Abstract
Background
Coronavirus disease 2019 (COVID-19) is a rapidly spreading disease that has caused an extensive burden to the world. Consequently, a large number of clinical trials have examined the efficacy of traditional Chinese medicine (TCM) for treating and preventing COVID-19, with coinciding proliferation of reviews summarizing these studies.
Objective
This study aimed to evaluate the methodological quality and evidence quality of systematic reviews and meta-analyses on the efficacy of TCM.
Search strategy
Seven electronic databases, including PubMed, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Chongqing VIP, Wanfang Data and SinoMed, were searched for systematic reviews and meta-analyses in October 2021. Search terms such as “Chinese medicine,” “Lianhua Qingwen” and “COVID-19” were used.
Inclusion criteria
Systematic reviews and meta-analyses of randomized controlled trials that evaluated the efficacy of TCM treatment of COVID-19 were included.
Data extraction and analysis
A Measurement Tool to Assess Systematic Reviews Version 2.0 (AMSTAR 2) was used to evaluate the methodological quality. The quality of evidence was graded using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. Data extraction and analysis were performed by two reviewers independently.
Results
There were 17 meta-analyses included in our overview. The intervention group was defined as TCM combined with Western medicine, while the control group was Western medicine alone. The methodological quality of all the included studies was moderate to poor. A total of 89 outcome indicators were evaluated, of which, 8 were rated as moderate quality, 39 as low quality, and 41 as very low quality. Only one outcome measure was graded as being of high quality. The moderate quality of evidence indicated that, for the treatment of COVID-19, the clinical efficacy of TCM in combination with Western medicine was better, in terms of lung recovery, rate of conversion to severe/critical cases, symptom scores, duration of symptoms, mortality, and length of hospital stay.
Conclusion
Evidence from the included studies shows that, compared with conventional Western medical therapy alone, the addition of TCM to COVID-19 treatment may improve clinical outcomes. Overall, the quality of evidence of TCM for COVID-19 was moderate to poor. Meta-analyses of the use of TCM in the treatment of COVID-19 can be used for clinical decision making by accounting for the experiences of clinical experts, medical policies, and other factors.
Keywords: Medicine, Chinese traditional, Integrative medicine, COVID-19, Systematic review, Meta-analysis
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first report of COVID-19 in December 2019, the epidemic has rapidly swept throughout the world [1]. According to the World Health Organization, up to February 2022, there have been 404,910,528 confirmed cases of COVID-19, including 5,783,776 deaths [2].
Traditional Chinese medicine (TCM) has been widely used in the treatment of infectious diseases in China for thousands of years, including SARS, influenza, and community-acquired pneumonia [3], [4], [5], [6]. Since the outbreak of COVID-19, numerous clinical trials studying the effects of TCM in the treatment of COVID-19 have been launched. Based on the existence of these clinical trials, numerous systematic reviews and meta-analyses on the effects of TCM for COVID-19 have also been published between 2020 and 2021 [7], [8], [9], [10], [11], [12], [13], [14]. Research shows that TCM, such as Lianhua Qingwen, can significantly reduce the rate of clinical COVID-19 cases worsening to the classifications of severe or critical cases, with a risk ratio of 0.38 [15].
Despite the explosion of review literature on the use of TCM in the treatment of COVID-19, the overall efficacy of this approach was evaluated across different populations, using different compound Chinese medicines, and focusing on different outcome measures. Furthermore, few of these review studies were prepared strictly following the standards and the conclusions were limited by the quality of included trials and high heterogeneity [16], [17]. Although systematic reviews are recognized as evidence of the highest level for clinical decision making [18], the reliability of the results was greatly affected by the quality of included trials.
A Measurement Tool to Assess Systematic Reviews Version 2.0 (AMSTAR 2) is a tool for critical appraisal of systematic reviews and meta-analyses of healthcare interventions; it allows researchers to assess methodological quality and assists decision makers in the identification of high-quality systematic reviews [19]. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system is widely used to evaluate the quality of evidence, and the strength of its recommendations facilitates use by patients, clinicians, and policy makers [20].
There are now such a large number of systematic reviews and meta-analyses evaluating the clinical efficacy of TCM for the treatment of COVID-19 that identification, appraisal and consideration of each individual paper are not feasible for practitioners. Furthermore, these reviews vary in quality and scope (and include different types of preparations from the Chinese materia medica). Thus, there is a need to collate these high-level analyses and systematically evaluate their quality.
Therefore, we conducted this overview to systematically evaluate the methodological quality and quality of evidence in the published systematic reviews and meta-analyses on the use of TCM in the treatment of COVID-19, by using the AMSTAR 2 and GRADE. Through this analysis, we provide guidance for understanding the quality of current evidence, which should benefit individuals responsible for clinical decision making.
2. Methods
2.1. Search strategy
Seven electronic databases, including PubMed, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Chongqing VIP (VIP), Wanfang Data and SinoMed, were searched for systematic reviews and meta-analyses in October 2021. Search terms, such as “Chinese medicine,” “integrated traditional Chinese and Western medicine,” “herbal medicine,” “Lianhua Qingwen,” “Shufeng Jiedu Capsule,” “COVID-19,” “2019-nCoV,” “coronavirus,” “SARS-CoV-2,” “coronavirus pneumonia,” “systematic review” and “meta-analysis,” and their near-synonym words were used. The search included both medical subject-heading terms and text-word terms. Additional references from identified studies, reviews, unpublished work, or relevant citations provided by experts were manually checked to include potentially missed studies. Systematic reviews published from 2019-12-01 to 2021-10-31 were included. There were no restrictions on language. The full search strategy is presented in the supplementary file.
