Abstract
Background
Prospective studies regarding tuberculous myelitis are lacking. We aimed to prospectively evaluate patients with tuberculous myelitis to identify the features that distinguish tuberculous myelitis from other myelitis.
Methods
This was a prospective study. Patients presenting with paraparesis/quadriparesis, and MRI showing myelitis were included. All patients were subjected to clinical, neuroimaging, and laboratory evaluation. Diagnosis of definite tuberculous myelitis was made if GeneXpert test in CSF was positive. Probable tuberculous myelitis was diagnosed if there was evidence of tuberculosis elsewhere in the body. Patients were treated with methylprednisolone and antituberculosis treatment. Patients were followed for 6 months. We compared the clinical, laboratory, and neuroimaging parameters and response to treatment of tuberculous myelitis with other myelitis. P values were adjusted using the Benjamini-Hochberg (BH) procedure to control false discovery rate.
Results
We enrolled 52 patients. Eighteen (34.6%) patients had tuberculous myelitis. Headache (P = 0.018) was significantly more common in tuberculous myelitis. The CSF protein (P < 0.001), and CSF cell count (P < 0.001) were significantly higher in tuberculous myelitis. On neuroimaging, a LETM was common in tuberculous myelitis. Spinal meningeal enhancement (14; 77.8%), extra-axial collection, and CSF loculation (6; 33.4%), arachnoiditis (3;16.7%), and concomitant spinal tuberculoma (2;11.1%) were other common imaging features of tuberculous myelitis. Tuberculous myelitis patients showed a better response (P = 0.025) to treatment.
Conclusion
Tuberculous myelitis was seen in approximately 35% of all myelitis cases, in a high tuberculosis endemic zone. Headache, markedly elevated CSF protein and spinal meningeal enhancement were distinguishing features. Tuberculous myelitis patients responded well to corticosteroids.
Keywords: Tuberculous myelitis; Tuberculous meningitis, Neuromyelitis optica, Myelin oligodendrocyte glycoprotein
Introduction
The spinal cord can be affected by tuberculosis in a wide variety of ways [1]. The involvement of vertebrae can lead to spinal cord compression, also non-osseous spinal tuberculosis can manifest as spinal arachnoiditis, spinal tuberculoma, and myelitis [1].
Tuberculous myelitis or inflammation of the spinal cord due to tuberculosis is considered a rare entity and most of the information regarding tuberculous myelitis comes from case reports and case series [2]. In PubMed, the earliest case of myelitis with pulmonary tuberculosis was recorded in 1905 [3]. Rigdon, in 1947, described a case of tuberculous myelitis in a patient with tuberculous meningitis [4]. In many cases, neuromyelitis optica spectrum disease (NMOSD), an autoimmune inflammatory disease affecting the central nervous system, has been described in association with pulmonary tuberculosis or central nervous system tuberculosis [5–8]. In a retrospective analysis done in India, Synmon, and colleagues, reported that approximately 19% of longitudinal extensive myelitis (LETM) cases were associated with either pulmonary or meningeal tuberculosis [9].
We aimed to prospectively evaluate the clinical, laboratory, neuroimaging features and response to treatment of tuberculous myelitis.
Material and methods
Study design and settings
The study design was a prospective observational study. The study was conducted at the Department of Neurology, King George's Medical University, Lucknow, India. Our institute is a tertiary care teaching hospital, which is located in a highly endemic area for tuberculosis. The study was approved by the Institutional Ethics Committee. A written and informed consent was obtained from every patient/guardian before enrollment.
Inclusion criteria
Patients admitted to neurology wards with predominant complaints of paraparesis/quadriparesis of ≤ 4 weeks duration, with/without other complaints and MRI of the spine suggestive of myelitis were included in our study. Myelitis was defined as an MRI spine showing an intramedullary hyperintensity on T2 weighted imaging, with or without cord swelling or contrast enhancement [10].
Exclusion criteria
Patients with MRI evidence of spinal cord compression and spinal vertebral tuberculosis were not included. MRI spine, which was compatible with other diagnoses like spinal cord infarction and surface flow voids suggesting spinal cord arteriovenous malformations were also excluded from our study. Comatose patients, in whom proper motor system assessment could not be done were not considered.
Clinical evaluation
All patients were subjected to a detailed clinical evaluation. History of previous tuberculosis, and anti-tuberculosis drug intake were also recorded. Symptoms suggestive of tuberculosis in other organs like fever, cough, headache, altered sensorium, and seizures were also recorded. Records related to COVID-19 and COVID-19 vaccination were also reviewed.
