Abstract
The high cost of viral load (VL) testing limits its use for antiretroviral therapy (ART) adherence support. A low-cost lateral flow urine tenofovir (TFV) rapid assay predicts pre-exposure prophylaxis breakthroughs, but has not yet been investigated in HIV treatment. We therefore evaluated its utility in a pilot cross-sectional study of TFV-containing ART recipients at an increased risk of virologic failure (VF). Participants who had a treatment interruption ≥30 days or had ≥1 episode of viremia (VL ≥400 copies/mL) in the previous year were recruited from a public health setting in Cape Town, South Africa. Self-reported adherence data were collected, the urine TFV assay performed, and concurrent TFV-diphosphate analyzed in dried blood spots. VL testing was done concurrently and, if viremic, genotypic HIV drug resistance testing was performed. Of 48 participants, 18 (37.5%) had VL (>400 copies/mL) at the time of the study, including 16 of 39 receiving efavirenz (EFV), 2 of 6 receiving protease inhibitors, and 0 of 3 receiving dolutegravir. Resistance testing succeeded in 17/18, of which 14 had significant mutations compromising ≥2 agents of the current EFV-based regimen. Of these 14, all had detected urine TFV. Urine TFV was undetectable in two out of three without regimen-relevant resistance; p = .02. In participants on EFV-based regimens returning to care, VF was largely due to viral resistance, where detectable urine TFV had 100% sensitivity (14/14 participants) in predicting resistance. Conversely, when undetectable, the urine-based assay could be used to preclude participants with poor adherence from undergoing costly HIV drug resistance testing.
Keywords: point of care, adherence, urine, resistance, real time
Background
The effectiveness of antiretroviral therapy (ART) is confirmed by undetectable plasma HIV-1 RNA viral load (VL), which is of both individual and public health importance. On a patient level, an undetectable VL allows immune reconstitution and reduces the risk of HIV-1 associated conditions, especially with early treatment.1–3 From a public health perspective, an undetectable VL (<200 copies/mL) equals an untransmittable infection.4 Achieving and maintaining undetectable VL depend on life-long adherence to ART.
Unfortunately, the high costs of VL assays limit their frequent use in large public health programs of high burden resource-limited settings. VL testing is therefore performed infrequently as per the World Health Organization (WHO) guidelines (at 6 months after initiating ART and then annually)5 or when patients have clinical or immunologic failure. Patient advocacy groups emphasize a patient-centered approach to support treatment adherence by providing immediate feedback to patients,6 but rapid VL testing is not always affordable. However, point of care (POC) adherence metrics that are low cost for frequent use are being developed. As tenofovir (TFV) is included in most first-line WHO-recommended regimens, a noninvasive inexpensive real-time assay to assess recent TFV exposure would be of great value to reinforce adherence and has recently been developed.7
The utility of adherence reinforcement may, however, be dependent on the genetic barrier of the ART regimen and whether episodes of reduced adherence are detected and managed early. Periods of treatment interruption are strongly predictive of treatment failure and the development of drug resistance on non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens.8,9 Indeed, the majority of these patients may already have drug resistance when identified with virologic failure (VF)10 and may not subsequently achieve VL suppression, despite high levels of adherence.11
In contrast, drug resistance may take much longer to develop in patients treated with agents with a high genetic barrier to resistance, such as protease inhibitors (PI) and second-generation integrase strand transfer inhibitors (INSTI), “forgiving regimens” where real-time adherence support would allow for subsequent VL re-suppression and protection from the development of viral resistance.12,13
A low-cost lateral flow assay to detect TFV in urine at the POC was recently developed and validated by our group in conjunction with Abbott Diagnostics.14,15 Other assays to objectively monitor drug exposure exist in blood and hair, but these assays are largely unable to be harnessed for real-time testing.15 This urine tenofovir rapid assay (UTRA) has been shown to predict pre-exposure prophylaxis (PrEP) breakthroughs in analyses of PrEP trial data16,17 and is being studied to support adherence in the context of PrEP.18 However, the utility of UTRA in combination with VL testing to predict HIV drug resistance and support adherence has not yet been assessed in patients on ART.
We conducted a pilot cross-sectional study to evaluate the utility of the UTRA assay among people living with HIV (PLWH) at increased risk of VF in South Africa, thereby assessing the urine-based adherence test in the context of treatment for the first time.