2.2. Inclusion and exclusion criteria
The inclusion criteria for this overview were as follows: (1) the study included must be a systematic review or meta-analysis of randomized controlled trials (RCTs), where systematic review was defined as a literature review that used a clearly defined search strategy in at least one electronic database to identify all studies that met pre-defined eligibility criteria along with a study selection progress; (2) the efficacy or safety of TCM (herbal decoction, patent medicine or herbal injections) for the treatment of COVID-19 was evaluated, and combined interventions using Chinese medicine and Western medicine were also eligible; (3) included patients diagnosed with COVID-19, with no restrictions on sex, age, race, occupation, course of the disease or the severity of disease; (4) the efficacy of the treatment was measured by at least one experimentally quantifiable outcome.
Articles were excluded if: (1) they were duplicate publications; (2) they were only published in conference proceedings or protocols; (3) they evaluated interventions that included TCM combined with non-drug therapies such as point thread embedding, acupuncture, Taichi and Qigong, etc.; (4) control groups also received TCM or proprietary Chinese medicine treatment.
2.3. Study selection
Search results were compiled, and duplicates were removed using EndNote X9.1 (Clarivate Analytics, Philadelphia, USA). Two reviewers independently screened study titles and abstracts retrieved from the literature search and then read the full text of studies that passed the inclusion and exclusion criteria. Any disagreements were resolved through discussion or, if necessary, with the involvement of a third reviewer.
2.4. Data extraction
Data extraction was performed independently by two reviewers using a custom data extraction form. For each systematic review or meta-analysis included, the following characteristics were extracted: first author, year of publication, intervention, control, number of included trials, total sample size, outcome measures and main conclusions. The third review author checked for accuracy and resolved any inconsistencies in the extracted data through discussion with the reviewing authors.
2.5. Assessment of methodological quality
AMSTAR 2 was used to assess the quality of the systematic reviews included by our two independent reviewers. The scale contains 16 items [18], graded as “Yes,” “Partial Yes,” and “No.” To be specific, when the reporting and implementation for an item fully met the standards, it was graded as “Yes.” “Partial Yes” was selected when the reporting and implementation of an item was insufficient. Finally, “No” was selected when there was no reporting or implementation of the item. Among the 16 items of the AMSTAR 2, seven items (2, 4, 7, 9, 11, 13 and 15) were critical to the evaluation of systematic reviews.
Criteria for rating overall confidence in the results of the systematic review by AMSTAR 2 guideline were as follows. “High” had no or one non-critical weakness; “Moderate” had more than one non-critical weakness; “Low” had one critical flaw with or without non-critical weaknesses; “Critically low” had more than one critical flaw with or without non-critical weaknesses.
2.6. Assessment of evidence quality
Furthermore, the GRADE system [19] was used to evaluate the quality of evidence for each of our chosen outcome measures. According to the five indicators of reduced quality (limitations, inconsistency, indirectness, imprecision and publication bias) and three indicators of enhanced quality (large effect size, dose–response relationship, all plausible residual confounding), we evaluated the methodological quality of each outcome synthesized with meta-analysis of included studies, and our scores were reported as “very low” (total scores < –2), “low” (total scores = –2), “moderate” (total scores = –1), or “high” (total scores = 0). Disagreements were resolved by discussion or consultation with a third author.
3. Results
3.1. Study selection
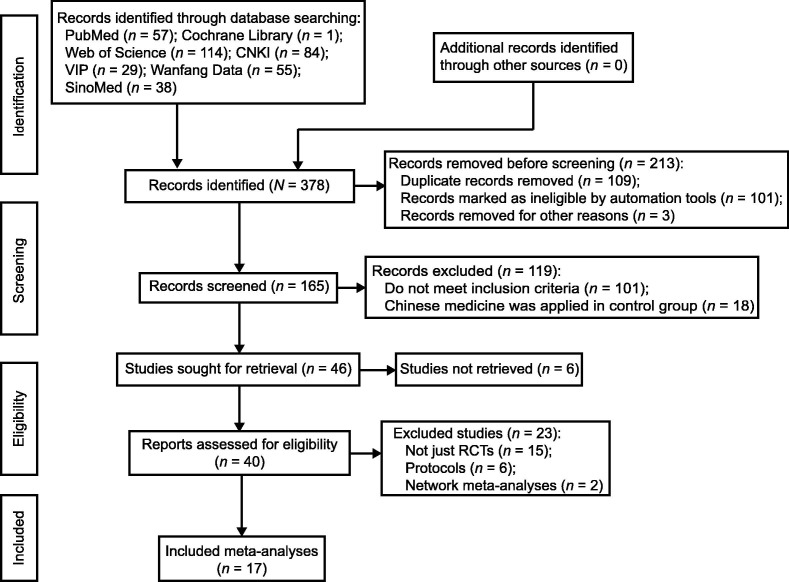
Through a search of electronic databases, 378 articles were identified. After the exclusion of duplicate articles, 165 articles remained. After screening the titles and abstracts, we selected to read the full text of 40 articles. Finally, 17 meta-analyses were included in our overview [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Most of the studies were excluded because their analysis included a combination of RCTs and other study types, or because their interventions were combined with non-drug therapies. The selection process of studies is summarized and shown in Fig. 1 .