Laboratory evaluation
Routine hematological and biochemical investigations were done on all the patients. Before inclusion, RT-PCR tests for SARS-COV-2 and enzyme-linked immunosorbent assay for human immunodeficiency virus were performed. Tests for IgG-anti-aquaporin 4 (AQP-4) antibodies and Myelin oligodendrocyte glycoprotein (MOG) were done. Tests for connective tissue disorders and vasculitides like antinuclear antibodies, extractable nuclear antigens, and antineutrophil cytoplasmic antibodies were also performed. A lumbar puncture was performed and a cerebrospinal fluid (CSF) examination was done on all subjects. CSF was subjected to protein, glucose, total and differential leukocyte count, gram staining, Ziehl Neelsen staining, and India ink staining. CSF was also sent for culture on Lowenstein Jensen medium for Mycobacterium tuberculosis. A GeneXpert MTB/RIF was performed on all CSF samples for detection of M. tuberculosis DNA as well as rifampicin resistance. Sputum samples, if available, were subjected to Ziehl Neelsen staining and GeneXpert MTB/RIF.
Neuroimaging
A contrast-enhanced MRI of the spine was done on all the patients. The MRI was done using Signa Excite 1.5 Tesla instrument (General Electric Medical Systems, Milwaukee, WI, USA). T2 weighted as well as pre- and post-contrast T1 weighted images were obtained. MRI was reviewed by an independent neuroradiologist. Myelitis was defined as the presence of hyperintensity on T2 weighted images within the substance of the spinal cord, with or without cord swelling and contrast enhancement [11]. Longitudinally extensive transverse myelitis (LETM) was defined as the involvement of 3 or more contagious vertebral segments [11]. Other features that were noted in the spinal MRI were meningeal enhancement, lumbosacral arachnoiditis, CSF loculation, spinal tuberculoma, and syrinx formation.
Contrast-enhanced MRI of the brain was performed in all cases, MRI of the brain was specifically analyzed to look for typical lesions compatible with common demyelinating disorders [11]. Typical imaging features of tuberculous meningitis like hydrocephalus, basal exudates, optochiasmatic arachnoiditis, tuberculomas, meningeal enhancement, and infarcts, were recorded [12].
Other imaging
A chest X-ray was done in all patients, and a CT scan of the thorax was done in selected cases if indicated. Chest imaging findings suggestive of tuberculosis like miliary pattern, cavitation, fibro-consolidation, pleural effusion, and lymphadenopathy were noted [13].
Definitions
Tuberculous myelitis was diagnosed using the following criteria: (1) MRI evidence of myelitis plus (2) evidence of tuberculosis. It was further subdivided into definite and probable tuberculous myelitis. Definite tuberculous myelitis was diagnosed only if an MRI suggestive of myelitis was documented along with a CSF GeneXpert positive for M. tuberculosis. A diagnosis of probable tuberculous myelitis was made if an MRI suggestive of myelitis was present along with evidence of tuberculosis anywhere else in the body, like the concomitant presence of tuberculous meningitis, pulmonary tuberculosis (sputum positivity for M. tuberculosis or chest radiology suggestive of pulmonary tuberculosis) or proven extra-pulmonary tuberculosis in any other organ.
Tuberculous meningitis was diagnosed based on a consensus case definition [14]. Pulmonary tuberculosis was diagnosed as per the standard World Health Organization case definition [15]. Neuromyelitis optica spectrum disorders (NMOSD) were diagnosed based on international consensus diagnostic criteria [16]. Myelitis along with seropositivity for MOG-IgG as detected employing a cell-based assay, lead to the diagnosis of MOG-myelitis [17]. Idiopathic acute transverse myelitis was diagnosed based on standard diagnostic criteria [18]. Post-infectious and post-vaccination myelitis were diagnosed if there was a clear temporal relationship between the onset of myelitis and a known infection/vaccination.
The development of new myelitis in a patient, who was taking antituberculosis treatment, was diagnosed as paradoxical tuberculous myelitis [12, 19].
Treatment
All the patients with myelitis were treated with intravenous pulse methylprednisolone (500–1000 mg/day) for 5 days. Patients having tuberculous myelitis also received standard World Health Organization-recommended anti-tuberculosis drug treatment [20]. Patients suffering from tuberculous meningitis were given oral corticosteroids tapered over 8 weeks after completion of pulse methylprednisolone therapy.
Follow-up and outcome assessment
All the patients were followed up for 6 months. Disability assessment at the end of 6 months was done using the modified Rankin scale (mRS). At 6 months an mRS of 0–2 was considered a good outcome, whereas an mRS of 3–6 was considered a poor outcome [21].
Statistical analysis
Statistical analysis was done using the IBM SPSS version 24.0. The categorical variables were expressed as percentages and the continuous variables were expressed as mean ± standard deviation as well as median (inter-quartile) range. The categorical variables were compared using the Chi-square/Fisher exact test. The continuous variables were compared using the Mann–Whitney U test. A univariate followed by a multivariate analysis was performed. The multivariate analysis was performed using the binary logistic regression analysis. Post-univariate analysis multivariate binary logistic regression was performed to identify the independent predictors (chosen based on the level of significance found in the univariate analysis as well as clinical experience) of the dependent variable (tuberculous myelitis or poor outcome). In view of multiple comparisons, P values were adjusted using the Benjamini–Hochberg (BH) procedure for false discovery rate (FDR) control at 0.05 level [22].
