Granulocyte colony-stimulating factor (G-CSF), a protein that stimulates the growth of new blood cells, was isolated from human cells by Malcolm Moore and Karl Welte in 1984 [1]. It formed the basis for filgrastim, one of the most important drugs in cancer therapy [2]. In the hierarchical development of hematopoiesis, G-CSF predominantly stimulates the myeloid cell series from committed progenitor cells to mature neutrophil granulocytes. There are several important effects of G-CSF: maintaining the viability of progenitor cells and their mature progeny, blocking apoptosis, stimulating cell division, determining lineage affiliation (granulocytes or macrophage monocytes), influencing the maturation process, and stimulating phagocytosis activity [3]. Natural G-CSF is O-glycosylated with a molecular weight of 19,000 Da. Derived from the amino acid sequence, molecular cloning of the cDNA and expression in Escherichia coli was successful [4]. G-CSF acts through a homodimeric G-CSF receptor (GCSFR) expressed on myeloid cells from myeloblasts to mature neutrophils. It transduces the signals that trigger the effects described [5]. The GCSFR occurs at a relatively low density of 700–1500 per cell on the cell surface and has a high affinity for G-CSF. A low occupancy at receptors is sufficient to obtain the maximal biological response.
Many body cells can produce G-CSF after appropriate stimulation; cells from the monocyte and macrophage series are the most significant G-CSF source; however, normal cells of mesodermal origin can also produce G-CSF. In healthy individuals, G-CSF levels are not measurable or are below 100 pg/ml [5]. Under stress such as infection or high-dose chemotherapy, levels can increase to over 2000 pg/ml. There is an inverse correlation between neutrophil levels and G-CSF levels. Physiologically produced G-CSF acts as a neutrophil mobilizer at the relatively late stage of acute inflammation. In the presence of bacterial or fungal infection and normal bone marrow function, granulocytes from the bone marrow reserve are rapidly mobilized into the blood. However, CXCR2 ligands mobilize 10 × more neutrophils within 30 min than endogenous G-CSF, which requires hours to days to do so [6].
G-CSF and granulocyte macrophage colony-stimulating factor (GM-CSF) have pleiotropic and overlapping effects. It required gene knockout studies in mice in order to establish that each CSF did, in fact, have actions that were exclusive to that CSF. G-CSF is clearly responsible for the formation of 75% of granulocytes under basal conditions [7]. GM-CSF, on the other hand, did not appear to affect the number of mature granulocytes, but instead was essential for the functional activity of macrophages, particularly those in the lung [8].
GM-CSF also stimulated eosinophil colony formation and, at high concentrations, megakaryocyte colony formation.
CSF has the ability to stimulate the functional activity of mature cells. Thus, in mature neutrophils, GM-CSF can induce chemotaxis, promote oxidative metabolism, enhance antibody-dependent phagocytosis and killing of microorganisms, and produce a variety of regulatory proteins. Similar effects have also been demonstrated in eosinophils and monocytes. These effects were found both in vitro and in vivo. A similar spectrum of effects has been documented for G-CSF and other cytokines [9]. For the clinical use of GM-CSF, sargramostim and molgramostim have been developed and approved.
G-CSF and clinical application
In chemotherapy-induced neutropenia, the bone marrow reserve of granulocytes is decreased. Exogenous G-CSF can accelerate proliferation and differentiation of progenitor cells, making neutrophil replenishment more rapidly available and thus shortening the neutropenia phase.
The non-glycosylated filgrastim was first produced by Amgen and later as a biosimilar by other companies. The glycosylated version, lenograstim, produced in CHO cells, was approved in Europe in 1993. They are eliminated in vivo by renal excretion, degradation via G-CSF receptors, or serum elastase. The serum half-life of filgrastim is 3.5 h, with a concentration of 10 ng/ml over 8–16 h [10].
Long-acting G-CSF
To prolong the half-life after subcutaneous injection to 30–53 h in patients after chemotherapy, filgrastim was combined with polyethylene glycol (pegfilgrastim, lipegfilgrastim) to prevent renal excretion [11, 12]. This made it possible to apply filgrastim after chemotherapy only once instead of daily until neutrophil regeneration.
Mobilization of hematopoietic stem cells
Hematopoietic progenitor and stem cells characterized by the CD34 + cells also express the CXCR4 receptor, whose ligand chemokine CXCL12 is also called stromal-derived growth factor (SDF-1). G-CSF suppresses the CXCL12 -CXCR4 axis [13], allowing hematopoietic progenitor cells to be mobilized into the blood in high numbers, where they can be collected with leukapheresis and used for allogeneic and autologous stem cell transplantation [14–18]. Details of stem cell mobilization are discussed elsewhere [19, 20].
Transplantation of hematopoietic stem and progenitor cells
After autologous stem cell transplantation, G-CSF significantly accelerates regeneration of granulopoiesis, making it the standard of care [17, 21]. To prevent infections, G-CSF prophylaxis is clearly preferable to antibiotic prophylaxis because of increased bacterial resistance rates and is recommended by the ASCO guidelines [22, 23]. After allogeneic stem cell transplantation, G-CSF accelerates neutrophil regeneration. Some studies have described increased rates of acute and chronic graft versus host disease. However, meta-analyses showed no significant increase in the rate of GvHD, and a 1-day benefit in neutrophil regeneration. Survival and length of hospital stay were not affected [24].
Neutropenia after chemotherapy
Chemotherapy-induced neutropenia can cause complications such as febrile neutropenia or other infections, which can also be life-threatening. Strategies to prevent these complications, such as cycle postponement or dose reduction, may worsen the efficacy of chemotherapy. Shortening neutropenia duration and reducing neutropenia depth were therefore the first applications of G-CSF.
G-CSF after chemotherapy significantly decreases neutropenia and febrile neutropenia by stimulating granulopoiesis. The initial studies after MVAC chemotherapy for patients with urothelial carcinoma significantly reduced days below 1000 neutrophils/µl, reduced days of antibiotic therapy, febrile neutropenia (FN), and increased the number of patients on scheduled chemotherapy [25]. A subsequent prospective randomized trial in patients with small cell lung cancer found reductions in febrile neutropenia, infections with positive blood culture, incidence, duration and severity of grade IV neutropenia (neutrophils < 500 µl), antibiotic therapy duration, and days with hospitalization [26].
