Abstract
Pancreatic islet beta cells require a fine-tuned endoplasmic reticulum (ER) stress response for normal function; abnormal ER stress contributes to diabetes pathogenesis. Here, we identified a small molecule, SW016789, with time-dependent effects on beta cell ER stress and function. Acute treatment with SW016789 potentiated nutrient-induced calcium influx and insulin secretion, while chronic exposure to SW016789 transiently induced ER stress and shut down secretory function in a reversible manner. Distinct from the effects of thapsigargin, SW016789 did not affect beta cell viability or apoptosis, potentially due to a rapid induction of adaptive genes, weak signaling through the eIF2α kinase PERK, and lack of oxidative stress gene Txnip induction. We determined that SW016789 acted upstream of voltage-dependent calcium channels (VDCCs) and potentiated nutrient- but not KCl-stimulated calcium influx. Measurements of metabolomics, oxygen consumption rate, and G protein-coupled receptor signaling did not explain the potentiating effects of SW016789. In chemical cotreatment experiments, we discovered synergy between SW016789 and activators of protein kinase C and VDCCs, suggesting involvement of these pathways in the mechanism of action. Finally, chronically elevated calcium influx was required for the inhibitory impact of SW016789, as blockade of VDCCs protected human islets and MIN6 beta cells from hypersecretion-induced dysfunction. We conclude that beta cells undergoing this type of pharmacological hypersecretion have the capacity to suppress their function to mitigate ER stress and avoid apoptosis. These results have the potential to uncover beta cell ER stress mitigation factors and add support to beta cell rest strategies to preserve function.
Keywords: pancreatic islet beta cells, insulin secretion, endoplasmic reticulum stress
Pancreatic islet beta cells sense and control circulating glucose concentrations by secreting insulin. In type 1 diabetes, autoimmune attack on beta cells results in their death and thus a lack of insulin. In type 2 diabetes, beta cells initially compensate for insulin resistance but eventually fail to secrete sufficient insulin to maintain euglycemia (1). Interventions are aimed at restoring beta cell functionality, lowering insulin resistance, and reducing hyperglycemia. However, existing therapeutics have limitations, necessitating identification of new targets and pathways.
Beta cells sense elevated circulating blood glucose concentrations via the glucose transporters GLUT2 in mice and GLUT1/3 in humans (2). Upon cellular entry, glucose metabolism via glycolysis and the tricarboxylic acid cycle yields a relative increase in [adenosine 5′-triphosphates (ATP)/adenosine 5′-diphosphates (ADP)], causing ATP-sensitive potassium (KATP) channel closure. This closure reduces the plasma membrane permeability to potassium, causing membrane depolarization, opening of voltage-dependent Ca2+ channels (VDCCs) and Ca2+ influx (3). Ca2+ influx also stimulates oxidative phosphorylation in the mitochondria, driving a metabolic cycle that sustains ATP production and insulin secretion (4, 5). Ca2+ influx triggers the fusion of insulin secretory vesicles with the plasma membrane resulting in insulin exocytosis (6). Nutrient metabolism further enhances secretion without further Ca2+ influx via the amplifying pathway (7-9). To handle their specialized secretory function, beta cells engage the unfolded protein response of the endoplasmic reticulum (UPRER) (10). The UPRER is crucial in beta cells, as its disruption (eg, PERK loss of function) leads to beta cell failure and diabetes (11). Conversely, unmitigated UPRER is also detrimental to beta cell function and survival, eventually leading to induction of proapoptotic transcription factors including Ddit3 (CHOP) and Txnip (12, 13).
Many studies have elucidated aspects of beta cell biology that may be targeted to improve overall beta cell health and function, including the UPRER, beta cell dedifferentiation, and the role of immature beta cells in whole islet function (14-18). However, the high-throughput identification of novel small molecules that alter pancreatic beta cell function has lagged in comparison. Here, we used a secreted insulin-luciferase–based high-throughput screening tool to identify a small molecule, SW016789, which acutely and chronically alters beta cell secretory responses (19, 20). We hypothesized the chronic effects on function were a result of acute hypersecretion, which induced stress pathways (eg, ER stress) that led to a shutdown of beta cell function but not beta cell death. We investigated the mechanisms of action of SW016789 using a multipronged approach including measuring Ca2+ influx and insulin secretion, metabolomics and mitochondrial function, gene expression, and cotreatment with compounds that may interfere with the effects of SW016789. Our results with SW016789 agree with a model of beta cell plasticity wherein the effects of persistent secretory stress are mitigated to avoid cell death at the expense of function. Chemical tools such as SW016789 present an opportunity to discover previously unrecognized or poorly characterized genes and pathways involved in these processes in the beta cell.
Materials and Methods
Reagents
Complete source information for all small molecules used in this work are listed in Supplementary Table 4 (21).
Small Molecule Screening
High-throughput screening of small molecules in insulin-Gaussia luciferase (InsGLuc) MIN6 cells was as described (22). Briefly, cells were seeded at ~15e6 cells per T175 flask and grown 7 days until confluence and then trypsinized, resuspended in medium, passed through a 40-µm cell strainer, and diluted to 1.5e6 cells/mL. Cells were dispensed at 50 µL per well in 384-well opaque white cell culture dishes using a BioTek Multiflo FX liquid handler to yield 7.5e4 cells/well. Plates were placed in a tissue culture incubator for 72 hours. Next, 0.5 µL each of dimethyl sulfoxide (DMSO; negative control, 1%), thapsigargin (positive control, 1 mM), and small molecules (5 µM) were added robotically to the plates, and the cells were incubated 24 hours. On the day of the assay, using a BioTek Multiflo FX liquid handler, cells were washed twice with Krebs-Ringer bicarbonate HEPES (KRBH; 75 µL and then 50 µL) buffer. To remove medium and buffer between washes, plates were centrifuged upside-down in collection trays at 30 × g for 1 minute. Cells were then incubated in 25 µL of KRBH containing 200 mM diazoxide for 60 minutes. Next, 25 µL of 2× stimulation buffer in KRBH was added to yield a final concentration of 20 mM glucose, 35 mM KCl, and 250 mM diazoxide in a total volume of 50 µL. The plates were incubated for 60 minutes at 37°C in a tissue culture incubator. Afterward, 20 µL of GLuc assay working solution (5 mM KCl, 15 mM Hepes pH 7.4, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, 300 mM sodium ascorbate, and 3.54 µM coelenterazine) was added to each well. Within 10 to 20 minutes, the luminescence from each well of the plates was measured using a Perkin Elmer EnVision multimode plate reader with 0.1 second integration time. The raw data were corrected for plate effects using a GeneData proprietary algorithm, and then each value on a per plate basis was subjected to either single-point or 2-point normalization, followed by calculation of the robust Z (RZ) score for each well. Hit compounds with an |robust Z| > 3 were selected for structural clustering analysis. Representative compounds were then cherry picked and confirmed in triplicate in the same InsGLuc-MIN6 secretion assay and tested for toxicity in MIN6 cells by Cell Titer Glo assay after 72 hours of exposure.
Cell Culture and Treatments
Culture of MIN6 beta cells (RRID: CVCL_0431) has been described (20). MIN6 cells were cultured in high glucose DMEM containing 10% fetal bovine serum (FBS), 50 µM beta-mercaptoethanol, 1 mM pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Prior to glucose-stimulated insulin secretion experiments, MIN6 cells were washed twice with and incubated for 2 hours in freshly prepared glucose-free modified KRBH buffer [5 mM KCl, 120 mM NaCl, 15 mM HEPES, pH 7.4, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 1 mg/mL radioimmunoassay-grade bovine serum albumin (BSA)]. MIN6 cells were stimulated with glucose as indicated, and secreted insulin and insulin content were measured using the Cisbio Insulin High Range HTRF kit (RRID: AB_2890910). To generate lysates for immunoblotting, cells were lysed in 1% NP40 lysis buffer (25 mM HEPES, pH 7.4, 1% Nonidet P-40, 10% glycerol, 137 mM NaCl, 1 mM EDTA, 1 mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 1 μg/mL pepstatin, 5 μg/mL leupeptin), rotated (10 minutes, 4°C), centrifuged (10 000 × g; 10 minutes; 4°C), and stored at −80°C. InsGLuc-MIN6 cells were generated and characterized as previously described (20) and were determined to be negative for mycoplasma. Mouse intestinal L cell line GLUTag (RRID: CVCL_J406) was originally generated by Daniel Drucker (Mount Sinai Hospital) and were obtained from the lab of George G. Holz (Upstate Medical University). GLUTag cells were cultured in low glucose DMEM with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The human somatostatinoma line QGP-1 (RRID: CVCL_3143) was provided by Dawn E. Quelle (University of Iowa). QGP-1 cells were cultured in RPMI-1640 with 10% FBS, 1 mM pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. GLUTag and QGP-1 cells were plated in 12-well dishes and treated in medium as indicated in the figure legend [Supplementary Figure 4B-C (21)] before harvesting for RNA purification and downstream gene expression analysis.
Secreted Gaussia Luciferase Assays
InsGLuc-MIN6 cells were plated in 96-well dishes at 1e5 cells/well and cultured for 3 to 4 days before compound treatment either overnight (24 hours) in medium or acutely (1 hour) in KRBH. Treatments in 96-well assays were performed with at least 3 replicate wells and in at least 3 independent passages of cells. For assays, cells were washed twice with KRBH and preincubated in 100 µL of KRBH (250 µM diazoxide where indicated) for 1 hour. The solution was then removed, and cells were washed once with 100 µL of KRBH and then incubated in KRBH with or without the indicated stimulation and compound treatments for 1 hour. Fifty microliters of KRBH was collected from each well and pipetted into a white opaque 96-well plate Optiplate-96 (Perkin-Elmer). Fresh GLuc assay working solution was then prepared by adding coelenterazine stock solution into assay buffer to a final concentration of 10 µM. Fifty microliters of working solution was then rapidly added to the wells using an Integra Voyager 1250 µL electric multichannel pipette for a final concentration of 5 µM coelenterazine. After adding reagent to the plate, plates were spun briefly, and luminescence was measured on a Synergy H1M2 plate reader (BioTek). The Gen5 software protocol was set to shake the plate orbitally for 3 seconds and then read the luminescence of each well (integration, 100 ms; gain, 150).