Methods
Design, participants, and setting
Study participants were recruited from the Gugulethu Community Health Centre (CHC), a large ART service in the Klipfontein Health District of Cape Town, South Africa, between September 2020 and February 2021. The eligibility of potential participants was determined through screening and medical chart review, and participants were then invited to attend a single study visit at the adjacent research site. PLWH ≥18 years old were considered eligible to participate if they were currently using an ART regimen containing TFV for at least 30 days following an episode of recent increased risk of experiencing VF, as noted by either one or more episodes of viremia (≥400 copies/mL), while on their current regimen, or a treatment interruption ≥30 days in the previous year, confirmed by pharmacy refill (PR) collection data.
All participants provided written informed consent in their preferred language (English or isiXhosa).
Study procedures
Study procedures involved a single cross-sectional study visit. Demographic and disease data were collected, including age, gender, WHO clinical stage, current ART, and most recent CD4 cell count. Blood samples were drawn for plasma VL (EDTA plasma) and 50 μL ethylenediaminetetraacetic acid (EDTA) blood was pipetted on Whatman™ 903 protein saver cards (GE Healthcare Life Sciences, Cardiff, UK) to generate dried blood spots (DBS). After DBS had been left at room temperature to dry for at least 2 h, these were transported to the laboratory with Whatman® 1 g desiccant with indicator.
Plasma was reserved for HIV-1 drug resistance testing, should the VL be elevated. TFV in the urine was tested using the UTRA, a lateral flow assay that takes 2–3 min for a result to be determined. An UTRA test was classified as “negative” (no current drug exposure) if it indicated the absence of detectable urine TFV. The cutoff for the UTRA test is 1,500 ng/mL, indicating no recent dosing within the previous 4–5 days.1
Self-reported adherence was noted using the three-item scale below:
“In the last 30 days, on how many days did you miss at least one dose of your HIV medicines?” This was recorded as the number of days doses were missed; and adherence percentage calculated as [(30–number of doses missed)/30]*100 (days adherent %)
“In the last 30 days, how good a job did you do at taking your HIV medicines in the way that you were supposed to?” This was scored using a 6-point Likert scale from “very poor” to “excellent.” (Self-reported adherence score 2)
“In the last 30 days, how often did you take your HIV medicines in the way that you were supposed to?” This was scored using a 6-point Likert scale from “never” to “always.” (Self-reported adherence scale 3)
Finally, as one additional metric of adherence, PR data were derived from ART collections over the year before our study visit, using both chart review and the Gugulethu CHC central pharmacy database. Start date for the PR adherence calculation was taken as the date of PR immediately before a date one calendar year before our study visit; and the end date was the most recent date of PR before our visit. Total number of tablets collected within the start and end dates were quantified and adherence calculated as (total number of tablets/doses per day)/(number of days between start and end dates)*100.
Sample processing
Whole blood EDTA-derived plasma was collected and stored at −80°C and retrieved for HIV drug resistance testing, in participants with concurrent HIV-1 plasma VL >400 copies/mL. DBS were stored at −20°C until tenofovir diphosphate (TFV-DP) analysis by liquid chromatography/tandem mass spectrometry (LC-MS/MS) was performed through validated methods.19
Laboratory methods
VL testing was performed as part of routine VL monitoring as per National Health Laboratory Service (NHLS) National Tender with the Alinity m HIV-1 assay (Abbott Laboratories, Abbott Park, IL) or COBAS® AmpliPrep/COBAS TaqMan® HIV-1 Test, v2.0 (Roche Diagnostics, Basel, Switzerland). The limit of detection was dependent on the sample volume tested (Supplementary Table S1)
HIV drug resistance testing
HIV drug resistance testing was performed by Sanger Sequencing of HIV protease, reverse transcriptase, and integrase using previously published and validated in-house method20; mutations were scored with the Stanford HIV drug resistance database and reported back to the clinician.
TFV-DP testing with LC-MS/MS
The method previously described19 for the quantification of TFV-DP from DBS was adapted for optimized analysis on a Shimadzu 8040 triple quadrupole LC-MS/MS by extracting a whole 50 μL DBS and dephosphorylating with a proportionally increased amount of acid phosphatase. The TFV-DP quantitative range was from 27 to 13,848 fmol/punch per 3 mm punch.
