Abstract
Expanded access to HIV treatment services has improved outcomes for children and adolescents living with HIV in Kenya. Minimal data are available on these outcomes. We describe temporal trends in outcomes for children and adolescents initiating antiretroviral therapy (ART) from 2004 to 2014 at sites supported by Centre for Health Solutions—Kenya, in central Kenya. We retrospectively analyzed data from children 0–9 years of age (n = 3,519) and adolescents 10–19 years of age (n = 1,663) living with HIV, who newly initiated ART at 47 health facilities in central Kenya. Year cohorts were analyzed from the Comprehensive Patient Application Database (CPAD) and International Quality Care (IQCare) electronic medical databases, including temporal trends in outcomes and associated factors using multivariable competing risk regression analysis. There were more girls (2,453 [52.7%]) than boys, with most enrolled at World Health Organization (WHO) stage II (1,813 [37.7%]) or III disease (1,694 [35.1%]). Most of the children and adolescents (4,431 [96.4%]) did not have tuberculosis (TB) symptoms. Cumulative lost to follow-up (LTFU) incidence at 6, 12, 24, and 36 months were 5.0%, 9.9%, 22.9%, and 33.1%, respectively. Cumulative mortality incidence at 6, 12, 24, and 36 months were 0.7%, 1.0%, 1.2%, and 1.5%, respectively. The incidence of LTFU was higher among female children and adolescents, those initiated on tenofovir-based regimens, and those with presumptive TB symptoms. Mortality risk was higher among those with WHO stage III or IV disease, and children and adolescents on TB treatment or who had presumptive TB. Enrollment occurred at a young age and pediatric-friendly ART regimens were initiated at earlier WHO stages implying effective early infant diagnosis and treatment for all strategies, resulting in improved treatment outcomes. The higher retention rates in recent years as well as the lower retention after many years of follow-up underscore the importance of implementing longitudinal follow-up strategies targeting this population.
Keywords: adolescents, antiretroviral therapy, HIV positive, Kenya, pediatric, treatment outcomes
Introduction
In 2019, ∼1.5 million people in Kenya were living with HIV, including 110,000 children younger than 15 years1 with 68.0% of these receiving antiretroviral therapy (ART). An estimated 4,333 children younger than 15 years died of AIDS, and an estimated 2,275 adolescents 10–19 years of age died in 2019.1
Mother-to-child HIV transmission (MTCT) is the primary mode of infection for children living with HIV, who typically receive a diagnosis early in life. In southern Africa, median age at enrolment into HIV services for children, including those diagnosed through prevention of mother-to-child transmission programs, has been reported to be less than 3.3 years, with equal distribution between boys and girls.2
In 2005, Kenya ART guidelines recommended switching from a combination of stavudine (D4T) and lamivudine (3TC) to a combination of zidovudine (AZT) and lamivudine as the preferred first-line nucleoside reverse transcriptase inhibitor regimen for children and adolescents. However, it took several years to phase out stavudine-based ART regimens. In 2011, guidelines recommended abacavir (ABC)-based regimens as the preferred first-line treatment for children with zidovudine-based regimens as the preferred alternative; tenofovir (TDF) was also recommended as the first-line regimen for adolescents 10–19 years of age, who weigh more than 35 kg.3,4 Since 2014, Kenyan treatment guidelines have recommended ART initiation for all children younger than 10 years regardless of World Health Organization (WHO) clinical stage.3
Retention of children in HIV services is an important measure of the success of a pediatric HIV program. Few studies have examined temporal trends in the characteristics and outcomes of children initiating ART in Kenya, hence justifying this analysis. As HIV diagnoses and access to ART have increased, it is important to analyze the treatment outcomes of children receiving ART to help improve pediatric HIV programming.
Materials and Methods
Study setting
The study was conducted at all 47 government-owned health facilities in the former central province of Kenya, which provide HIV services to children and adolescents and are supported by Center for Health Solutions–Kenya (CHS), through funding from the U.S. President's Emergency Plan for AIDS/Centers for Disease Control and Prevention (PEPFAR/CDC). Per the national HIV treatment guidelines, CHS HIV services included providing HIV testing for children, conducting clinical evaluations, obtaining baseline CD4 cell counts/percentages, and treatment monitoring through viral load testing.
All children are screened for tuberculosis (TB) symptoms: all children received cotrimoxazole to prevent opportunistic infections, and those with no presumptive or active TB also received isoniazid prophylaxis to prevent TB. CHS facilities also provide adherence support, including HIV education, pre-ART counseling and education, adherence counseling at ART initiation and at every visit, and disclosure support for both children and caregivers. Further details of CHS projects in Kenya have been described elsewhere.5–7
Study design, population, and sample
We retrospectively analyzed medical records from a cohort (n = 5,182) of children 0–9 years of age and adolescents 10–19 years of age living with HIV, who initiated ART between January 2004 and December 2014 and who were followed for treatment outcomes up to February 2017.
Data sources
We analyzed data collected between January 2004 and December 2014 using the Kenya Ministry of Health Comprehensive Care Clinic patient card (MOH 257). Clinicians captured the data during routine clinical visits, and then trained data clerks uploaded the data into the Comprehensive Patient Application Database (CPAD) and International Quality Care (IQCare) electronic medical record (EMR) system. These two EMR systems were in use at supported health facilities for patient-data capture and management. Data collected in the two EMR systems were subject to routine review for errors and completeness. The study team prepared the analysis dataset from data extracted from the CPAD and IQCare EMR.
Variables
Baseline characteristics collected included age at enrollment, sex, WHO clinical stage, date of ART initiation, regimen at ART initiation, and TB status. The backbone regimens at ART initiation containing ABC, AZT, D4T, and TDF were mutually exclusive. Retention was defined as children who were enrolled into ART and who were alive and active in the clinic (not lost to follow-up [LTFU] or dead, as indicated in the patient's chart) as of February 2017. LTFU was determined when the interval between the last clinic visit registered as attended in the database was more than 90 days by time of analysis.8,9 Transfer-out status was determined based on documentation in the patient charts. Patients who transferred out were included as active patients in our analysis.
Time to LTFU or mortality outcome was recorded in months. Patients still active in care were censored at 36 months of follow-up. Those who transferred out to other facilities were considered active, but censored at their transfer-out date to utilize their information up to that point. No sign of TB was defined as patients screening negative using the ministry of health symptomatic TB screening tool and presumptive TB was defined as patients screening positive for TB using the symptomatic screening tool, but with pending confirmatory investigations for TB, while presence of TB was defined as patients with confirmed TB based on positive GeneXpert, TB lipoarabinomannan, and/or chest X-ray.