Results
Baseline characteristics
Fifty-two patients with myelitis were enrolled. The mean age of the patients was 26.94 ± 11.86 years, and 26 (50%) patients were males. All the patients were having paraparesis (80.8%) or quadriparesis (19.2%), sensory involvement was seen in 35 (67.3%), and bladder/bowel involvement was observed in 47(90.4%) (Table1). Tuberculous myelitis was diagnosed in 18 (34.6%), NMOSD was diagnosed in 15 (28.8%), and MOG-myelitis was diagnosed in 3 (5.8%) patients. None of the patients had HIV or SARS-COV-2 infection (Fig. 1).
Table 1.
Comparison of clinical, neuroimaging and laboratory characteristics of tuberculous myelitis versus other causes of myelitis
| Variables | All patients N = 52 | Tuberculous myelitis N = 18 | Other causes of myelitis N = 34 | P | B-H adjusted P values | Relative risk (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| Age in years | 0.810 | 0.810 | NA | |||
| Mean ± SD | 26.94 ± 11.86 | 25.83 ± 6.48 | 27.53 ± 13.96 | |||
| Median (IQR) | 26.5 (13.8) | 26.00 (5.5) | 27.00 (20.5) | |||
| Gender Male N (%) | 26 (50) | 8 (44.4) | 18 (52.9) | 0.560 | 0.632 | 0.8 (0.38–1.70) |
| Paraparesis N (%) | 42 (80.8) | 17 (94.4) | 25 (73.5) | 0.136 | 0.216 | 4.05 (0.61–26.93) |
| Quadriparesis N (%) | 10 (19.2) | 1 (5.6) | 9 (26.5) | 0.136 | 0.238 | 0.25 (0.04–1.64) |
| Sensory involvement N (%) | 35 (67.3) | 8 (44.4) | 27 (79.4) | 0.011 | 0.032 | 0.39 (0.19–0.804) |
| Sphincter involvement N (%) | 47 (90.4) | 15 (83.3) | 32 (94.1) | 0.327 | 0.424 | 0.53 (0.23–1.22) |
| Fever N (%) | 18 (34.6) | 10 (55.6) | 8 (23.5) | 0.021 | 0.057 | 2.36 (1.13–4.92) |
| Headache N (%) | 11 (21.2) | 8 (44.4) | 3 (8.8) | 0.005 | 0.018 | 2.98 (1.56–5.71) |
| Seizure N (%) | 03 (5.8) | 2 (11.1) | 1 (2.9) | 0.543 | 0.634 | 2.04 (0.84–5.00) |
| Vomiting N (%) | 09 (17.3) | 5 (27.8) | 4 (11.8) | 0.247 | 0.360 | 1.84 (0.88–3.85) |
| Hiccough N (%) | 03 (5.8) | 0 (0) | 3 (8.8) | 0.308 | 0.414 | NA |
| Vision loss N (%) | 04 (7.7) | 0 (0) | 4 (11.8) | 0.285 | 0.399 | NA |
| Chest X-ray abnormalities (s/o TB) N (%) | 9 (17.3) | 9 (50.0) | 0 (0) | 0.001 | 0.004 | NA |
| T2 hyperintensity in spinal cord N (%) | 52 (100.0) | 18 (100) | 34 (100) | NA | NA | NA |
| Corresponding T1 hypointensity N (%) | 21 (40.4) | 8 (44.4) | 13 (38.2) | 0.664 | 0.704 | 1.18 (0.56–2.49) |
| Cord swelling N (%) | 10 (19.2) | 1 (5.6) | 9 (26.5) | 0.136 | 0.227 | 0.25 (0.04–1.64) |
| Spinal meningeal enhancement N (%) | 14 (26.9) | 14 (77.8) | 0 (0) | < 0.001 | < 0.001 | NA |
| Arachnoiditis N (%) | 3 (5.8) | 3 (16.7) | 0 (0) | 0.037 | 0.086 | NA |
| CSF loculation N (%) | 3 (5.8) | 3 (16.7) | 0 (0) | 0.037 | 0.081 | NA |
| Extra axial collection | 3 (5.8) | 3 (16.7) | 0 (0) | 0.037 | 0.093 | NA |
| Spinal tuberculoma | 2 (3.8) | 2 (11.1) | 0 (0) | 0.115 | 0.211 | NA |