Statistical analysis
Statistics and graphics were implemented in R version 4.0.2.21 Wilcoxon rank sum tests were used to compare continuous variables between groups and Fisher exact test for categorical variables; correlation was assessed with Spearman's rank order correlation. Confidence intervals for percentages were calculated using exact binomial probabilities. One-way analysis of variance was used to assess differences between multiple groups.
Ethics
The study was approved by the University of Cape Town Human Research Ethics Committee. All participants provided written informed consent.
Results
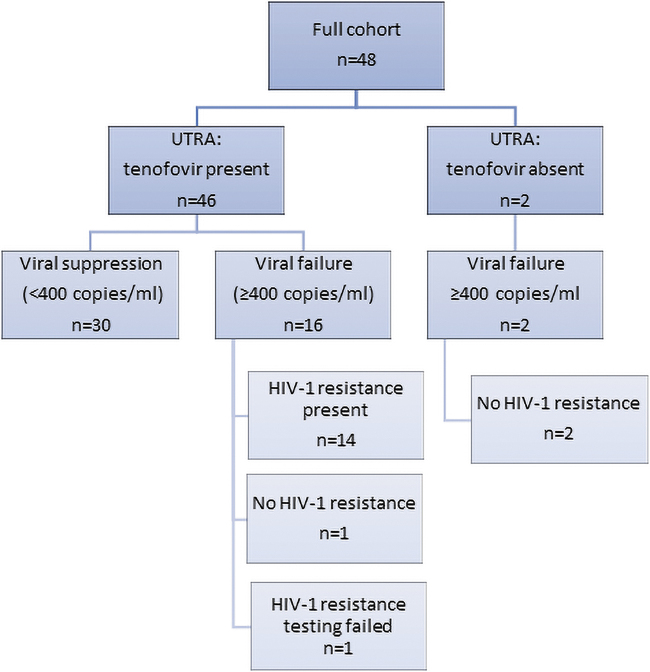
Fifty participants who were eligible and provided written consent were recruited, of whom 48 had urine TFV, VL, and DBS TFV-DP results available to be included in this analysis (Fig. 1). TFV-DP testing failed in 2/50 cases due to laboratory bench errors during sample extraction. All participants were on TFV based on eligibility criteria. Baseline characteristics and demographics of study participants are summarized in Table 1.
FIG. 1.
The full cohort of 48 participants who had results for UTRA, VL, and TFV-DP concentration in DBS categorized by (1) UTRA results, (2) VL results, and (3) HIV drug resistance results. DP, diphosphate.
Table 1.
Baseline Characteristics and Main Results by Virologic Failure
| Characteristic | Current VL ≥400 copies/mL (n = 18) | Current VL <400 copies/mL (n = 30) | Total (n = 48) |
|---|---|---|---|
| WHO stage | |||
| Stage 1 | 6 | 19 | 25 |
| Stage 2 | 2 | 5 | 7 |
| Stage 3 | 9 | 5 | 14 |
| Stage 4 | 1 | 1 | 2 |
| CD4 count (cells/μL) median (IQR) | 217.5 (94.75–393.25) | 306.5 (112.2–488.2) | 255.5 (106.8–456.8) |
| Enrolment reason | |||
| RTC | 3 | 22 | 25 |
| VL ≥400 copies/mL | 14 | 6 | 20 |
| RTC &VL ≥400 copies/mL | 1 | 2 | 3 |
| Regimen | |||
| TDF/3TC/DTG | 0 | 3 | 3 |
| TDF/FTC/ATV/r | 1 | 0 | 1 |
| TDF/FTC/EFV | 16 | 23 | 39 |
| TDF/FTC/LPV/r | 1 | 4 | 5 |
| Gender | |||
| Female | 11 | 20 | 31 |
| Male | 7 | 10 | 17 |
| Age (years) | |||
| Median age (IQR) | 39 (34.5–45.25) | 37 (28.5–41) | 37.5 (31.75–42) |
| TNF-exposure | |||
| UTRA—negative | 2 | 0 | 2 |
| TFV-DP in DBS (fmol/punch): median (IQR) | 399 (193–643) | 402 (342–648) | 402 (319–652) |
| Adherence | |||
| Self-reported adherence % of 30 most recent days Median (IQR) |
97 (93—100) | 97 (91–100) | 97 (93–100) |
| Self-reported adherence score 2 Likert scale: Median (IQR) |
4 (4–4) | 4 (4–4.75) | 4 (4–4) |
| Self-reported adherence score 3 Likert scale: Median (IQR) |
5 (4–5) | 5 (5–5) | 5 (4–5) |
| PR adherence (%) | 68.45 (32.51–96.54) | 34.43 (30.60–59.63) | 45.06 (30.60–75.46) |
CD4 counts did not differ significantly between those who had VF versus not (p = .18). Patients were enrolled, who had returned to care (RTC) after having or ≥30 days of being out of care in the previous year, confirmed by PR collection data and/or having had one or more episodes of viremia (≥400 copies/mL), while on their current regimen. Regimens included fixed dose combination therapy of tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and efavirenz (EFV) or TDF, lamivudine (3TC), and dolutegravir (DTG) or regimens of lopinavir (LPV) boosted with low-dose ritonavir tables (LPV/r) combined with fixed-dose TDF and FTC or atazanavir boosted with low-dose ritonavir tablets (ATV/r) combined with fixed-dose TDF and FTC.