Ethics
Local ethical approval was obtained from Kenyatta National Hospital—University of Nairobi Ethics Review Committee (P339/06/2013). This study was also reviewed according to the U.S. Centers for Disease Control and Prevention human research protection procedures and was determined to be research, but the CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. As this research used available retrospective data, consent from study participants was waived. All study procedures were done in accordance with Kenya Government, Ministry of Health, CDC and local IRB regulations.
Data analysis
Descriptive analysis of demographic and baseline clinical characteristics was done using counts and percentages for all categorical variables. Median and interquartile intervals (IQIs) were presented for time to treatment outcome event. To assess temporal trends in outcomes and characteristics across the ART cohort years, we used the trend function, which is a modified Wilcoxon rank-sum nonparametric test for trends across ordered groups with corresponding p values. The person years of observation (pyo) were also calculated and used to determine the LTFU and mortality rates per 100 pyo.
Competing risk regression analysis (Fine and Gray modeling) was done to assess the factors associated with time on ART to LTFU or mortality among the children and adolescents.10,11 Mortality outcome was treated as a competing event for being LTFU and likewise, LTFU was treated as a competing event for mortality.
The following variables had missing data: regimen (9.8%), WHO Stage (7.1%), and TB Status (11.3%). CD4 data were missing for over 60% of the subjects and therefore were not included. We assumed the data were missing at random and that missing values could be accounted for by complete measured covariates based on Little's missing completely at random and covariate-dependent missing tests. To deal with missing data, we estimated all the regression models after multiple imputations of missing values using chained equations.12 Ten imputed datasets were then used to calculate mean estimates of covariates of interest using Rubin's rules during the regression analysis.12 We assessed the observed and imputed values using diagnostic plots to ensure the imputations were reasonable. A sensitivity analysis was done, excluding transfer outs, and this did not change results.
The multivariate models included a priori all demographic and clinical characteristics collected. To account for any variability in outcomes by year of ART initiation, we also adjusted for the clustering within the patients' cohort year. The estimated cumulative incidence of mortality and LTFU was based on the multivariable models. The cumulative incidence function plots and subhazard ratios (sHR 95% confidence intervals [CIs]) are presented. Model assumptions were assessed for and met. Statistical significance was evaluated at 5.0% level. All analyses were done using Stata, version 15.1 (StataCorp 2017. Stata Statistical Software, Release 15; College Station, TX).
Results
Characteristics of study participants
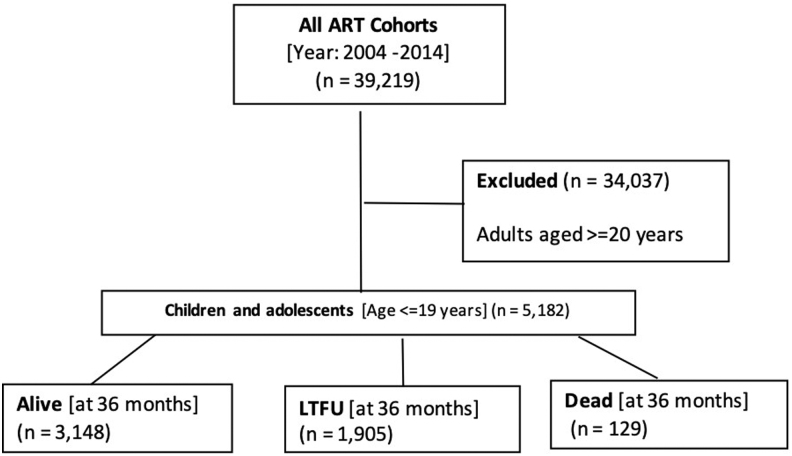
The cohort analysis included 5,182 children and adolescents (Fig. 1). Most of the cohort were children (3,519 [67.9%]) at ART initiation (Table 1). Overall, our cohort included slightly more girls (2,729 [52.7%]) than boys. Most of the patients initiated zidovudine-based (1,827 [39.1%]) or ABC-based regimens, (1,506 [32.2%]). Most children and adolescents had WHO stage II (1,813 [37.7%]) or III (1,694 [35.2%]) disease at ART initiation.
FIG. 1.
Flow diagram of children and adolescents initiating ART in central Kenya (2004–2014). ART, antiretroviral therapy.
Table 1.