Human Islet Culture, Drug Treatments, and Static Culture Secretion Assays
Cadaveric human islets were obtained through the Integrated Islet Distribution Program. Islets were isolated by the affiliated islet isolation center and cultured in PIM medium (PIM-R001GMP, Prodo Labs) supplemented with glutamine/glutathione (PIM-G001GMP, Prodo Labs), 5% human AB serum (100512, Gemini Bio Products), and ciprofloxacin (61-277RG, Cellgro, Inc) at 37°C and 5% CO2 until shipping at 4°C overnight. Human islets were cultured upon receipt in complete CMRL-1066 (10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin). For drug treatments and static culture, human islets (50-75) were handpicked under a dissection microscope and transferred to low-binding 1.5 mL tubes (50 islets/tube). Islets were cultured 24 hours in 500 µL of complete CMRL-1066 medium containing indicated drug treatments. For static culture insulin secretion experiments, the islets were washed twice with 500 µL of KRBH (134 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM HEPES pH 7.4, 0.1% radioimmunoassay-grade BSA) and preincubated in KRBH (500 µL, 1 mM glucose; 1 hour) before switching to 500 µL KRBH containing either 1 or 16 mM glucose for 1 hour. Supernatants were collected, centrifuged (10 000 × g; 5 minutes) and transferred to fresh tubes for storage at −80°C. Total insulin content was extracted by acid-ethanol (1.5% HCl in 70% ethanol) overnight at −80°C, and the solution was neutralized with an equal volume of 1 M Tris pH 7.4 prior to insulin assays. Secreted insulin and insulin content were measured using the Cisbio Insulin High Range HTRF kit. Human islet characteristics and donor information are listed in Supplementary ESM Human Islet Checklist (21).
Cell Death and Viability Assays
To measure live and dead cells as well as the live/dead cell ratio, MultiTox-Fluor (Promega) was used according to the manufacturer’s instructions. Briefly, MIN6 cells were seeded into 96-well black-walled clear bottom tissue culture dishes at 5e4-1e5 cells/well and culture for 3 to 5 days before treatment with compounds as indicated in the figure legend (Fig. 1G-I). On the day of the assay, cell-permeant GF-AFC substrate (live cell readout) and cell-impermeant bis-AAF-R110 substrate (dead cell readout) reagents were diluted into kit assay buffer as a 2× working solution. One hundred microliters of 2× working solution was added to each well of cells in 100 µL of medium and incubated 30 minutes at 37°C. Plates were read on a BioTek Synergy H1M2 plate reader (400Ex/505Em for live cells; 485Ex/520Em for dead cells). Digitonin was a positive control for cell death, which lysed cells immediately prior to addition of 2× working solution. Viability and death were normalized to DMSO control.
Ca2+ Influx Assays
Ca2+ influx measurements were completed in MIN6 cells plated in triplicate in black-walled clear-bottom 96-well tissue culture imaging plates. Cells were preincubated for 1 hour in glucose-free KRBH, followed by loading with Fura-2-LR-AM (5 µM) for 25 minutes and a 5 minutes washout. Using a BioTek Synergy H1 plate reader equipped with dual monochromators, relative Ca2+ levels were measured (Ex340/380/Em510 gain = 150). Recordings included 5 minutes baseline followed by 5 to 30 minutes postaddition of stimulation as indicated. Data were normalized by subtracting the mean of the respective baseline reads. Areas under the curves were calculated from peaks detected in GraphPad Prism using a baseline of 0 ΔRFU, restricted to peaks that go above baseline by more than 0.05 ΔRFU and defined by at least 10 adjacent points. For Ca2+ measurements in the presence of diazoxide, after loading with Ca2+ indicator dye, cells were incubated in KRBH containing 250 µM diazoxide for 5 minutes prior to the baseline read and subsequent stimulation with 35 mM KCl and/or 20 mM glucose with DMSO or SW016789 in the continued presence of diazoxide. For measurements of ER Ca2+ and store-operated Ca2+ entry (SOCE), beta cells were incubated in normal glucose-free KRBH for 1 hour and then loaded with Fura-2-LR-AM (5 µM) in Ca2+-free KRBH (5 mM KCl, 120 mM NaCl, 15 mM HEPES, pH 7.4, 24 mM NaHCO3, 3 mM MgCl2, and 1 mg/mL radioimmunoassay-grade BSA) for 25 minutes, followed by a 5-minute washout in the same buffer. Cells were placed in the BioTek Synergy H1 for a baseline read (30 seconds), followed by injection of thapsigargin (10 µM) for a 5 minutes read and CaCl2 (2.5 mM) for another 5 minutes read.
3′,5′-Cyclic Adenosine 5′-Monophosphate Reporter Assays
For relative 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) measurements, the genetically encoded Epac-based bioluminescence-resonance energy transfer (BRET) reporter for cAMP (CAMYEL) was used as previously described (23). CAMYEL MIN6 cells were preincubated in glucose-free KRBH for 1.5 hours. Cells were incubated for 15 minutes at room temperature with coelenterazine-h (20 µM). After a 3-minute baseline recording, stimulations were added, and plates were read for an additional 15 minutes. Using the BRET1 cube from BioTek, emission signals at 460/40 and 540/25 nm (center/bandpass) were measured every 0.8 seconds. Data were normalized by subtracting the mean of the respective baseline reads. Areas under the curve were calculated in GraphPad Prism using a baseline of 0 ΔRFU and ignoring peaks that are <10% of the distance from minimum to maximum ΔRFU and those defined by <15 adjacent points. All peaks must go above the baseline.
Chiral Separation and Structure-Activity Analysis
Separated enantiomers of racemic SW016789 were obtained from Lotus Separations using supercritical fluid chromatography (24). Purified enantiomers were resuspended in DMSO and subjected to acute and chronic InsGLuc-MIN6 experiments as described. For structure-activity analysis, structures of SW016789 analogs and their respective activity scores were analyzed in Datawarrior software (25). Glucose-stimulated InsGLuc secretory activity normalized to unstimulated secretion was input along with structures and activity-cliff analysis was performed.
Antibodies and Immunoblotting
A total of 40 to 50 µg of cleared cell lysates were separated on 10% gels by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunoblotting. All membranes were blocked in Odyssey blocking buffer (Licor) for 1 hour before overnight incubation with primary antibodies diluted in blocking buffer. Mouse anti-CHOP (AB_2089254), mouse anti-PARP (cleaved) (AB_2160592), rabbit anti-BclXL (AB_2228008), rabbit anti-PERK (pThr980) (AB_2095853), rabbit anti-total PERK (AB_2095847), rabbit anti-BiP (AB_2119845), rabbit anti-ATF3 (AB_2799039), rabbit anti-ATF4 (AB_2616025), rabbit anti-eIF2α (pS51) (AB_2096481), and mouse anti-GAPDH (AB_2756824) were all from Cell Signaling Technology. Mouse anti-actin (AB_476697) was from Sigma. Goat anti-MafA (AB_2133149) and goat anti-Pdx1(AB_653988) were from Santa Cruz Biotechnologies. Complete antibody information available in Supplementary Table 4 (21). After three 10-minute washes in Tris-buffered saline with 0.1% Tween® 20 (20 mM Tris-HCl pH 7.6, 150 mM NaCl, 0.1% Tween-20), membranes were incubated with fluorescent secondary antibodies for 1 hour at room temperature. After three 10-minute washes in Tris-buffered saline with 0.1% Tween® 20 , membranes were imaged on a Licor Odyssey scanner.
Relative Gene Expression
RNA was isolated from MIN6 cells using the PureLink RNA Mini Kit (Life Technologies) or the Aurum Total RNA Mini kit (Bio-Rad). Briefly, after indicated treatments, medium was removed from cells, and lysis buffer (with β-mercaptoethanol) was added to cells. Cells were scraped, and lysates were transferred to 1.5 mL tubes on ice and then transferred to −80°C for storage. Samples were processed according to the kit manufacturer’s instructions, including on-column digestion with RNase-free DNase. RNA concentration was measured using an Implen Nanophotometer or Nanodrop spectrophotometer and verified to have A260/280 ratios > 2.0. 1000 ng of RNA was converted into complementary DNA (cDNA) using the iScript cDNA synthesis kit (Bio-Rad) following manufacturer instructions, and the resulting cDNA was diluted 20-fold with water. One microliter of diluted cDNA was used in 10 μL quantitative polymerase chain reactions using 2× SYBR Bio-Rad master mix and 250 nM of each primer. Reactions were run in 384-well format on a CFX384 (Bio-Rad) or QuantStudio 5 (Thermo). Quantitative polymerase chain reaction data were analyzed using CFX Manager (Bio-Rad) with 18S RNA as the reference gene. Relative expression was calculated by the 2-ΔΔCt method. All primer details are listed in Supplementary Table 4 (21).
Bioenergetics
Oxygen consumption rates were measured using a Seahorse XFe96 (Agilent). MIN6 cells were seeded at a density of 5e4 cells/well in XF96 cell culture microplates and cultured for 48 hours. On the day before the assay a Seahorse cartridge was hydrated with calibrant solution and incubated overnight. On the day of the assay, the XFe96 PrepStation was set to 37°C, and a hydrated cartridge was placed inside. MIN6 cells were washed twice with Seahorse-modified KRBH (5 mM KCl, 114 mM NaCl, 20 mM Hepes pH 7.4, 1.2 mM MgSO4, 2.5 mM CaCl2, 1.2 mM KH2PO4, and pH carefully adjusted to 7.4, BSA and NaHCO3 are not present) and placed in the PrepStation to equilibrate for 1 hour. The hydrated Seahorse cartridge was placed on an empty utility plate to be loaded with compounds diluted in Seahorse-modified KRBH. The drug cartridge was returned to the utility plate with calibrant solution and inserted into the XFe96 for calibration. After calibration, the cell culture plate was inserted into the XFe96, and MIN6 cells were subjected to 3 cycles of mixing (2 minutes) and measuring (3 minutes) to measure baseline oxygen consumption rate and extracellular acidification rates. Stimulations were injected as indicated, and after each stimulation, cells were monitored for 3 cycles as during baseline measurements. Data were analyzed in the Seahorse WAVE software (version 2.61.53).