| Cervical cord involvement N (%) | 4 (7.7) | 2 (11.1) | 2 (5.9) | 0.602 | 0.658 | 1.50 (0.52–4.32) |
| Cervico-Dorsal cord involvement N (%) | 38 (73.1) | 12 (66.7) | 26 (76.5) | 0.448 | 0.541 | 0.74 (0.34–1.58) |
| Dorsal cord involvement N (%) | 10 (19.2) | 4 (22.2) | 6 (17.6) | 0.723 | 0.744 | 1.2 (0.50–2.87) |
| Area postrema involvement N (%) | 12 (23.1) | 1 (5.6) | 11 (32.4) | 0.039 | 0.080 | 0.20 (0.03–1.32) |
| LETM N (%) | 46 (88.5) | 17 (94.4) | 29 (85.3) | 0.412 | 0.515 | 2.22 (0.36–13.80) |
| Hydrocephalus N (%) | 7 (13.5) | 7 (38.9) | 0 (0) | < 0.001 | < 0.001 | NA |
| Basal exudates N (%) | 8 (15.4) | 8 (44.4) | 0 (0) | < 0.001 | < 0.001 | NA |
| Tuberculoma N (%) | 9 (17.3) | 9 (50.0) | 0 (0) | < 0.001 | < 0.001 | NA |
| Infarct N (%) | 2 (3.8) | 2 (11.1) | 0 (0) | 0.113 | 0.212 | NA |
| Meningeal enhancement N (%) | 11 (21.2) | 11 (61.1) | 0 (0) | < 0.001 | < 0.001 | NA |
| Brain demyelination N (%) | 5 (9.6) | 0 (0) | 3 (14.7) | 0.150 | 0.228 | NA |
| CSF protein mg/dl | < 0.001 | < 0.001 | NA | |||
| Mean ± SD | 204.30 ± 368.20 | 403.67 ± 534.15 | 98.76 ± 171.41 | |||
| Median (IQR) | 70.00 (98.3) | 148.50 (501.00) | 58.00 (45.8) | |||
| CSF glucose mg/dl | < 0.001 | < 0.001 | NA | |||
| Mean ± SD | 69.29 ± 30.97 | 49.89 ± 29.79 | 79.56 ± 26.66 | |||
| Median (IQR) | 63.50 (44.8) | 45.50 (25.8) | 69.50 (39.8) | |||
| CSF cell/mm3 | < 0.001 | < 0.001 | NA | |||
| Mean ± SD | 72.75 ± 129.26 | 159.72 ± 185.09 | 26.71 ± 43.19 | |||
| Median (IQR) | 17.50 (62.5) | 60.00 (274.5) | 5 (16.3) | |||
| Poor outcome N (%) | 22 (42.3) | 3 (16.7) | 19 (55.9) | 0.008 | 0.025 | 0.27 (0.09–0.82) |
B-H, Benjamini–Hochberg Adjusted P value where multiple comparisons were done. SD, standard deviation, IQR, inter-quartile range, CSF, cerebrospinal fluid, LETM, longitudinally extensive transverse myelitis, TLC, total leukocyte count
P values showing statistical significant results are marked in bold
Fig. 1.
Flow diagram of the study. (TBM = tuberculous meningitis, PTB = pulmonary tuberculosis, NMO-SD = Neuromyelitis Optica spectrum disorder, MOG = Myelin Oligodendrocyte Glycoprotein)
Clinical, neuroimaging, and laboratory features of tuberculous myelitis
Tuberculous myelitis was classified as definite in 3 patients (based on a positive GeneXpert MTB/RIF in CSF) and probable in the rest of the 15 patients. Tuberculous myelitis was diagnosed as an isolated entity in only one patient, other 17 patients were having myelitis along with evidence of tuberculous meningitis or pulmonary tuberculosis (Fig. 1)(Table 2). Six patients (33.3%) developed paradoxical myelitis; these patients were already taking antituberculosis drugs for tuberculous meningitis or pulmonary tuberculosis (Table 2). The duration of antituberculosis drug intake in these patients before the development of myelitis ranged from 30 to 120 days (median 75 days).
Table 2.