Characteristics of Children and Adolescents Who Initiated Antiretroviral Therapy in Central Kenya (2004–2014)
| ART cohort |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 36 | 138 | 413 | 608 | 662 | 832 | 641 | 586 | 425 | 368 | 473 | 5,182 |
| Age, n (%) | ||||||||||||
| 0–4 (n = 1,815) | 7 (19.4) | 43 (31.2) | 100 (24.2) | 208 (34.2) | 230 (34.7) | 334 (40.1) | 236 (36.8) | 213 (36.3) | 162 (38.1) | 127 (34.5) | 155 (32.8) | 1,815 (35.0) |
| 5–9 (n = 1,704) | 22 (61.1) | 51 (37.0) | 184 (44.6) | 212 (34.9) | 234 (35.3) | 261 (31.4) | 213 (33.2) | 171 (29.2) | 118 (27.8) | 96 (26.1) | 142 (30.0) | 1,704 (32.9) |
| 10–14 (n = 997) | 4 (11.1) | 27 (19.6) | 86 (20.8) | 130 (21.4) | 122 (18.4) | 164 (19.7) | 112 (17.5) | 109 (18.6) | 88 (20.7) | 71 (19.3) | 84 (17.8) | 997 (19.2) |
| 15–19 (n = 666) | 3 (8.3) | 17 (12.3) | 43 (10.4) | 58 (9.5) | 76 (11.5) | 73 (8.8) | 80 (12.5) | 93 (15.9) | 57 (13.4) | 74 (20.1) | 92 (19.5) | 666 (12.9) |
| Trend test, p value = .099 | ||||||||||||
| Sex, n (%) | ||||||||||||
| Male (n = 2,453) | 19 (52.8) | 54 (39.1) | 193 (46.7) | 287 (47.2) | 328 (49.5) | 452 (54.3) | 300 (46.8) | 272 (46.4) | 216 (50.8) | 148 (40.2) | 184 (38.9) | 2,453 (47.3) |
| Female (n = 2,729) | 17 (47.2) | 84 (60.9) | 220 (53.3) | 321 (52.8) | 334 (50.5) | 380 (45.7) | 341 (53.2) | 314 (53.6) | 209 (49.2) | 220 (59.8) | 289 (61.1) | 2,729 (52.7) |
| Trend test, p value = .006* | ||||||||||||
| Regimen, n (%) | ||||||||||||
| D4T (n = 1,103) | 27 (79.4) | 92 (68.1) | 235 (58.2) | 281 (48.5) | 251 (38.9) | 164 (24.0) | 47 (8.9) | 5 (1.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1,103 (23.6) |
| AZT (n = 1,827) | 7 (20.6) | 40 (29.6) | 164 (40.6) | 285 (49.2) | 360 (55.8) | 266 (38.9) | 195 (36.8) | 182 (38.0) | 128 (32.0) | 113 (31.7) | 87 (20.2) | 1,827 (39.1) |
| TDF (n = 239) | 0 (0.0) | 1 (0.7) | 3 (0.7) | 1 (0.2) | 4 (0.6) | 4 (0.6) | 18 (3.4) | 46 (9.6) | 37 (9.2) | 51 (14.3) | 74 (17.2) | 239 (5.1) |
| ABC (n = 1,506) | 0 (0.0) | 2 (1.5) | 2 (0.5) | 12 (2.1) | 30 (4.7) | 249 (36.5) | 270 (50.9) | 246 (51.4) | 234 (58.5) | 192 (53.9) | 269 (62.6) | 1,506 (32.2) |
| Trend test, p value <.001* | ||||||||||||
| WHO stage, n (%) | ||||||||||||
| Stage I (n = 1,050) | 6 (20.0) | 7 (5.4) | 35 (10.0) | 41 (7.8) | 79 (13.1) | 145 (18.4) | 153 (24.7) | 145 (25.6) | 116 (29.4) | 130 (36.6) | 193 (42.8) | 1,050 (21.8) |
| Stage II (n = 1,813) | 0 (0.0) | 27 (20.9) | 85 (24.2) | 195 (36.9) | 206 (34.2) | 311 (39.6) | 268 (43.3) | 240 (42.4) | 173 (43.8) | 132 (37.2) | 176 (39.0) | 1,813 (37.7) |
| Stage III (n = 1,694) | 18 (60.0) | 69 (53.5) | 177 (50.4) | 249 (47.2) | 276 (45.8) | 302 (38.4) | 184 (29.7) | 172 (30.4) | 86 (21.8) | 86 (24.2) | 75 (16.6) | 1,694 (35.2) |
| Stage IV (n = 256) | 6 (20.0) | 26 (20.2) | 54 (15.4) | 43 (8.1) | 42 (7.0) | 28 (3.6) | 14 (2.3) | 9 (1.6) | 20 (5.1) | 7 (2.0) | 7 (1.6) | 256 (5.3) |
| Trend test, p value <.001* | ||||||||||||
| TB status, n (%) | ||||||||||||
| No TB signs (n = 4,431) | 27 (90.0) | 108 (92.3) | 348 (95.3) | 511 (94.3) | 565 (95.1) | 711 (97.0) | 553 (96.8) | 510 (97.5) | 374 (97.4) | 311 (98.4) | 413 (97.9) | 4,431 (96.4) |
| Presumptive TB (n = 42) | 0 (0.0) | 3 (2.6) | 5 (1.4) | 6 (1.1) | 9 (1.5) | 6 (0.8) | 7 (1.2) | 2 (0.4) | 0 (0.0) | 1 (0.3) | 3 (0.7) | 42 (0.9) |
| On TB TX (n = 124) | 3 (10.0) | 6 (5.1) | 12 (3.3) | 25 (4.6) | 20 (3.4) | 16 (2.2) | 11 (1.9) | 11 (2.1) | 10 (2.6) | 4 (1.3) | 6 (1.4) | 124 (2.7) |
Trend test, p value <.001*.
ART, antiretroviral therapy; ABC, abacavir; TB, tuberculosis; TDF, tenofovir.
The proportion of children and adolescents with WHO stage I or II disease at ART initiation increased over the study period, from a low of 6 (20%) in 2004 to a peak of 369 (81.8%) in 2014, while the proportion of those with WHO stage III or IV disease at ART initiation decreased over time, from a high of 80.0% (24) in 2004 to a low of 18.2% (82) in 2014. Most of the children and adolescents (4,431 [96.4%]) did not have signs of TB. This was similar across all the year cohorts: 27 (90.0%) in 2004 and 413 (97.9) in 2014. Overall, 124 (2.7%) of children and adolescents were receiving TB treatment; however, TB treatment rates at ART initiation were higher in earlier cohorts (3 [10.0%] in 2004) compared to later cohorts (6 [1.4%] in 2014; Table 1).
Treatment outcomes
Overall LTFU was 36.8% (1,905) and mortality was 2.5% (129), respectively. The median time on follow-up was 16.00 months (IQI: 8.00; 25.00) among those LTFU and 4.00 months (IQI: 1.00; 15.00) among those who died (Table 2). There were 1,781 cases of those LTFU and 105 mortality cases observed over 12,153.25 person years at risk. Overall incidence rate (IR) of being LTFU and mortality was 14.65 (95% CI: 13.99–15.35) and 0.86 (95% CI: 0.71–1.05) per 100 pyo.
Table 2.