Metabolomics
Metabolomic analysis was performed as described (26, 27). MIN6 cells grown to confluence in 6-well dishes were preincubated in glucose-free KRBH for 2 hours at 37°C. DMSO (0.1%), SW016789 (5 µM), and/or glucose (20 mM) were added as indicated and cells were incubated a further 30 minutes at 37°C. After indicated treatments, cells were washed with 1 mL ice-cold saline and harvested in 1 mL of 80% methanol at −80°C. Cells were scraped and transferred to 1.5 mL tubes for 3 freeze-thaw cycles in liquid nitrogen. Lysates were clarified at 14 000 rpm for 15 minutes at 4°C. Metabolite containing supernatants were transferred to a new 1.5 mL tube and lyophilized using a SpeedVac. Breathable membranes were placed on top of the open tubes to prevent cross contamination and the SpeedVac was operated without heat for 4 hours. Pellets were stored at −80°C prior to analysis by the Metabolomics Facility at Children’s Medical Center Research Institute at UT Southwestern (Dallas, TX, USA). Metabolites were reconstituted in 100 mL of 0.03% formic acid in analytical grade water, vortex mixed, and centrifuged to remove debris. Thereafter, the supernatant was transferred to a high-performance liquid chromatography (HPLC) vial for the metabolomics study. Targeted metabolite profiling was performed using a liquid chromatography-tandem mass spectrometry approach. Separation was achieved on a Phenomenex Synergi Polar RP HPLC column (150 × 2 mm, 4 µm, 80 Å) using a Nexera ultra HPLC system (Shimadzu Corporation). The mobile phases employed were 0.03% formic acid in water (A) and 0.03% formic acid in acetonitrile (B). The gradient program was as follows: 0 to 3 minutes, 100% A; 3 to 15 minutes, 100% to 0% A; 15 to 21 minutes, 0% A; 21 to 21.1 minutes, 0% to 100% A; and 21.1 to 30 minutes, 100% A. The column was maintained at 35°C and the samples kept in the autosampler at 4°C. The flow rate was 0.5 mL/min and injection volume 10 µL. The mass spectrometer was an AB QTRAP 5500 (Applied Biosystems SCIEX) with electrospray ionization source in multiple reaction monitoring (MRM) mode. Sample analysis was performed in positive/negative switching mode. Declustering potential and collision energy were optimized for each metabolite by direct infusion of reference standards using a syringe pump prior to sample analysis. The MRM tandem /mass spectrometry detector conditions were set as follows: curtain gas 30 psi, ion spray voltages 5000 V (positive) and 1500 V (negative), temperature 650°C, ion source gas 150 psi, ion source gas 250 psi, interface heater on, and entrance potential 10 V. Dwell time for each transition was set at 3 msec. Samples were analyzed in a randomized order, and MRM data were acquired using Analyst 1.6.1 software (Applied Biosystems SCIEX). Chromatogram review and peak area integration were performed using MultiQuant software version 2.1 (Applied Biosystems SCIEX). Although the numbers of cells were similar and each sample was processed identically and randomly, the peak area for each detected metabolite was normalized against the protein content of that sample to correct any variations introduced from sample handling through instrument analysis. The normalized area values were used as variables for the multivariate and univariate statistical data analysis. The chromatographically coeluted metabolites with shared MRM transitions were shown in a grouped format (ie, leucine/isoleucine). Principle component analysis was completed using SIMCA-P software (version 13.0.1, Umetrics). To generate heat maps, metabolite TIC values were normalized to the average of “DMSO Basal” and expressed in log2. Heatmap was generated using MetaboAnalyst 5.0 (28) using Euclidean distance with the Ward clustering algorithm, without autoscaling of the features. Statistical differences were assessed by 1-way analysis of variance with Fisher’s least significant difference post hoc test with a false discovery rate < 0.05 in MetaboAnalyst 5.0. Raw TIC values and table of significantly altered metabolites are provided in Supplementary Table 3 (21).
Statistical Analysis
Quantitated data are expressed as mean ± SD. Data were evaluated using 1- or 2-way analysis of variance as indicated and considered significant if P < 0.05. Graphs were made in GraphPad Prism 9, and figures were assembled in Affinity Designer (Serif).
Results
High-throughput Screen Identifies Small Molecule Modulator of Insulin Secretion
To discover small molecules that alter beta cell function, we utilized the InsGLuc fusion reporter expressed in MIN6 beta cells (InsGLuc-MIN6) (20). InsGLuc is processed and leads to cosecretion of GLuc with insulin. Secreted GLuc activity in this manner has been well-established as a proxy for insulin release (19, 29-33). Our approach involved treating InsGLuc-MIN6 beta cells for 24 hours with compounds followed by a brief washout prior to testing their sustained impact on insulin secretion. This strategy probes a distinct phenotypic space and is complementary to results from studies utilizing acute treatments during stimulation (19). We screened an 8320-compound diversity subset approximately representing the chemical space of the >300 000 compound library at the UT Southwestern Medical Center High-Throughput Screening Core. InsGLuc-MIN6 beta cells were treated with control and test compounds for 24 hours in normal medium followed by a washout and stimulation with glucose and KCl in the presence of diazoxide (Fig. 1A). Among the hits, chronic exposure to SW016789 caused inhibition of secretion in InsGLuc-MIN6 cells with an IC50 < 1 µM (Fig. 1B) and was chosen for further study. Glucose-stimulated InsGLuc secretion was blunted in MIN6 cells chronically exposed to resupplied stocks of SW016789, validating screening results (Fig. 1C). Chronic pretreatment (24 hours) of isolated human islets with SW016789 also impaired glucose-stimulated insulin secretion (GSIS) (Fig. 1D and 1E), without a significant impact on insulin content (Fig. 1F). SW016789 did not impact cell viability (Fig. 1G), cell death (Fig. 1H), or the live/dead cell ratio (Fig. 1I) in MIN6 cells treated for up to 72 hours. During this time course, SW016789-treated cells remained unresponsive to stimulation with glucose or KCl (Fig. 1J) but were able to mount significant, although blunted, responses to glucose if combined with stimulators of cAMP generation or protein kinase C (PKC) activation (Fig. 1J). Beta cells exposed to SW016789 for 24 hours recovered function after a 48-hour washout (Fig. 1K), indicating the effects are reversible.
Figure 1.
Discovery of SW016789 as a small molecule chronic suppressor of insulin secretion. (A) High-throughput screening workflow leading to the discovery of SW016789. Screening was performed on an 8320-compound diversity subset representing the chemical space of the full compound library at the UT Southwestern Medical Center High-Throughput Screening Core. Cells were treated with control and test compounds for 24 hours in normal medium followed by a washout and stimulation with glucose and KCl in the presence of diazoxide and finally subjected to InsGLuc assays. These maximal stimulatory conditions afforded the assay a large dynamic range (average Z-score > 0.5) and resulted in 168 primary hits, which after structural clustering were narrowed down to 82, of which 32 confirmed in triplicate. (B) Concentration response curve testing to determine EC50 of SW016789 InsGLuc-MIN6 after a 24-hour exposure. Data are the mean ± SD of 3 independent assays. (C and D) In mouse InsGLuc-MIN6 beta cells (C) and human islets (D), 24-hour exposure to SW016789 (5 µM) resulted in suppression of glucose-stimulated insulin secretion during assays in the absence of compound. Data are the mean ± SD of 3 or 4 independent experiments. (G-I) Exposure of MIN6 beta cells to dimethyl sulfoxide (DMSO; 0.1%) or SW016789 (5 µM) for up to 72 hours did not alter relative amounts of (G) live cells, (H) dead cells, or (I) the live/dead cell ratio. Digitonin (Dgt) treatment was a positive control for cell death added just prior to the assay. (J) InsGLuc-MIN6 cells were exposed to SW016789 (5 µM) for 24 to 72 hours followed by a 1-hour washout in glucose-free Krebs-Ringer bicarbonate HEPES buffer. Cells were then stimulated for 1 hour with the indicated treatments (0G, glucose-free; 20G, glucose 20 mM; Fsk, forskolin 10 µM; 3-isobutyl-1-methylxanthine 100 µM; PMA, phorbol myristate acetate 100 nM). *P < 0.05 vs respective Basal; †P < 0.05 vs respective DMSO. (K) InsGLuc-MIN6 cells were exposed to SW016789 (5 µM) for 24 hours followed by a 48-hour washout in complete medium. Cells were then assayed for glucose-stimulated InsGLuc secretion. All data are the mean ± SD of at least 3 independent experiments. *P < 0.05 by 2-way analysis of variance.