Summary of all 18 patients diagnosed as tuberculous myelitis
| Age/gender | Clinical features | MRI spine | MRI brain | Cerebrospinal fluid | Sputum | Pulmonary TB | Tuberculous meningitis | Paradoxically developed myelitis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 30/M | Paraparesis Sphincter involvement Fever Altered sensorium | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement Extra axial collection | Hydrocephalus Basal exudate Meningeal enhancement Tuberculoma | Protein-1219 mg/dl glucose-36 mg/dl TLC-40/mm3 | Negative | Yes | Yes | No | Good |
| 28/F | Paraparesis Sphincter involvement Fever Headache Seizure | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement Extra axial collection | Basal exudate Tuberculoma | Protein-68 mg/dl glucose -46 mg/dl TLC-15/mm3 | Negative | Yes | Yes | No | Good |
| 25/F | Paraparesis Sensory involvement vomiting | Dorsal myelitis (LETM) Spinal meningeal enhancement CSF loculations | Tuberculoma | Protein-1825 mg/dl glucose -87 mg/dl TLC-35/mm3 | Negative | Yes | Yes | Yes | Poor |
| 19/F | Paraparesis Sensory involvement Sphincter involvement | Cervico-dorsal myelitis (LETM) | Normal | Protein-58 mg/dl glucose -57 mg/dl TLC-50/mm3 | Positive | Yes | No | Yes | Good |
| 25/F | Paraparesis Sensory involvement Sphincter involvement Fever Headache | Dorsal myelitis (LETM) Spinal meningeal enhancement | Meningeal enhancement Tuberculoma | Protein-249 mg/dl glucose -44 mg/dl TLC-318/mm3 | Negative | No | Yes | Yes | Good |
| 16/F | Paraparesis Fever Headache Altered sensorium Vomiting | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement CSF loculations Extra axial collection | Basal exudate Meningeal enhancement Tuberculoma | Protein-153 mg/dl glucose -7 mg/dl TLC-50/mm3 | Negative | No | Yes | Yes | Good |
| 24/F | Paraparesis Sphincter involvement | Cervical myelitis (LETM) Spinal meningeal enhancement Arachnoiditis | Hydrocephalus Basal exudate Meningeal enhancement | Protein-221 mg/dl glucose-20 mg/dl TLC-460/mm3GeneXpert-Positive | Negative | No | Yes | Yes | Good |
| 28/F | Paraparesis Sensory involvement Sphincter involvement | Dorsal myelitis (LETM) Spinal meningeal enhancement Spinal tuberculoma | Tuberculoma | Protein-46 mg/dl glucose -56 mg/dl TLC-70/ mm3 | Negative | No | Yes | No | Good |
| 35/M | Quadriparesis Fever Vomiting Altered sensorium | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement | Hydrocephalus Basal exudates Tuberculoma Optochiasmatic arachnoiditis Meningeal enhancement | Protein-206 mg/dl glucose -56 mg/dl TLC-510/mm3 | Negative | No | Yes | No | Good |
| 12/F | Paraparesis Sphincter involvement | Cervico-dorsal myelitis (LETM) Cord swelling Cervico-medullary area postrema involvement | Normal | Protein-89 mg/dl glucose -46 mg/dl TLC-80/ mm3 | Negative | Yes | No | Yes | Good |
| 30/M | Paraparesis Sensory involvement Sphincter involvement Headache Altered sensorium | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement | Hydrocephalus Basal exudates Infract Meningeal enhancement | Protein-501 mg/dl glucose -37 mg/dl TLC-5/ mm3 | Negative | No | Yes | No | Poor |
| 28/M | Paraparesis Sensory involvement Sphincter involvement | Cervical myelitis (LETM) Spinal meningeal enhancement | Normal | Protein-120 mg/dl glucose -42 mg/dl TLC-25/ mm3 | Negative | Yes | No | No | Good |
| 28/M | Paraparesis Sensory involvement Sphincter involvement | Cervico-dorsal myelitis (LETM) Spinal tuberculoma | Normal | Protein-35 mg/dl glucose -116 mg/dl TLC-5/mm3 | Positive | Yes | No | No | Good |
| 25/M | Paraparesis Sensory involvement Sphincter involvement fever | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement Arachnoiditis | Normal | Protein-144 mg/dl glucose-45 mg/dl TLC-100/mm3 GeneXpert-Positive | Negative | No | No | No | Poor |
| 25/F | Paraparesis Sphincter involvement Fever Headache | Dorsal myelitis (LETM) Spinal meningeal enhancement Arachnoiditis | Hydrocephalus Basal exudate Infract Meningeal enhancement | Protein-1371 mg/dl glucose -110 mg/dl TLC-290/mm3 | Negative | No | Yes | No | Good |
| 40/M | Paraparesis Sphincter involvement Fever Headache Altered sensorium | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement | Hydrocephalus Basal exudate Tuberculoma Optochiasmatic arachnoiditis Meningeal enhancement | Protein-140 mg/dl glucose-20 mg/dl TLC-290/mm3 GeneXpert-Positive | Positive | Yes | Yes | No | Good |
| 27/F | Paraparesis Sphincter involvement Fever Headache vomiting | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement CSF loculation | Tuberculoma Meningeal enhancement | Protein-763 mg/dl glucose -13 mg/dl TLC-520/mm3 | Negative | No | Yes | No | Good |
| 20/M | Paraparesis Sphincter involvement Fever Headache Seizure Altered sensorium vomiting | Cervico-dorsal myelitis (LETM) Spinal meningeal enhancement | Hydrocephalus Meningeal enhancement | Protein-58 mg/dl glucose -60 mg/dl TLC-12/mm3 | Negative | Yes | Yes | No | Good |
LETM, longitudinally extensive transverse myelitis; TLC, total leukocyte count
On neuroimaging, 17 (94.4%) patients were having an LETM. The cervico-dorsal spinal cord was the most commonly involved area, seen in 12 (66.7%). The myelitis extended to involve the area Postrema in 1 (5.6%) patient. Apart from myelitis, the MRI of the spine showed additional imaging features in these patients in the form of spinal meningeal enhancement 14 (77.8%), extra-axial collection 3 (16.7%), CSF loculation 3 (16.7%), arachnoiditis 3 (16.7%), and spinal tuberculoma 2(11.1%). MRI of the brain showed hydrocephalus 7 (38.9%), basal exudates 8 (44.4%), meningeal enhancement 11(61.1%), tuberculomas 9 (50%), and infarct 2 (11.1%) (Tables 1 and 2) (Fig. 2).