Treatment Outcomes of Children and Adolescents Initiating Antiretroviral Therapy in Central Kenya (2004–2014)
| 36-month follow-up ART outcome |
Total |
LTFU |
Dead |
Censored (Retained) |
|---|---|---|---|---|
| n (%) | 5,182 (100.0) | 1,905 (36.8) | 129 (2.5) | 3,148 (60.7) |
| Age, n (%) | ||||
| 0–4 (n = 1,815) | 1,815 (35.0) | 694 (36.4) | 41 (31.8) | 1,080 (34.3) |
| 5–9 (n = 1,704) | 1,704 (32.9) | 558 (29.3) | 43 (33.3) | 1,103 (35.0) |
| 10–14 (n = 997) | 997 (19.2) | 341 (17.9) | 33 (25.6) | 623 (19.8) |
| 15–19 (n = 666) | 666 (12.9) | 312 (16.4) | 12 (9.3) | 342 (10.9) |
| Sex, n (%) | ||||
| Male (n = 2,453) | 2,453 (47.3) | 817 (42.9) | 75 (58.1) | 1,561 (49.6) |
| Female (n = 2,729) | 2,729 (52.7) | 1,088 (57.1) | 54 (41.9) | 1,587 (50.4) |
| Regimen, n (%) | ||||
| D4T (n = 1,103) | 1,103 (23.6) | 315 (18.7) | 45 (40.2) | 743 (25.8) |
| AZT (n = 1,827) | 1,827 (39.1) | 539 (32.0) | 39 (34.8) | 1,249 (43.4) |
| TDF (n = 239) | 239 (5.1) | 161 (9.5) | 4 (3.6) | 74 (2.6) |
| ABC (n = 1,506) | 1,506 (32.2) | 672 (39.8) | 24 (21.4) | 810 (28.2) |
| WHO Stage, n (%) | ||||
| Stage I (n = 1,050) | 1,050 (21.8) | 478 (27.0) | 9 (7.6) | 563 (19.2) |
| Stage II (n = 1,813) | 1,813 (37.7) | 666 (37.7) | 33 (28.0) | 1,114 (38.1) |
| Stage III (n = 1,694) | 1,694 (35.2) | 535 (30.3) | 58 (49.2) | 1,101 (37.6) |
| Stage IV (n = 256) | 256 (5.3) | 89 (5.0) | 18 (15.3) | 149 (5.1) |
| TB status, n (%) | ||||
| TB status, n (%) | 4,431 (96.4) | 1,592 (94.3) | 85 (74.6) | 2,754 (98.5) |
| No TB signs (n = 4,431) | 42 (0.9) | 17 (1.0) | 11 (9.6) | 14 (0.5) |
| Presumptive TB (n = 42) | 124 (2.7) | 79 (4.7) | 18 (15.8) | 27 (1.0) |
| ART Cohort, n (%) | ||||
| 2004 (n = 36) | 36 (0.7) | 13 (0.7) | 0 (0.0) | 23 (0.7) |
| 2005 (n = 138) | 138 (2.7) | 30 (1.6) | 2 (1.6) | 106 (3.4) |
| 2006 (n = 413) | 413 (8.0) | 82 (4.3) | 11 (8.5) | 320 (10.2) |
| 2007 (n = 608) | 608 (11.7) | 168 (8.8) | 29 (22.5) | 411 (13.1) |
| 2008 (n = 662) | 662 (12.8) | 150 (7.9) | 24 (18.6) | 488 (15.5) |
| 2009 (n = 832) | 832 (16.1) | 188 (9.9) | 27 (20.9) | 617 (19.6) |
| 2010 (n = 641) | 641 (12.4) | 158 (8.3) | 16 (12.4) | 467 (14.8) |
| 2011 (n = 586) | 586 (11.3) | 155 (8.1) | 8 (6.2) | 423 (13.4) |
| 2012 (n = 425) | 425 (8.2) | 132 (6.9) | 7 (5.4) | 286 (9.1) |
| 2013 (n = 368) | 368 (7.1) | 358 (18.8) | 3 (2.3) | 7 (0.2) |
| 2014 (n = 473) | 473 (9.1) | 471 (24.7) | 2 (1.6) | 0 (0.0) |
| Months to event, median (IQI) | 36.00 (20.00; 36.00) | 16.00 (8.00; 25.00) | 4.00 (1.00; 15.00) | 36.00 (36.00; 36.00) |
LTFU, lost to follow-up; IQI, interquartile interval.
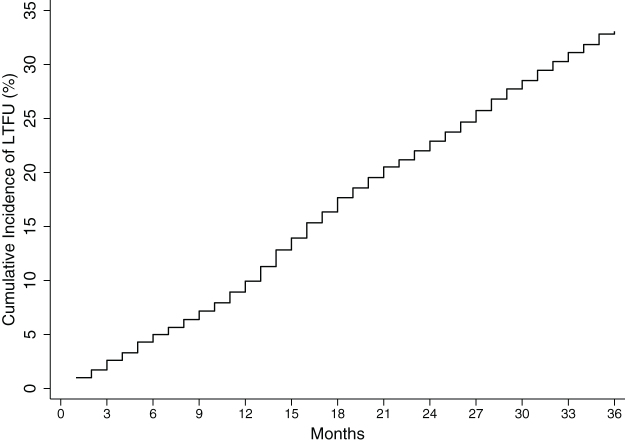
The rate of being LTFU was highest between 13 and 24 months and mortality rate within the first 6 months of follow-up; 17.22 (95% CI: 15.98–18.55) per 100 pyo and 2.09 (95% CI: 1.59–2.76) per 100 pyo, respectively. Overall cumulative incidence of mortality as estimated by the regression model at 6, 12, 24, and 36 months was 0.7%, 1.0%, 1.2%, and 1.5%, respectively (Table 3 and Fig. 2). The overall cumulative incidence of being LTFU as estimated by the regression model was 5.0% at 6 months, 9.9% at 12 months, 22.9% at 24 months, and 33.1% at 36 months of follow-up (Table 3 and Fig. 3).
Table 3.
Incidence Rate of Being Lost to Follow-Up and Mortality of Children and Adolescents Initiating Antiretroviral Therapy in Central Kenya (2004–2014).
| Time (months) | pyo | No. of events (n) | Rate per 100 pyo (95% CI) | Cumulative incidence (%)a |
|---|---|---|---|---|
| LTFU outcome | ||||
| 6 months | 2,435.25 | 305 | 12.52 (11.19–14.01) | 5.0 |
| 12 months | 2,284.17 | 275 | 12.04 (10.70–13.55) | 9.9 |
| 24 months | 4,019.33 | 692 | 17.22 (15.98–18.55) | 22.9 |
| 36 months | 3,414.5 | 509 | 14.91 (13.67–16.26) | 33.1 |
| Total | 12,153.25 | 1,781 | 14.65 (13.99–15.35) | 33.1 |
| Mortality Outcome | ||||
| 6 months | 2,435.25 | 51 | 2.09 (1.59–2.76) | 0.7 |
| 12 months | 2,284.17 | 16 | 0.70 (0.43–1.14) | 1.0 |
| 24 months | 4,019.33 | 14 | 0.35 (0.21–0.59) | 1.2 |
| 36 months | 3,414.5 | 24 | 0.70 (0.47–1.05) | 1.5 |
| Total | 12,153.25 | 105 | 0.86 (0.71–1.05) | 1.5 |
Estimated based on the cumulative incidence function of the multivariable regression model.