Fig. 1.
Flow diagram. CNKI: China National Knowledge Infrastructure; VIP: Chongqing VIP; RCT: randomized controlled trial.
3.2. Study characteristics
All included meta-analyses were conducted on the Chinese mainland between 2020 and 2021. Seven of the 17 articles [21], [24], [30], [31], [33], [34], [37] were published in 2020 and 10 were published in 2021 [22], [23], [25], [26], [27], [28], [29], [32], [35], [36]. Thirteen were published in English [21], [22], [23], [24], [25], [27], [28], [30], [31], [32], [34], [36], [37] and 4 were published in Chinese [26], [29], [33], [35]. One meta-analysis included both patients diagnosed with COVID-19 and patients suspected of having COVID-19 [24], while the rest of the meta-analyses included only patients with confirmed cases of COVID-19. Of the 17 reviews, 13 (13/17, 72.2%) [21], [22], [23], [24], [26], [27], [28], [29], [30], [31], [32], [34], [36] comprehensively examined the efficacy and safety of TCM without differentiating between specific formulas. Four (4/17, 23.5%) of the included reviews evaluated the effects of the Lianhua Qingwen preparation [25], [33], [35], [37]. Among the 17 included reviews, the number of included trials ranged from 2 to 25, and the number of included participants ranged from 154 to 2257. Two reviews limited the clinical classification of COVID-19 to mild and moderate, and one limited it to moderate. Study characteristics are presented in Table 1 .
Table 1.
Characteristics of included systematic reviews and meta-analyses.
| Study | Publication year | Clinical status of participants | Number of included trials | Total sample size | Treatment group | Control group | Risk of bias evaluation | Main conclusion |
|---|---|---|---|---|---|---|---|---|
| Ang et al. [21] | 2020 | Diagnosed | 7 | 855 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | Significant effects of the combined therapy of herbal medicine with WM were found. |
| Du et al. [22] | 2021 | Diagnosed (mild and moderate) | 12 | 1393 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | Chinese herbal medicine combined with conventional therapy may be effective and safe in the treatment of mild to moderate COVID-19. |
| Du et al. [23] | 2021 | Diagnosed | 9 | 1286 | TCM contains honeysuckle + WM | Conventional WM | Cochrane risk of bias assessment tool | Honeysuckle combined with conventional therapy may be beneficial for the treatment of COVID-19 in improving lung CT, clinical cure rate, clinical symptoms, and laboratory indicators, and reducing the rate of conversion to severe cases. Combination therapy did not increase adverse events. |
| Fan et al. [24] | 2020 | Diagnosed | 7 | 732 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | TCM, as an adjunct treatment with standard care, helps to improve treatment outcomes in COVID-19 cases. |
| Fan et al. [25] | 2021 | Diagnosed and suspected (mild and moderate) | 5 | 824 | LH preparation + WM | Conventional WM | Cochrane risk of bias assessment tool | LH in combination with usual treatment may improve the clinical efficacy in patients with mild or moderate COVID-19 without increasing adverse events. |
| Zhou et al. [26] | 2021 | Diagnosed | 6 | 470 | TCM/CPM + WM | Conventional WM | Cochrane risk of bias assessment tool | Chinese herbal decoction combined with conventional WM has some advantages in relieving clinical symptoms of cough and fatigue and can shorten the hospital stay. |
| Li et al. [27] | 2021 | Diagnosed | 8 | 750 | Oral TCM/TCM injection + WM | Conventional WM | NOS/Jadad | The integration of TCM with WM significantly improves the treatment for COVID-19 patients compared to WM treatment alone. |
| Liang et al. [28] | 2021 | Diagnosed | 7 | 1079 | Oral TCM + WM | Conventional WM | Cochrane risk of bias assessment tool | Oral TCM may have add-on potential therapeutic effects for patients with non-serious COVID-19. There are some differences in therapeutic effects between different oral TCM for the same COVID-19 outcome. |
| Liu et al. [29] | 2021 | Diagnosed | 7 | 588 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | The effectiveness of the combination of TCM and WM in treating COVID-19, in terms of total effective rate, syndrome scores, disappearance rate of clinical symptoms, lung CT, and risk of adverse effects, was better than that of the control group that received only WM. |
| Pang et al. [30] | 2020 | Diagnosed | 11 | 1259 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | TCM may bring potential benefits to patients suffering from COVID-19. However, the quality of included trials is not good enough. High-quality studies with a core outcome set are still required. |
| Sun et al. [31] | 2020 | Diagnosed | 7 | 681 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | TCM combined with conventional treatment was the better treatment choice for COVID-19. |
| Wang et al. [32] | 2021 | Diagnosed | 25 | 2222 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool 2 | TCM treatment plus routine care may promote a clinical cure and chest image improvement compared to routine care alone, while reducing clinical deterioration, development of ARDS, use of mechanical ventilation, and death in patients with COVID-19. TCM treatment plus routine care may not change the rate of negativity on the SARS-CoV-2 nucleic acid test, compared to routine care alone. TCM treatment was found to be safe for patients with COVID-19. |
| Zhang et al. [33] | 2020 | Diagnosed (Moderate) | 5 | 600 | LH preparation + WM | Conventional WM | Cochrane risk of bias assessment tool | LH preparation in combination with WM is effective and has few adverse effects in the treatment of patients with the moderate COVID-19. |
| Xiong et al. [34] | 2020 | Diagnosed | 18 | 2257 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | TCM may be beneficial for the treatment of COVID-19 and appeared to improve clinical symptoms, imaging, and laboratory indicators, shorten the course of the disease, and reduce the number of severe cases. |
| Tang et al. [35] | 2021 | Diagnosed | 5 | 824 | LH preparation + WM | Conventional WM | Cochrane risk of bias assessment tool | Compared with the conventional WM, the use of LH in combination with WM can produce an intervention effect on clinical symptoms, lung CT, and inflammatory indicators, and can shorten the duration of fever. Its safety profile remains to be confirmed by further studies. |
| Yin et al. [36] | 2021 | Diagnosed | 19 | 1853 | TCM/CPM/TCM injection + WM | Conventional WM | Cochrane risk of bias assessment tool | The integrated medicine can improve the clinical symptoms, chest CT and infection indicators of COVID-19 patients. |
| Zeng et al. [37] | 2020 | Diagnosed | 2 | 154 | LH preparation + WM | Conventional WM | Cochrane risk of bias assessment tool | The treatment of new pneumonia with LH can be used as an effective therapy to improve the clinical symptoms of new coronary pneumonia. |
ARDS: acute respiratory distress syndrome; COVID-19: coronavirus disease 2019; CPM: Chinese patent medicine; CT: computerized tomography; LH: Lianhua Qingwen; NOS: Newcastle–Ottawa Scale; TCM: traditional Chinese medicine; WM: Western medicine.