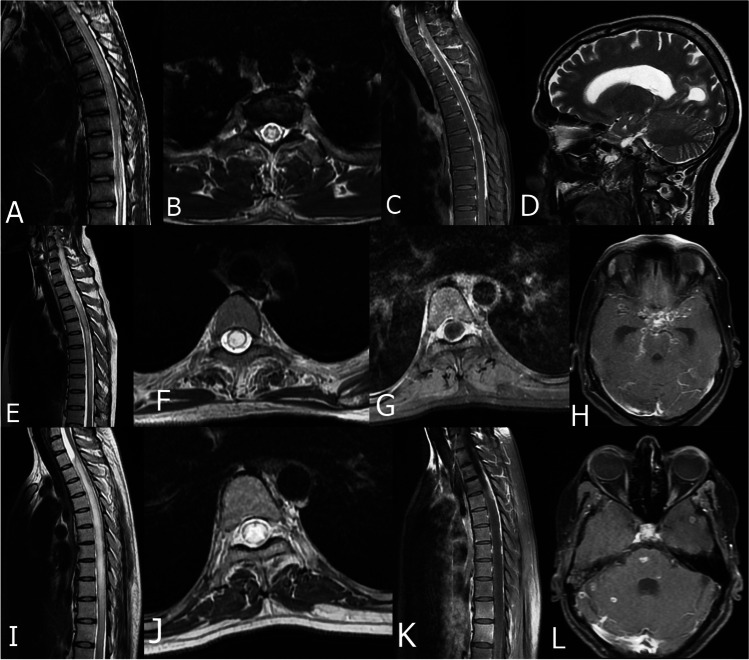
Fig. 2.
MRI of 3 patients with tuberculous myelitis. (A–D) Patient 1, sagittal T2 image showing LETM involving dorsal spine (A), Axial T2 image showing central hyperintensity within the spinal cord (B), post contrast sagittal image showing spinal meningeal enhancement (C), MRI brain of the same patient showing hydrocephalus. (E–H) Patient 2, (E) MRI spine, sagittal T2 image showing LETM involving cervico-dorsal spine, (F) axial T2 image showing myelitis involving the whole transverse diameter of the cord, (G) Post contrast axial image showing meningeal enhancement, (H) MRI brain of the same patient showing opto-chiasmatic tuberculomas. (I–L) Patient 3, (I) sagittal T2 image showing LETM with CSF loculation, (J) Axial T2 image showing myelitis involving the whole transverse diameter, (K) Post contrast image showing spinal meningeal enhancement, (L) MRI brain of the same patient showing multiple tuberculoma
Chest X-ray abnormalities consistent with tuberculosis were seen in 9 (50%), and 3 of them were sputum positive for acid-fast bacilli. The chest X-ray showed a miliary pattern in 2 of these 9 patients. CSF GeneXpert MTB/RIF was positive in 3 (16.7%). The CSF examination showed a raised protein, low glucose, and pleocytosis (Tables 1 and 2).
Comparison of tuberculous myelitis with other myelitis
Among the clinical features, headache (B-H adjusted P value = 0.018) was significantly more common in tuberculous myelitis; fever was also seen commonly in tuberculous myelitis patients, although it does not reach statistical significance (B-H adjusted P = 0.057). The CSF protein (B-H adjusted P < 0.001), and CSF cell count (B-H adjusted P < 0.001) was significantly higher in tuberculous myelitis as compared to other causes of myelitis. The CSF glucose was significantly lower (B-H adjusted P < 0.001) in tuberculous myelitis. Neuroimaging findings like spinal meningeal enhancement, CSF loculations, arachnoiditis, exudates, hydrocephalus, and tuberculomas were seen in the tuberculous myelitis patients (Table 1). On multivariate analysis, none of the factors were significantly associated with tuberculous myelitis.
Outcome
Twenty-two (42.3%) patients experienced a poor outcome at 6 months. On univariate analysis, patients with tuberculous myelitis had a significantly better outcome. Three out of 18 (16.7%) patients with tuberculous myelitis experienced a poor outcome, as compared to 19/34 (55.9%) patients of the other group (B-H adjusted P = 0.025, RR = 0.27 (0.09–0.82). On multivariate analysis, none of the factors predicted a poor outcome.