CI, confidence interval; pyo, person years of observation.
FIG. 2.
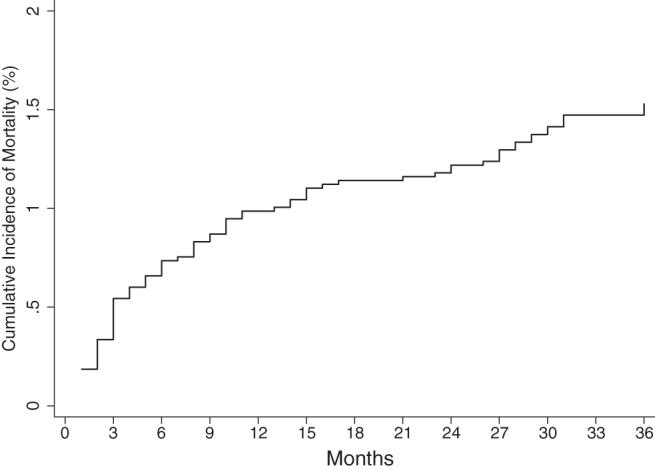
Overall cumulative incidence of mortality among children and adolescents initiating ART in central Kenya (2004–2014).
FIG. 3.
Overall cumulative incidence of being lost to follow-up among children and adolescents initiating ART in central Kenya (2004–2014).
Associations between patient characteristics and mortality
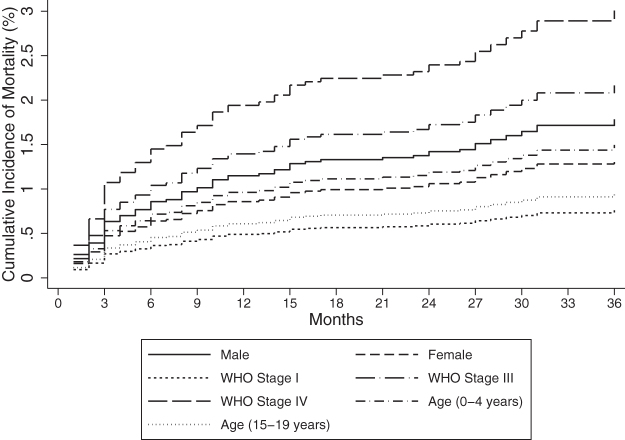
The univariable and multivariable models for the risk of mortality are presented in Table 4. On multivariable analysis, there was significantly lower risk of mortality among children and adolescents 10–19 years of age compared to those 0–4 years of age, (sHR, 0.56 [95% CI: 0.38–0.81]). Compared with those classified as WHO stage I disease, the risk of mortality for those with WHO stage III and IV disease was significantly over twofold higher, (sHR, 2.44 [95% CI: 1.15–5.16]) and (sHR, 3.62 [95% CI: 1.20–10.89]), respectively. Compared to those with no sign of TB, children and adolescents on TB treatment and had presumptive TB had significantly higher risk of mortality (aSHR, 11.89 [95% CI: 5.51–25.65]), and (sHR, 5.76 [95% CI: 2.82–11.76]), respectively (Table 4 and Fig. 4).
Table 4.
Regression Analysis—Mortality Risk Among Children and Adolescents Initiating Antiretroviral Therapy in Central Kenya (2004–2014)
| Outcome: mortality |
Univariable results |
|
Multivariable results |
|
|---|---|---|---|---|
| Imputed models | sHR (95% CI) | p | sHR (95% CI) | p |
| Age groups, years | ||||
| 0–4 | Ref. | Ref. | ||
| 5–9 | 1.08 (0.63–1.87) | .007 | 0.89 (0.47–1.66) | .703 |
| 10–14 | 1.61 (1.10–2.37) | .015 | 1.31 (0.83–2.06) | .252 |
| 15–19 | 0.65 (0.41–1.03) | <.001 | 0.56 (0.38–0.81) | .002 |
| Sex | ||||
| Female | Ref. | Ref. | ||
| Male | 1.48 (1.02–2.15) | <.001 | 1.44 (0.93–2.23) | .099 |
| Regimen | ||||
| D4T Based | Ref. | Ref. | ||
| AZT Based | 0.72 (0.40–1.31) | .916 | 0.86 (0.45–1.65) | .655 |
| TDF based | 0.26 (0.07–0.97) | .004 | 0.50 (0.13–1.93) | .313 |
| ABC based | 0.51 (0.26–0.98) | .221 | 0.69 (0.31–1.54) | .363 |
| Baseline WHO stage | ||||
| Stage I | Ref. | Ref. | ||
| Stage II | 2.13 (0.99–4.57) | .902 | 1.87 (0.91–3.83) | .090 |
| Stage III | 3.44 (1.51–7.82) | .599 | 2.44 (1.15–5.16) | .020 |
| Stage IV | 5.49 (2.18–13.86) | .237 | 3.62 (1.20–10.89) | .022 |
| TB status | ||||
| No signs | Ref. | Ref. | ||
| On TB treatment | 13.85 (6.60–29.04) | .593 | 11.89 (5.51–25.65) | <.001 |
| Presumptive TB | 6.71 (3.37–13.37) | .001 | 5.76 (2.82–11.76) | <.001 |
AZT, zidovudine; D4T, stavudine; sHR, subhazard ratios; TX, treatment; WHO, World Health Organization.
FIG. 4.
Cumulative incidence of mortality by sex, age, and WHO stage among children and adolescents initiating ART in central Kenya (2004–2014).
Associations between patient characteristics and LTFU
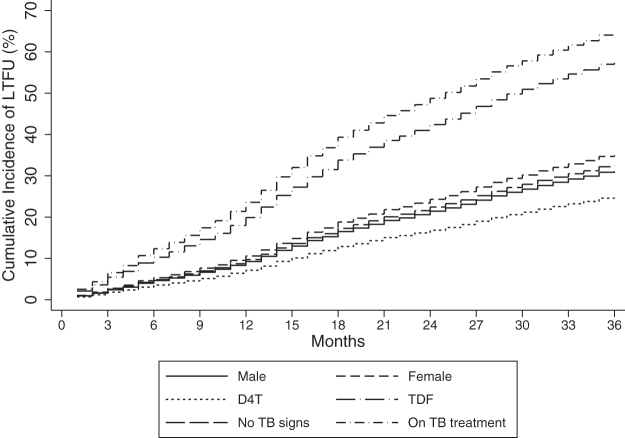
The univariable and multivariable models are presented in Table 5. The risk of being LTFU was significantly higher among girls than boys, (aSHR, 1.16 [95% CI: 1.05–1.29]). Children and adolescents who initiated TDF-based ART regimens had significantly higher risk of being LTFU compared to those who initiated stavudine, (aSHR, 2.37 [95% CI: 1.23–4.58]). Children and adolescents with presumptive TB had significantly higher risk of being LTFU compared to those with no sign of TB (aSHR, 2.59 [95% CI: 1.78–3.77]) (Table 5 and Fig. 5).