3.3. Assessment of methodological quality with AMSTAR 2
AMSTAR 2 was used to rate the overall confidence in the methodological quality of the meta-analyses included in this review. The methodologies of two reviews were graded as moderate, 11 as low quality, and 4 as critically low quality.
According to the recommendations of AMSTAR 2, none of the meta-analyses reported on the funding sources of the studies included in their reviews. Of the 17 reviews, 6 provided the registration number of study protocol, but the other 11 did not, which made it difficult to evaluate any inconsistencies between the protocol and the final analysis. All 17 reviews included only RCTs but they did not explain how they selected that study designs for inclusion in the analysis. All of the included reviews stated that they conducted the study screening process, and that data extraction was conducted by two independent reviewers. The Cochrane risk of bias assessment tool was used to evaluate all 17 reviews and only one study was found to have used an unsatisfactory technique for assessing the risk of bias. Nine reviews analyzed their included trials to explore the publication bias with a funnel plot. Three analyses failed to report potential conflicts of interest, including any funding they received for conducting the review. The AMSTAR 2 scoring is presented in Table 2 .
Table 2.
Assessment of methodological quality by A Measurement Tool to Assess Systematic Reviews Version 2.0.
| Author (year) | Item |
Methodological quality | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Ang et al. [21] (2020) | Y | Y | Y | Y | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | N | Y | Low |
| Du et al. [22] (2021) | Y | Y | N | Y | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | Y | Y | Moderate |
| Du et al. [23] (2021) | Y | Y | N | Y | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | Y | Low |
| Fan et al. [24] (2020) | Y | Y | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | Y | Low |
| Fan et al. [25] (2021) | Y | N | N | Y | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | Y | Critical low |
| Zhou et al. [26] (2021) | Y | N | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | N | Critical low |
| Li et al. [27] (2021) | Y | N | N | PY | Y | Y | PY | Y | N | N | Y | Y | Y | Y | Y | Y | Low |
| Liang et al. [28] (2021) | Y | N | N | PY | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | N | Y | Critical low |
| Liu et al. [29] (2021) | Y | N | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | Y | Y | Low |
| Pang et al. [30] (2020) | Y | Y | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | Y | Y | Moderate |
| Sun et al. [31] (2020) | Y | N | N | PY | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Low |
| Wang et al. [32] (2021) | Y | Y | N | Y | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | N | Y | Low |
| Zhang et al. [33] (2020) | Y | N | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | N | Critical low |
| Xiong et al. [34] (2020) | Y | N | N | Y | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Low |
| Tang et al. [35] (2021) | Y | N | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | N | N | Critical low |
| Yin et al. [36] (2021) | Y | N | N | PY | Y | Y | PY | Y | Y | N | Y | Y | Y | Y | Y | Y | Low |
| Zeng et al. [37] (2020) | Y | N | N | PY | Y | Y | PY | PY | Y | N | Y | Y | Y | Y | Y | Y | Low |
Y: yes; N: no; PY: partial yes.
3.4. Assessment of quality of evidence using GRADE
One of the 17 reviews was excluded from the GRADE evaluation because it lacked a forest plot, sample size report and appraisal of the heterogeneity of individual outcomes. A total of 89 outcome indicators were identified in the 16 included reviews. The results showed that evidence was of moderate quality for 8 outcome indicators, of low quality for 39 outcome indicators, and of very low quality for 41 outcome measures. Only one of the outcomes was deemed to have high-quality evidence. There were no indicators of enhanced quality in any of the 89 outcome indicators. Table 3 describes the quality of evidence for each outcome measure.
Table 3.
