidemiological studies have shown that circulating neutrophil count positively correlates with coronary atherosclerotic risk [25]. A higher neutrophil count is an independent predictor of adverse cardiovascular events [26]. Infiltration of neutrophils into advanced lesions has been shown to induce collagen degradation and necrosis [27]. Histopathologic studies show that neutrophil count is strongly associated with the formation of rupture-prone lesions, suggesting a role in plaque destabilization [28]. A lower lymphocyte level has been associated with poor cardiovascular outcomes in ACS [29]. Lymphocytopenia is independently associated with the occurrence of mechanical complications after MI [30]. Patients with heart failure who have a lower lymphocyte count have a higher mortality rate [31]. Higher MLR has been associated with the occurrence of heart failure, myocarditis, and coronary severity [32–34]. In stable patients with CAD, lower lymphocyte count is associated with a higher mortality rate [35].
Inflammation plays an important role in the pathogenesis of CAD and especially ACS. At present, many inflammatory indicators such as hs-CRP, neutrophil count, and monocyte count are associated with patient prognosis in CAD. However, a single indicator of inflammation is not sufficient to predict the severity of inflammation. The SIRI, which combines three inflammatory biomarkers, is a comprehensive, easily accessible, and inexpensive indicator of chronic low-grade inflammation. SIRI also includes the NLR and MLR, which might be a more sensitive and useful inflammatory biomarkers than any of these alone. Indeed, SIRI is associated with many diseases. A higher SIRI is positively associated with the risk and poor prognosis of stroke [17,19]. SIRI has also been evaluated as a biomarker in the diagnosis and assessment of rheumatoid arthritis [18]. In patients with cancer, SIRI could predict postoperative survival and disease recurrence [36,37]. In this study, we showed that a higher SIRI was correlated with poor clinical presentation, where patients with a higher SIRI had higher percentages of smoking, dyslipidemia, history of MI, heart failure, and STEMI and a higher SYNTAX score. These patients also had lower LVEF, HDL-C and eGFR. A higher SIRI may be associated with more severe low-grade inflammation. Similarly, NLR is negatively correlated with HDL-C and positively correlated with the SYNTAX score [38,39]. Higher NLR is associated with the presence of diabetes and CKD [40,41]. MLR is also positively correlated with the SYNTAX score and vulnerability of coronary lesions [42,43].
As the combination of NLR and MLR, SIRI may have similar scenarios of treatment. Previous studies investigated treatment for patients with a high NLR. Neither proprotein convertase subtilisin/Kexin type-9 inhibitors nor rosuvastatin or bococizumab could significantly decrease the NLR [12]. Nebivolol, but not metoprolol, has antioxidant and anti-inflammatory properties and could decrease the NLR [44]. Additionally, amlodipine and valsartan could decrease the NLR in patients with newly diagnosed hypertension [45]. In patients with hypercholesterolaemia, atorvastatin could significantly decrease the NLR [46]. Inhibition of interleukin-1β with canakinumab could lower the NLR in a dose-dependent response and reduce cardiovascular events [47,48]. Colchicine, which can inhibit the inflammatory response mediated by monocytes and neutrophils, could reduce MACE in patients with CAD [49,50]. Methotrexate cannot reduce the inflammatory response and has no effects on cardiovascular events [51]. No studies have investigated interventions in terms of a high SIRI; therefore, further studies on lowering the SIRI are needed.
Different kinds of predictive risk scores have been developed in ACS, among which the GRACE and Thrombolysis in Myocardial Infarction (TIMI) risk scores are the most widely used. In particular, studies have shown that the GRACE score is more efficient than the TIMI score [52,53]. However, the GRACE risk score does not include inflammatory indicators. Some potential risk factors beyond the GRACE risk score system have been identified, and these factors improve the predictive ability of adverse events after ACS. Considering the inflammatory biomarkers, hs-CRP and monocyte to HDL-C ratio could improve the predictive power of the GRACE risk score [14,54]. In this study, we showed that the novel biomarker SIRI could also improve the prognostic value of the GRACE risk score in patients with ACS undergoing PCI. Several bleeding risk scores include the Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) and Acute Catheterisation and Urgent Intervention Triage Strategy (ACUITY) scores. However, whether SIRI has predictive value in bleeding and whether it can improve the predictive value of CRUSADE and ACUITY scores are still unknown; further investigation is needed.
