Abstract
Parallel processing is more stable than serial processing in many areas that employ interconnected activities. This hypothesis was tested for microbial community function using two quadruplicate sets of methanogenic communities, each set having substantially different populations. The two communities were maintained at a mean cell residence time of 16 days and a mean glucose loading rate of 0.34 g/liter-day in variable-volume reactors. To test stability to perturbation, they were subjected to an instantaneous glucose pulse that resulted in a 6.8-g/liter reactor concentration. The pattern of accumulated products in response to the perturbation was analyzed for various measures of functional stability, including resistance, resilience, and reactivity for each product. A new stability parameter, “moment of amplification envelope,” was used to compare the soluble compound stability. These parameters indicated that the communities with predominantly parallel substrate processing were functionally more stable in response to the perturbation than the communities with predominantly serial substrate processing. The data also indicated that there was good replication of function under perturbed conditions; the degrees of replication were 0.79 and 0.83 for the two test communities.
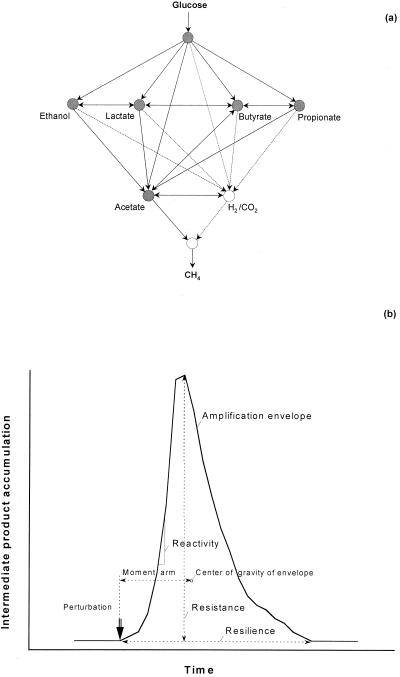
Parallel processing of complex interconnected activities has been proven to be a more efficient and robust approach in many systems, including information processing (5, 13), fluid flow (7), neurobiology (10), and communication (5). This phenomenon may also hold true for mixed microbial systems since functionally they are also equivalent to a network of metabolic activities of many interdependent populations. Methanogenic systems are good examples of networks in which a number of fermentative, syntrophic, and methanogenic populations work together as a community to convert organic substrates to methane via the well-recognized anaerobic food chain (Fig. 1a). If the above hypothesis is true, a substrate perturbation processed through multiple routes in parallel should result in a higher functional stability of the system because of the potential distribution of substrate to several populations or activities. Alternatively, if the same perturbation is processed through routes involving a smaller number of intermediate products in a serial manner, then the system should be less stable. The kinetic and thermodynamic constraints on each route may, in addition, modulate the outcome. Several issues emerge at the outset for testing such a hypothesis; these issues include establishment of the two systems, quantification of the functional stability, and replication of results. We address these issues as follows.
FIG. 1.
Anaerobic food chain as a network of substrate flow through a methanogenic community (a) and ecological parameters of functional stability (b).
Performance of the same overall function by different microbial communities under the referential state is perhaps ubiquitous. However, selecting two communities that perform the same overall function (viz., conversion of complex organic substrate to methane) via parallel and serial routes in both referential and perturbed states is extremely difficult, if not impossible, with our current knowledge of microbial systems. To approximate the processing differences, we used two methanogenic communities that originated from anaerobic digestor sludge from the same wastewater treatment plant but differed widely in their maintenance periods on glucose as well as in their dominant fermentative and methanogenic populations (4). One inoculum source was operated for 17.5 mean cell residence times (MCRTs), and the other was operated only for 5.8 MCRTs before a substrate perturbation was applied. Based on these differences, we expected that the two communities would also differ in their responses to the perturbation. Whether they would conform to the parallel or serial model of substrate flow under the perturbed conditions was not known a priori.
To measure functional stability, we adopted parameters described in ecology (6, 11) in terms of the amplification envelope of key intermediate products in response to a perturbation (Fig. 1b). The two main parameters obtained from this envelope are resistance and resilience. Resistance of a community with respect to an intermediate product is defined as the maximum accumulation of the product. It is a measure of the buffering capacity of the community with respect to the corresponding intermediate product (11). Resilience is defined as the time taken by the accumulated intermediate product to return to its referential state (11). As defined, a higher numerical value denotes lower resistance or resilience. A third component of the amplification envelope that may be useful in characterizing the stability is reactivity, which is defined by the maximum slope of the rising limb of the envelope (11).
For replication, we used four identical reactors for each inoculum source. Replication was an important part of this study because it is possible that two communities similar in function under a referential state may perform quite differently under perturbed conditions. This may be due to underlying differences that may exist in the microbial community structure or in the biochemical pathways that are operating in the two communities. It was anticipated, however, that multiple communities originating from the same source and maintained under identical conditions without contamination should be similar with respect to function under both referential state and perturbed conditions.
The responses of the two communities to the applied perturbation indicated that their functional stabilities were related to the substrate flow pattern. We observed greater stability in the communities that processed glucose by parallel pathways. In addition, the functional responses of the two communities were found to be replicable within each set under perturbed conditions.