DBS, dried blood spots; DP, diphosphate; IQR, interquartile range; PR, pharmacy refill; TFV-DP, tenofovir diphosphate; UTRA, urine tenofovir rapid assay; VL, viral load; WHO, World Health Organization.
Participants with undetectable urine TFV
Two of the 48 participants had undetectable TFV in their urine using the UTRA. Both of these participants were male and had virologic failure as defined by VL >400 copies/mL at the time of their study visit. One was on an efavirenz (EFV)-based regimen and had a very low, but detectable TFV-DP concentration in DBS of only 32.5 fmol/punch (limit of quantification is 27 fmol/punch); and one was on a lopinavir/ritonavir (LPV/r)-based regimen and had a TFV-DP concentration in DBS, which was unquantifiable. Neither of these participants had HIV drug resistance to more than one drug in their regimen.
Participants with detectable urine TFV
Of the 46 participants with detectable TFV in their urine using UTRA, 16 (35%) had VF. Fifteen of those with failure were on EFV and 1 was on a boosted PI-based regimen. Drug resistance testing was conducted for all participants with VF, but the testing failed in one participant due to a lack of amplification. Of the 15 remaining, all had NNRTI drug resistance mutations, none had major PI mutations, and all, but 1 participant had NRTI mutations: 14 participants had an M184V/I mutation [with K65R in 9, K70E in 2, and thymidine-associated mutations (TAMs) in 4 (3 in combination with K65R)]. Two participants did not have an M184V/I mutation (1 with no NRTI mutations and 1 with D67N and K70E) (Supplementary Table S3).
Of these 15 participants, all who were on an EFV-based regimen had viral resistance that would compromise at least 2 agents in the current regimen. For the participant on a boosted PI regimen, the only treatment-relevant mutations were NRTI mutations.
Sensitivity of UTRA to predict HIV resistance in those with VF
Of the 17 participants who had VF and HIV drug resistance testing, 100% (14/14) of those who had two-class regimen-specific HIV resistance had detectable TFV in their urine at their study visit, giving UTRA a 100% sensitivity for predicting resistance mutations in those with VF (Supplementary Table S2). Of the three participants who did not have two-class resistance, two had undetectable urine TFV levels, giving UTRA a 66% specificity for predicting resistance in those with VF.
DBS results
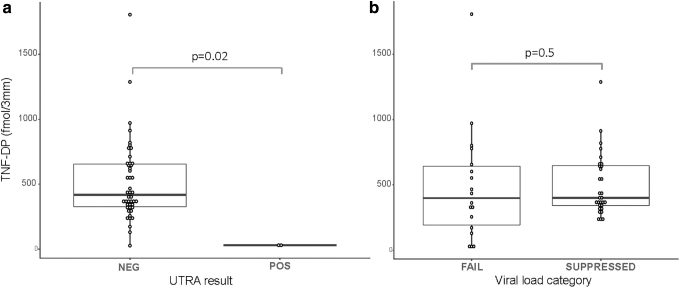
The median, interquartile range (IQR), TFV-DP concentration in DBS was 402 (319–652) fmol/punch; the mean TFV-DP [standard deviation (SD)] was 494 fmol/punch (321 fmol/punch). Only 5/48 participants had a TFV-DP concentration >1 SD below (below 173 fmol/punch). TFV-DP concentrations in DBS were significantly lower in the two participants with undetectable urine TFV compared to those with detectable urine TFV (p = .02; Fig. 2).