Discussion
We found that 18 (34.6%) patients fullfilled the definition of tuberculous myelitis. The existing knowledge about tuberculous myelitis is generally available in form of case reports and case series [2]. Our study shows that tuberculous myelitis, in a high tuberculosis burden zone, is commoner than expected. In our cohort of tuberculous myelitis, the majority of patients (17/18) had concomitant meningeal or pulmonary tuberculosis. In our study, 6 patients paradoxically developed myelitis while being treated with the antituberculosis drugs. We noted that tuberculous myelitis on outcome assessment fared better than other myelitis.
These findings are important as they shed some light on the pathogenesis of tuberculous myelitis. The proposed pathogenesis of tuberculous myelitis is an immune-mediated attack against mycobacterium leading to the inflammatory demyelination of the spinal cord. The occurrence of paradoxical tuberculous myelitis further strengthens the notion that immune-mediated mechanisms might be responsible for tuberculous myelitis. Several mycobacterial cell wall antigens are presented in the infected tissues, these antigens may stimulate an exaggerated inflammatory reaction in the host [19]. These inflammatory reactions may also lead to myelitis. Previous studies have also reported paradoxical tuberculous myelitis occurring during the treatment of tuberculous meningitis [23, 24]. Previous studies have also shown an association between the occurrence of pulmonary tuberculosis and inflammatory demyelination of the spinal cord and suggested underlying immune mechanisms as the cause of myelitis [25].
On neuroimaging, in patients with tuberculous myelitis, there is extensive spinal cord involvement. NMO is the most common cause of LETM but other infectious, neoplastic, autoimmune, and vascular conditions can lead to LETM [26]. Physicians and neurologists practicing in tuberculosis endemic areas must consider tuberculosis in the differential diagnosis of LETM. We found that the cervico-dorsal spinal cord was the most commonly involved area in cases of tuberculous myelitis. The findings of cervico-dorsal LETM as a common imaging manifestation of tuberculous myelitis are consistent with other recent studies [24]. Other neuroimaging findings were the presence of spinal meningeal enhancement, CSF loculation, tuberculoma, extra-axial collection, and arachnoiditis. These findings suggest that tuberculous myelitis may not be an isolated entity; rather, it can be a combination of meningo-myelitis. A similar combination of neuroimaging findings has also been described in other studies [24].
CSF in patients with tuberculous myelitis characteristically revealed markedly raised protein levels indicating the presence of tuberculous spinal involvement [27]. The CSF GeneXpert MTB/RIF was positive in 3 of our cases, the bacteriological confirmation of extra-pulmonary tuberculosis is often difficult due to the paucibacillary nature of the disease [28]. Thus in the absence of a bacteriological diagnosis in all the cases, one may have to rely on clinical, neuroimaging, and other laboratory features to make a diagnosis of tuberculous myelitis (Table 3).
Table 3.
Salient distinguishing features of tuberculous myelitis
| Clinical features |
| Fever |
| Headache |
| History of antituberculosis treatment |
| Neuroimaging features |
| Longitudinally Extensive transverse myelitis |
| Involvement of cervicodorsal spine |
| Spinal meningeal enhancement |
| Chest X-ray |
| Miliary shadows or typical findings of pulmonary tuberculosis |
| Cerebrospinal fluid examination |
| Markedly raised protein |
| Low glucose |
| GeneXpert MTB/RIF positive |
On parameters of the outcome, tuberculous myelitis fared significantly better, this further highlighted the importance of a timely diagnosis of this condition, as anti-tuberculosis treatment along with corticosteroids might lead to a better outcome. Although studies with a larger sample size may be required to fully explore the prognosis and its determinants in patients with tuberculous myelitis.
In conclusion, tuberculous myelitis can be seen in approximately 35% of all myelitis cases in tuberculosis endemic areas. Fever, headache, markedly elevated CSF protein and spinal meningeal enhancement indicated tuberculous myelitis. Tuberculous myelitis patients generally respond well to corticosteroids.
Declarations
Ethical approval and Informed consent
The study was approved by the institutional ethics committee. Informed consent was obtained from all the patients.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohd. Imran Khan, Ravindra Kumar Garg, Imran Rizvi, contributed equally to this work.