Limitations
First, this was a single centre study based on the Chinese population, and there was no external validation of SIRI in other populations; therefore, the general application of the findings should be made with caution. Second, future studies on long-term events are needed. Third, the predictive value of SIRI based on the variates measured at discharge was unknown. Finally, owing to a lack of continuous monitoring of blood tests in this study, admission SIRI was evaluated at one-time point, and fluctuation of SIRI was not considered.
Conclusion
SIRI was found to be a reliable and independent prognostic factor for patients with ACS undergoing PCI.
Supplementary Material
Funding Statement
This work was supported by China Postdoctoral Science Foundation [2021M692253]; Beijing Postdoctoral Research Foundation [2021-ZZ-023]; Beijing Municipal Administration of Hospitals Mission Plan [SML20180601].
Ethical approval
This study was conducted under the Declaration of Helsinki and was approved by the Local Ethics Committee of Beijing Anzhen Hospital, Capital Medical University.
Author contributions
Kangning Han performed the statistical analysis, interpreted the data and drafted the manuscript. Xiaoteng Ma and Yujie Zhou designed the study and revised the manuscript. All authors contributed to the acquisition of data and final approval of the version to be published. All authors agreed to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data of this study are available from the corresponding author upon request.
References
- 1.Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1667–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natorska J, Wypasek E, Grudzień G, et al. Does diabetes accelerate the progression of aortic stenosis through enhanced inflammatory response within aortic valves? Inflammation. 2012;35(3):834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäck M, Yurdagul A, Tabas I, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. [DOI] [PubMed] [Google Scholar]
- 5.Ensrud K, Grimm RH.. The white blood cell count and risk for coronary heart disease. Am Heart J. 1992;124(1):207–213. [DOI] [PubMed] [Google Scholar]
- 6.Gurm HS, Bhatt DL, Lincoff AM, et al. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89(10):1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Tabas I, Fredman G, et al. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114(12):1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25–31. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler JG, Mussolino ME, Gillum RF, et al. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30 374 individuals. Eur Heart J. 2004;25(15):1287–1292. [DOI] [PubMed] [Google Scholar]
- 10.Anrather J, Iadecola C.. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landman GWD, Kleefstra N, Groenier KH, et al. Inflammation biomarkers and mortality prediction in patients with type 2 diabetes (ZODIAC-27). Atherosclerosis. 2016;250:46–51. [DOI] [PubMed] [Google Scholar]
- 12.Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song FH, Zheng YY, Tang JN, et al. A correlation between monocyte to lymphocyte ratio and long-term prognosis in patients with coronary artery disease after PCI. Clin Appl Thromb Hemost. 2021;27:107602962199971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Han K, Yang L, et al. Adjustment of the GRACE risk score by monocyte to high-density lipoprotein ratio improves prediction of adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Front Cardiovasc Med. 2022;8:755806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MQ, Wang HY, Shi WR, et al. Estimate of prevalent hyperuricemia by systemic inflammation response index: results from a rural Chinese population. Postgrad Med. 2021;133(2):242–249. [DOI] [PubMed] [Google Scholar]
- 16.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Xing Z, Zhou K, et al. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;16:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, He H, Zang Y, et al. Systemic inflammation response index (SIRI) as a novel biomarker in patients with rheumatoid arthritis: a multi-center retrospective study. Clin Rheumatol. 2022. [DOI] [PubMed] [Google Scholar]
- 19.Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oude Nijhuis M, van Keulen J, Pasterkamp G, et al. Activation of the innate immune system in atherosclerotic disease. Curr Pharm Des. 2007;13(10):983–994. [DOI] [PubMed] [Google Scholar]
- 21.Han YP, Tuan TL, Wu H, et al. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altieri DC, Morrissey JH, Edgington TS.. Adhesive receptor mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation protease cascade. Proc Natl Acad Sci USA. 1988;85(20):7462–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–138. [DOI] [PubMed] [Google Scholar]
- 24.Lindau A, Härdtner C, Hergeth SP, et al. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res Cardiol. 2016;111(2):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostis JB, Turkevich D, Sharp J.. Association between leukocyte count and the presence and extent of coronary atherosclerosis as determined by coronary arteriography. Am J Cardiol. 1984;53(8):997–999. [DOI] [PubMed] [Google Scholar]