FIG. 2.
TFV-DP in DBS in patients categorized by (a) UTRA results and (b) VL failure category (above or below 400 copies/mL). There were significantly lower TFV-DP concentrations in participants who were UTRA negative (p = .02), but the difference in TFV-DP between participants with VF versus suppression was not significant (p = .5). DBS, dried blood spots; TFV-DP, tenofovir diphosphate; UTRA, urine tenofovir rapid assay; VF, virologic failure; VL, viral load.
When considering all antiretroviral treatment regimens, there was no significant difference in TFV-DP concentrations between participants with VF [median (IQR) of 399 (193–643) fmol/punch] versus no failure [401 (342–648) fmol/punch] (Fig. 1b: p = .5), and no correlation between VL and TFV-DP concentrations in DBS (rho = −0.03, p = .8). Similarly, there was no difference between those with failure or without, when considering those who were EFV treated (p = .9) or PI treated (p = .2). However, there were only six participants on PIs, of which the two with VF had undetectable TFV-DP. There was nevertheless no significant difference in TFV-DP concentrations in DBS between different treatment groups (p = .5).
Self-reported adherence and PR data
There was no difference in self-reported adherence (median 97% in both groups) between those with and without VF (p = .9), but PR adherence was significantly higher in participants with VL ≥400 copies/mL than in those with suppressed VLs (p = .03). Self-reported adherence percentage was also significantly lower in those with undetectable urine TFV (p = .03), but PR adherence was not significantly different (p = .76). There was no correlation between TFV-DP in DBS and PR (rho = −0.04, p = .8).
Discussion
In this study, we evaluated the utility of a POC assay to assess TFV adherence in urine in the context of HIV treatment for the first time. We conducted a pilot cross-sectional study to evaluate the urine-based test (UTRA) among PLWH at increased risk of VF and found that only 2 of 48 (4%) participants had undetectable UTRA tests (despite 38% experiencing VF). The combination of detected TFV in urine and VF was 100% sensitive in predicting two-class resistance on a low genetic barrier to resistance regimen, specifically, EFV-based ART. Therefore, the combination of UTRA and VL testing, especially in low-resource settings, could be used to target follow-up and inform the need for more expensive resistance testing or to prioritize transition to second-line regimens.
In contrast to urine TFV testing, which assesses exposure over the past 120 h, TFV-DP levels in DBS measure a longer duration of treatment exposure (up to 8 weeks), as the half-life of the TFV-DP metabolite is 17 days.22,23 In our cohort, one participant with detectable TFV with UTRA had unquantifiable TFV-DP in DBS, and another five participants with detectable TFV with UTRA had TFV-DP concentrations in DBS, which were at least one SD below the mean (<173 fmol/punch). The concentrations of TFV-DP in DBS were variable and largely overlapped between participants with or without VF (Fig. 1b).
This is in contrast to other studies that have shown moderately high TFV-DP in DBS, in persons with viral resistance.24,25 This may have been because some of our participants had only been back on treatment for as short as 30 days after their most recent treatment interruption and the time to steady state for TFV-DP is 8 weeks.22
Moreover, VF occurred, despite an almost twice as high overall level of PR adherence in those with VL ≥400 copies/mL. This finding is not surprising, given the high prevalence of two-class drug resistance in those with VF, a state that increasing adherence cannot alter. Among the three participants without two-class VF, in which adherence—rather than resistance—was expected to drive suppression, two participants had undetectable urine TFV. Two of these three participants were on higher barrier PI-based regimens (one detectable and one undetectable UTRA).
All participants with VF had NNRTI drug resistance and all, except one, had additional NRTI drug resistance. Many participants had extensive drug resistance, including a K65R and/or TAMs (Supplementary Table S3). Taken together, this suggests that the difference between the groups with and without VF is largely explained by drug resistance, already present, at the time when participants reinitiated treatment or attempted to improve their adherence. Median TFV-DP concentrations were low in participants who had suppressed VLs, when compared to other studies from the literature,26 and to a cohort from the same site27 (median 1041 fmol/punch; IQR: 727–1355). This may reflect our success in purposeful selection of a population with adherence challenges requiring additional adherence support (although insufficient time for TFV-DP levels to steady state may be playing a role).