Numerous phase III studies confirmed the reduction in febrile neutropenia. In 2013, Lyman published a meta-analysis of 59 randomized trials of chemotherapy with or without G-CSF prophylaxis involving nearly 25,000 patients with solid tumors or lymphomas. The relative risk (RR) with G-CSF support for all-cause mortality was 0.93 (p < 0.001). The reduced risk of death was seen in all cancer types and dose and schedule categories. Comparison of different forms of G-CSF found filgrastim and lenograstim to be similarly effective in reducing FN, while pegylated filgrastim “pegfilgrastim” reduced the risk of febrile neutropenia to a greater extent than filgrastim or lenograstim [27, 28]. An updated meta-analysis published in 2018 showed that survival was significantly better in patients who received primary G-CSF support compared to patients without primary G-CSF support (mortality RR 0.92; 95% CI 0.90–0.95; absolute risk reduction by 3.3%; 95% CI 4.2–2.4; p < 0.0001) [29]. The higher rate of secondary malignancies under G-CSF prophylaxis and higher-dose intensity of chemotherapy published there is outweighed by the higher survival rate. It is unclear whether the increased rate of secondary malignancies was caused by increased chemotherapy dose or by G-CSF. Although some reduction in the risk of all-cause mortality was noted with the addition of G-CSF to the chemotherapy regimen, improved disease control is the most likely explanation for the improved survival reported in this meta-analysis. Primary G-CSF support may enable the administration of dose-dense, dose escalation, and intensified chemotherapy regimens, which may lead to improved disease control and a resulting reduction in mortality compared with no G-CSF support [29, 30].
G-CSF may improve neutropenia complications and prognosis of patients receiving chemotherapy. Therefore, the question was in which chemotherapy G-CSF should be given. Over the years, it crystallized that patients with a risk of febrile neutropenia of 20% or more across all cycles of therapy benefit significantly from G-CSF prophylaxis. These tend to be more intensive chemotherapy protocols.
It should be noted that the additional use of monoclonal antibodies in chemotherapy regimens may increase the risk of FN. Particularly critical is rituximab, an anti-CD20 monoclonal antibody used primarily in the treatment of CD20 + hematologic malignancies, which is known to cause severe neutropenia independently [31].
However, additional patient-specific risk factors must also be considered, such that if a treatment protocol has a 10–20% risk of FN, the individual patient’s risk may increase to > 20% and G-CSF prophylaxis is indicated.
Based on a multivariable risk model, the NCCN uses the following risk factors [32] and recommends G-CSF prophylaxis if one or more of these are present [31]: prior chemotherapy or radiation therapy, persistent neutropenia, bone marrow involvement from tumor, recent surgery and/or open wounds, liver dysfunction (bilirubin > 2.0), renal dysfunction (creatinine clearance < 50), and age > 65 years at full chemotherapy dose intensity.
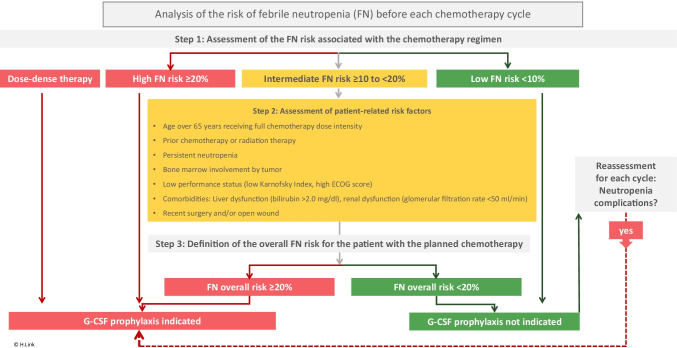
Guidelines on supportive therapy make appropriate recommendations [23, 31, 33] (Fig. 1).
Fig. 1.
Synopsis of international guidelines on the prophylactic use of G-CSF; additional risk factors mentioned by ASCO guidelines: advanced disease, poor performance status or poor nutritional status, cardiovascular disease multiple comorbid conditions HIV infection [23]. Secondary prophylaxis is indicated if neutropenia complications occurred without G-CSF or neutropenia-related cycle delays were necessary. Assessment is required after each cycle of chemotherapy.
Problematically, often the FN risk of a particular chemotherapy is not known to users. These data on the risk of febrile neutropenia can be extracted from the original publications, obtained from some guidelines, or from protocol databases such as NCCN (www.nccn.org), Oncopti (www.oncopti.com), and others. Guideline adherence has been investigated in several studies that showed inadequate G-CSF prophylaxis when chemotherapy FN risk was 10–20% and patients with risk factors [34, 35]. Thus, educational interventions are needed to increase guideline implementation of G-CSF prophylaxis.
Neutropenia complications can lead to chemotherapy dose reduction or cycle deferral in subsequent cycles, thereby decreasing planned dose intensity and significantly worsening patient survival probability [36, 37]. G-CSF enables dose-dense therapy by shortening chemotherapy intervals, thereby contributing to improved prognosis in some diseases [36, 38, 39].
G-CSF therefore offers the opportunity to maintain dose intensity and achieve the primary goal of tumor therapy [30, 31, 33, 40].
G-CSF prophylaxis without indication is not useful as it only adds unnecessary cost and potential side effects; ASCO states in this regard [41]:
“Don’t use prophylactic white cell stimulating factors unless the expected risk of febrile neutropenia associated with a chemotherapy agent or regimen is equal to or greater than 20%.
ASCO guidelines recommend using prophylactic white cell-stimulating factors when the risk of febrile neutropenia, secondary to a chemotherapy regimen, is equal to or greater than approximately 20%, and equally effective, alternative chemotherapy options that do not require white cell-stimulating factors are unavailable.
Exceptions should be made when using chemotherapy regimens that are typically associated with lower risk of febrile neutropenia, if it is determined that the patient is at high risk for this complication (due to age, comorbidities, or disease characteristics).”
Acute myeloid leukemia
Since the malignant cells in acute myeloid leukemia (AML) can be stimulated via the G-CSF receptor (colony-stimulating factor 3 receptor (CSF3R)), physicians were initially skeptical with G-CSF therapy based on in vitro data. On the other hand, infections and very long and pronounced neutropenias are major problems of AML chemotherapy.
G-CSF is approved for patients with acute myeloid leukemia (AML) and is indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment. Neutrophils regenerate significantly faster with G-CSF after both induction and consolidation therapy [42]. No effect was seen in infection rates, while less antibiotic use was noted after consolidation [43–47]. Patient survival was longer after consolidation therapy in some studies with G-CSF [42], and no negative effect was found in any study. In some studies, hospitalization time was significantly shorter with G-CSF.
We are keen to see whether additional therapy with expanded allogeneic myeloid progenitor cells after AML chemotherapy can further shorten neutropenia duration and, in particular, reduce infection rates, as shown in initial clinical trials [48].