SW016789 Acutely Potentiates Nutrient-stimulated Insulin Secretion
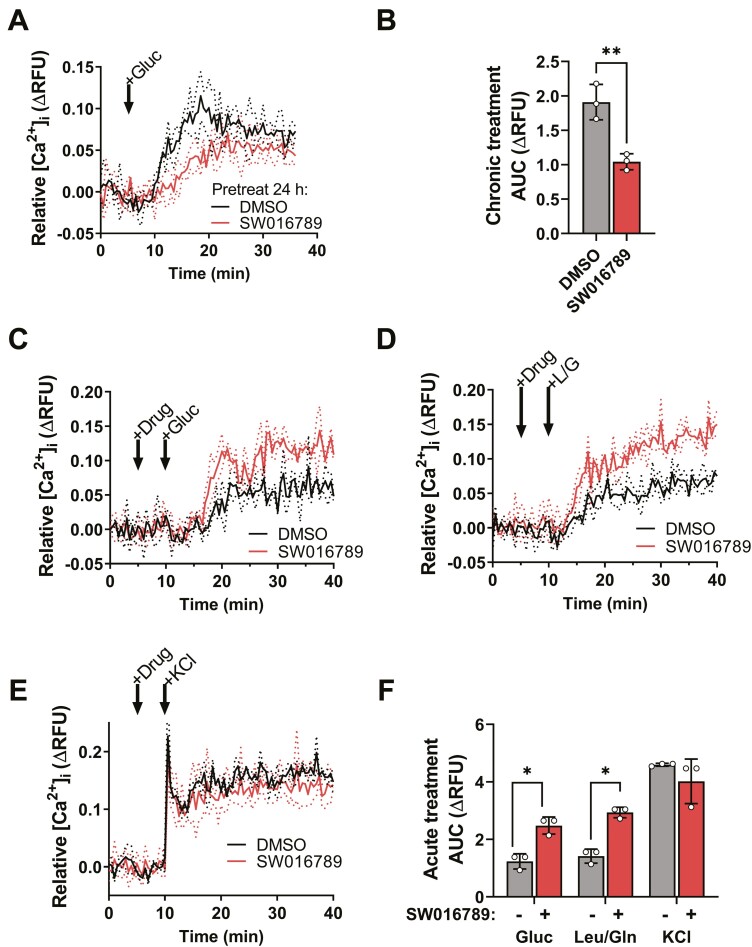
We determined that exposure of InsGLuc-MIN6 cells to SW016789 for 4 to 8 hours resulted in significant inhibition of GSIS while acute (≤2 hours) exposure caused a marked potentiation of GSIS (EC50 = 2.7 µM) [Fig. 2A; Supplementary Figure 1A and 1B (21)]. Structure-activity relationship studies revealed that while SW016789 is chiral, both enantiomers retain activity [Supplementary Figure 1C-1F (21)], and the alcohol at the chiral center is important for activity [Supplementary Figure 1G (21)]. SW016789 potentiated amino acid-induced secretion (Fig. 2B) but had no effect in cells treated with diazoxide and depolarizing KCl (Fig. 2C). However, SW016789 elicited a response from cells treated with diazoxide and glucose without elevated KCl. These data suggested the pathway activated by SW016789 requires nutrient metabolism and may impact Ca2+ influx. Accordingly, chronic SW016789 treatment suppressed glucose-stimulated Ca2+ influx (GSCa) (Fig. 3A and 3B), and acute SW016789 treatment potentiated nutrient- but not KCl-stimulated Ca2+ influx (Fig. 3C-3F). Considering these results, we determined the effects of SW016789 on Ca2+ influx in the diazoxide paradigm. Beta cells were either pretreated for 24 hours or treated with compound simultaneously with stimulation. With chronic SW016789, stimulation with depolarizing KCl or KCl/glucose caused a rapid and sustained rise in Ca2+ influx, which was significantly lower than control by ~40% [Supplementary Figure 2A (21)], while acute SW016789 had no impact [Supplementary Figure 2B (21)]. We also observed a slight increase in GSCa even in the presence of diazoxide and low KCl [Supplementary Figure 2C (21)], which was consistent with secretion data (Fig. 2C).
Figure 2.
Time-course studies and phenotypic assays reveal SW016789 as an acute potentiator of nutrient-stimulated insulin secretion. (A) InsGLuc-MIN6 cells were treated with SW016789 (5 µM) in a time course as indicated in the diagram. At the end of the time course, glucose-stimulated InsGLuc secretion was assayed. Data are the mean ± SD of n = 4. (B) InsGLuc-MIN6 cells were exposed to SW016789 (5 µM) for 1 hour during stimulation with glucose (20 mM), leucine (5 mM), or leucine/glutamine (Leu/Gln; 5 mM each) or (C) in the presence of diazoxide (250 µM) with or without KCl (35 mM) or glucose (20 mM). Data are the mean ± SD of n = 3. *P < 0.05 by 2-way analysis of variance.
Figure 3.
SW016789 treatment modulates nutrient-stimulated Ca2+ influx. (A) MIN6 beta cells pretreated 24 hours with SW016789 (5 µM) or dimethyl sulfoxide (DMSO; 0.1%) followed by washout prior to glucose-stimulated Ca2+ influx assays and (B) area under the curve (AUC) calculation. *P < 0.05 by unpaired Student’s t-test. (C-F) MIN6 beta cells acutely treated with SW016789 (5 µM) or DMSO (0.1%) followed by (C) glucose-, (D) leucine/glutamine-, or (E) KCl-stimulated Ca2+ influx assays and (F) associated AUCs. Data are the mean ± SD of 3 independent experiments. *P < 0.05 by 2-way analysis of variance.
A potential source of the SW016789-enhanced Ca2+ influx is the ER. We tested whether SW016789 impacted ER Ca2+ stores or SOCE. MIN6 beta cells were pretreated with SW016789 for 2, 6, and 24 hours and subjected to Ca2+ assays including thapsigargin stimulation to block SERCA2, leading to an observable Ca2+ leak into the cytosol [Supplementary Figure 2D (21)]. Treatment with SW016789 did not alter this indirect measure of ER Ca2+. Subsequently, Ca2+ was reintroduced to measure SOCE. Preexposure to SW016789 for 2 to 6 hours caused a small but significant decrease in SOCE, but this effect was absent in cells treated for 24 hours, suggesting that in beta cells exposed to SW016789, ER Ca2+ and SOCE responses were unmodified and may not underlie the acute potentiating or chronic inhibitory effects of SW016789. Additionally, acute SW016789 exposure did not impact muscarinic receptor-linked Gαq-stimulated Ca2+ influx [Supplementary Figure 2E (21)].
SW016789 Is Unlikely to Exert Its Beta Cell Effects via G Protein-coupled Receptors
Because of its acute potentiating activity, we considered SW016789 may act through G protein-coupled receptors (GPCRs). Acute potentiation of insulin secretion can occur via activation of Gαs-linked GPCRs and resultant cAMP generation (34). To monitor relative cAMP production, we used the BRET reporter CAMYEL stably expressed in MIN6 cells (23). The positive control (forskolin + 3-isobutyl-1-methylxanthine) caused a marked increase in cAMP, while acute stimulation with SW016789, with or without glucose, yielded no consistent alterations in signal [Supplementary Figure 3A and 3B (21)]. To test a wider range of GPCRs, we also submitted SW016789 for screening against the GPCR library at the NIMH Psychoactive Drug Screening Program at University of North Carolina (35) [Supplementary Figure 3C (21)]. Overall, SW016789 exhibited little to no activity against the GPCRs in the panel, including well-known beta cell GPCRs [Supplementary Table 1 (21)]. Only CXCR7 (ACKR3) out of 320 GPCRs was a substantial primary hit; however, confirmation studies determined the EC50 of SW016789 on CXCR7 was ~26 µM [Supplementary Figure 3D (21)], well above the EC50 for beta cell activity [Fig. 1B; Supplementary Figure 1A (21)]. In line with this result, the CXCR7 agonist VUF11207 (36) had a negligible impact on InsGLuc secretion during chronic or acute treatments [Supplementary Table 2 (21)]. These data suggested CXCR7 is unlikely to mediate the effects of SW016789 in beta cells where its activity is observed in the 1 to 5 µM range. Together, these data suggested SW016789 does not exhibit substantial off-target activity on GPCRs and that other mechanisms may be involved in its effects in beta cells.
Beta Cell Metabolomic and Mitochondrial Responses Are Normal Following Acute SW016789 Exposure
Because SW016789 required nutrient costimulation to elicit effects on Ca2+ influx, we hypothesized altered metabolism may contribute to the mechanism of action. To test this, we performed targeted metabolomics on normal MIN6 beta cells stimulated with or without glucose in the presence of DMSO or SW016789 for 30 minutes (Fig. 4A). The amplifying effect of SW016789 on GSIS was confirmed by insulin measurements, and the cellular extracts were submitted for metabolomic analysis [Fig. 4B; Supplementary Table 3 (21)]. Expectedly, glucose induced glycolysis and tricarboxylic acid cycle intermediates and the reduced/oxidized glutathione ratio (Fig. 4C and 4D). In each of these cases, SW016789 had no impact. These results suggested SW016789 is unlikely to exert effects by acutely altering well-known metabolic pathways. Beta cell oxygen consumption rates and extracellular acidification rates were also unaffected by acute exposure to SW016789 as measured by Seahorse assays (Fig. 4E-4G).
Figure 4.
SW016789 does not impact oxygen consumption rate or canonical glycolysis and tricarboxylic acid (TCA) cycle metabolites in MIN6 beta cells. (A) Experimental design for targeted metabolomics in MIN6 cells preincubated in glucose-free Krebs-Ringer bicarbonate HEPES (KRBH) for 2 hours and then treated with or without glucose (0 vs 20 mM) in the presence of dimethyl sulfoxide (DMSO; 0.1%; DMSO_0G and DMSO_20G) or SW016789 (5 µM; SW_0G and SW_20G). (B) KRBH supernatants were collected for insulin enzyme-linked immunosorbent assay to verify that SW016789 potentiated insulin secretion under these conditions. (C) Metabolomic data are represented as the log2 fold-change with respect to DMSO_0G of 3 independent experiments. (D) Representative metabolites showing glucose induced changes in glycolysis (glucose 6-phosphate, fructose-1,6-bisphosphate), TCA cycle (citrate, α-ketoglutarate, and malate), amino acids (cysteine, lysine), adenosine monophosphate, and redox potential [reduced glutathione (GSH)/oxidized glutathione (GSSG)] in MIN6 cells. (E-G) MIN6 beta cells were subjected to Seahorse analysis. Cells were preincubated in glucose-free KRBH and (E) oxygen consumption rate, (F) extracellular acidification rate, and (G) calculated Seahorse parameters were measured during stimulation with glucose (20 mM) (G20) in the presence or absence of SW016789 (5 µM) or DMSO (0.1%). Subsequently all cells were sequentially stimulated with oligomycin (oligo), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP), and antimycin A (AntA). Data represent the mean ± SD of 3 independent experiments.