Table 5.
Regression Analysis—Lost to Follow-Up Risk Among Children and Adolescents Initiating Antiretroviral Therapy in Central Kenya (2004–2014)
| Outcome: LTFU |
Univariable results |
|
Multivariable results |
|
|---|---|---|---|---|
| Imputed models | sHR (95% CI) | p | sHR (95% CI) | p |
| Age groups, years | ||||
| 0–4 | Ref. | Ref. | ||
| 5–9 | 0.84 (0.75–0.95) | .007 | 0.93 (0.74–1.17) | .551 |
| 10–14 | 0.91 (0.84–0.98) | .015 | 1.05 (0.80–1.38) | .735 |
| 15–19 | 1.35 (1.19–1.54) | <.001 | 1.26 (1.00–1.59) | .051 |
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 1.25 (1.12–1.40) | <.001 | 1.16 (1.05–1.29) | .003 |
| Regimen | ||||
| D4T based | Ref. | Ref. | ||
| AZT based | 1.03 (0.64–1.64) | .916 | 1.02 (0.65–1.59) | .933 |
| TDF based | 3.03 (1.43–6.42) | .004 | 2.37 (1.23–4.58) | .010 |
| ABC based | 1.66 (0.74–3.75) | .221 | 1.64 (0.74–3.66) | .225 |
| Baseline WHO stage | ||||
| Stage I | 0.98 (0.65–1.46) | .902 | 0.92 (0.63–1.35) | .676 |
| Stage II | 1.16 (0.67–2.02) | .599 | 1.08 (0.71–1.63) | .722 |
| Stage III | 1.56 (0.75–3.25) | .237 | 1.35 (0.83–2.22) | .228 |
| Stage IV | Ref. | Ref. | ||
| TB status | ||||
| No signs | Ref. | Ref. | ||
| On TB treatment | 1.22 (0.59–2.53) | .593 | 1.37 (0.69–2.75) | .372 |
| Presumptive TB | 2.30 (1.39–3.79) | .001 | 2.59 (1.78–3.77) | <.001 |
AZT, zidovudine; D4T, stavudine; WHO, World Health Organization; TX, treatment.
FIG. 5.
Cumulative incidence of being LTFU by sex, D4T, TDF, and TB status among children and adolescents initiating ART in central Kenya (2004–2014). LTFU, lost to follow-up; TB, tuberculosis; TDF, tenofovir.
Discussion
Most patients were younger than 10 years at enrolment across all the cohorts studied. The higher number of young children enrolled was likely due to easier access to HIV services, young age at HIV diagnosis, and the natural progression of HIV among children (most perinatally infected children present with signs and symptoms of HIV within the first decade of life). Our findings support those of a multicenter study conducted in sub-Saharan Africa, which reported a median age of 4.9 years for children initiating ART.13 Studies in sub-Saharan Africa also reported that the age at ART enrolment for children progressively decreased for later cohorts.14 Among older children and adolescents, youth-friendly models of care could help increase ART initiation.15
Most children and adolescents in our cohort initiated stavudine-based or ABC-based regimens. This finding is expected because of changes in national treatment guidelines recommending simplified and more tolerable antiretroviral agents for children and adolescents.3,4
The number of children and adolescents with baseline WHO stage I disease increased in later cohorts, whereas the number of children and adolescents with WHO stage III disease decreased. Our finding supports the results of several studies. A study conducted in four provinces in South Africa reported that the proportion of children with baseline WHO stage III or IV disease decreased from 72.9% to 49.0% between 2006 and 2009.16 In Tanzania, a study of 44 HIV clinics between 2005 and 2011 reported a decreasing trend of patients with baseline WHO stage IV disease at ART enrollment.17
In contrast, another study reported that 57.0% of children presented at WHO clinical stage III and IV at ART enrolment.18 Enrolment into HIV treatment programs at early stages of HIV disease could be due to the expansion of early infant diagnosis and enhanced provider-initiated testing in Kenya.19,20 This may also be due to progressively more inclusive ART initiation guidelines.3,4
In our study, few children and adolescents had a documented positive screen for TB at the time of ART enrollment, and the number of children and adolescents receiving treatment for TB at ART enrollment decreased in later cohorts. More than half the study population in earlier cohorts had WHO stage III or IV disease; we were not able to determine how many children and adolescents had documented completion of TB treatment before ART enrollment, which could have informed their WHO classification. We also were unable to determine how many children and adolescents received a TB diagnosis while in care. This may partly explain the low number of children and adolescents with documented TB/HIV co-infection in our study.
Another possibility is that the TB symptom screening tool used for HIV-positive patients is highly dependent on provider skill and therefore may have resulted in missed TB diagnoses in this population.21 Furthermore, health care workers run busy clinics and may not screen children adequately for TB. Since TB symptoms are nonspecific, symptoms of TB may have been attributed to other diseases, including HIV infection.22
To help avoid missing TB diagnoses, clinicians could ensure that patients are screened more thoroughly for TB, due to overlap in the clinical manifestations of both diseases.23 There is also need to improve the capacity of ART programs in low-income and middle-income countries to exclude and diagnose TB in children living with HIV. Our finding of high mortality rates among study participants with no sign of TB conflicts with results from other studies in sub-Saharan Africa, which have consistently shown that TB is a leading cause of death among children and adolescents living with HIV.24
The conflicting result could be due to missed TB diagnosis using the symptomatic TB screening tool, resulting in more children dying from undiagnosed TB, thus resulting in an underestimation of childhood TB mortality in our study. This is compounded by the fact that current diagnostic tests for TB in children, even when done correctly, underperform and may result in children with TB being misclassified as having no TB.