The relationship between treatment failure and drug resistance is likely to be different for high genetic barrier regimens such as ritonavir-boosted PI-based regimens and second-generation INSTIs such as dolutegravir.12,28–30 The majority of the participants in this study at risk of failure received tenofovir, emtricitabine, and EFV, as tenofovir, lamivudine, and dolutegravir (TLD)31 rollout was only initiated in the South African public sector in mid-2020. Patients with failure on boosted PI regimens had low or undetectable TFV-DP in DBS, in contrast to studies that have shown increased TFV-DP concentrations with boosted PI regimens.25 This suggests that adherence is the most important predictor of failure on high genetic barrier regimens (such as PIs), which concur with earlier findings that low LPV plasma or hair concentrations predicted failure.30
As high genetic barrier regimens retain efficacy in the presence of NRTI drug resistance mutations, participants on these regimens may benefit most from adherence support, without requiring a treatment switch.13 Finally, two patients without M184V/I in this study had very low or undetectable TFV-DP in DBS. The high fitness cost of these mutations explains why patients may become undetectable during periods of treatment interruption and could be a marker of inadequate adherence.32–34
Our study has a number of limitations. First, the sample size of our pilot study is small. Of six participants on high genetic barrier PI regimens, only two had VF and none of the three had failure on TLD. The current treatment cohort includes few patients receiving TLD, which is largely a result of the delayed rollout of TLD due to initial concerns with congenital malformation and interruption of HIV services by the coronavirus disease 2019 (COVID-19) pandemic.35,36 Moreover, the expected rate of failure and treatment interruption on first-line TLD is low, resulting in few eligible participants.37 Since the UTRA assay measures recent TFV exposure, we could not exclude social desirability bias or the “white coat effect,” contributing to participants having higher urine TFV concentrations.38
As this cross-sectional study purposefully included participants who had prior episodes of failure or treatment lapses, the findings are not generalizable to patients at earlier stages in their treatment history. Participants were only required to have been on their regimen for at least 30 days after a treatment interruption. This may have resulted in insufficient time to reach steady-state concentrations for TFV-DP in DBS, which limits the interpretation of these results. Finally, one of the three participants without two-class resistance had EFV resistance without detected NRTI resistance. Given the presence of only two fully active NRTIs, it is possible that viral resistance would have compromised this regimen as well. If this were the case, the sensitivity for significant HIVDR would decrease from 100% to 93%.
Our cross-sectional study purposefully enriched for participants at high risk of failure, having previous treatment interruption or failure without regimen switch. This enrichment was successful since 37.5% of our participants had VF, which is much higher than the baseline prevalence.39 However, these patients may have had long periods of failure, as evident in the extensive drug resistance observed. Prior studies of resuppression after detectable VLs suggest that adherence interventions may be most effective in achieving resuppression when initiated early after failure onset on low-barrier regimens.40,41
Conclusion
We found that, in participants at high risk of VF who had periods of previous failure or treatment interruption on NNRTI-based regimens, few demonstrated very low drug exposure (indicating inadequate adherence) by objective measures. Despite current improved adherence, participants receiving low genetic barrier EFV-based regimens with prior treatment interruption or episodes of high VLs are at high risk of having treatment-compromising drug resistance when reinitiating ART.42,43 In contrast, concurrent failure on boosted PIs could be largely explained by inadequate adherence.
Among those with VF in the setting of two-class viral resistance to the current regimen, detectable TFV levels in urine had 100% sensitivity in predicting concurrent resistance. Among participants on low genetic barrier regimens, the combination of VF and detectable urine TFV using a POC test should raise suspicion for resistance. Indeed, health systems seeking to prioritize costly resistance testing for those with the highest likelihood of resistance could use UTRA to target resistance testing.
Finally, in the case of high genetic barrier regimens or low genetic barrier regimens early after initiation, noninvasive POC testing of urine for the presence of TFV with UTRA could allow immediate adherence interventions to be deployed. This will help prevent the emergence of subsequent drug resistance through rapid, objective screening for adherence. Our study demonstrated the utility of the urine-based POC adherence test in ART for the first time, either in targeting resistance testing or triggering adherence support. Further investigations of this low-cost real-time objective metric of adherence in larger prospective studies are ongoing.
Supplementary Material
Acknowledgments
We would like to thank the participants and clinic staff.
Disclaimer
The content and findings reported/illustrated herein are the sole deduction, view, and responsibility of the researchers and do not reflect the official position and sentiments of the NIH and SAMRC.