Chronic Amplification of Secretion Leads to Transient UPRER and Shutdown of Beta Cell Function
Acute SW016789-induced insulin hypersecretion eventually caused beta cells to lose normal GSCa and GSIS responses, which we hypothesized was due to the UPRER. To test this, we treated MIN6 cells in a time course with SW016789 or thapsigargin and collected RNA and protein for analysis (Fig. 5A). Acute SW016789 increased immediate early gene (IEG) expression within 1 hour, which decreased to near baseline by 2 to 6 hours (Fig. 5B), in agreement with observed GSCa effects. Thapsigargin did not affect the expression of these IEGs. SW016789 also induced expression of UPRER genes by ~1 hour with a peak from 2 to 6 hours, depending on the specific gene (Fig. 5C), followed by a return to near baseline by 24 hours. This was in stark contrast to thapsigargin, which caused a similar fold induction but a delayed peak of UPRER gene expression, which persisted at 24 hours. Notably, the oxidative stress transcription factor Txnip was induced only by thapsigargin. Over the time course, SW016789 did not significantly alter insulin gene expression, but thapsigargin caused a decrease after 24 hours (Fig. 5D). SW016789 decreased insulin protein content at 6 hours but caused no further decrease by 24 hours (Fig. 5E), suggesting the effect may be due to the induced hypersecretion and implied that after shutdown of beta cell function the drop in insulin content halted. We observed a similar pattern of induction and return to baseline of UPRER genes with higher doses of SW016789 [Supplementary Figure 4A (21)], indicating SW016789 did not induce terminal UPRER as occurs with thapsigargin. We also tested other endocrine cells that express stimulus-secretion coupling machinery similar to beta cells, including a human delta cell line and a mouse intestinal L cell line. SW016789 had minimal effects on IEGs or UPRER genes in these lines, while thapsigargin robustly induced the UPRER [Supplementary Figure 4B and 4C (21)].
Figure 5.
SW016789 transiently induces the unfolded protein response (UPRER). (A) MIN6 beta cells were treated with dimethyl sulfoxide (DMSO; 0.1%), SW016789 (5 µM), or thapsigargin (100 nM) in complete medium for 0 to 24 hours as indicated. (B-D) Compound additions were staggered to allow simultaneous sample harvesting. Expression of (B) immediate early genes and (C) adaptive and maladaptive UPRER genes are shown. (D) Expression of Ins1/2 in the time course and (E) insulin content. All data are the mean ± SD of 3 independent experiments. *P < 0.05 vs DMSO.
We also observed differences at the protein level between SW016789 and thapsigargin for UPRER and apoptotic components (Fig. 6A). Thapsigargin, but not SW016789, significantly induced the apoptosis markers CHOP and cleaved PARP by 24 hours. Phosphorylated PERK was significantly induced by thapsigargin at both 6 and 24 hours, while the effects of SW01789 were minimal. Alterations in phosphorylated eIF2α were variable and not significantly different with any treatment at 6 and 24 hours; however, thapsigargin induced BiP by 24 hours. Although the effects of SW016789 on BiP at 6 hours were variable, a relative increase in BiP would agree with the gene expression time course in Figure 5C. ATF3 and ATF4 protein expression was induced only at 6 hours by SW016789 and to a lesser extent than with thapsigargin (Fig. 6B). Amounts of beta cell transcription factors Pdx1 and MafA were unaltered by 6 or 24 hours of SW016789 or thapsigargin (Fig. 6C). Together, these results partially explain why even though SW016789 amplified secretion and caused ER stress, beta cells only ceased their secretory function and did not undergo terminal UPRER.
Figure 6.
SW016789 and thapsigargin have distinct effects on UPRER-related proteins. (A) MIN6 beta cells were treated with SW016789 (5 µM) or thapsigargin (500 nM) for either 6 or 24 hours in complete medium, and lysates were immunoblotted. Thapsigargin (Tg), but not SW016789, induced proapoptotic markers CHOP and cleaved-PARP (cl-PARP). Alterations in UPRER markers pS51-eIF2α, pT980-PERK, and BiP are shown. (B) SW016789 and Tg increased the stress-induced transcription factors ATF4 and ATF3. Data are the mean ± SD of 3 independent experiments. (C) Beta cell transcription factors Pdx1 and MafA were not significantly affected by either drug. Data are the mean ± SD of 2 or 3 independent experiments. In all immunoblots either actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as loading controls. *P < 0.05 by 2-way analysis of variance.
Acute Effects of SW016789 are Mediated via Ca2+ Influx and PKCs
The UPRER effects of SW016789 in beta cells appeared to stem from the enhancement of nutrient-stimulated Ca2+ influx and insulin secretion. To elucidate the mechanisms underlying SW016789-induced hypersecretion of insulin and subsequent UPRER induction, we took 2 chemical approaches. In the first approach, we cotreated beta cells acutely with SW016789 and candidate compounds to identify any synergy or disruption in SW016789 actions. We were surprised to discover that in the absence of nutrients, combining SW016789 with either phorbol esters or Ca2+ channel openers stimulated insulin secretion. PKC activation by the phorbol ester phorbol 12-myristate 13-acetate (PMA) has well known effects on beta cells (37, 38). Phorbol esters, including PMA, activate a subset of PKCs, as well as other targets (39), to potentiate nutrient-stimulated insulin secretion. SW016789 and PMA each given alone had little impact on secretion in the absence of nutrients and each amplified GSIS (Fig. 7A). However, the combination of PMA and SW016789 elicited a marked synergistic insulin secretion response in the absence of nutrients (Fig. 7A, DMSO pretreated). To further explore the role of PMA-activated PKCs in the response to SW016789, we exploited the property of chronic (>16 hours) treatment with PMA, which causes the degradation of the PMA-responsive PKCs (38). According to this paradigm, we pretreated beta cells overnight with PMA and repeated the acute stimulation experiment. Under these conditions, SW016789 and PMA no longer stimulated secretion in the absence of nutrients and the effect of SW016789 on GSIS was no longer significant (Fig. 7A, PMA pretreated). These results indicated SW016789 may act in part though PKCs to elicit its acute effects. We also tested the effects of directly opening VDCCs in the presence or absence of SW016789. The potent VDCC activator FPL64176 phenocopied SW016789 in chronic (Fig. 7B) and acute (Fig. 7C) assays in beta cells. FPL64176 had distinct characteristics, including acutely synergizing with SW016789 in the absence of nutrients to induce Ca2+ influx and insulin secretion (Fig. 7C and 7D) and impacting GSCa almost immediately compared to the more delayed effect of SW016789 (Fig. 7D). These data suggested that FPL64176 and SW016789 act by different mechanisms.
Figure 7.
SW016789 acutely synergizes with activators of protein kinase C and voltage-dependent calcium channels (VDCCs) to induce secretion. (A) InsGLuc-MIN6 cells were treated 24 hours with either dimethyl sulfoxide (DMSO; 0.1%) or phorbol 12-myristate 13-acetate (PMA; 100 nM) in complete medium. Cells were then washed and preincubated in glucose-free Krebs-Ringer bicarbonate HEPES for 1 hour prior to stimulation with SW016789 (5 µM), PMA (100 nM), or both in the presence or absence of glucose (20 mM) for 1 hour. Afterward, buffer was collected for InsGLuc assays. Data are the mean ± SD of 8 independent experiments. *P < 0.05 by 2-way analysis of variance. (B) InsGLuc-MIN6 cells were treated for 24 hours with DMSO or the VDCC activator FPL64176 followed by glucose-stimulated InsGLuc secretion assay in the absence of compounds. Data are the mean ± SD of 4 independent experiments. *P < 0.05 basal vs glucose. (C) InsGLuc-MIN6 cells were acutely treated for 1 hour with DMSO, FPL64176, or FPL64176 + SW016789 in the presence or absence of glucose. Data are the mean ± SD of 4 independent experiments. *P < 0.05 vs DMSO. (D) Normal glucose-stimulated Ca2+ can be observed with glucose and DMSO (peach) and is enhanced by SW016789 (red). FPL64176 rapidly potentiates glucose-stimulated Ca2+ influx in MIN6 cells (green). In the absence of glucose, DMSO (black), FPL64176 (grey), and SW016789 (blue) have little effect individually, but together FPL64176 and SW016789 synergize to stimulate Ca2+ influx (pink). Data are the mean ± SD of 3 independent experiments.
In a second approach, we used a chemical suppressor screen to disrupt potentially involved pathways by cotreating cells with SW016789 and with compounds having known mechanisms. Compounds were tested for their ability to protect against chronic inhibitory effects of SW016789 [Fig. 8A; Supplementary Table 2 (21)]. The VDCC blocker nifedipine was identified as a hit and was selected for further studies. Cotreatment of InsGLuc-MIN6 beta cells with SW016789 and nifedipine significantly blocked the chronic inhibitory effects of SW016789 on GSIS (Fig. 8B) and partially mitigated the induction of Ddit3 (CHOP) expression at 6 hours (Fig. 8C). We confirmed the acute potentiating effects of SW016789 on GSIS were indeed dependent on VDCC-mediated Ca2+ influx, as cotreatment with nifedipine completely blocked all stimulated secretion in the presence or absence of SW016789 (Fig. 8D). Furthermore, the protective effects of nifedipine against chronic SW016789 exposure were confirmed in human islet static culture GSIS experiments (Fig. 8E) without significant impact on islet insulin content [Supplementary Figure 5 (21)].
Figure 8.