Quality of evidence in the included studies by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
| Author (year) | Outcome indicators | Number of included trials | Study limitations | Inconsistency of results | Indirectness of evidence | Imprecision | Reporting bias | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| Ang et al. [21] (2020) | Overall efficacy | 4 | −1 | 0 | 0 | 0 | −1 | Low |
| Rate of clinical symptom disappearance | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Clinical symptom scores | 3 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Laboratory indicators | 4 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Time to viral assay conversion | 5 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Du et al. [22] (2021) | Lung computerized tomography | 7 | −1 | 0 | 0 | 0 | −1 | Low |
| Clinical cure rate | 5 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 9 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of viral assay conversion | 4 | −1 | −1 | 0 | −1 | −1 | Very low | |
| Rate of clinical symptom disappearance | 3 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Clinical symptom scores | 4 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Laboratory indicators | 6 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Adverse events | 10 | −1 | −2 | 0 | 0 | 0 | Very low | |
| Du et al. [23] (2021) | Lung computerized tomography | 4 | −1 | 0 | 0 | 0 | −1 | Low |
| Clinical cure rate | 5 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of viral assay conversion | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 6 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of clinical symptom disappearance | 3 | −1 | 0 | 0 | −1 | −1 | Very low | |
| Clinical symptom scores | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Laboratory indicators | 5 | −1 | 0 | 0 | 0 | −1 | Low | |
| Adverse events | 5 | −1 | 0 | 0 | 0 | −1 | Low | |
| Fan et al. [24] (2020) | Clinical symptom scores | 3 | −1 | −2 | 0 | −1 | −1 | Very low |
| Laboratory indicators | 5 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Lung computerized tomography | 4 | −1 | 0 | 0 | −1 | −1 | Very low | |
| Fan et al. [25] (2021) | Overall efficacy | 5 | −1 | 0 | 0 | 0 | −1 | Low |
| Rate of viral assay conversion | 4 | −1 | 0 | 0 | 0 | −1 | Low | |
| Lung computerized tomography | 3 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Duration of clinical symptoms | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Adverse events | 2 | −1 | −2 | 0 | −1 | −1 | Very Low | |
| Li et al. [27] (2021) | Overall efficacy | 3 | −1 | 0 | 0 | 0 | 0 | Moderate |
| Lung computerized tomography | 4 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 3 | −1 | 0 | 0 | 0 | 0 | Moderate | |
| Rate of clinical symptom disappearance | 5 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Duration of clinical symptoms | 5 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Liang et al. [28] (2021) | Clinical cure rate | 2 | −2 | 0 | 0 | 0 | −1 | Very low |
| Rate of conversion to severe/critical cases | 6 | −2 | 0 | 0 | −1 | −1 | Very low | |
| Rate of clinical symptom disappearance | 2 | −2 | −1 | 0 | −1 | −1 | Very low | |
| Lung computerized tomography | 4 | −2 | 0 | 0 | 0 | −1 | Very low | |
| Liu et al. [29] (2021) | Overall efficacy | 3 | −1 | 0 | 0 | −1 | −1 | Very low |
| Clinical symptom scores | 2 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Rate of clinical symptom disappearance | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Lung computerized tomography | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 4 | −1 | 0 | 0 | 0 | −1 | Low | |
| Adverse events | 3 | −1 | 0 | 0 | −1 | −1 | Very low | |
| Pang et al. [30] (2020) | Rate of conversion to severe/critical cases | 8 | −1 | 0 | 0 | 0 | −1 | Low |
| Mortality rate | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Adverse events | 8 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Clinical symptom scores | 2 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Rate of clinical symptom disappearance | 3 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Duration of clinical symptoms | 2 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Sun et al. [31] (2020) | Overall efficacy | 2 | −1 | 0 | 0 | 0 | −1 | Low |
| Adverse events | 7 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Rate of viral assay conversion | 7 | −1 | −1 | 0 | −2 | −1 | Very low | |
| Lung computerized tomography | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Laboratory indicators | 5 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Wang et al. [32] 2021 | Clinical cure rate | 2 | 0 | 0 | 0 | 0 | −1 | Moderate |
| Rate of viral assay conversion | 2 | 0 | 0 | 0 | −1 | −1 | Low | |
| Rate of conversion to severe/critical cases | 3 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| The incidence of clinical exacerbation | 3 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Lung computerized tomography | 3 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Mortality rate | 3 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Adverse events | 17 | −1 | 0 | 0 | 0 | −1 | Low | |
| Zhang et al. [33] (2020) | Rate of clinical symptom disappearance | 3 | −1 | 0 | 0 | 0 | −1 | Low |
| Xiong et al. [34] (2020) | Lung computerized tomography | 13 | −1 | −1 | 0 | 0 | −1 | Very low |
| Mortality rate | 4 | −1 | 0 | 0 | −1 | −1 | Very low | |
| Clinical cure rate | 7 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to mild cases | 2 | −1 | 0 | 0 | −1 | −1 | Very low | |
| Rate of conversion to severe/critical cases | 11 | −1 | 0 | 0 | 0 | −1 | Low | |
| The length of hospital stay | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Clinical symptom scores | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of clinical symptom disappearance | 15 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Duration of clinical symptoms | 15 | −1 | −2 | 0 | 0 | −1 | Very low | |
| Rate of viral assay conversion | 4 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Laboratory indicators | 6 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Adverse events | 9 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Tang et al. [35] (2021) | Duration of clinical symptoms | 4 | −1 | 0 | 0 | 0 | −1 | Low |
| Rate of clinical symptom disappearance | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Overall efficacy | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Lung computerized tomography | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 4 | −1 | 0 | 0 | 0 | −1 | Low | |
| Yin et al. [36] (2021) | Overall efficacy | 6 | −1 | 0 | 0 | 0 | −1 | Low |
| Rate of clinical symptom disappearance | 8 | −1 | −1 | 0 | 0 | −1 | Very low | |
| Lung computerized tomography | 9 | −1 | 0 | 0 | 0 | −1 | Low | |
| Laboratory indicators | 9 | −1 | −2 | 0 | −1 | −1 | Very low | |
| Zeng et al. [37] (2020) | Rate of clinical symptom disappearance | 2 | 0 | −1 | 0 | 0 | 0 | Moderate |
| Duration of clinical symptoms | 2 | 0 | 0 | 0 | 0 | 0 | High | |
3.5. Main outcomes
According to the GRADE guideline, high-quality evidence means that the true effect lies close to that of the estimate of the effect and findings have high confidence. For moderate quality of evidence, findings can have moderate confidence, and the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Thus, here we summarize evidence of high and moderate quality and recommend these data for clinical use. The summary of evidence quality and results is shown in Table 4, Table 5 .