G-CSF may therefore be beneficial in AML therapy. A common counterargument is the lack of reduction in febrile neutropenia and infections and the positive effect on survival demonstrated in only a few studies. Major arguments in favor of G-CSF prophylaxis are the reduction of neutropenia duration and hospitalization time and the reduced antibiotic consumption.
G-CSF stimulation of leukemic cells aims to sensitize them to cytotoxic chemotherapy and thereby achieve a better therapeutic outcome. A meta-analysis showed significantly better survival and progression-free survival [49]. A typical application is the commonly used FLAG-IDA protocol [50].
In summary, G-CSF has a firm place in AML therapy as both a supportive and disease-specific drug.
Myelodysplastic syndrome
In myelodysplastic syndrome (MDS), hematopoietic cytokines, including G-CSF, should be considered for refractory symptomatic cytopenias, such as repeated or resistant bacterial infections. G-CSF may additionally be used in the anemia therapy of low-risk MDS with erythropoiesis-stimulating agents. An increased rate of leukemia progression has not been described. Further details can be found, for example, in the NCCN MDS Guidelines.
Aplastic anemia
Especially severe and very severe aplastic anemia have a high mortality and are therefore an emergency. Therapy with G-CSF, even in combination with immunosuppressive therapy, has no effect on long-term outcomes [51]. A higher rate of MDS, AML, isolated cytogenetic abnormalities, solid cancer, clinical paroxysmal nocturnal hemoglobinuria, aseptic osteonecrosis, chronic kidney disease, and relapse was not demonstrated [51].
In combination with antithymocyte globulin, cyclosporine, and eltrombopag, G-CSF may be used as clinically indicated but is not a standard component of the treatment protocol [52]. G-CSF as monotherapy is not effective and therefore not indicated [53].
Treatment of persistent neutropenia in advanced HIV infection
In Europe, filgrastim is approved by the EMA for the treatment of persistent neutropenia (ANC less than or equal to 1.0 × 109/l) in patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to manage neutropenia are inappropriate. The recommended starting dose of filgrastim is 0.1 MIU (1 μg)/kg/day with titration up to a maximum of 0.4 MIU (4 μg)/kg/day until a normal neutrophil count is reached and can be maintained (ANC > 2.0 × 109/l). Further details can be found in the respective product information.
Radiation-induced myelosuppression following a radiological/nuclear incident (H-ARS)
As demonstrated in animal experiments, both filgrastim and pegfilgrastim can improve survival after radiation-induced myelosuppression [54–56]. Both agents are FDA approved for this purpose and can be used to treat adult and pediatric patients acutely exposed to myelosuppressive radiation doses.
G-CSF and Covid-19
The risk of Covid-19 is significantly increased for patients with malignancies and neutropenia [57]. Therefore, ASCO [58], the National Comprehensive Cancer Network (NCCN) [59], and ESMO [60] recommend the following: It may be reasonable for patients at risk for neutropenic fever to be prescribed growth factor for treatment regimens at a lower level of expected risk (e.g., > 10% risk) or < 10% expected risk in patients whose comorbidities or age place them at intrinsically higher risk for FN due to poor bone marrow reserve [59].
Secondary G-CSF prophylaxis should be given whenever complications have occurred without G-CSF in neutropenia [31].
Patients with active Covid-19 and concurrent G-CSF therapy may worsen clinically [61, 62]. Because lung injury in Covid-19 results from a hyperinflammatory immune system response in which neutrophils are also involved, G-CSF should be avoided in this situation [63]. However, this issue should not lead to the omission of routine prophylaxis after chemotherapy with G-CSF in Covid-19-negative patients; rather, the goal here is to avoid neutropenia complications with G-CSF [59, 64].
Timing of application and duration of therapy
With G-CSF preparations to be given daily, it is often not considered that administration must continue until neutrophils have returned to normal, as has been done in most studies. It has been well known for many years that with daily G-CSF prophylaxis, the risk of hospitalization for neutropenia or infection declines with each additional day of filgrastim prophylaxis among patients with NHL and breast cancer and, possibly, among those with lung cancer. Patients with at least 7 days of G-CSF prophylaxis had a significantly lower risk of hospitalization than patients with fewer treatment days [65].
In everyday clinical practice, this daily therapy is usually administered for a much shorter period of time. Accordingly, clinical efficacy is not assured [66]. G-CSF should be applied the day after chemotherapy but after no more than 3–4 days, and until the nadir is crossed and the proximity of the neutrophil normal range is reached [31]. G-CSF should be applied the day after chemotherapy but after no more than 3–4 days, and until the nadir is crossed and the proximity of the neutrophil normal range is reached [31]. The laboratory control of the neutrophil count should be adapted to the therapy situation; a daily control would not be necessary.
The problem of G-CSF prophylaxis after chemotherapy being too short can be solved by using long-acting G-CSF, which only needs to be injected once. These pegylated filgrastim preparations should be administered no earlier than 24 h but no later than 3 days after the end of cytostatic administration. It is suggestive to give long-acting G-CSF on the last day of chemotherapy to reduce the burden on the patient and the team. However, the rate of neutropenia and febrile neutropenia is then higher [67]. Therefore, it is not recommended to give pegfilgrastim on the same day of chemotherapy or 4–5 days after [31].
Patients can inject pegfilgrastim themselves at home or, if that is not possible, use an on body injector [68, 69]. This is filled with pegfilgrastim, applied to the patient with a subcutaneous needle, and taped to the skin. Approximately 27 h after application, the device automatically delivers the pegfilgrastim dose over 45 min. Thus, the patient can also be cared for by the treatment team without having to come back for an extra pegfilgrastim injection.
Biosimilars of filgrastim and pegfilgrastim
Several biosimilars of the originator drugs filgrastim and pegfilgrastim are now approved in different regions of the world [70–72], several of them according to the strict requirements of the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). There are no differences in clinical efficacy and side effects compared to the original filgrastim or pegfilgrastim preparation [73]. Approved biosimilars of filgrastim can also be used for stem cell mobilization in healthy individuals [20].
Elderly patients
The median age of most patients with malignant disease is approximately 65 years, meaning that half of the patients have at least one relevant risk factor of febrile neutropenia. This patient group benefits significantly from G-CSF prophylaxis, with a significant 5.7% reduction in the absolute risk of fatal complications after chemotherapy [27]. Dose intensity can be better maintained [29, 74].