Chemical suppressor screen uncovers voltage-dependent calcium channel inhibitors as protective agents in chronic SW016789 treatment in MIN6 beta cells and human islets. (A) InsGLuc-MIN6 cells were coincubated with SW016789 (5 µM) and candidate small molecules as indicated. Following a 24-hour incubation, all treatments were removed, and glucose-stimulated InsGLuc secretion responses were assessed. (B) InsGLuc-MIN6 cells pretreated in complete medium for 24 hours with dimethyl sulfoxide (DMSO; 0.1%) or SW016789 (5 µM) in the presence or absence of nifedipine (10 µM). After compound washout cells were stimulated with or without glucose (20 mM) for 1 hour for InsGLuc assays. Data are the mean ± SD of 5 independent experiments. (C) Cells were treated as in (B), and Ddit3 gene expression was measured. Data are the mean ± SD of 4 independent experiments. (D) InsGLuc-MIN6 cells were acutely incubated with DMSO (0.1%), nifedipine (10µM), SW016789 (5µM), or nifedipine + SW016789 for 1 hour in the presence or absence of glucose (20 mM). Data are the mean ± SD of 4 independent experiments. (E) Human islets were treated 24 hours in complete medium with DMSO (0.1%) and SW016789 (5 µM) in the presence or absence of nifedipine (10 µM). Islets were washed and incubated in low glucose (1 mM) Krebs-Ringer bicarbonate HEPES (KRBH) for 1 hour, buffer discarded, then incubated 1 hour in low (1 mM) or high (16 mM) glucose KRBH for 1 hour. Data represent the mean ± SD of insulin measured in supernatant normalized to total insulin content from 6 different donor islet preparations. *P < 0.05 by 2-way analysis of variance.
Discussion
Deconvolving the Mechanism of Action of SW016789
A few conclusions can be made about the mechanism of SW016789 based on the acute phenotype in beta cells. GSCa and GSIS were enhanced by SW016789, but KCl-induced responses were unaffected, suggesting the involvement of metabolism. Metabolic measurements suggested that SW016789 affects nutrient-induced insulin secretion downstream of ATP generation to enhance Ca2+ influx. We discovered intersections of SW016789-mediated actions with the PKC activator PMA and VDCC activator FPL64176. This raised concerns about the requirement for nutrient-stimulated ATP production given that SW016789, PMA, and FPL64176 are not nutrients and individually do not stimulate substantial Ca2+ influx or insulin secretion (Fig. 7). In beta cells, phorbol esters potentiate GSIS through PKC activation (40, 41), but PKCs are not required for normal GSIS (38, 42). Synergy between phorbol esters and SW016789 may occur through PKC-mediated phosphorylation of substrates involved in insulin exocytosis that lower the threshold for SW016789 to cause enhanced Ca2+ influx (43). We also observed a small but clear impact of SW016789 on Ca2+ influx and InsGLuc secretion in the presence of diazoxide and low KCl, which was glucose-dependent [Fig. 2C; Supplementary Figure 2C (21)]. This suggests SW016789 can bypass KATP to cause membrane depolarization or VDCC opening. SW016789 could potentially outcompete the effect of diazoxide on KATP, although we think this is unlikely because of estimations that >90% of KATP channels must close before beta cells depolarize (3, 44, 45).
Given the prominent role of cAMP in the potentiation of insulin secretion, we had initially suspected SW016789 might act through a Gαs-coupled GPCR. However, we did not observe a major impact of SW016789 in cAMP reporter assays [Supplementary Figure 3A and 3B (21)]. We also did not observe protection from chronic SW016789 effects by cotreatment with inhibitors of cAMP pathways (eg, PKA inhibitor H89, adenylyl cyclase inhibitor ddAd) (Fig. 8A). While previous work has shown that the cAMP-PKA pathway can protect against thapsigargin-mediated beta cell death (46), SW016789 did not cause cell death (Fig. 1G-1I), and the UPRER dynamics induced by SW016789 appear to be quite different compared to thapsigargin.
Because of the nutrient-dependent impacts of SW016789 on Ca2+ influx through VDCCs, it is worth noting that mitochondrial Ca2+ also plays a critical role in the coupling of metabolism and secretion in beta cells (47). Although not investigated here, the mitochondrial Ca2+ uniporter takes up cytosolic Ca2+ into the mitochondria, and alterations in this uptake could potentially contribute to generation of metabolic coupling factors, enhanced cytosolic Ca2+ in acute SW016789 treatments (Fig. 3C), or suppressed Ca2+ influx after chronic SW016789 treatments (Fig. 3A). In our metabolomics screen, SW016789 did not alter common metabolites in the presence or absence of glucose for 30 minutes (Fig. 4). While glucose stimulation begins inducing alterations in metabolites within a few minutes (48), we did not observe alterations in oxygen consumption rate or extracellular acidification rates in Seahorse assay measurements at 5 minutes intervals after acute exposure to SW016789 (Fig. 4E and 4F). However, it is possible that SW016789 altered metabolites at an earlier time during the stimulation, and by 30 minutes, the concentrations have reached steady state, and the difference was no longer apparent. Alternatively, a metabolite may be altered, which was not measured in the panel. Future investigations using shorter time points or metabolic flux analysis may be informative about potential mechanisms of SW016789 action.
Among compounds tested for protection against SW016789, the most consistent hits were from the dihydropyridine VDCC inhibitor family. We surmise that preventing increased Ca2+ influx abrogates SW016789-enhanced insulin secretion, thereby preventing downstream UPRER and preserving beta cell function. As blocking VDCCs prevents both Ca2+ influx and subsequent insulin secretion, it is unclear whether downstream stress and functional loss are induced specifically because of chronically increased Ca2+, enhanced insulin release, or both. Indeed, genetically reducing insulin production by 50% mitigated stress in mouse beta cells (49), suggesting the possibility that in our system, blocking insulin secretion itself using VDCC inhibitors may be responsible for mitigating hypersecretion-induced stress.
Beta Cell Adaptation and Dysfunction in Response to Chronic Secretory Demand
Chronic metabolic stress leads to beta cell dysfunction, and 2 recent studies provide prime examples (50, 51). Sustained physiological hyperglycemia (72 hours) increases absolute insulin secretion but impairs beta cell function as gauged by the disposition index (50). Accordingly, human islets exposed to glucolipotoxic stress for 48 hours were unable to recover GSIS, while islets exposed to milder stress (high glucose or palmitate alone) were able to recover function (51). Under our culture conditions, SW016789 initially potentiated beta cell function, but the secretory response shut down by 4 hours. Although UPRER induction was transient, returning to baseline after 24 hours, beta cell function in terms of insulin secretion remained impaired. Beta cells recovered from this inhibited state after a 48-hour washout, indicating the changes were reversible. A potential explanation is that a protein(s) affected by SW016789 signaling are degraded/reduced and require the washout period to recover. Recently, multiple groups have shown that transient UPRER, either downstream of partial pancreatectomy (52) or via inhibition of salt-inducible kinase (53), is associated with enhanced beta cell proliferation. While we did not observe significant alterations in cell viability or death over a 72-hour exposure to SW016789, it is possible the mechanism of UPRER induction due to hypersecretion in this case is different from that of pancreatectomy or salt-inducible kinase inhibition.
SW016789 selectively and transiently induced canonical UPRER stress response genes, but with a distinct temporal pattern from that of thapsigargin. A lesser induction of ATF3/4, p-PERK, and p-eIF2α may indicate an overall weaker induction of the PERK arm of the UPRER by SW016789. Our findings also suggest a lack of oxidative stress induction by SW016789 ascertained by no observed changes in glutathione redox state or Txnip expression. SW016789-treated beta cells were therefore able to downregulate their response to transiently enhanced Ca2+ influx. Relative induction of prosurvival vs proapoptotic genes may underlie the effects of SW016789-mediated UPRER. For example, the Ca2+-activated gene Npas4 was shown to have a protective role in beta cells and was highly induced by SW016789, but not by thapsigargin (54, 55). A gene program that mitigates stress within the first 6 hours of drug treatment may allow SW016789-treated beta cells to downregulate UPRER pathway before the point of no return. SW016789 will be a useful tool compound for discovering genes involved in adaptive versus maladaptive responses to chronic Ca2+ influx or secretory pressure.
Role of Pharmacological Modulation of VDCC Activity for Beta Cell Protective Effects
Ca2+ channel blockers have been in the spotlight for their therapeutic value in both type 1 (56) and type 2 diabetes (57). For example, verapamil improved beta cell function in a clinical trial of recent-onset type 1 diabetes (56). The proposed mechanism of action is that suppressing beta cell Ca2+ influx reduces expression of the cellular redox regulator Txnip, leading to protection from apoptosis (58). In our studies SW016789 did not induce Txnip, suggesting an alternate mechanism. In addition, the VDCC blockers of the dihydropyridine class (nifedipine, nimodipine, amlodipine), as well as the phenylalkylamine class (verapamil) protected beta cells from SW016789-induced dysfunction. Our results showing the protective effects of nifedipine agree with previous findings in beta cells using other stressors including hyperglycemia and palmitate (59-61). Distinct VDCC inhibitor mechanisms of action may underlie the differences between dihydropyridine and phenylalkylamine-mediated beta cell protection (62) and may imply that SW016789 acts through a mechanism or pathway that is more dependent on VDCCs in the inactivated state. Because amplified GSCa was confirmed as a major mechanism underlying the effects of SW016789, we hypothesized that directly activating VDCCs using FPL64176 could phenocopy SW016789 (63). While SW016789 activity on Ca2+ influx was inhibited by nifedipine, it also synergized with FPL64176 to enhance Ca2+ influx and secretion. The kinetics of Ca2+ influx induced by FPL64176 or SW016789 in the presence of glucose differed greatly. In agreement with our findings with FPL64176 and SW016789, prior work showed that chronic treatment with VDCC activator BayK8644 caused insulin secretion defects attributed to decreased abundance of KATP channel and VDCC subunits (64). Although not assessed in those studies, beta cells likely underwent transient UPRER concomitant with or prior to losing function.