MATERIALS AND METHODS
Reactor operation, inoculum source, and characteristics.
Two sets of variable-volume methanogenic reactors were operated with an effective MCRT of 16 days. One set, designated the high-spirochete (HS) set (reactors 5 through 8), was dominated by spirochetes, short rods, and methanosarcinas (4), and the other set, designated the low-spirochete (LS) set (reactors 1 through 4), was dominated by cocci and methanosaetas. Each reactor consisted of a 500-ml reagent bottle (Fisher Scientific, Pittsburg, Pa.) sealed with a butyl rubber stopper (Fisher Scientific) and maintained at 35°C in a temperature-controlled water bath (Lab Line Instruments, Inc., Melrose Park, Ill.). Reactor contents were completely mixed with stir bars and were protected from light. Feeding and sampling ports for the liquid and headspace were made by using 2- to 6-in.-long 18-gauge syringe needles (Fisher Scientific), 3/8-in.-diameter tubing (low O2 permeability; Cole Parmer Instrument Co., Vernon Hills, Ill.), and plastic connectors and valves (Cole Parmer). A sterile glucose solution (8 g of glucose per liter, 7 g of sodium bicarbonate per liter) and a sterile nutrient solution (40× diluted) (16) were fed at rates of 12.5 and 5 ml/day, respectively, by using 140-ml syringes (Sherwood Medical Co., Norfolk, Nebr.) and syringe pumps (Harvard Apparatus, Inc., Holliston, Mass.). A 3.5-ml sample of each suspended culture was removed daily for measurement of pH, optical density, and soluble intermediate products.
After a period of 8 days, the 112 ml of suspended culture that accumulated was removed from each reactor and used for substrate utilization and community characterization assays. As a result, the liquid volume of the reactors varied from 250 to 362 ml over a period of 8 days (causing the glucose loading rate to cycle between 0.40 and 0.28 g/liter-day over an 8-day period). This sampling and wasting schedule resulted in an MCRT of 16 days. Each wasted volume was replaced by an equivalent volume of an oxygen-free CO2-N2 (50:50) gas mixture from a 4-liter Tedlar gas collection bag (Cole Parmer) that was filled immediately prior to wasting. These bags were also used to collect the gases produced over the 8-day period and to provide replacement gas for daily sampling. The source and purity of all chemicals used in this study for feeding or standardization were the same as those described previously (16).
Inocula for both sets of reactors were obtained from two 18-liter mother reactors fed glucose and seeded with anaerobic digester sludge from the municipal wastewater treatment facility at Jackson, Mich. The mother reactor for the HS set operated for more than 4 years under two different operating conditions. For the first 3.5 years, it operated at a loading rate of 0.8 g/liter-day and at an MCRT of 10 days. Nearly 200 days prior to its use as the inoculum source for the HS set, it received additional anaerobic digester sludge from the same source and was switched to a loading rate of 0.5 g/liter-day and an MCRT of 16 days. The mother reactor for the LS set was operated for 60 days at an MCRT of 16 days and at a loading rate of 0.5 g/liter-day.
At the time of inoculation of the two sets of reactors, the soluble organic carbon removal efficiency measured by chemical oxygen demand (COD) for both mother reactors was greater than 90%. The maximum substrate utilization rates for the mother reactor of the HS set were 29.1 ± 3.2 μmol/mg of protein/h for glucose, 7.6 ± 3.3 μmol/mg of protein/h for lactate, 2.5 ± 0.45 μmol/mg of protein/h for acetate, and 0.92 ± 0.18 μmol/mg of protein/h for butyrate. For hydrogen, the maximum substrate utilization rate, measured as the rate of production of CH4, was 11.4 ± 4.6 μmol/mg of protein/h. A methanogenic inhibition assay performed with bromoethanesulfonic acid (12) indicated that at 1 mM glucose fed in batch, the mother reactor for the HS set accumulated mostly acetate while the mother reactor for the LS set accumulated both acetate and butyrate.
Analytical techniques and assays.