There were patients in our study classified as having presumptive TB. The presence of presumptive TB cases could be due to delays in conducting confirmatory tests for TB or even challenges in accessing health care. The higher risk of mortality noted among presumptive TB cases could be due to delays in initiating TB treatment in these suspected, but not confirmed TB cases. At the time of the study, Kenyan guidelines did not recommend treatment of presumptive TB cases with anti-TB medicines.
In this study, the overall incidence of LTFU was 36.8% and overall mortality was 2.5% after a median follow-up time of 16 months for being LTFU, which was similar to a study conducted in South Africa that reported a cumulative LTFU incidence of 36% after a median follow-up time of 3.3 years.25 Overall IR of being LTFU and mortality was 14.65 and 0.86 per 100 person years compared to IR of LTFU of 10.8 per 100 person years in South Africa.25 Overall cumulative incidence of being LTFU and mortality increased with the number of years of follow-up.
Studies conducted among adults also have reported higher incidence of being LTFU in successive years of the ART program.26–29
Mortality rate was low in our study. Similarly, other studies have recorded decreased mortality rates in children who initiated ART.30 A systematic review in 15 sub-Saharan African countries among 51,619 pediatric patients receiving first-line ART from 2014 to 2018 reported low mortality rates of 3.0%, 5.0%, 6.0%, and 7.0% at 3, 6, 12, and 24 months after ART initiation, respectively.31 In contrast, a study in Mozambique reported a high mortality rate (29.0%) among 735 children after 2 years of ART.31 These differences could be due to disparities in the data, such as undocumented deaths reported as LTFU.32
We found higher risk of death among children 0–4 years of age, those with WHO stage III or IV disease, those on TB treatment, and those with presumptive TB.
Studies elsewhere have identified young age as a risk factor for mortality.33,34 In contrast, a study conducted in four HIV programs in Kenya, Uganda, and Malawi reported high mortality rates among children 5–14 years of age compared to those 2–4 years of age.35
Similar to our study, WHO stage III and IV disease and TB co-infection are associated with increased likelihood of death.31,35–37 The higher risk of mortality in children and adolescents with presumptive TB could be due to delayed TB diagnosis.
In our study, girls, children, and adolescents receiving TDF, and those with presumptive TB had higher risk of being LTFU. Transition of children to adolescents could have resulted in higher risk of being LTFU among those on TDF. Studies have shown greater LTFU during this transition.38
Our finding that children with advanced HIV had higher risk of being LTFU could be because of associated opportunistic infections which may result in undocumented deaths.36,37 Identifying children who miss scheduled appointments early and developing strategies directed at retaining them in care are critical to improving long-term pediatric HIV outcomes.
Our study had several limitations. The retrospective design of the study could have resulted in missing data that were not possible to retrieve. Poor documentation in patient records resulted in missing data from the electronic database. Some instances of being LTFU could have been patients who enrolled in other facilities following self-referral or could have been unreported deaths, resulting in falsely higher LTFU rates.38 Viral load data were not analyzed because routine viral load monitoring was not available in Kenya before 2014. TB data were suboptimal since it did not analyze for diagnosis of TB during follow-up period nor for completion of TB treatment. Furthermore, poor quality screening for TB as well as lack of treatment for presumptive TB could have skewed our outcome data.
Conclusion
In conclusion, children were enrolled into care at a young age. This implies that early infant diagnostic approaches are effective. Over time, children and adolescents initiated ART at earlier WHO clinical stages, implying that PITC is a useful strategy in implementing prompt HIV testing and treatment of HIV in children and adolescents and should continue to be scaled up. This is especially important because children and adolescents who were enrolled at advanced WHO stages had higher risk of getting LTFU and mortality. The study found that children were switched from initial regimens at enrolment to pediatric friendly regimens in line with both WHO and Kenya HIV guideline recommendations. We did not analyze whether the use of pediatric-friendly regimens resulted in an improvement in early ART initiation.
Retention rates reduced with longer follow-up periods, underscoring the importance of strong systems to ensure longitudinal follow-up of patients on cART. The higher risk of mortality and getting LTFU among children and adolescents with presumptive TB could have been due to delayed TB diagnosis and initiation of TB treatment. Higher risk of mortality among WHO stage III and IV patients also implies the need to establish systems to identify and manage opportunistic infections early in this population. These results are generalizable to other HIV programs within Kenya and sub-Saharan Africa, which implement similar CDC/PEPFAR-funded programs.
Ethics Approval and Consent to Participate
The Kenyatta National Hospital—University of Nairobi Ethics Review Committee. This study was reviewed according to the U.S. Centers for Disease Control and Prevention human research protection procedures and was determined to be research, but the CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. As this research was retrospective, consent from study participants was not required.
PEPFAR/CDC Disclaimer
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the funding agency.
Acknowledgments
The authors acknowledge the help in data collection and management by all staff at the CDC-funded CHS Tegemeza project in central Kenya and Ministry of Health health care workers, the monitoring and evaluation advisor, data officers, data manager, and leadership. We also thank the children and adolescents living with HIV in central Kenya, whose data were analyzed in this study.
Authors' Contributions
All authors contributed to the development of this article (A.M., P.W., K.O., I.M., J.M., and L.I.). P.W. and A.M. developed the idea and were in charge of study implementation, including data collection. K.O. and P.W. were involved in data management, analysis, and result write-up. P.W., A.M., and K.O. contributed to drafting and revising the article with guidance and intellectual input from co-authors (J.M., I.M., and L.I.). P.W. and K.O. have access to data and take responsibility for the integrity and accuracy of data. All authors (A.M., P.W., K.O., I.M., J.M., and L.I.) contributed substantially to the interpretation of data and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This article has been supported by funding from the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreement NU2GGH002024-01-00. The funding body did not play any role in the study design and collection, analysis, and interpretation of data and in writing of this article.