Authors' Contributions
M.G. and M.S. co-developed and validated the UTRA assay. L.J., C.O., and Z.N. oversaw the clinical site and participant recruitment. T.K. performed and oversaw the DBS TFV-DP testing. D.C. developed the site database. G.v.Z. oversaw drug resistance testing, performed statistical analyses, and drafted the article with inputs from C.O., L.J., E.D., M.v.S., M.S., and M.G. All authors approved the final article.
Author Disclosure Statement
All authors have no competing interests, and all work was funded by the NIH/NIAID R01AI152119 and R01AI143340.
Funding Information
Research reported in this publication/poster/article was supported by the U.S. National Institutes of Health (NIH) and South African Medical Research Council through its U.S.-SA Program for Collaborative Biomedical Research, Grant R01AI152119 (PIs van Zyl, Orrell, Gandhi). The NIH further supported the urine-based TFV test through R01AI143340 (Gandhi PI).
Supplementary Material
References
- 1. The TEMPRANO ANRS 12136 Study Group: A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–822. [DOI] [PubMed] [Google Scholar]
- 2. The INSIGHT START Study Group: Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. : Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc 2019;22:e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodger AJ, Cambiano V, Phillips AN, et al. : Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd Ed. World Health Organization, 2016. Available at www.who.int/hiv/pub/arv/arv-2016/en/, accessed February 5, 2016. [PubMed]
- 6. Byrd KK, Hou JG, Bush T, et al. : Adherence and viral suppression among participants of the patient-centered human immunodeficiency virus (HIV) care model project: A collaboration between community-based pharmacists and HIV clinical providers. Clin Infect Dis 2019;70:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gandhi M, Bacchetti P, Rodrigues WC, et al. : Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinicalMedicine 2018;2–3:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parienti J-J, Das-Douglas M, Massari V, et al. : Not all missed doses are the same: Sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. Carr JK, ed. PLoS One 2008;3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haberer JE, Musinguzi N, Boum Y, et al. : Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr 2015;70:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steegen K, Bronze M, Papathanasopoulos MA, et al. : HIV-1 antiretroviral drug resistance patterns in patients failing NNRTI-based treatment: Results from a national survey in South Africa. J Antimicrob Chemother 2017;72:210–219. [DOI] [PubMed] [Google Scholar]
- 11. Hoffmann CJ, Charalambous S, Sim J, et al. : Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis 2009;49:1928–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garone DB, Conradie K, Patten G, Cornell M, Goemaere E, Kunene J, et al. : High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: A model of care in Khayelitsha, South Africa. South Afr J HIV Med 2013;14:166–169. [Google Scholar]
- 13. Charpentier C, Peytavin G, Le MP, et al. : High virological suppression regardless of the genotypic susceptibility score after switching to a dolutegravir-based regimen: Week 48 results in an observational cohort. J Antimicrob Chemother 2018;73:1665–1671. [DOI] [PubMed] [Google Scholar]
- 14. Gandhi M, Bacchetti P, SpinelliI MA, et al. : Validation of a urine tenofovir immunoassay for adherence monitoring to PrEP and ART and establishing the cut-off for a point-of-care test. JAIDS J Acquir Immune Defic Syndr 2019:81:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi M, Wang G, King R, et al. : Development and validation of the first point-of-care assay to objectively monitor adherence to HIV treatment and prevention in real-time in routine settings. AIDS 2020;34:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spinelli MA, Glidden D V., Rodrigues WC, et al. : Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a preexposure prophylaxis demonstration project. AIDS 2019;33:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stalter RM, Baeten JM, Donnell D, et al. : Urine tenofovir levels measured using a novel immunoassay predict human immunodeficiency virus protection. Clin Infect Dis 2021;72:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drain P, Ngure K, Mugo N, et al. : Testing a real-time tenofovir urine adherence assay for monitoring and providing feedback to preexposure prophylaxis in Kenya (PUMA): Protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2020;9:e15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bushman LR, Kiser JJ, Rower JE, et al. : Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011;56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Zyl GU, Liu TF, Claassen M, et al. : Trends in genotypic HIV-1 antiretroviral resistance between 2006 and 2012 in South African patients receiving first- and second-line antiretroviral treatment regimens. Landay A, ed. PLoS One 2013;8:e67188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing 2020. Available at https://www.r-project.org/.