The efficiency of soluble organic carbon removal was monitored by analyzing COD and intermediate soluble products, including acetate, butyrate, isobutyrate, propionate, formate, lactate, and ethanol. Cells were removed by centrifugation at 6,000 × g for 15 min, and an appropriate amount of the supernatant fluid was digested for COD analysis by using Hach 0- to 1,500-ppm-range COD vials (Hach Company, Loveland, Colo.). Glucose and soluble intermediate products were analyzed with a high-performance liquid chromatograph (HPLC) equipped with a 300-mm HPX-87H ion exclusion column (Bio-Rad Laboratories, Hercules, Calif.) connected to a UV/Vis absorbance detector (Shimadzu, Wood Dale, Ill.) set at 210 nm and a refractive index detector (Waters, Inc., Milford, Mass.). The mobile phase was 0.013 N H2SO4 at a flow rate of 0.7 ml/min, and the column temperature was set at 65°C. Centrifuged reactor samples were filtered through a 0.22-μm-pore-size filter (Whatman, Inc., Ann Arbor, Mich.), acidified with 0.1 M H2SO4, and injected (100 μl) into the HPLC. All compounds detectable with either the UV/Vis absorbance detector or the refractive index detector within 120 min were monitored. Volatile fatty acids were identified by their retention times compared to those of authentic standards on the HPX-87H column and also by gas chromatography-flame ionization detector analysis performed with a 4-mm-inside-diameter, 183-cm-long glass column (GP10%, SP-1200/1% H3PO4) and a Hewlett-Packard model 5890A series II gas chromatograph. The detection limits were 34 μM for glucose, 3 μM for lactate and ethanol, 4 μM for butyrate and propionate, 5 μM for acetate, 6 μM for isobutyrate, 2 μM for succinate, and 0.2 μM for formate. Headspace hydrogen was measured with a Trace Analytical RGA3 reduction gas analyzer, and headspace methane was analyzed by gas chromatography (Hewlett-Packard model 5890 series II). The detection limits for hydrogen and methane were 2.7 × 10−6 and 0.01 atm, respectively. The gas production rate in the two reactor sets was not monitored. The measures of biomass used included optical density at 650 nm with a 1-cm light path (OD650, 1 cm), total protein content (Comassie blue protein assay of alkaline extract), and content of volatile suspended solids (3). OD650, 1 cm values were experimentally related to the content of volatile suspended solids (assumed to be biomass) by the following relationship: biomass (in milligrams per liter) = 442 × OD650, 1 cm.
Substrate utilization assays were conducted in duplicate 60-ml serum bottles containing 20 ml of culture, 0.5 mM phosphate buffer (pH 7.2), and 20 mM substrate at 35°C (9, 15). Specific inhibitor assays were conducted in duplicate 155-ml serum bottles containing 20 ml of culture, 1 mM glucose, and 0, 4.5, 8, and 17 mM bromoethanesulfonic acid at 35°C. Inhibition of methanogenic activity was quantified by measuring soluble products and methane accumulation after six consecutive additions of glucose.
Glucose perturbation.
After the HS set was operated for 5 MCRTs (80 days) and the LS set was operated for 2 MCRTs (32 days) at an average glucose loading rate of 0.34 g/liter-day, glucose perturbation was applied simultaneously to both sets by increasing the concentration of glucose in each reactor to approximately 6.8 g/liter (38 mM). The concentrated glucose stock solution used for this purpose also contained an equivalent amount of a 50:50 mixture of sodium and potassium bicarbonates in order to provide buffer for the additional organic acids expected from fermentation of glucose. Samples were collected at appropriate intervals for soluble intermediate product analysis, as well as pH, optical density, protein, H2, and CH4 analyses. For the initial 48 h after the perturbation, a rigorous sampling schedule was used to closely monitor the accumulation of intermediate products.
The time of perturbation for each set is designated time zero, and preperturbation times are referred to by negative values (days −80 to 0 for the HS set and days −32 to 0 for the LS set). The amounts of all soluble intermediate products were normalized to the amount of added glucose by expressing the levels of compounds in terms of the available electron equivalents (ee) and are expressed as percentages. Hydrogen and methane concentrations are expressed as reactor headspace concentrations (in atmospheres).
RESULTS AND DISCUSSION
Dynamics of the referential state.
The COD removal efficiency before the perturbation (days −80 to 0 for the HS set and days −32 to 0 for the LS set) was 93% ± 3% for both sets of reactors. For the HS set, the remaining COD included 1.5 ± 1.3 mM acetate, 1.2 ± 1.5 mM propionate, and 0.12 ± 0.23 mM butyrate. For the LS set, the remaining COD included 0.89 ± 1.2 mM acetate, 0.39 ± 0.42 mM propionate, and 0.05 ± 0.13 mM butyrate. The steady-state glucose concentrations in both reactor sets under these conditions were below the detection limit (less than 34 μM). The headspace methane and hydrogen concentrations in both sets were 0.50 ± 0.04 and 9 × 10−5 ± 0.51 × 10−5 atm, respectively, and the pH of the reactor liquid was 6.91 ± 0.09. No lactate, ethanol, or any other soluble product was detected during this period in both reactor sets by the analytical techniques used, indicating efficient methanogenic conversion of glucose. At the time of perturbation, the concentrations of the intermediate soluble products in all reactors were below 1 mM each for acetate, butyrate, and propionate. The maximum substrate utilization rate determined only for the HS set over this period was 11.8 ± 5.5 μmol/mg of protein/h for glucose, 4.6 ± 3.0 μmol/mg of protein/h for lactate, 1.6 ± 0.65 μmol/mg of protein/h for acetate, and 0.32 ± 0.43 μmol/mg of protein/h for butyrate. For hydrogen, this rate was measured by monitoring methane production and was found be 7.0 ± 1.2 μmol/mg of protein/h. This indicates that there was a somewhat lower range of activities compared to the mother reactor activities. The biomass concentration prior to the perturbation was 428 ± 14 mg/liter in the HS set and 410 ± 27 mg/liter in the LS set, and there was no significant difference between the two sets (P = 0.372). Hence, the stability analysis results are presented without normalization.