Side effects of G-CSF prophylaxis
Incidence rates of adverse events in each category compare closely between filgrastim- and pegfilgrastim-treated patients. See the full package inserts for specific product information. In terms of relevant pegfilgrastim side effects, mild to moderate bone pain and joint and muscle pain are reported in about 10–30% of patients, which are mostly well treatable with non-steroidal anti-inflammatory drugs (NSAIDs) 5 to 7 days after GCSF administration [75]. Alternatively, prophylaxis with the antihistamine loratadine reduces bone pain and with fewer side effects than the equally effective NSAID naproxen [76]. Other side effects are described, for example, by the EMA in the summary of product characteristics of pegfilgrastim (Neulasta): Hypersensitivity reactions, including rash, urticaria, angioedema, dyspnea, erythema, flushing, and hypotension, occurred during initial or subsequent treatment with pegfilgrastim (occasionally [≥ 1/1000, < 1/100]). Serious allergic reactions, including anaphylaxis, may occur in patients receiving pegfilgrastim (occasional). Capillary leak syndrome, which can be life-threatening if not treated promptly, has been reported occasionally (≥ 1/1000, < 1/100) in cancer patients undergoing chemotherapy followed by granulocyte colony-stimulating factor treatment. Splenomegaly, generally asymptomatic, occurs occasionally. Splenic ruptures, fatal in some cases, are occasionally reported after treatment with G-CSF.
Therefore in case of pain in the left upper abdomen and/or shoulder tip, the patients should be evaluated for an enlarged spleen or splenic rupture. Because severe sickle cell crisis can be induced by G-CSF, only physicians experienced in the treatment of sickle cell disease should prescribe G-CSF in these patients after careful consideration and risk assessment.
Not unchallenged is the alleged increase in pulmonary toxicity when G-CSF is used after bleomycin-containing chemotherapy [31, 77]. There are several publications that see no increased risk of interstitial pulmonary fibrosis when patients receive bleomycin and G-CSF therapy [78, 79]. On the other hand, in patients with Hodgkin lymphoma and ABVD chemotherapy, a correlation is described in patients older than 45 years between G-CSF therapy and bleomycin-induced pulmonary toxicity [80].
Neutropenic patients with pulmonary infections may develop acute respiratory distress syndrome (ARDS) during the neutrophil regeneration phase, which may worsen with G-CSF therapy (see also the “G-CSF and Covid-19” section).
Quality of life and social value
By preventing complications, quality of life can be improved with G-CSF prophylaxis [81, 82]. The total social value of G-CSF therapy was analyzed in 314,440 patients treated in the USA in 2014. Compared with what they would have experienced without G-CSF, patients receiving G-CSF prophylaxis were less likely to be hospitalized or die of FN, to have their relative dose of chemotherapy reduced, to receive antibiotics, to be unable to work, or to have reduced health-related quality of life. The estimated total societal value (TSV) of G-CSFs in 2014 was $8.5 billion. Industry profits related to G-CSFs were estimated at $1.3 billion, or about 15% of TSV [83].
G-CSF and radiation therapy
Previously, G-CSF prophylaxis was discouraged during combined radiation chemotherapy. An older randomized study showed a significant increase in grade 3/4 thrombocytopenia and an excess of pulmonary toxic deaths in the use of GM-CSF during concomitant mediastinal chemoradiotherapy in conventional two-dimensional technique [84]. In modern three-dimensional conformal radiotherapy, G-CSF administration concurrently with chemoradiotherapy does not result in the increased risk of pulmonary toxicity, but increases the risk of thrombocytopenia [85].
In an unplanned analysis, one study showed that rates of febrile neutropenia were 60% lower with secondary G-CSF prophylaxis (10% versus 22%) than without prophylaxis. It is also interesting to note a higher-dose intensity of cisplatin with G-CSF prophylaxis. It is possible that the higher-dose intensity increases the rate of thrombocytopenia and anemia in patients with already reduced bone marrow reserve [86]. G-CSF prophylaxis for radiation chemotherapy could therefore be justified with these new findings [87].
Therapeutic use of G-CSF
Treatment of febrile patients with neutropenia
G-CSF should not be routinely used in addition to antibiotics in FN. However, G-CSF is indicated in patients without G-CSF prophylaxis who do not respond to adequate antibiotic treatment or develop a life-threatening infection [33]. According to ASCO and NCCN recommendations [88], G-CSF should also be administered to patients with a high risk of infection-associated complications or unfavorable prognostic factors—such as sepsis syndrome, age greater than 65 years, absolute neutrophil count < 100/µl, neutropenia expected to last more than 10 days, pneumonia or other clinically documented infections, invasive fungal infection, hospitalization at the time of fever, and a previous episode of febrile neutropenia [23, 31].
If fever has occurred while on daily short-acting G-CSF, then therapy should be continued until neutrophil recovery. If pegfilgrastim was previously used, then no re-administration is required unless neutropenia has already been prolonged (beyond 12–14 days), as pegylated G-CSF is unlikely to act beyond this period. [31].
Neutropenia in critically ill cancer patients and in documented infection
In critically ill cancer patients, neutropenia is known to be a risk factor for increased mortality. However, when neutropenia is treated with G-CSF, it is no longer a risk factor, according to a meta-analysis involving 7515 patients [89].
With documented infection in neutropenia, interventional G-CSF has no effect on mortality but shortens the duration of neutropenia, antibiotic therapy, and hospitalization [90, 91].
Special situations of treatment-associated neutropenia
CDK-4/6 inhibitors
CDK-4/6 inhibitors such as palbociclib lead to neutropenia, which is not caused by apoptotic cell death as in chemotherapy, but by an arrest of the cell cycle whereby the proliferation of hematopoetic stem cells decreases and resumed proliferation following CDK4/6 dose reduction or interruption [92]. Accordingly, G-CSF prophylaxis is not required. In case of neutropenia below 1000/µl, therapy should be interrupted. Depending on the substance and the extent of myelotoxicity, there are recommendations for the continuation of therapy from the approvals of the substances [93].
Short chemotherapy intervals
There are few studies on neutropenia prophylaxis, with shorter chemotherapy intervals than 14 days, so pragmatic short-acting G-CSF is often given here on the days without chemotherapy.
Example of such protocols is R-MPV induction chemotherapy in CNS lymphoma with rituximab, methotrexate, procarbazine, and vincristine, in which filgrastim is given for 3–5 days after each cycle [94], weekly chemotherapy in breast carcinoma [95–97]. This is to avoid neutropenia-related treatment deferrals or dose reductions, thus maintaining the planned dose intensity of therapy [36].
If the intensity and myelosuppressive potential of weekly chemotherapy per dose is relatively low, then the respective G-CSF neutropenia prophylaxis with a maximum of 6 days after each administration is also sufficient. Although there are no prospective controlled studies on this question, this variant of short-term prophylaxis is not excluded by the approval.