Nifedipine or newer generation dihydropyridines such as amlodipine may also be useful therapeutics for conditions that cause hyperinsulinemia or where beta cell VDCCs are dysfunctional. A recent example is an individual with congenital hyperinsulinism with a gain-of-function mutation at a conserved residue in the α1 pore-forming subunit of Cav1.3 (L271H), encoded by CACNA1D (65). Hyperinsulinism in this individual was successfully treated with nifedipine and diazoxide. However, chronic blockade of beta cell VDCCs should be carefully considered, as preliminary findings with genetic deletion of Cav1.3 in mice suggest a positive role in beta cell mass (66). Auxiliary channel subunits can also impact beta cell function in diabetes. For example, the VDCC β 3 subunit (Cacnb3) is upregulated in islets from diabetic animals and was associated with decreased channel function. Deletion of Cacnb3 improved beta cell function in a mouse model of diabetes (67, 68). Precision targeting of VDCCs or their regulatory partners may be a promising avenue of research in diseases of beta cell dysfunction.
In conclusion, we discovered a new compound and potentially novel pathway that modulates beta cell function and may expand the cohort of targets for disease mitigation. Future experiments to identify mechanisms of SW016789 action may require strategies including time course transcriptomics, or cross-linkable, click chemistry-enabled probes (69). Simultaneously, this work also generates new knowledge about the mechanics of nutrient- and hormone-regulated exocytosis from specialized secretory cell types, including insights into the metabolic amplifying pathway.
Acknowledgments
We thank past and present members of the Cobb and Whitehurst labs, D. Rosenbaum at UT Southwestern, D. Eizirik and M. Cnop at Université libre de Bruxelles, and A. Templin at IBRI for helpful discussions. Thanks to C. Cheng in the Whitehurst lab and C. Llamas in the Mishra lab at UT Southwestern for training and advice on Seahorse experiments. Thanks to the L. Zacharias and the DeBerardinis lab at UT Southwestern for advice on metabolomics analyses. Agonist and antagonist functional data on GPCRs for SW016789 were generously provided by B. Roth’s group through the National Institute of Mental Health’s Psychoactive Drug Screening Program, contract no. HHSN-271-2018-00023-C (NIMH PDSP). MIN6 beta cells were originally generated by J. Miyazaki (70) and were kindly shared with us via the late J.C. Hutton. Human QGP1 somatostatinoma cells were a gift from D. Quelle (University of Iowa Carver College of Medicine). Mouse intestinal GLUTag L cells were originally generated by D. Drucker (Mount Sinai Hospital) and were shared with us by the lab of G.G. Holz (Upstate Medical University).
Contributor Information
Karina Rodrigues-dos-Santos, Lilly Diabetes Center of Excellence, Indiana Biosciences Research Institute, Indianapolis, IN, USA.
Gitanjali Roy, Lilly Diabetes Center of Excellence, Indiana Biosciences Research Institute, Indianapolis, IN, USA.
Derk D Binns, Departments of Pharmacology, UT Southwestern Medical Center, Dallas, TX, USA.
Magdalena G Grzemska, Departments of Pharmacology, UT Southwestern Medical Center, Dallas, TX, USA.
Luiz F Barella, Lilly Diabetes Center of Excellence, Indiana Biosciences Research Institute, Indianapolis, IN, USA.
Fiona Armoo, Lilly Diabetes Center of Excellence, Indiana Biosciences Research Institute, Indianapolis, IN, USA.
Melissa K McCoy, Departments of Biochemistry, UT Southwestern Medical Center, Dallas, TX, USA.
Andy V Huynh, Departments of Pharmacology, UT Southwestern Medical Center, Dallas, TX, USA.
Jonathan Z Yang, Departments of Pharmacology, UT Southwestern Medical Center, Dallas, TX, USA.
Bruce A Posner, Departments of Biochemistry, UT Southwestern Medical Center, Dallas, TX, USA.
Melanie H Cobb, Departments of Pharmacology, UT Southwestern Medical Center, Dallas, TX, USA.
Michael A Kalwat, Lilly Diabetes Center of Excellence, Indiana Biosciences Research Institute, Indianapolis, IN, USA; Center for Diabetes and Metabolic Diseases, Indiana University School of Medicine, Indianapolis, IN, USA.
Funding
This work was supported by the Juvenile Diabetes Research Foundation (JDRF) (2-SRA-2019-702-Q-R to MAK), the Diabetes Research Connection #33 (to M.A.K.), internal funding from the Indiana Biosciences Research Institute (to M.A.K.), and the Welch Foundation (I1243 to M.H.C.). Human pancreatic islets were provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded Integrated Islet Distribution Program (IIDP) (RRID:SCR_014387) at City of Hope (National Institutes of Health grant no. 2UC4DK098085) and the JDRF-funded IIDP Islet Award Initiative (BS438P to M.A.K.).
Author Contributions
Conceptualization: M.A.K. Data curation: M.A.K. Formal analysis: K.R-S., G.R., D.D.B., F.A., and M.A.K. Funding acquisition: M.A.K. and M.H.C. Investigation: K.R-S., G.R., D.D.B., M.G.G., F.A., M.K.M., A.H., J.Z.Y., and M.A.K. Methodology: M.A.K. Project administration: M.A.K. Resources: B.A.P., M.H.C., and M.A.K. Supervision: M.H.C. and M.A.K. Validation: K.R-S., G.R., D.D.B., M.G.G., J.Z.Y., and M.A.K. Visualization: K.R-S., G.R., and M.A.K. Writing—original draft: M.A.K. Writing—review and editing: K.R-S., G.R., D.D.B., L.B., M.H.C., and M.A.K. M.A.K. is the guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Disclosure Statement
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the references.
References
- 1. Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Meneur C, Bernal-Mizrachi E. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol Aspects Med. 2015;42:19-41. Doi: 10.1016/j.mam.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger C, Zdzieblo D. Glucose transporters in pancreatic islets. Pflugers Arch. 2020;472(9):1249-1272. Doi: 10.1007/s00424-020-02383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rorsman P, Ashcroft FM. Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98(1):117-214. Doi: 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewandowski SL, Cardone RL, Foster HR, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab. 2020;32(5):736-750.e5. Doi: 10.1016/j.cmet.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nicholls DG. The pancreatic beta-cell: a bioenergetic perspective. Physiol Rev. 2016;96(4):1385-1 447. Doi: 10.1152/physrev.00009.2016 [DOI] [PubMed] [Google Scholar]
- 6. Thurmond DC, Gaisano HY. Recent insights into beta-cell exocytosis in type 2 diabetes. J Mol Biol. 2020;432(5):1310-1325. Doi: 10.1016/j.jmb.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751-17 60. Doi: 10.2337/diabetes.49.11.1751 [DOI] [PubMed] [Google Scholar]
- 8. Kalwat MA, Cobb MH. Mechanisms of the amplifying pathway of insulin secretion in the beta cell. Pharmacol Ther. 2017;179:17-30. Doi: 10.1016/j.pharmthera.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162-1 85. Doi: 10.1016/j.cmet.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 10. Kalwat MA, Scheuner D, Rodrigues Dos Santos K, Eizirik DL, Cobb MH. The pancreatic ß cell response to secretory demands and adaption to stress. Endocrinology. 2021;162(11):bqab173. Doi: 10.1210/endocr/bqab173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25(4):406-40 9. Doi: 10.1038/78085 [DOI] [PubMed] [Google Scholar]
- 12. Shalev A, Pise-Masison CA, Radonovich M, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143(9):3695-369 8. Doi: 10.1210/en.2002-220564 [DOI] [PubMed] [Google Scholar]
- 13. Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress–mediated diabetes. J Clin Investig. 2002;109(4):525-532. Doi: 10.1172/jci0214550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma RB, O’Donnell AC, Stamateris RE, et al. Insulin demand regulates beta cell number via the unfolded protein response. J Clin Invest. 2015;125(10):3831-38 46. Doi: 10.1172/JCI79264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H, Lee YS, Harenda Q, et al. Beta cell dedifferentiation induced by IRE1alpha deletion prevents type 1 diabetes. Cell Metab. 2020;31(4):822-836.e5. Doi: 10.1016/j.cmet.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eizirik DL, Pasquali L, Cnop M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349-362. Doi: 10.1038/s41574-020-0355-7 [DOI] [PubMed] [Google Scholar]
- 17. Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64(1):2-6. Doi: 10.1097/JIM.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herbert TP, Laybutt DR. A reevaluation of the role of the unfolded protein response in islet dysfunction: maladaptation or a failure to adapt? Diabetes. 2016;65(6):1472-14 80. Doi: 10.2337/db15-1633 [DOI] [PubMed] [Google Scholar]
- 19. Burns SM, Vetere A, Walpita D, et al. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 2015;21(1):126-1 37. Doi: 10.1016/j.cmet.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 20. Kalwat MA, Wichaidit C, Nava Garcia AY, et al. Insulin promoter-driven Gaussia luciferase-based insulin secretion biosensor assay for discovery of beta-cell glucose-sensing pathways. ACS Sens. 2016;1(10):1208-1212. Doi: 10.1021/acssensors.6b00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues-dos-Santos K, Roy G, Binns DD, et al. ESM Figures and Tables. figshare. Dataset. Deposition May 16, 2022. 10.6084/m9.figshare.19776208.v1 [DOI] [Google Scholar]
- 22. Kalwat MA. High-throughput screening for insulin secretion modulators. Methods Mol Biol. 2021;2233:131-138. Doi: 10.1007/978-1-0716-1044-2_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang LI, Collins J, Davis R, et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem. 2007;282(14):10576-105 84. Doi: 10.1074/jbc.M609695200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson LJ, Mi C, Kraml CM. A preparative chiral separation of hydroxychloroquine using supercritical fluid chromatography. J Chromatogr A. 2020;1634:461661. Doi: 10.1016/j.chroma.2020.461661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sander T, Freyss J, von Korff M, Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model. 2015;55(2):460-4 73. Doi: 10.1021/ci500588j [DOI] [PubMed] [Google Scholar]