Resistance, resilience, and stability.
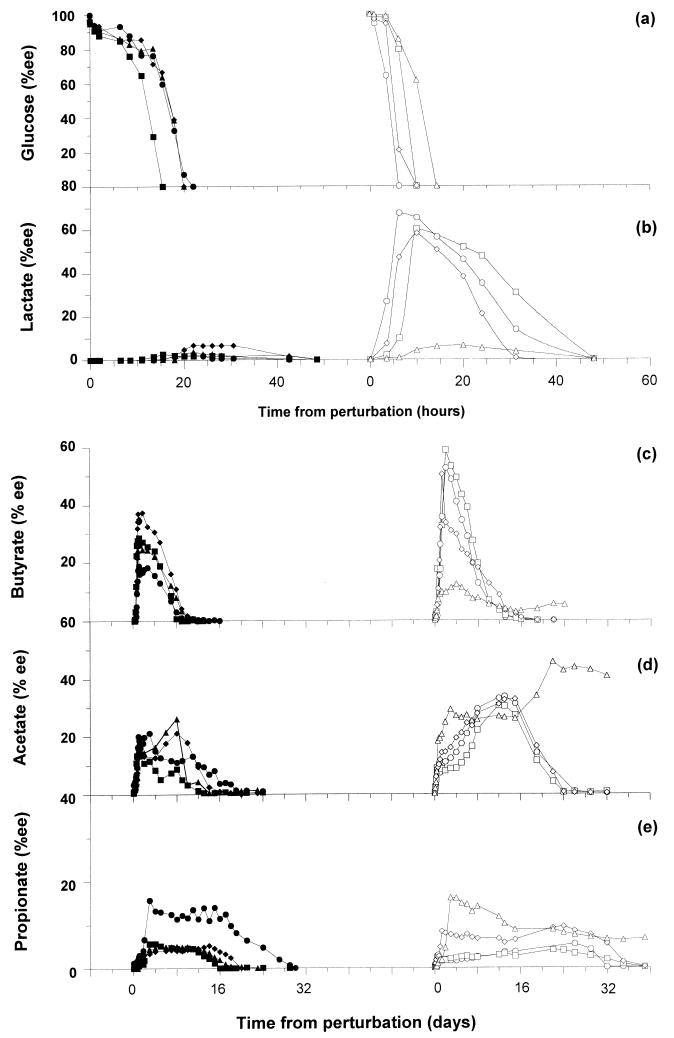
The utilization of glucose and the accumulation of major soluble products in response to the perturbation are shown in Fig. 2. Various functional stability parameters were computed by analyzing the amplification envelope for glucose and the four dominant intermediate soluble products: lactate, butyrate, acetate, and propionate. Formate and isobutyrate accumulated to less than 8% each and were consumed within 2 days; therefore, these compounds were not included in the stability analysis. No other soluble intermediate products were observed by the analytical techniques used. Total COD analysis conducted on a subset of the reactor samples indicated that the soluble products measured constituted most of the COD. Reactor 5 of the HS set and reactor 4 of the LS set had anomalous performance either under referential state or perturbed state conditions compared to the other three reactors in the corresponding sets. This is apparent from the accumulation of lactate, butyrate, and especially acetate in reactor 4 (Fig. 2b, c, and d) and from the accumulation of propionate in reactor 5 (Fig. 2e). The startup of reactor 5 was also poor (data not shown). Hence, reactors 4 and 5 are excluded from the stability analysis.
FIG. 2.
Disappearance of glucose (a) and accumulation of major soluble products (b through e) in response to the perturbation. The data for HS reactors are on the left, and the data for LS reactors are on the right. Symbols: ●, reactor 5; ■, reactor 6; ⧫, reactor 7; ▴, reactor 8; ○, reactor 1; □, reactor 2; ◊, reactor 3; ▵, reactor 4.
Since a higher numerical value for resistance indicates lower system resistance, the data for lactate and butyrate support the hypothesis that the HS set was more resistant than the LS set (Table 1). For the remaining two intermediate products, the difference was statistically insignificant for the two sets. When the resilience results were compared, however, the HS set was more resilient with respect to all four intermediate products. The two sets also differed in terms of their reactivity towards lactate and acetate. The LS set was more reactive with respect to lactate and less reactive with respect to acetate, and there was no significant difference for the other two products. A fourth stability parameter, declivity, defined by the average slope of the declining limb of the envelope, was also calculated in order to compare the net rates of utilization of the soluble compounds. This parameter indicated that the net rates of utilization of all compounds in the LS set were either equal to or higher than those in the HS set (Table 1).
TABLE 1.