References
- 1.Garg RK, Malhotra HS, Gupta R. Spinal cord involvement in tuberculous meningitis. Spinal Cord. 2015;53(9):649–657. doi: 10.1038/sc.2015.58. [DOI] [PubMed] [Google Scholar]
- 2.Wasay M, Arif H, Khealani B, Ahsan H. Neuroimaging of tuberculous myelitis: analysis of ten cases and review of literature. J Neuroimaging Off J Am Soc Neuroimaging. 2006;16(3):197–205. doi: 10.1111/j.1552-6569.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous (1905) Myelitis and pulmonary tuberculosis. Hospital (Lond 1886) 9;38(986):359–360 [PMC free article] [PubMed]
- 4.Rigdon RH. Transverse myelitis accompanying tuberculous meningitis. Am Rev Tuberc. 1947;55(4):332–340. doi: 10.1164/art.1947.55.4.332. [DOI] [PubMed] [Google Scholar]
- 5.Jorge de Saráchaga A, Rivera-Chávez LF, López-Martínez MS (2020) Neuromyelitis optica spectrum disorder (NMOSD) with hypothalamic involvement and central nervous system tuberculosis: a case report. Clin Neurol Neurosurg 193:105751 [DOI] [PubMed]
- 6.Zhang Y, Zhu M, Wang L, Shi M, Deng H. Longitudinally extensive transverse myelitis with pulmonary tuberculosis: two case reports. Medicine (Baltimore) 2018;97(3):e9676. doi: 10.1097/MD.0000000000009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang L, Gong Y, Han K, Lv Y, Li M, Wang J (2021) Longitudinally extensive transverse myelitis with mycobacterium tuberculosis infection. Acta Neurol Belg. 10.1007/s13760-021-01723-0. Epub ahead of print [DOI] [PMC free article] [PubMed]
- 8.Zayet S, Zaghdoudi A, Harrabi H, Goubantini A, Tiouiri BH. Devic’s neuromyelitis optica associated with active pulmonary tuberculosis. Tunisia New Microbes New Infect. 2021;39:100828. doi: 10.1016/j.nmni.2020.100828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Synmon B, Phukan P, Sharma SR, Hussain M. Etiological and radiological spectrum of longitudinal myelitis: a hospital-based study in North East India. J Neurosci Rural Pract. 2021;12(4):739–744. doi: 10.1055/s-0041-1735826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KJ, Brunberg JA, Quint DJ, Kazanjian PH. Spinal cord infection: myelitis and abscess formation. AJNR Am J Neuroradiol. 1998;19(2):341–348. [PMC free article] [PubMed] [Google Scholar]
- 11.Dutra BG, da Rocha AJ, Nunes RH, Maia ACM (2018) Neuromyelitis optica spectrum disorders: spectrum of MR imaging findings and their differential diagnosis. Radiogr Rev Publ Radiol Soc N Am Inc 38(1):169–93 [DOI] [PubMed]
- 12.Singh AK, Malhotra HS, Garg RK, Jain A, Kumar N, Kohli N, et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect Dis. 2016;21(16):306. doi: 10.1186/s12879-016-1625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uniyal R, Garg RK, Malhotra HS, Kumar N, Jain A, Kohli N, et al. Computed tomography thorax abnormalities in immunocompetent patients with tuberculous meningitis: an observational study. J Neurol Sci. 2019;15(397):11–15. doi: 10.1016/j.jns.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (2014) Definitions and reporting framework for tuberculosis: 2013 revision [Internet] Geneva: World Health Organization; 2013. Available from: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf?ua=1
- 16.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;3(15):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4):499–505. doi: 10.1212/WNL.59.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Garg RK, Malhotra HS, Kumar N. Paradoxical reaction in HIV negative tuberculous meningitis. J Neurol Sci. 2014;340(1–2):26–36. doi: 10.1016/j.jns.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (2010) Guidelines for treatment of tuberculosis fourth edition. Downloaded from http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf?ua=1&ua=1
- 21.Lee SY, Kim DY, Sohn MK, Lee J, Lee SG, Shin YI, et al. Determining the cut-off score for the Modified Barthel Index and the Modified Rankin Scale for assessment of functional independence and residual disability after stroke. PLoS ONE. 2020;15(1):e0226324. doi: 10.1371/journal.pone.0226324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 23.Gupta R, Garg RK, Jain A, Malhotra HS, Verma R, Sharma PK. Spinal cord and spinal nerve root involvement (myeloradiculopathy) in tuberculous meningitis. Medicine (Baltimore) 2015;94(3):e404. doi: 10.1097/MD.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Xu X, Guo Z, Liu Y, Lin J, Suo L, et al. Myelitis: a common complication of tuberculous meningitis predicting poor outcome. Front Neurol. 2022;13:830029. doi: 10.3389/fneur.2022.830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zatjirua V, Butler J, Carr J, Henning F. Neuromyelitis optica and pulmonary tuberculosis: a case-control study. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2011;15(12):1675–1680. doi: 10.5588/ijtld.10.0780. [DOI] [PubMed] [Google Scholar]
- 26.Trebst C, Raab P, Voss EV, Rommer P, Abu-Mugheisib M, Zettl UK, et al. Longitudinal extensive transverse myelitis–it’s not all neuromyelitis optica. Nat Rev Neurol. 2011;7(12):688–698. doi: 10.1038/nrneurol.2011.176. [DOI] [PubMed] [Google Scholar]
- 27.Mantese CE, Lubini R (2022) Froin’s syndrome with tuberculosis myelitis and spinal block. Rev Assoc Medica Bras 1992 68(1):10–2 [DOI] [PubMed]
- 28.Purohit M, Mustafa T (2015) Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource-constrained setting: state of the art, challenges and the need. J Clin Diagn Res JCDR 9(4):EE01–06 [DOI] [PMC free article] [PubMed]