Continuous antineoplastic chemotherapy
To date, there are no prospective studies and no guideline recommendation on the issue of G-CSF prophylaxis during continuous antineoplastic therapy. Few studies are available for therapy protocols with oral agents over multiple days, nor for tyrosine kinase inhibitors and other newer agents [98, 99].
For example, in multiple myeloma and oral lenalidomide treatment with intermittent G-CSF 300 μg subcutaneously 2–3 times weekly during weeks 3–4 of a 28-day cycle [98], this allowed better adherence to the lenalidomide dose in this retrospective study, resulting in better response and outcome.
Venetoclax therapy for chronic lymphocytic leukemia (CLL) results in neutropenia; to maintain the target dose, neutrophil levels below 1000/µl should be avoided. When ventoclax was combined with obinutuzumab, 43.5% of CLL patients received growth factor support [100]. In practice, intermittent administration of pegylated filgrastim has proven effective during venetoclax treatment; intermittent administration of filgrastim is also useful, but frequent blood count checks must then be performed [99].
Neutropenia in chimeric antigen receptor T cell therapy
Chimeric antigen receptor (CAR) T cell therapy has opened a new era in immunotherapy of hematologic diseases [101]. Besides cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) and hypogammaglobulinemia have been well-described with CAR T cell therapy. In addition, cytopenias of all cell series occur, with neutropenias of severity grades 3–4 in 60 to 100% of mostly very heavily pretreated patients [102, 103].
After therapy with chimeric antigen receptor T cells, pancytopenia occurs in many patients, with 95% having grade 3–4 neutropenia, and 62% of patients required G-CSF therapy [102]. Neutropenia can be biphasic and persist for a very long time [104]. Concerns exist with G-CSF use because of the potential for exacerbation of ICANS or CRS. However, there are studies showing that G-CSF does not increase the toxicity of CAR T cell therapy [105].
The 2021 guidelines of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA) recommend the following G-CSF prophylaxis [106]:
G-CSF to shorten duration of neutropenia can be used from day + 14 or after resolution of CRS or ICANS; can consider starting earlier, e.g., day 5, if patient is at high risk of infection, e.g., ALL, post-allogeneic hematopoietic stem cell transplantation, high-dose steroids. For persistent neutropenia (< 0.5 × 109/l) following day + 28, consider G-CSF. The guideline notes that contrary to previous statements, G-CSF did not show an increase in CRS or ICANS on day 5 after CAR-T infusion, suggesting that earlier use may be safe and shorten the duration of neutropenia.
Neutropenia in therapy with immune checkpoint inhibitors
Many malignancies are treated with immune checkpoint inhibitors, with a low rate of neutropenia. Increasingly, however, this therapy is combined with conventional chemotherapy, with a significantly increased risk of infection, especially in neutropenia. G-CSF prophylaxis is therefore indicated with the combination especially in patients with risk factors of infection during neutropenia, such as shown in Fig. 1 [107].
Conclusion
Prophylaxis of neutropenia with G-CSF after chemotherapy is an essential supportive therapy that reduces neutropenia complications and improves patient survival and quality of life.
Footnotes
The author is the previous head of Hematology, Oncology, Westpfalz‑Klinikum.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci. 1985;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): The first 10 years. Blood. 1996;88(6):1907–1929. doi: 10.1182/blood.V88.6.1907.bloodjournal8861907. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf D. The colony-stimulating factors and cancer. Cancer Immunol Res. 2013;1(6):351–356. doi: 10.1158/2326-6066.cir-13-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78(11):2791–2808. doi: 10.1182/blood.v78.11.2791.2791. [DOI] [PubMed] [Google Scholar]
- 6.Bajrami B, Zhu H, Kwak HJ, Mondal S, Hou Q, Geng G, et al. G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J Exp Med. 2016;213(10):1999–2018. doi: 10.1084/jem.20160393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. doi: 10.1182/blood.V84.6.1737.1737. [DOI] [PubMed] [Google Scholar]
- 8.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10(6):425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratiograstim Epar Product Information . Filgrastim. London: European Medicines Agency; 2021. [Google Scholar]
- 11.Molineux G. Pegfilgrastim: using pegylation technology to improve neutropenia support in cancer patients. Anti-Cancer Drugs. 2003;14(4):259–264. doi: 10.1097/00001813-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Buchner A, Lammerich A, Abdolzade-Bavil A, Muller U, Bias P. Lipegfilgrastim: pharmacodynamics and pharmacokinetics for body-weight-adjusted and 6 mg fixed doses in two randomized studies in healthy volunteers. Curr Med Res Opin. 2014;30(12):2523–2533. doi: 10.1185/03007995.2014.962131. [DOI] [PubMed] [Google Scholar]
- 13.Lévesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Investig. 2003;111(2):187–196. doi: 10.1172/jci15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1(8596):1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 15.Lapidot T, Petit I. Current understanding of stem cell mobilization. Exp Hematol. 2002;30(9):973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 16.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/nejmoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link H, Arseniev L, Bähre O, Kadar J, Diedrich H, Poliwoda H (1996) Transplantation of allogeneic CD34+ blood cells. Blood 87(11):4903–4909 [PubMed]
- 18.Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–357. doi: 10.1016/S0140-6736(96)90536-X. [DOI] [PubMed] [Google Scholar]
- 19.Pahnke S, Egeland T, Halter J, Hägglund H, Shaw BE, Woolfrey AE, et al. Current use of biosimilar G-CSF for haematopoietic stem cell mobilisation. Bone Marrow Transplant. 2019;54(6):858–866. doi: 10.1038/s41409-018-0350-y. [DOI] [PubMed] [Google Scholar]
- 20.Hübel K. Mobilization and Collection of HSC. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. Cham (CH): Springer; 2019. pp. 117–122. [PubMed] [Google Scholar]
- 21.Trivedi M, Martinez S, Corringham S, Medley K, Ball ED. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant. 2009;43(12):895–908. doi: 10.1038/bmt.2009.75. [DOI] [PubMed] [Google Scholar]