Stability parameters for soluble compounds in the HS and LS sets of reactors
| Parameter | Reactors | Glucose | Lactate | Butyrate | Acetate | Propionate |
|---|---|---|---|---|---|---|
| Resistance (% of ee of total glucose added) | HS-6, -7, -8 | 2.6, 6.6, 3.7 | 29, 37, 28 | 14, 21, 26 | 5.7, 5.1, 5.0 | |
| HS mean ± SD | (4.3 ± 2.1) | 31 ± 4.9 | 20 ± 6.0 | 5.3 ± 0.38 | ||
| LS-1, -2, -3 | 68, 60, 58 | 53, 58, 50 | 34, 31, 33 | 5.5, 4.1, 8.4 | ||
| LS mean ± SD | 62 ± 5.3 (0.003)a | 54 ± 4.0 (0.009) | 33 ± 1.5 (0.075) | 6.0 ± 2.2 (0.626) | ||
| Resilience (days) | HS-6, -7, -8 | 0.63, 0.83, 0.83 | 0.65, 0.92, 0.92 | 8, 10, 10 | 13, 16, 14 | 16, 20, 18 |
| HS mean ± SD | 0.76 ± 0.12 (0.010) | 0.83 ± 0.16 | 9.3 ± 1.2 | 14 ± 1.5 | 18 ± 2.0 | |
| LS-1, -2, -3 | 0.25, 0.38, 0.33 | 0.26, 0.42, 0.42 | 16, 16, 16 | 26, 24, 26 | 32, 35, 35 | |
| LS mean ± SD | 0.32 ± 0.07 | 0.37 ± 0.09 (0.021) | 16 ± 0.0 (0.010) | 25 ± 1.2 (0.002) | 34 ± 1.7 (0.002) | |
| Reactivity (% ee · day−1) | HS-6, -7, -8 | 12, 23, 16 | 71, 102, 81 | 31, 27, 32 | 2.7, 2.2, 1.7 | |
| HS mean ± SD | 17 ± 5.6 | 85 ± 16 | 30 ± 2.7 | 2.2 ± 0.50 | ||
| LS-1, -2, -3 | 256, 221, 181 | 41, 42, 52 | 3.1, 2.0, 2.0 | 4.5, 1.6, 12 | ||
| LS mean ± SD | 219 ± 38 (0.012) | 45 ± 6.1 (0.056) | 2.4 ± 0.64 (0.003) | 6.0 ± 5.4 (0.343) | ||
| Declivity (% ee · day−1) | HS-6, -7, -8 | 155, 120, 120 | 1.9, 6.0, 3.4 | 4.2, 4.1, 3.2 | 1.2, 1.4, 2.0 | 0.95, 0.85, 0.50 |
| HS mean ± SD | 132 ± 20 | 3.8 ± 2.1 | 3.8 ± 0.55 | 1.5 ± 0.42 | 0.77 ± 0.24 | |
| LS-1, -2, -3 | 385, 240, 240 | 39, 38, 37 | 3.8, 4.1, 3.4 | 2.6, 2.6, 3.0 | 0.92, 0.32, 0.76 | |
| LS mean ± SD | 288 ± 84 (0.088) | 38 ± 1.0 (0.002) | 3.8 ± 0.35 (0.871) | 2.7 ± 0.23 (0.022) | 0.67 ± 0.31 (0.687) | |
| Stability (% ee · day2)b | HS-6, -7, -8 | 12, 22, 23 | 1.1, 6.6, 3.0 | 383, 819, 617 | 1,078, 1,424, 1,118 | 406, 552, 540 |
| HS mean ± SD | 19 ± 6.1 | 3.6 ± 2.8 | 606 ± 218 | 1,207 ± 189 | 499 ± 81 | |
| LS-1, -2, -3 | 5.3, 5.3, 3.0 | 42, 55, 25 | 1,412, 1,750, 1,544 | 5,981, 4,825, 5,863 | 2,111, 1,819, 4,446 | |
| LS mean ± SD | 4.5 ± 1.3 (0.057) | 41 ± 15 (0.052) | 1,569 ± 170 (0.009) | 5,556 ± 636 (0.008) | 2,792 ± 1,440 (0.110) |
The values in parentheses are the P values for 95% confidence intervals for two-sample t tests conducted on the corresponding stability parameters for the two reactor sets. A P value less than 0.05 implies that the difference in the means for the two sets was significant at the 95% confidence interval.
The total soluble compound stability for the HS set was 2,335% ± 473% ee · day2, and the total soluble compound stability for the LS set was 9,962% ± 1,750% ee · day2 (P = 0.018).