Table 4.
Summary of evidence quality in the included studies by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
| Treatment | Outcome measure | Number of included reviews | Study limitations | Inconsistency of results | Indirectness of evidence | Imprecision | Reporting bias | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| Traditional Chinese medicine + Western medicine vs Western medicine | Lung computerized tomography | 10 | 0 | 0 | 0 | 0 | −1 | Moderate |
| Rate of clinical symptom disappearance | 9 | 0 | −1 | 0 | 0 | 0 | Low | |
| Adverse events | 8 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 8 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Clinical symptom scores | 7 | −1 | 0 | 0 | 0 | −1 | Low | |
| Laboratory indicators | 7 | −1 | 0 | 0 | 0 | −1 | Low | |
| Clinical cure rate | 5 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Overall efficacy | 5 | −1 | 0 | 0 | 0 | 0 | Moderate | |
| Rate of viral assay conversion | 5 | −1 | 0 | 0 | 0 | −1 | Low | |
| Duration of clinical symptoms | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Mortality rate | 3 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| Rate of conversion to mild cases | 1 | −1 | 0 | 0 | −1 | −1 | Very low | |
| The incidence of clinical exacerbation | 1 | 0 | 0 | 0 | 0 | −1 | Moderate | |
| The length of hospital stay | 1 | −1 | 0 | 0 | 0 | −1 | Low | |
| Lianhua Qingwen + Western medicine vs Western medicine | Duration of clinical symptoms | 4 | 0 | 0 | 0 | 0 | 0 | High |
| Overall efficacy | 3 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of clinical symptom disappearance | 3 | 0 | −1 | 0 | 0 | 0 | Moderate | |
| Adverse events | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Lung computerized tomography | 2 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of conversion to severe/critical cases | 1 | −1 | 0 | 0 | 0 | −1 | Low | |
| Rate of viral assay conversion | 1 | −1 | 0 | 0 | 0 | −1 | Low |
Table 5.
Studies with high-quality and moderate-quality results.
| Author (year) | Treatment | Outcome indicator | Effect size, [95% CI], and P-value (if available) | Total participants in both groups | Number of included trials | Quality of evidence |
|---|---|---|---|---|---|---|
| Li et al. [27] (2021) | TCM + WM vs WM | Overall efficacy | OR 2.50 [1.46, 4.29] | 100/73 | 3 | Moderate |
| Rate of conversion to severe/critical cases | OR 0.35 [0.18, 0.69] | 196/130 | 3 | Moderate | ||
| Wang et al. [32] (2021) | TCM + WM vs WM | Clinical cure rate | RR 1.20, [1.04, 1.38], P = 0.01 | 173/173 | 2 | Moderate |
| Rate of conversion to severe/critical cases | RR 0.39, [0.18, 0.86], P = 0.02 | 208/206 | 3 | Moderate | ||
| The incidence of clinical exacerbation | RR 0.30, [0.12, 0.77], P = 0.01 | 81/65 | 3 | Moderate | ||
| Lung computerized tomography | RR 1.22, [1.07, 1.39], P = 0.01 | 313/314 | 3 | Moderate | ||
| Mortality rate | RR 0.28, [0.09, 0.84], P = 0.02 | 241/241 | 3 | Moderate | ||
| Zeng et al. [37] (2020) | LH + WM vs WM | Rate of clinical symptom disappearance | OR 3.34, [2.06, 5.44], P < 0.001 | 72/72 | 2 | Moderate |
| Duration of clinical symptoms | OR − 1.04, [−1.60, −0.49], P < 0.001 | 72/72 | 2 | High | ||
TCM: traditional Chinese medicine; WM: Western medicine; LH: Lianhua Qingwen; CI: confidence interval; RR: relative risk; OR: odds ratio.
3.5.1. Lung computerized tomography
Twelve pieces of literature quantitatively analyzed the recovery of the lungs based on data from computerized tomography (CT) scans. Moderate-quality evidence suggested that TCM combined with Western medicine significantly improved the recovery rate of lung CT in patients diagnosed with COVID-19.
3.5.2. Disappearance rate of clinical symptoms
Twelve reviews reported the rate of recovery from clinical symptoms, including fever, cough, and fatigue. Moderate-quality evidence indicated that Lianhua Qingwen in combination with Western medicine significantly enhanced the rate of recovery from clinical symptoms.