- 22.Klein E-M, Sauer S, Klein S, Tichy D, Benner A, Bertsch U, et al. Antibiotic prophylaxis or granulocyte-colony stimulating factor support in multiple myeloma patients undergoing autologous stem cell transplantation. Cancers. 2021;13(14):3439. doi: 10.3390/cancers13143439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Meena JP, Haldar P, Tanwar P, Seth R. Impact of G-CSF administration post-allogeneic hematopoietic stem-cell transplantation on outcomes: a systematic review and meta-analysis. Am J Blood Res. 2021;11(5):544–563. [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, Grous J, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988;318:1414–1422. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- 26.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 27.Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24(10):2475–2484. doi: 10.1093/annonc/mdt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeil AM, Allcott K, Pettengell R, von Minckwitz G, Schwenkglenks M, Szabo Z. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer. 2015;23(2):525–545. doi: 10.1007/s00520-014-2457-z. [DOI] [PubMed] [Google Scholar]
- 29.Lyman GH, Yau L, Nakov R, Krendyukov A. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol. 2018;29(9):1903–1910. doi: 10.1093/annonc/mdy311. [DOI] [PubMed] [Google Scholar]
- 30.Lyman GH, Dale DC, Wolff DA, Culakova E, Poniewierski MS, Kuderer NM, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28(17):2914–2924. doi: 10.1200/jco.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths EA (2021) NCCN Guidelines Version 3.2021, Hematopoietic growth factors. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)
- 32.Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199. doi: 10.1016/j.critrevonc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Aapro MS, Bohlius J, Cameron DA, Dal LL, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Link H, Kerkmann M, Holtmann L, Ortner P. Working Groups Supportive C, Medical Oncology within the German Cancer S. G-CSF guideline adherence in Germany, an update with a retrospective and representative sample survey. Support Care Cancer. 2019;27(4):1459–1469. doi: 10.1007/s00520-018-4481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Link H, Nietsch J, Kerkmann M, Ortner P. Supportive Care Group of the German Cancer S. Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy-a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367–376. doi: 10.1007/s00520-015-2779-5. [DOI] [PubMed] [Google Scholar]
- 36.Nielson CM, Bylsma LC, Fryzek JP, Saad HA, Crawford J. Relative dose intensity of chemotherapy and survival in patients with advanced stage solid tumor cancer: a systematic review and meta-analysis. The Oncologist. 2021;26(9):e1609–e1e18. doi: 10.1002/onco.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol. 2008;87(5):429–430. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray R, Bradley R, Braybrooke J, Liu Z, Peto R, Davies L, et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. The Lancet. 2019;393(10179):1440–1452. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfister C, Gravis G, Flechon A, Chevreau CM, Mahammedi H, Laguerre B, et al. 652O Dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for patients with muscle-invasive bladder cancer (MIBC): Results of the GETUG/AFU VESPER V05 phase III trial. Ann Oncol. 2021;32:S678. doi: 10.1016/j.annonc.2021.08.048. [DOI] [PubMed] [Google Scholar]
- 40.Lyman GH, Yau L, Nakov R, Krendyukov A. a systematic literature review of overall survival and delivered dose intensity in cancer patients receiving chemotherapy and G-CSF in randomized control trials. Blood. 2017;130(Suppl 1):3424. [Google Scholar]
- 41.ASCO's 2021 top five list in oncology (2021). https://www.asco.org/news-initiatives/current-initiatives/cancer-care-initiatives/value-cancer-care/choosing-wisely. Accessed 20.12.2021
- 42.Dale DC, Crawford J, Klippel Z, Reiner M, Osslund T, Fan E, et al. A systematic literature review of the efficacy, effectiveness, and safety of filgrastim. Support Care Cancer. 2018;26(1):7–20. doi: 10.1007/s00520-017-3854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harousseau JL, Witz B, Lioure B, Hunault-Berger M, Desablens B, Delain M, et al. Granulocyte colony-stimulating factor after intensive consolidation chemotherapy in acute myeloid leukemia: results of a randomized trial of the Groupe Ouest-Est Leucemies Aigues Myeloblastiques. J Clin Oncol. 2000;18(4):780–787. doi: 10.1200/JCO.2000.18.4.780. [DOI] [PubMed] [Google Scholar]
- 44.Link H, Wandt H, Schönrock-Nabulsi P, Ehninger G, Franke A, Gäckle R, et al. G-CSF (Lenograstim) after chemotherapy for acute myeloid leukemia: A placebo controlled trial. Blood. 1996;88(10, Suppl. 1):666a. [Google Scholar]
- 45.Ohno R, Tomonaga M, Kobayashi T, Kanamaru A, Shirakawa S, Masaoka T, et al. Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia. N Engl J Med. 1990;323(13):871–877. doi: 10.1056/NEJM199009273231304. [DOI] [PubMed] [Google Scholar]
- 46.Dombret H, Chastang C, Fenaux P, Reiffers J, Bordessoule D, Bouabdallah R, et al. A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia. AML Cooperative Study Group. N Engl J Med. 1995;332(25):1678–1683. doi: 10.1056/NEJM199506223322504. [DOI] [PubMed] [Google Scholar]
- 47.Gurion R, Belnik-Plitman Y, Gafter-Gvili A, Paul M, Vidal L, Ben-Bassat I et al (2012) Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database Syst Rev. 10.1002/14651858.CD008238.pub3 [DOI] [PMC free article] [PubMed]
- 48.Desai PM, Brown J, Gill S, Solh MM, Akard LP, Hsu JW, et al. Open-label phase II prospective, randomized, controlled study of romyelocel-L Myeloid progenitor cells to reduce infection during induction chemotherapy for acute myeloid leukemia. J Clin Oncol. 2021;39(29):3261–3272. doi: 10.1200/jco.20.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng X, Lan H, Ruan Y, Li C. Impact on acute myeloid leukemia relapse in granulocyte colony-stimulating factor application: a meta-analysis. Hematology. 2018;23(9):581–589. doi: 10.1080/10245332.2018.1446811. [DOI] [PubMed] [Google Scholar]