While the above analysis enables one to compare the stability of a portion of the community, it does not allow an assessment of the total stability with respect to soluble compounds. This is because measures of stability are always associated with a specific compound. Thus, it is likely that a system is less resistant or resilient with respect to one compound and more resistant or resilient with respect to another. Hence, a measure of the total soluble compound stability was also needed. Some possible candidates were the sum of the resistance or resilience values for all soluble compounds, the sum of the areas under the envelopes of all soluble compounds, and the sum of the total moments of areas under the envelopes of all soluble compounds. The third option was chosen as the most appropriate measure because it emphasizes the products that accumulate to higher levels for longer periods. It is defined by:
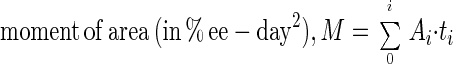 |
where Ai is the area under the envelope of the ith compound and ti (in days) is the moment arm for the envelope of the ith compound given by the distance from the center of gravity of the envelope to the perturbation axis. This equation indirectly incorporates all the parameters of stability, including the effect of the shape of the amplification envelope. It also allows summation of all compounds (often reflecting a separate guild), making it possible to compare the stabilities of two systems. However, all compounds must be expressed in the same units. Like resistance and resilience, a higher numerical moment of area denotes lower system stability. The total soluble compound stability calculated using the above equation for the two sets of communities indicated that the HS set was indeed more stable than the LS set (Table 1). The contribution of each intermediate product to the total soluble compound stability can also be obtained as a percentage defined by:
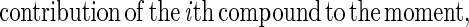 |
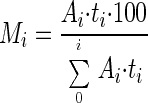 |
The results obtained with this equation indicated that acetate accumulation in the LS set was the most dominant reason (56% ± 6.4%) for the lower stability of this set, followed by propionate accumulation (28% ± 14%) and butyrate accumulation (16% ± 1.7%). Use of this equation also indicated that lactate accumulation contributed only 0.4% ± 0.2% by itself to the moment of area of the LS set. However, as discussed below, the serial conversion of lactate to butyrate and then to acetate became the primary manifestation of instability for this community.
Parallel versus serial substrate processing and functional stability.
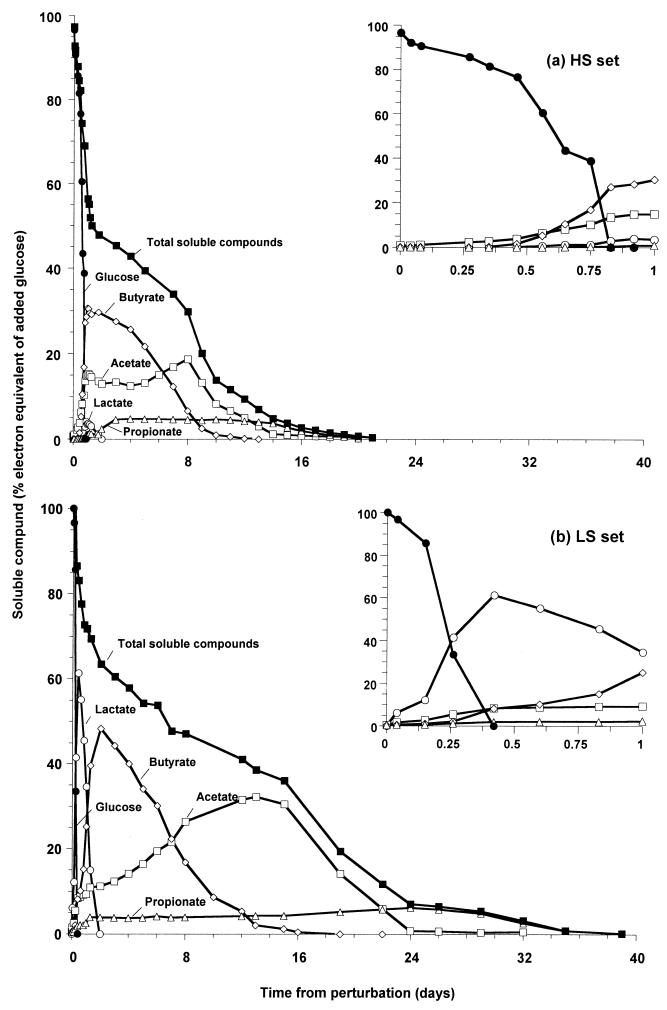
The sequence of accumulation of the four major soluble intermediate products in response to the glucose perturbation is shown in Fig. 3 (average of three reactors from each set). In the HS set, the accumulations of the four intermediate products were simultaneous in response to the glucose perturbation, indicating a predominantly parallel flow of substrate (Fig. 3a). In the LS set, the depletion of glucose (occurring within 20 h [Fig. 2]) coincided with the rapid accumulation of lactate, which was then converted predominantly to butyrate within 2 days (Fig. 3b). The utilization of butyrate occurred over the next 10 days, and there was significantly greater accumulation of acetate than that in the HS set. Accumulation of the intermediate products in the LS set was predominantly serial (Fig. 3b).
FIG. 3.
Utilization of glucose and accumulation of the four major soluble intermediate products in the HS set (a) and the LS set (b). The lines indicate averages for three reactors. Insets show magnified data for the first 24 h.
One of the major differences between the two sets was the smaller amount of total reducing equivalents present in the soluble compounds in the HS set (Fig. 3). The reason for this may have been the initially lower glucose utilization rate in the HS set (Fig. 3a, inset) (4 μmol/mg of protein/h for the first 11 h versus 16 μmol/mg of protein/h for the remaining 9 h). During this initial period, nearly 24% of the glucose was utilized, but only 4.5% was accounted for in soluble intermediates, indicating that the remaining 19.5% was most likely converted to methane, biomass, and H2 during this period. When the same data for the LS set (Fig. 3b, inset) were compared, all of the glucose was utilized within 10 h (at a rate of 19 μmol/mg of protein/h) and nearly 83% of the total reducing equivalents could be accounted for in soluble intermediates. A maximum of 7.2% ± 1.3% of the ee could be attributed to biomass in both sets; the concentrations increased to maximum values of 611 ± 33 mg/liter in the HS set and 588 ± 54 mg/liter in the LS set in response to the perturbation by day 1. These values gradually declined to 439 ± 32 mg/liter in the HS set and 436 ± 29 mg/liter in the LS set by day 16. Hence, differences in biomass concentration cannot account for the difference in soluble product accumulation. Since no other soluble compounds were detected in both sets by HPLC (using both UV and refractive index detectors for up to 120-min runs), the remainder was most likely distributed between methane and hydrogen.