- 26.Guasti L, Dentali F, Castiglioni L, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106(4):591–599. [DOI] [PubMed] [Google Scholar]
- 27.Mawhin MA, Tilly P, Zirka G, et al. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc Res. 2018;114(12):1656–1666. [DOI] [PubMed] [Google Scholar]
- 28.Ionita MG, Van Den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. ATVB. 2010;30(9):1842–1848. [DOI] [PubMed] [Google Scholar]
- 29.Bian C, Wu Y, Shi Y, et al. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessels. 2010;25(6):469–473. [DOI] [PubMed] [Google Scholar]
- 30.Widmer A, Linka AZ, Attenhofer Jost CH, et al. Mechanical complications after myocardial infarction reliably predicted using C-reactive protein levels and lymphocytopenia. Cardiology. 2003;99(1):25–31. [DOI] [PubMed] [Google Scholar]
- 31.Acanfora D, Gheorghiade M, Trojano L, et al. Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J. 2001;142(1):167–173. [DOI] [PubMed] [Google Scholar]
- 32.Gijsberts CM, Ellenbroek GHJM, ten Berg MJ, et al. Effect of monocyte-to-lymphocyte ratio on heart failure characteristics and hospitalizations in a coronary angiography cohort. Am J Cardiol. 2017;120(6):911–916. [DOI] [PubMed] [Google Scholar]
- 33.Mirna M, Schmutzler L, Topf A, et al. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. 2021;11(1):18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong S, Gao X, Xu F, et al. Association of lymphocyte to monocyte ratio with severity of coronary artery disease. Medicine. 2018;97(43):e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ommen SR, Gibbons RJ, Hodge DO, et al. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79(6):812–814. [DOI] [PubMed] [Google Scholar]
- 36.Geng Y, Zhu D, Wu C, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018;65:503–510. [DOI] [PubMed] [Google Scholar]
- 37.Xin Y, Zhang X, Li Y, et al. A systemic inflammation response index (SIRI)-based nomogram for predicting the recurrence of early stage hepatocellular carcinoma after radiofrequency ablation. Cardiovasc Intervent Radiol. 2022;45(1):43–53. [DOI] [PubMed] [Google Scholar]
- 38.Varol E, Bas HA, Aksoy F, et al. Relationship between neutrophil-lymphocyte ratio and isolated low high-density lipoprotein cholesterol. Angiology. 2014;65(7):630–633. [DOI] [PubMed] [Google Scholar]
- 39.Altun B, Turkon H, Tasolar H, et al. The relationship between high-sensitive troponin T, neutrophil lymphocyte ratio and SYNTAX score. Scand J Clin Lab Invest. 2014;74(2):108–115. [DOI] [PubMed] [Google Scholar]
- 40.Ulu SM, Dogan M, Ahsen A, et al. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15(11):942–947. [DOI] [PubMed] [Google Scholar]
- 41.Solak Y, Yilmaz MI, Sonmez A, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(4):532–540. [DOI] [PubMed] [Google Scholar]
- 42.Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord. 2017;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan Z, Ji H, Li Y, et al. Relationship between monocyte-to-lymphocyte ratio and coronary plaque vulnerability in patients with stable angina. Biomark Med. 2017;11(11):979–990. [DOI] [PubMed] [Google Scholar]
- 44.Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62:388–393. [DOI] [PubMed] [Google Scholar]
- 45.Karaman M, Balta S, Ay SA, et al. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin Exp Hypertens. 2013;35(7):516–522. [DOI] [PubMed] [Google Scholar]
- 46.Akin F, Ayça B, Köse N, et al. Effect of atorvastatin on hematologic parameters in patients with hypercholesterolemia. Angiology. 2013;64(8):621–625. [DOI] [PubMed] [Google Scholar]
- 47.Everett BM, MacFadyen JG, Thuren T, et al. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J Am Coll Cardiol. 2020;76(14):1660–1670. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 49.Imazio M, Nidorf M.. Colchicine and the heart. Eur Heart J. 2021;42(28):2745–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsivgoulis G, Katsanos AH, Giannopoulos G, et al. The role of colchicine in the prevention of cerebrovascular ischemia. Curr Pharm Des. 2018;24(6):668–674. [DOI] [PubMed] [Google Scholar]
- 51.Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan AT, Yan RT, Tan M, et al. Risk scores for risk stratification in acute coronary syndromes: useful but simpler is not necessarily better. Eur Heart J. 2007;28(9):1072–1078. [DOI] [PubMed] [Google Scholar]
- 53.Aragam KG, Tamhane UU, Kline-Rogers E, et al. Does simplicity compromise accuracy in ACS risk prediction? A retrospective analysis of the TIMI and GRACE risk scores. PLoS One. 2009;4(11):e7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin XL, Sun HX, Li FQ, et al. Admission high-sensitivity C-reactive protein levels improve the grace risk score prediction on in-hospital outcomes in acute myocardial infarction patients. Clin Cardiol. 2022;45(3):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of this study are available from the corresponding author upon request.