- 50.Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360–3368. doi: 10.1200/jco.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 51.Tichelli A, De Latour RP, Passweg J, Knol-Bout C, Socié G, Marsh J, et al. Long-term outcome of a randomized controlled study in patients with newly diagnosed severe aplastic anemia treated with antithymocyte globulin and cyclosporine, with or without granulocyte colony-stimulating factor: a severe aplastic anemia working party. Haematologica. 2020;105(5):1223–1231. doi: 10.3324/haematol.2019.222562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187–207. doi: 10.1111/bjh.13853. [DOI] [PubMed] [Google Scholar]
- 54.DiCarlo AL, Horta ZP, Aldrich JT, Jakubowski AA, Skinner WK, Case CM., Jr Use of growth factors and other cytokines for treatment of injuries during a radiation public health emergency. Radiat Res. 2019;192(1):99–120. doi: 10.1667/rr15363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc) 2015;51(9):537–548. doi: 10.1358/dot.2015.51.9.2386730. [DOI] [PubMed] [Google Scholar]
- 56.Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, et al. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 2015;183(6):643–655. doi: 10.1667/rr13940.1. [DOI] [PubMed] [Google Scholar]
- 57.Yarza R, Bover M, Paredes D, López-López F, Jara-Casas D, Castelo-Loureiro A, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020;135:242–250. doi: 10.1016/j.ejca.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.COVID-19 Patient Care Information - Cancer Treatment & Supportive Care (2021). https://www.asco.org/covid-resources/patient-care-info/cancer-treatment-supportive-care. Accessed 01/31/2022 2021
- 59.Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, Curtin P et al (2020) Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic. J Natl Compr Canc Netw 1-4. 10.6004/jnccn.2020.7610 [DOI] [PMC free article] [PubMed]
- 60.Aapro M, Lyman GH, Bokemeyer C, Rapoport BL, Mathieson N, Koptelova N et al (2021) Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open 6(1). 10.1016/j.esmoop.2020.100038 [DOI] [PMC free article] [PubMed]
- 61.Zhang AW, Morjaria S, Kaltsas A, Hohl TM, Parameswaran R, Patel D et al (2021) The Effect of Neutropenia and Filgrastim (G-CSF) in Cancer Patients With COVID-19 Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America: ciab534. 10.1093/cid/ciab534 [DOI] [PMC free article] [PubMed]
- 62.Sereno M, Jimenez-Gordo AM, Baena-Espinar J, Aguado C, Mielgo X, Pertejo A et al (2021) A multicenter analysis of the outcome of cancer patients with neutropenia and COVID-19 optionally treated with Granulocyte-Colony Stimulating Factor (G-CSF): A comparative analysis. Cancers 13(16). 10.3390/cancers13164205 [DOI] [PMC free article] [PubMed]
- 63.Lasagna A, Muzzana M, Pedrazzoli P. Lights and shadows on the role of rhG-CSF in cancer patients during the COVID-19 pandemic and future perspectives of research. Immunotherapy. 2021;13(17):1369–1372. doi: 10.2217/imt-2021-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooksley T, Font C, Scotte F, Escalante C, Johnson L, Anderson R, et al. Emerging challenges in the evaluation of fever in cancer patients at risk of febrile neutropenia in the era of COVID-19: a MASCC position paper. Support Care Cancer. 2021;29(2):1129–1138. doi: 10.1007/s00520-020-05906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402–407. doi: 10.1345/aph.1G516. [DOI] [PubMed] [Google Scholar]
- 66.Weycker D, Li X, Tzivelekis S, Atwood M, Garcia J, Li Y, et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in us clinical practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer. 2017;25(2):439–447. doi: 10.1007/s00520-016-3421-x. [DOI] [PubMed] [Google Scholar]
- 67.Lyman GH, Allcott K, Garcia J, Stryker S, Li Y, Reiner MT, et al. The effectiveness and safety of same-day versus next-day administration of long-acting granulocyte colony-stimulating factors for the prophylaxis of chemotherapy-induced neutropenia: a systematic review. Support Care Cancer. 2017;25(8):2619–2629. doi: 10.1007/s00520-017-3703-y. [DOI] [PubMed] [Google Scholar]
- 68.Neulasta -Pegfilgrastim. European Medicines Agency; 2007
- 69.NEULASTA® (pegfilgrastim) injection. Silver Spring, MD 2099: European Medicines Agency: U.S. Food and Drug Administration
- 70.Weise M, Kurki P, Wolff-Holz E, Bielsky MC, Schneider CK. Biosimilars: the science of extrapolation. Blood. 2014;124(22):3191–3196. doi: 10.1182/blood-2014-06-583617. [DOI] [PubMed] [Google Scholar]
- 71.Biosimilars of filgrastim (2021). https://www.gabionline.net/biosimilars/general/Biosimilars-of-filgrastim. Accessed 20.12.2021 2021
- 72.Pettengell R, Bias P, Mueller U, Lang N. Clinical safety of tbo-filgrastim, a short-acting human granulocyte colony-stimulating factor. Support Care Cancer. 2016;24(6):2677–2684. doi: 10.1007/s00520-015-3057-2. [DOI] [PubMed] [Google Scholar]
- 73.Ghidini M, Indini A, Nigro O, Polito S, Rijavec E, Petrelli F, et al. Advances in the pharmacological management of neutropenia in solid tumors: the advent of biosimilars. Expert Opin Pharmacother. 2021;22(7):857–865. doi: 10.1080/14656566.2021.1873950. [DOI] [PubMed] [Google Scholar]
- 74.Link H, Illerhaus G, Martens UM, Salar A, Depenbusch R, Kohler A, et al. Efficacy and safety of lipegfilgrastim versus pegfilgrastim in elderly patients with aggressive B cell non-Hodgkin lymphoma (B-NHL): results of the randomized, open-label, non-inferiority AVOID neutropenia study. Support Care Cancer. 2021;29(5):2519–2527. doi: 10.1007/s00520-020-05711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirshner JJ, Heckler CE, Janelsins MC, Dakhil SR, Hopkins JO, Coles C, et al. Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the university of rochester cancer center clinical community oncology program research base. J Clin Oncol. 2012;30(16):1974–1979. doi: 10.1200/JCO.2011.37.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirshner JJ, McDonald MC, Kruter F, Guinigundo AS, Vanni L, Maxwell CL, et al. NOLAN: a randomized, phase 2 study to estimate the effect of prophylactic naproxen or loratadine vs no prophylactic treatment on bone pain in patients with early-stage breast cancer receiving chemotherapy and pegfilgrastim. Support Care Cancer. 2018;26(4):1323–1334. doi: 10.1007/s00520-017-3959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwan EM, Beck S, Amir E, Jewett MA, Sturgeon JF, Anson-Cartwright L et al (2017) Impact of granulocyte-colony stimulating factor on bleomycin-induced pneumonitis in chemotherapy-treated germ cell tumors. Clin genitourinary cancer. 10.1016/j.clgc.2017.08.012 [DOI] [PubMed]