The rates of utilization of total soluble compounds in the two sets were similar, with 2.4% ee/day utilized in the LS set and 2.7% ee/day utilized in the HS set (Fig. 3). Also, the individual rates of utilization of lactate, butyrate, and acetate in the LS set were either equal to or higher than the corresponding rates in the HS set (indicated by their declivity [Table 1]). Therefore, the greater accumulation of soluble products in the LS set was attributed to a higher production rate and channeling of all the excess ee into predominantly one intermediate at a time. For acetate, it is also possible that the rate of utilization by methanogens was low for the first 12 days, resulting in greater accumulation of acetate, and gradually rose after this to the rate observed in the HS set. Such an increase in activity was indeed observed in the HS set, where the acetate utilization rate doubled due to the perturbation (data not shown). The utilization of butyrate was also thermodynamically favorable after the initial 2 days. This is also supported by the net utilization of butyrate (Fig. 3b). The concentration of the volatile fatty acids present in the LS set was below the inhibitory level (1). Hence, greater sequential production of lactate, butyrate, and acetate coupled with possibly a lower initial rate of acetate utilization resulting in delayed accumulation of acetate is the most likely reason for the lower stability of the LS set.
Production of lactate and its serial conversion to butyrate are not common in full-scale anaerobic digesters even under perturbed conditions. However, lactic acid bacteria play a crucial role in silage making, cassava flour production, and cheese blowing. It is probable that the LS set contained a higher proportion of lactic acid-producing bacteria (consistent with a high proportion of coccus morphotypes [4]) and these organisms responded to the glucose perturbation. The subsequent conversion of lactate to butyrate observed in the LS set is also energetically favorable, as shown by the following equations: ΔG0′, kJ/molLactate + 0.4 Acetate + 0.7 H+ ⇒ 0.7 Butyrate + −183.9 0.6 H2+ CO2 + 0.4 H2O Lactate + Acetate + H+ ⇒ Butyrate + 0.8 H2 + −59.4 1.4 CO2 + 0.6 H2O 2 Lactate + H+ ⇒ Butyrate + 2 H2 + 2 CO2 −64.1
A number of organisms, including Clostridium tyrobutyricum, Clostridium acetobutylicum, and Butyribacterium methylotrophicum, have been implicated in the above reactions (2, 8, 14). Since the mother reactor for the LS set was started at a different time of the year than the mother reactor for the HS set was started, it is possible that it contained significantly larger populations of organisms that can produce lactate and convert lactate to butyrate.
Replication of function under perturbed states.
As evident from the data in Table 1 and Fig. 2, replication of the substrate flow pattern under perturbed conditions by mixed microbial communities is possible. It should be noted, however, that one reactor from each set of four reactors was not included in the analysis. This could be considered the limit to replication of complex microbial systems. Moreover, even for the three reactors in each set, exact replication for all intermediate products was never observed, indicating that there is a need to define a “degree of replication.” We combined the replication in all the stability parameters using the following equation:
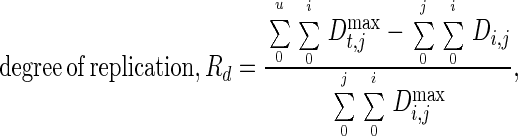 |
where Dij is the absolute percent deviation from the mean value for the ith intermediate product and jth stability parameter and Di,jmax is the maximum value possible for this absolute deviation. A deviation of 100% in each parameter and product was taken as the upper limit for Di,jmax in this analysis. Thus, for a given intermediate product i, deviation from the mean in the stability parameter j was calculated for all three reactors in each set. The deviations were then added together for all four stability parameters and intermediate products to compare the overall replication. Accordingly, an average absolute deviation of 5% from the mean in all four stability parameters, four intermediate products, and three reactors in each set results in an overall degree of replication of 0.95, a 10% deviation results in an overall degree of replication of 0.90, and so on. Using this approach, the degree of replication for the HS set was 0.79 and the degree of replication for the LS set was 0.83, indicating that both sets replicated reasonably well, varying on average by 21 and 17%, respectively.