References
- 1. National AIDS Control Council: Kenya HIV Estimates Report 2018. Nairobi, Kenya, 2018. [Google Scholar]
- 2. Schomaker M, Egger M, Ndirangu J, et al. : When to start antiretroviral therapy in children aged 2–5 years: A collaborative causal modelling analysis of cohort studies from Southern Africa. PLoS Med 2013;10:e1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National AIDS and STI Control Programme, Ministry of Health: Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: A rapid advice. Nairobi, Kenya, 2014. [Google Scholar]
- 4. National AIDS and STI Control Program (NASCOP): Guidelines for antiretroviral therapy in Kenya (4th edition). Nairobi, Kenya, 2011. [Google Scholar]
- 5. Wekesa P, McLigeyo A, Owuor K, Mwangi J, Nganga E, Masamaro K: Factors associated with 36-month loss to follow-up and mortality outcomes among HIV-infected adults on antiretroviral therapy in Central Kenya. BMC Public Health 2020;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wekesa P, McLigeyo A, Owuor K, Mwangi J, Isavwa L, Katana A: Temporal trends in pre-ART patient characteristics and outcomes before the test and treat era in Central Kenya. BMC Infect Dis 2021;21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wekesa P, Kataka J, Owuor K, et al. : Time to HIV testing of sexual contacts identified by HIV-positive index clients in Siaya County, Kenya. PLoS One 2020;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenyan Ministry of Health: Guidelines for Antiretroviral Therapy in Kenya 4th Edition. Nairobi, Kenya: 2011 Re-print. In: (NASCOP) (NASCP, ed). National AIDS/STI Control Program (NASCOP), Nairobi, 2012. [Google Scholar]
- 9. World Health Organizations: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. In: (HIV, ed). World Health Organizations, Geneva, 2016 [Google Scholar]
- 10. Bakoyannis G, Touloumi G: Practical methods for competing risks data: A review. Stat Methods Med Res 2012;21:257–272. [DOI] [PubMed] [Google Scholar]
- 11. Gooley TA, Leisenring W, Crowley J, Storer BE: Estimation of failure probabilities in the presence of competing risks. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 12. Rubin DB: Multiple imputation for non response in surveys. John Wiley & Sons, 1987. [Google Scholar]
- 13. Arrive E, Marquis B, Timwesigye N, et al. : Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr 2008;49:523–531. [DOI] [PubMed] [Google Scholar]
- 14. Adedimeji A, Edmonds A, Hoover D, et al. : Characteristics of HIV-infected children at enrollment into care and at antiretroviral therapy initiation in Central Africa. PLoS One 2017;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goga AE, Singh Y, Singh M, et al. : Enhancing HIV treatment access and outcomes amongst HIV infected children and adolescents in resource limited settings. Matern Child Health J 2017;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fatti G, Bock P, Eley B, Eula M, Grimwood A: Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: An analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr 2011;58:60–67. [DOI] [PubMed] [Google Scholar]
- 17. Nuwagaba-Biribonwoha H, Kilama B, Antelman G, et al. : Reviewing progress: 7 year trends in characteristics of adults and children enrolled at HIV care and treatment clinics in the United Republic of Tanzania. BMC Public Health 2013;13:1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koye DN, Ayele TA, Zeleke BM: Predictors of mortality among children on Antiretroviral Therapy at a referral hospital, Northwest Ethiopia: A retrospective follow up study. BMC Pediatr 2012;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National AIDS and STI Control Programme (NASCOP), Ministry of Public Health and Sanitation: Guidelines for HIV Testing and Counselling in Kenya. Nairobi, Kenya, 2010. [Google Scholar]
- 20. Ministry of Health: Kenya AIDS Response progress Report, 2016. Nairobi, Kenya, 2016. [Google Scholar]
- 21. Modi S, Cavanaugh JS, Shiraishi RW, et al. : Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with hiv in Western Kenya. PLoS One 2016;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Anne K: Importance of tuberculosis control to address child survival. Lancet 2017;383:1605–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Venturini E, Turkova A, Chiappini E, Galli L, de Martino M, Thorne C: Tuberculosis and HIV co-infection in children. BMC Infect Dis 2014;14:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayalew MB: Mortality and its predictors among HIV infected patients taking antiretroviral treatment in ethiopia: A systematic review. AIDS Res Treat 2017;2017:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chandiwana N, Sawry S, Chersich M, Kachingwe E, Makhathini B, Fairlie L: High loss to follow-up of children on antiretroviral treatment in a primary care HIV clinic in Johannesburg, South Africa. Medicine (Baltimore) 2018;97:e10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ditekemena J, Luhata C, Bonane W, et al. : Antiretroviral treatment program retention among HIV-infected children in the Democratic Republic of Congo. PLoS One 2014;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ekouevi DK, Azondekon A, Dicko F, et al. : 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health 2011;11:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giddy J, Wood R, Prozesky H, Mohapi L, Graber C: Temporal changes in programme outcomes among adult initiating antiretroviral therapy acrosss South Africa 2011;24:2263–2270. [DOI] [PMC free article] [PubMed]
- 29. Boulle A, Van Cutsem G, Hilderbrand K, et al. : Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. Aids 2010;24:563–572. [DOI] [PubMed] [Google Scholar]
- 30. Davies MA, Keiser O, Technau K, et al. : Outcomes of the South African national antiretroviral treatment programme for children: The IeDEA southern Africa collaboration. South African Med J 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmed I, Lemma S: Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: A systematic review and meta-analysis. BMC Public Health 2019;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGuire M, Munyenyembe T, Szumilin E, et al. : Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health 2010;15:55–62. [DOI] [PubMed] [Google Scholar]
- 33. Sauvageot D, Schaefer M, Olson D, Pujades-Rodriguez M, O'Brien DP: Antiretroviral therapy outcomes in resource-limited settings for HIV-infected children <5 years of age. Pediatrics 2010;125:e1039–e1047. [DOI] [PubMed] [Google Scholar]
- 34. Fetzer BC, Hosseinipour MC, Kamthuzi P, et al. : Predictors for mortality and loss to follow-up among children receiving anti-retroviral therapy in Lilongwe, Malawi. Trop Med Int Health 2009;14:862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ben-Farhat J, Schramm B, Nicolay N, et al. : Mortality and clinical outcomes in children treated with antiretroviral therapy in four African vertical programmes during the first decade of paediatric HIV care, 2001–2010. Trop Med Int Health 2017;22:340–350. [DOI] [PubMed] [Google Scholar]
- 36. Collins IJ, Jourdain G, Hansudewechakul R, et al. : Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: A 5-year observational cohort study. Clin Infect Dis 2010;51:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Auld AF, Tuho MZ, Ekra KA, et al. : Temporal trends in mortality and loss to follow-up among children enrolled in côte d'ivoire's national antiretroviral therapy program. Pediatr Infect Dis J 2014;33:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kranzer K, Bradley J, Musaazi J, et al. : Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc 2017;20:21737. [DOI] [PMC free article] [PubMed] [Google Scholar]