- 78.Laprise-Lachance M, Lemieux P, Grégoire JP. Risk of pulmonary toxicity of bleomycin and filgrastim. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2019;25(7):1638–1644. doi: 10.1177/1078155218804293. [DOI] [PubMed] [Google Scholar]
- 79.Nakagawa R, Iwamoto H, Makino T, Kadomoto S, Yaegashi H, Iijima M et al (2022) Analysis of the safety of pegfilgrastim addition in bleomycin, etoposide, and cisplatin treatment patients with germ cell tumors. Front Oncol:11. 10.3389/fonc.2021.770067 [DOI] [PMC free article] [PubMed]
- 80.Andersen MD, Kamper P, d’Amore A, Clausen M, Bentzen H, d'Amore F. The incidence of bleomycin induced lung toxicity is increased in Hodgkin lymphoma patients over 45 years exposed to granulocyte-colony stimulating growth factor. Leuk Lymphoma. 2019;60(4):927–933. doi: 10.1080/10428194.2018.1515939. [DOI] [PubMed] [Google Scholar]
- 81.Lyman GH, Kuderer NM. Filgrastim in patients with neutropenia: potential effects on quality of life. Drugs. 2002;62(Suppl 1):65–78. doi: 10.2165/00003495-200262001-00005. [DOI] [PubMed] [Google Scholar]
- 82.Lapidari P, Vaz-Luis I, Di Meglio A. Side effects of using granulocyte-colony stimulating factors as prophylaxis of febrile neutropenia in cancer patients: A systematic review. Crit Rev Oncol Hematol. 2021;157:103193. doi: 10.1016/j.critrevonc.2020.103193. [DOI] [PubMed] [Google Scholar]
- 83.Vanderpuye-Orgle J, Sexton Ward A, Huber C, Kamson C, Jena AB. Estimating the social value of G-CSF therapies in the United States. Am J Manag Care. 2016;22(10):e343–e3e9. [PubMed] [Google Scholar]
- 84.Bunn PA, Jr, Crowley J, Kelly K, Hazuka MB, Beasley K, Upchurch C, et al. Chemoradiotherapy with or without granulocyte-macrophage colony- stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol. 1995;13(7):1632–1641. doi: 10.1200/JCO.1995.13.7.1632. [DOI] [PubMed] [Google Scholar]
- 85.Sheikh H, Colaco R, Lorigan P, Blackhall F, Califano R, Ashcroft L, et al. Use of G-CSF during concurrent chemotherapy and thoracic radiotherapy in patients with limited-stage small-cell lung cancer safety data from a phase II trial. Lung Cancer. 2011;74(1):75–79. doi: 10.1016/j.lungcan.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Gomes F, Faivre-Finn C, Mistry H, Bezjak A, Pourel N, Fournel P, et al. Safety of G-CSF with concurrent chemo-radiotherapy in limited-stage small cell lung cancer - Secondary analysis of the randomised phase 3 CONVERT trial. Lung Cancer. 2021;153:165–170. doi: 10.1016/j.lungcan.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 87.Benna M, Guy J-B, Bosacki C, Jmour O, Ben Mrad M, Ogorodniitchouk O, et al. Chemoradiation and granulocyte-colony or granulocyte macrophage-colony stimulating factors (G-CSF or GM-CSF): time to think out of the box? Br J Radiol. 2020;93(1109):20190147. doi: 10.1259/bjr.20190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 89.Georges Q, Azoulay E, Mokart D, Soares M, Jeon K, Oeyen S, et al. Influence of neutropenia on mortality of critically ill cancer patients: results of a meta-analysis on individual data. Critical Care. 2018;22(1):326. doi: 10.1186/s13054-018-2076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev 2014(10):Cd003039. 10.1002/14651858.CD003039.pub2. [DOI] [PMC free article] [PubMed]
- 91.Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23(18):4198–4214. doi: 10.1200/JCO.2005.05.645. [DOI] [PubMed] [Google Scholar]
- 92.Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clinical Cancer Research : an official Journal of the American Association for Cancer Research. 2016;22(8):2000–2008. doi: 10.1158/1078-0432.Ccr-15-1421. [DOI] [PubMed] [Google Scholar]
- 93.Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. The Oncologist. 2017;22(9):1039–1048. doi: 10.1634/theoncologist.2017-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wesolowski R, Peereboom D, Weiss P, Elson P, Thomas BG. Phase I trial of weekly docetaxel, weekly doxorubicin, daily oral cyclophosphamide, and G-CSF (ConTAC regimen) in advanced malignancies. Invest New Drugs. 2010;28(4):502–508. doi: 10.1007/s10637-009-9258-0. [DOI] [PubMed] [Google Scholar]
- 96.Frasci G, Comella P, D'Aiuto G, Budillon A, Barbarulo D, Thomas R, et al. Weekly paclitaxel-cisplatin administration with G-CSF support in advanced breast cancer. A phase II study. Breast Cancer Res Treat. 1998;49(1):13–26. doi: 10.1023/a:1005945218155. [DOI] [PubMed] [Google Scholar]
- 97.Ellis GK, Gralow JR, Pierce HI, Williams MA, Livingston RB. Infusional paclitaxel and weekly vinorelbine chemotherapy with concurrent filgrastim for metastatic breast cancer: high complete response rate in a phase I-II study of doxorubicin-treated patients. J Clin Oncol. 1999;17(5):1407. doi: 10.1200/jco.1999.17.5.1407. [DOI] [PubMed] [Google Scholar]
- 98.Sun HL, Atenafu EG, Yeboah E, Reece DE, Trudel S, Kukreti V, et al. Intermittent granulocyte colony-stimulating factor for neutropenia management in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leuk Lymphoma. 2015;56(2):407–414. doi: 10.3109/10428194.2014.915544. [DOI] [PubMed] [Google Scholar]
- 99.Wierda WG, Tambaro FP. How I manage CLL with venetoclax-based treatments. Blood. 2020;135(17):1421–1427. doi: 10.1182/blood.2019002841. [DOI] [PubMed] [Google Scholar]
- 100.Fischer K, Al-Sawaf O, Bahlo J, Fink A-M, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 101.Roschewski M, Longo DL, Wilson WH. CAR T-Cell Therapy for Large B-Cell Lymphoma — Who, When, and How? N Engl J Med. 2021;386(7):692–696. doi: 10.1056/NEJMe2118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. doi: 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taneja A, CAR-T-OPENIA JT. Chimeric antigen receptor T-cell therapy-associated cytopenias. eJHaem. 2021;3(S1):32–38. doi: 10.1002/jha2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. doi: 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
- 105.Galli E, Allain V, Di Blasi R, Bernard S, Vercellino L, Morin F, et al. G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transplant. 2020;55(12):2347–2349. doi: 10.1038/s41409-020-01006-x. [DOI] [PubMed] [Google Scholar]
- 106.Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA) Ann Oncol. 2022;33(3):259–275. doi: 10.1016/j.annonc.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Petrelli F, Morelli AM, Luciani A, Ghidini A, Solinas C. Risk of infection with immune checkpoint inhibitors: a systematic review and meta-analysis. Targ Oncol. 2021;16(5):553–568. doi: 10.1007/s11523-021-00824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]