3.5.3. Duration of clinical symptoms
Seven reviews reported the duration of clinical symptoms, including fever, cough, and fatigue. High-quality evidence indicated that Lianhua Qingwen in combination with Western medicine significantly shortened the duration of clinical symptoms.
3.5.4. Rate of conversion to severe/critical cases
The results of the rate of conversion to severe/critical cases were pooled in nine meta-analyses. The synthesized results showed that the application of Chinese medicine could help to reduce the rate of conversion to severe/critical cases, with moderate-quality evidence.
3.5.5. Clinical cure rate
The clinical cure rate was reported in five studies. Moderate-quality evidence suggested that TCM plus routine care could increase the clinical cure rate better than routine care alone.
3.5.6. Overall efficacy
Three studies assessed the overall efficacy of the Chinese medical treatment for COVID-19. Pooling of results from these studies showed that patients treated with combined TCM and Western medicine had an overall better effect. The level of evidence was moderate.
3.5.7. Mortality rate
Cases of death were reported in three systematic reviews. Moderate-quality evidence showed that, compared with routine care alone, TCM with routine care could decrease the death rate.
3.5.8. Risk of clinical exacerbation
Incidence of unfavorable clinical events, such as acute respiratory distress syndrome and mechanical ventilation, was analyzed in one systematic review. Moderate quality of evidence suggested that adjuvant treatment of Chinese medicine to Western medicine could decrease the incidence of unfavorable clinical events better than Western medicine alone.
4. Discussion
With the rapid transmission and worldwide spread of COVID-19 since 2019, researchers around the world have sought information from COVID-19 trials from pathophysiological basics to treatment and vaccines [38], [39], [40], [41]. Thus, a large number of clinical trials, systematic reviews and meta-analyses were generated. In the field of evidence-based medicine, data from systematic reviews based on RCTs are generally considered the highest level of information [42]. However, through literature screening, it was found that some methodological weaknesses should be noted in the published systematic reviews and meta-analyses.
Therefore, the present review uses the AMSTAR 2 scale and GRADE system to evaluate the methodological quality, quality of evidence and strength of recommendations of the included meta-analyses. This work describes the general characteristics, methodological quality, and quality of evidence of 17 meta-analyses that investigated the use of TCM in the treatment of COVID-19.
4.1. Summary of evidence
A total of 17 systematic reviews were identified in this overview. Moderate-quality evidence showed that the combination of Chinese medicine and Western medicine could help to enhance the clinical efficacy of COVID-19 treatment, mainly in lung CT, recovery from clinical symptoms, duration of clinical symptoms, rate of conversion to severe/critical cases, clinical cure rate, overall efficacy, mortality rate and risk of clinical exacerbation.
For reviews that evaluated the efficacy of Lianhua Qingwen, high-quality evidence suggested that taking the Lianhua Qingwen preparation in conjunction with conventional Western medicine could shorten the duration of clinical COVID-19 symptoms. Further, Lianhua Qingwen improved the rate of recovery from clinical symptoms, which was supported by moderate-quality evidence.
4.2. Methodological quality of included meta-analyses
We found that 89% of included studies were of low or critically low methodological quality. Systematic reviews and meta-analyses of high quality are still needed going forward. The following problems existed in the rating of methodological quality. (1) Most of the studies did not register their reviews or analyses or failed to provide the registration information in the article. This was the main reason for the lower quality of evidence. Protocols and registration are of vital importance to increase rigor and transparency of the systematic review literature [43]. (2) Common omissions in the reviews were lack of testing for publication bias and not searching grey literature. Relevant studies that had not been published yet were excluded from our research, which might have effect on the results of the meta-analyses [44]. (3) All reviews included only RCTs, but the justification for using this study design was not commonly explained. Reviews of RCTs offer the highest level of evidence, but the reason for selecting only RCTs is recommended in the AMSTAR 2 system. (4) The sources of funding for the studies included in the review were not reported in all reviews, making it impossible to judge whether the final result is objective [45].
4.3. Quality of evidence
Most of the outcome indicators were graded as being of low or very low quality. Within all the degraded factors, study limitation was the main factor. In this overview, limitations of the included systematic reviews mainly reflected the unclear risk of bias. The total risk of bias of most clinical trials was categorized as unclear since some of the items were not reported. On the other hand, some of the systematic reviews were conducted in the early stage of the COVID-19 pandemic and were limited by the number of included trials. The risk of bias was not considered in the process of quantitative synthesis, but most reviews account for the risk of bias in individual studies when interpreting the results of the review.
Moreover, high statistical heterogeneity accounted for the degradation of the inconsistency of results. Twenty-three percent of the included meta-analyses focused on an individual Chinese medical formula (Lianhua Qingwen), and the other studies synthesized data from different Chinese medicines together. Furthermore, only 18% of meta-analyses set inclusion criteria for the clinical stage of COVID-19 patients, including patients with mild to moderate symptoms. These factors might all contribute to the high heterogeneity and inconsistency of results.
Adequate investigation of publication bias was not carried out for most of the included reviews, and its potential impact on the results of the review was also ignored.