- 26. Mullen AR, Hu Z, Shi X, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7(5):1679-16 90. Doi: 10.1016/j.celrep.2014.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalwat MA, Huang Z, Wichaidit C, et al. Isoxazole alters metabolites and gene expression, decreasing proliferation and promoting a neuroendocrine phenotype in beta-cells. ACS Chem Biol. 2016;11(4):1128-11 36. Doi: 10.1021/acschembio.5b00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486-W494. Doi: 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalwat MA, Hwang IH, Macho J, et al. Chromomycin A2 potently inhibits glucose-stimulated insulin secretion from pancreatic beta cells. J Gen Physiol. 2018;150(12):1747-17 57. Doi: 10.1085/jgp.201812177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hager R, Pitsch J, Kerbl-Knapp J, et al. A high-content screen for the identification of plant extracts with insulin secretion-modulating activity. Pharmaceuticals (Basel). 2021;14(8):809. Doi: 10.3390/ph14080809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Praznik A, Fink T, Franko N, et al. Regulation of protein secretion through chemical regulation of endoplasmic reticulum retention signal cleavage. Nat Commun. 2022;13(1):1323. Doi: 10.1038/s41467-022-28971-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cardenas-Diaz FL, Leavens KF, Kishore S, et al. A dual reporter EndoC-βH1 human β-cell line for efficient quantification of calcium flux and insulin secretion. Endocrinology. 2020;161(2):bqaa005. Doi: 10.1210/endocr/bqaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shariati K, Pappalardo Z, Chopra DG, Yiv N, Sheen R, Ku G. Selective monitoring of insulin secretion after CRISPR interference in intact pancreatic islets despite submaximal infection. Islets. 2020;12(3):59-69. Doi: 10.1080/19382014.2020.1752072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barella LF, Jain S, Kimura T, Pydi SP. Metabolic roles of G protein-coupled receptor signaling in obesity and type 2 diabetes. FEBS J. 2021;288(8):2622-2644. Doi: 10.1111/febs.15800 [DOI] [PubMed] [Google Scholar]
- 35. Kroeze WK, Sassano MF, Huang XP, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22(5):362-36 9. Doi: 10.1038/nsmb.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wijtmans M, Maussang D, Sirci F, et al. Synthesis, modeling and functional activity of substituted styrene-amides as small-molecule CXCR7 agonists. Eur J Med Chem. 2012;51:184-192. Doi: 10.1016/j.ejmech.2012.02.041 [DOI] [PubMed] [Google Scholar]
- 37. Santo-Domingo J, Chareyron I, Dayon L, et al. Coordinated activation of mitochondrial respiration and exocytosis mediated by PKC signaling in pancreatic beta-cells. FASEB J. 2017;31(3):1028-1045. Doi: 10.1096/fj.201600837R [DOI] [PubMed] [Google Scholar]
- 38. Yaney GC, Fairbanks JM, Deeney JT, Korchak HM, Tornheim K, Corkey BE. Potentiation of insulin secretion by phorbol esters is mediated by PKC-alpha and nPKC isoforms. Am J Physiol Endocrinol Metab. 2002;283(5):E880-E88 8. Doi: 10.1152/ajpendo.00474.2001 [DOI] [PubMed] [Google Scholar]
- 39. Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115(Pt 23):4399-4 411. Doi: 10.1242/jcs.00122 [DOI] [PubMed] [Google Scholar]
- 40. Newton AC. Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol. 2018;53(2):208-2 30. Doi: 10.1080/10409238.2018.1442408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hii CS, Jones PM, Persaud SJ, Howell SL. A re-assessment of the role of protein kinase C in glucose-stimulated insulin secretion. Biochem J. 1987;246(2):489-4 93. Doi: 10.1042/bj2460489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones PM, Persaud SJ, Howell SL. Insulin secretion and protein phosphorylation in PKC-depleted islets of Langerhans. Life Sci. 1992;50(11):761-767. Doi: 10.1016/0024-3205(92)90180-w [DOI] [PubMed] [Google Scholar]
- 43. Uchida T, Iwashita N, Ohara-Imaizumi M, et al. Protein kinase Cdelta plays a non-redundant role in insulin secretion in pancreatic beta cells. J Biol Chem. 2007;282(4):2707-16. Doi: 10.1074/jbc.M610482200 [DOI] [PubMed] [Google Scholar]
- 44. Thompson B, Satin LS. Beta-cell ion channels and their role in regulating insulin secretion. Compr Physiol. 2021;11(4):1-21. Doi: 10.1002/cphy.c210004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cook DL, Satin LS, Ashford ML, Hales CN. ATP-sensitive K+ channels in pancreatic beta-cells: spare-channel hypothesis. Diabetes. 1988;37(5):495-8. Doi: 10.2337/diab.37.5.495 [DOI] [PubMed] [Google Scholar]
- 46. Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4(5):391-406. Doi: 10.1016/j.cmet.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 47. Weiser A, Feige JN, De Marchi U. Mitochondrial calcium signaling in pancreatic beta-cell. Int J Mol Sci. 2021;22(5):2515. Doi: 10.3390/ijms22052515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spegel P, Sharoyko VV, Goehring I, et al. Time-resolved metabolomics analysis of beta-cells implicates the pentose phosphate pathway in the control of insulin release. Biochem J. 2013;450(3):595-605. Doi: 10.1042/BJ20121349 [DOI] [PubMed] [Google Scholar]
- 49. Szabat M, Page MM, Panzhinskiy E, et al. Reduced insulin production relieves endoplasmic reticulum stress and induces beta cell proliferation. Cell Metab. 2016;23(1):179-1 93. Doi: 10.1016/j.cmet.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 50. Merovci A, Tripathy D, Chen X, et al. Effect of mild physiologic hyperglycemia on insulin secretion, insulin clearance, and insulin sensitivity in healthy glucose-tolerant subjects. Diabetes. 2021;70(1):204-2 13. Doi: 10.2337/db20-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marselli L, Piron A, Suleiman M, et al. Persistent or transient human beta cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with type 2 diabetes. Cell Rep. 2020;33(9):108466. Doi: 10.1016/j.celrep.2020.108466 [DOI] [PubMed] [Google Scholar]
- 52. Tatsuoka H, Sakamoto S, Yabe D, et al. Single-cell transcriptome analysis dissects the replicating process of pancreatic beta cells in partial pancreatectomy model. iScience. 2020;23(12):101774. Doi: 10.1016/j.isci.2020.101774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Charbord J, Ren L, Sharma RB, et al. In vivo screen identifies a SIK inhibitor that induces β cell proliferation through a transient UPR. Nat Metab. 2021;3(5):682-700. Doi: 10.1038/s42255-021-00391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Speckmann T, Sabatini PV, Nian C, Smith RG, Lynn FC. Npas4 transcription factor expression is regulated by calcium signaling pathways and prevents tacrolimus-induced cytotoxicity in pancreatic beta cells. J Biol Chem. 2016;291(6):2682-26 95. Doi: 10.1074/jbc.M115.704098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sabatini PV, Krentz NA, Zarrouki B, et al. Npas4 is a novel activity-regulated cytoprotective factor in pancreatic beta-cells. Diabetes. 2013;62(8):2808-28 20. Doi: 10.2337/db12-1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24(8):1108-11 12. Doi: 10.1038/s41591-018-0089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhao D, Cao Y, Yu CG, et al. The association of calcium channel blockers with beta-cell function in type 2 diabetic patients: a cross-sectional study. J Clin Hypertens (Greenwich). 2019;21(5):638-647. Doi: 10.1111/jch.13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu G, Chen J, Jing G, Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848-8 56. Doi: 10.2337/db11-0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Syeda K, Mohammed AM, Arora DK, Kowluru A. Glucotoxic conditions induce endoplasmic reticulum stress to cause caspase 3 mediated lamin B degradation in pancreatic beta-cells: protection by nifedipine. Biochem Pharmacol. 2013;86(9):1338-13 46. Doi: 10.1016/j.bcp.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jeffrey KD, Alejandro EU, Luciani DS, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A. 2008;105(24):8452-845 7. Doi: 10.1073/pnas.0711232105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Gao L, Li Y, Chen H, Sun Z. Nifedipine protects INS-1 beta-cell from high glucose-induced ER stress and apoptosis. Int J Mol Sci. 2011;12(11):7569-75 80. Doi: 10.3390/ijms12117569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang L, Gamal El-Din TM, Swanson TM, et al. Structural basis for inhibition of a voltage-gated Ca(2+) channel by Ca(2+) antagonist drugs. Nature. 2016;537(7618):117-1 21. Doi: 10.1038/nature19102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zheng W, Rampe D, Triggle D. Pharmacological, radioligand binding, and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol Pharm. 1991;40(5):734-7 41. [PubMed] [Google Scholar]
- 64. Minami K, Yokokura M, Ishizuka N, Seino S. Normalization of intracellular Ca(2+) induces a glucose-responsive state in glucose-unresponsive beta-cells. J Biol Chem. 2002;277(28):25277-252 82. Doi: 10.1074/jbc.M203988200 [DOI] [PubMed] [Google Scholar]
- 65. De Mingo Alemany MC, Mifsud Grau L, Moreno Macian F, Ferrer Lorente B, Leon Carinena S. A de novo CACNA1D missense mutation in a patient with congenital hyperinsulinism, primary hyperaldosteronism and hypotonia. Channels (Austin). 2020;14(1):175-1 80. Doi: 10.1080/19336950.2020.1761171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Theiner T, Jacobo-Piqueras N, Ortner NJ, Geisler SM, Tuluc P. Cav1.3 L-type Ca2+ channel modulates pancreatic beta-cell electrical activity and survival. J Gen Physiol. 2022;154(9):e2021ecc34. Doi: 10.1085/jgp.2021ecc34 [DOI] [Google Scholar]
- 67. Lee K, Kim J, Kohler M, et al. Blocking Ca(2+) channel beta3 subunit reverses diabetes. Cell Rep. 2018;24(4):922-9 34. Doi: 10.1016/j.celrep.2018.06.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Berggren PO, Yang SN, Murakami M, et al. Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119(2):273-2 84. Doi: 10.1016/j.cell.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 69. Ha J, Park H, Park J, Park SB. Recent advances in identifying protein targets in drug discovery. Cell Chem Biol. 2021;28(3):394-423. Doi: 10.1016/j.chembiol.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 70. Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127(1):126-1 32. Doi: 10.1210/endo-127-1-12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the references.