As mentioned before, one reactor in each set (reactors 4 and 5 [Fig. 2]) differed considerably in its response to perturbation. The differences may have been due to the presence of minor populations. The community in the HS set spent at least 17.5 MCRTs under laboratory conditions, while that in the LS set spent only 5.8 MCRTs under such conditions. Operating for longer times may reduce the likelihood of finding minor populations. For example, assuming no net growth of a population that is initially present in the inoculum at some level, the percentage of the original population remaining in a bioreactor is 5% after 3 MCRTs, 0.6% after 5.8 MCRTs, and 2.7 × 10−6% after 17.5 MCRTs. For these conditions, a minor population representing 1% of an inoculum consisting of 109 cells/ml is initially present at a concentration of 107 cells/ml. After 5.8 MCRTs, 6 × 105 cells/ml are still present even with no net growth. Since such a small population can exist in a microbial system and perhaps even find a niche and grow, the significant deviations observed within a set in response to the perturbation are not surprising. This also emphasizes the point that operating a mixed-community reactor for 3 MCRTs may be sufficient to achieve stable performance under referential state conditions but it is evidently inadequate to achieve a high degree of replication of stability of function under perturbed conditions.
This study supports the hypothesis that parallel processing of substrate may confer greater functional stability in response to a substrate perturbation. In this context, parallel should not be taken as absolutely parallel as in some fields, but as a network of multiple routes for substrate flow. The results also indicated that ecological functions of similar microbial communities are replicable within the uncertainties associated with complex systems. However, under perturbed conditions significant deviations are possible and are most likely due to the presence of numerically minor but important populations, as shown in the companion paper (4). These deviations can have a profound impact on the functional stability of such systems during perturbations.
ACKNOWLEDGMENTS
This research was supported by NSF grant DEB 9120006 to the Center for Microbial Ecology. A.S.F. was partially supported by an OAS grant and by the Comisión Secretorial de Investigación Cientifica, Universidad de República, Montevideo, Uruguay.
We are grateful to Kay Gross (Kellogg Biological Station, Michigan State University) and Lugarde Raskin (University of Illinois at Urbana-Champaign) for their valuable discussions about ecological concepts throughout the course of this work.
REFERENCES
- 1.Borzacconi L, Lopez I, Anido C. Hydrolysis constant and VFA inhibition in acidogenic phase of MSW anaerobic degradation. Water Sci Technol. 1997;36:479–484. [Google Scholar]
- 2.Brauman A, Keleke S, Malonga M, Miambi E, Ampe F. Microbiological and biochemical characterization of cassava retting, a traditional lactic acid fermentation for foo-foo (cassava flour) production. Appl Environ Microbiol. 1996;62:2854–2858. doi: 10.1128/aem.62.8.2854-2858.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clesceri L S, Greenberg A E, Eaton A D, editors. Standard methods for the examination of water and wastewater. 20th ed. Washington, D.C.: American Public Health Association; 1999. [Google Scholar]
- 4.Fernandez A S, Hashsham S A, Dolhopf S L, Raskin L, Glagoleva O, Dazzo F B, Hickey R F, Criddle C S, Tiedje J M. Flexible community structure correlates with stable community function in methanogenic bioreactor communities perturbed by glucose. Appl Environ Microbiol. 2000;66:4058–4067. doi: 10.1128/aem.66.9.4058-4067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrich M. Communication-efficient parallel sorting. SIAM (Soc Ind Appl Math) J Comput. 1999;29:416–432. [Google Scholar]
- 6.Grimm V, Schmidt E, Wissel C. On the application of stability concepts in ecology. Ecol Model. 1992;63:143–161. [Google Scholar]
- 7.Joseph D, Bai R, Liao T, Huang A, Hu H. Parallel pipelining. J Fluids Eng Trans Am Soc Mech Eng. 1995;117:446–449. [Google Scholar]
- 8.Klijn N, Bovie C, Dommes J, Hoolworf J D, Waals C B V D, Weerkamp A H, Nieuwenhof F F J. Identification of Clostridium tyrobutyricum and related species using sugar fermentation, organic acid formation and DNA probes based on specific 16S rRNA sequences. Syst Appl Microbiol. 1994;17:249–256. [Google Scholar]
- 9.Mendez M S, Lema J M. Methanogenic and non-methanogenic activity tests. Theoretical basis and experimental set up. Water Res. 1993;27:1361–1376. [Google Scholar]
- 10.Morris R G M. Parallel distributed processing: implications for psychology and neurobiology. New York, N.Y: Oxford University Press; 1989. [Google Scholar]
- 11.Neubert M G, Caswell H. Alternatives to resilience for measuring the responses of ecological systems to perturbation. Ecology. 1997;78:653–665. [Google Scholar]
- 12.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemisrty and microbial ecology. Adv Microb Ecol. 1990;11:285–383. [Google Scholar]
- 13.Scheiman C, Schauser K. Evaluating the benefits of communication coprocessors. J Parallel Distributed Comput. 1999;57:236–256. [Google Scholar]
- 14.Shen G-J, Annous B A, Lovitt R W, Jain M K, Zeikus J G. Biochemical route and control of butyrate synthesis in Butyribacterium methylotrophicum. Appl Microbiol Biotechnol. 1996;45:355–362. [Google Scholar]
- 15.Vavilin V A, Lokshina L Y. Modeling of volatile fatty acids degradation kinetics and evaluation of microorganism activity. Biores Technol. 1996;57:69–80. [Google Scholar]
- 16.Xing J, Criddle C, Hickey R. Effects of a long-term periodic substrate perturbation on an anaerobic community. Water Res. 1997;31:2195–2204. [Google Scholar]