- 22. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. : Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018;62:1710–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spinelli M, Haberer J, … PC-CH: Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Available at pubmed.ncbi.nlm.nih.gov. https://pubmed.ncbi.nlm.nih.gov/32424549/, accessed March 31, 2021. [DOI] [PMC free article] [PubMed]
- 24. Yager JL, Coyle RP, Coleman SS, et al. : Moderately high tenofovir diphosphate in dried blood spots indicates drug resistance in viremic persons living with HIV. J Int Assoc Provid AIDS Care 2019;18:2325958219888457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. : Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019;68:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pu F, Pandey S, Bushman LR, Anderson PL, Ouyang Z, Cooks RG: Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem 2020;412:1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robbins RN, Nguyen N, Ferraris C, et al. : Tenofovir diphosphate in dried blood spots predicts future viremia in South Africa | CROI Conference. In: Conference on Retroviruses and Opportunistic Infections (CROI 2021), March 6–10. Virtual Conference, 2021. Available at https://www.croiconference.org/search-abstracts/.
- 28. Pepperrell T, Venter WDF, McCann K, et al. : Participants on dolutegravir re-suppress HIV RNA after virologic failure: Updated data from the ADVANCE trial. Clin Infect Dis 2021;73:e1008–e1010. [DOI] [PubMed] [Google Scholar]
- 29. Wallis CL, Mellors JW, Venter WDF, Sanne I, Stevens W: Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat 2011;2011:769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Zyl GU, van Mens TE, McIlleron H, et al. : Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011;56:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vitoria M, Hill A, Ford N, et al. : The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries. AIDS 2018;32:1551–1561. [DOI] [PubMed] [Google Scholar]
- 32. Gandhi RT, Wurcel A, Rosenberg ES, et al. : Progressive reversion of human immunodeficiency virus type 1 resistance mutations in vivo after transmission of a multiply drug-resistant virus. Clin Infect Dis 2003;37:1693–1698. [DOI] [PubMed] [Google Scholar]
- 33. Petropoulos S, Kuritzkes D, Hoh J, Virol NP-J: 2009. undefined. In vivo Fitness cost of the M184V mutation. academia.edu. Available at https://www.academia.edu/download/34329304/2038.pdf, accessed April 2, 2021. [DOI] [PMC free article] [PubMed]
- 34. Hoffmann CJCJ, Maritz J, van Zyl GUGU: CD4 count-based failure criteria combined with viral load monitoring may trigger worse switch decisions than viral load monitoring alone. Trop Med Int Heal 2016;21:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alhassan Y, Twimukye A, Malaba T, et al. : Engendering health systems in response to national rollout of dolutegravir-based regimens among women of childbearing potential: A qualitative study with stakeholders in South Africa and Uganda. BMC Health Serv Res 2020;20:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mendelsohn AS, Ritchwood T: COVID-19 and antiretroviral therapies: South Africa's charge towards 90–90–90 in the midst of a second pandemic. AIDS Behav 2020;24:2754–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inzaule SC, Hamers RL, Doherty M, Shafer RW, Bertagnolio S, Rinke de Wit TF: Curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect Dis 2019;19:e246-e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ: “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008;9:238–246. [DOI] [PubMed] [Google Scholar]
- 39. Fatti G, Grimwood A, Nachega JB, et al. : Better virological outcomes among people living with human immunodeficiency virus (HIV) initiating early antiretroviral treatment (CD4 Counts ≥500 Cells/μL) in the HIV prevention trials network 071 (PopART) Trial in South Africa. Clin Infect Dis 2020;70:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basson AE, Charalambous S, Hoffmann CJ, Morris L: HIV-1 re-suppression on a first-line regimen despite the presence of phenotypic drug resistance. Apetrei C, ed. PLoS One 2020;15:e0234937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Orrell C, Harling G, Lawn SD, et al. : Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther 2007;12:83–88. [PubMed] [Google Scholar]
- 42. Steegen K, Carmona S, Bronze M, et al. : Moderate levels of pre-treatment HIV-1 antiretroviral drug resistance detected in the first south african national survey. PLoS One 2016;11:e0166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunt GM, Dokubo EK, Takuva S, et al. : Rates of virological suppression and drug resistance in adult HIV-1-positive patients attending primary healthcare facilities in KwaZulu-Natal, South Africa. J Antimicrob Chemother 2017;72:3141–3148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.