4.4. Recommendations for the future reviews
Based on the results of this overview, we found that the main problems of the included reviews were methodological. Registration and publication of protocols are important in development of systematic reviews, but many of the reviews we looked at neglected this step. It is recommended that authors register their reviews on the relevant registration platform such as international prospective register of systematic reviews (PROSPERO) [46] prior to their preparation, and report the registration information according to the guidelines for systematic reviews. Further, the financial support and conflict of interest statements of the included studies were insufficient. In fact, at the time these reviews were conducted, there were already recommended reporting guidelines for systematic reviews and meta-analyses, such as the Preferred Reporting Items for Systematic reviews and Meta-Analyses checklist. Conducting systematic reviews and meta-analyses in compliance with these guidelines would help to ensure that they receive a higher-quality rating and have greater use to researchers and clinicians. With the expansion of clinical trials, we also suggested that future systematic reviews could focus on the efficacy of individual Chinese medical formulas to reduce potential inconsistencies among therapies.
4.5. Limitations
There were some limitations in this overview. We were limited by the number of published trials. Many reviews pooled the data from different Chinese medical formulas, so we also could not distinguish the effects of individual Chinese medical formulas. Our overview was conducted based on the information reported in the included analyses. Some of these analyses did not provide adequate details, and it was difficult to determine whether the reviews were designed and conducted well.
5. Conclusion
Our overview shows that, compared with the use of conventional Western medicine alone, the addition of TCM, such as Lianhua Qingwen and Chinese herbal compounds, may improve the clinical efficacy of COVID-19 treatment and decrease the risk of unfavorable clinical events. Overall, the quality of evidence supporting the use of TCM in the treatment of COVID-19 was not very high. It is still necessary to conduct high-quality systematic reviews.
Funding
This work was supported by the Key Research and Development Projects from the Department of Science and Technology of Zhejiang Province (No. 2020C03126), and the Health Commission of Zhejiang Province (No. 2017KY502), China.
Acknowledgments
Acknowledgements
Thanks to all the participants and clinical researchers involved in the publications cited in this review. Thanks to all the peer reviewers who contributed to the continuous improvement of this article.
Authors’ contributions
HTW and CHJ coordinated the study and drafted the study design. JL and PJH were responsible for data collection. RCD and QSL organized the data. All authors participated in data interpretation and manuscript review and writing. HTW and CHJ were responsible for preparation of the manuscript, tables, and figures. XQW, JCY, WM and QG contributed to the scientific discussion of the data and of the manuscript.
Declaration of competing interest
The authors declare no financial or other conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joim.2022.06.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S., et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. (2022) [2022-06-22]. https://covid19.who.int/.
- 3.Leung P.C. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am J Chin Med. 2007;35(4):575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Yu X., Li S., Wang H., Bai Y., Wang M., et al. Randomized controlled multicenter clinical trial for integrated treatment of community-acquired pneumonia based on traditional Chinese medicine syndrome differentiation. J Tradit Chin Med. 2012;32(4):554–560. doi: 10.1016/s0254-6272(13)60070-9. [DOI] [PubMed] [Google Scholar]
- 5.Li J.H., Wang R.Q., Guo W.J., Li J.S. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): a meta-analysis. J Chin Med Assoc. 2016;79(5):281–291. doi: 10.1016/j.jcma.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y., Li N.X., Duan N., Liu B., Zhu H., Zhang C., et al. Traditional Chinese medicine in treating influenza: from basic science to clinical applications. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.575803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Tian J., et al. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10(1) doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Zhu H., Li M., Liu Y., Lai H., Yang Q., et al. Efficacy and safety of Qingfei Paidu decoction for treating COVID-19: a systematic review and meta-analysis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.688857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Greenhalgh T., Wardle J. Chinese herbal medicine (“3 medicines and 3 formulations”) for COVID-19: rapid systematic review and meta-analysis. J Eval Clin Pract. 2022;28(1):13–32. doi: 10.1111/jep.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X., Li W., Qin Z., Xue L., Huang G., Luo Z., et al. Traditional Chinese medicine as an adjunctive therapy for mild and common COVID-19: a systematic review and network meta-analysis. Medicine (Baltimore) 2021;100(40):e27372. doi: 10.1097/MD.0000000000027372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J.B., Wang Z.X., Jing J., Zhao P., Dong J.H., Zhou Y.F., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26(9):648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H., Dai R., Wu X., Li Q., Lu H., Yang J., et al. Efficacy and safety of Chinese medicine for COVID-19: a systematic review and meta-analysis. Am J Chin Med. 2022;50(2):333–349. doi: 10.1142/S0192415X22500136. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang J., Dai X., Wu Q., Cai H., Fu X., Zhang W., et al. A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19) Complement Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo X., Ni X., Lin J., Zhang Y., Wu L., Huang D., et al. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S., Wang F., Li J., Li Y., Li W., Wu X., et al. The effect of Chinese herbal medicine on digestive system and liver functions should not be neglected in COVID-19: an updated systematic review and meta-analysis. IUBMB Life. 2021;73(5):739–760. doi: 10.1002/iub.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OCEBM Levels of Evidence Working Group. The Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine. (2011) [2022-05-10]. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 19.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins D., Eccles M., Flottorp S., Guyatt G.H., Henry D., Hill S., et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang L., Song E., Lee H.W., Lee M.S. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(5):1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du X., Shi L., Cao W., Zuo B., Zhou A. Add-on effect of Chinese herbal medicine in the treatment of mild to moderate COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0256429. doi: 10.1371/journal.pone.0256429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X.Q., Shi L.P., Cao W.F., Chen Z.W., Zuo B., Hu J.Y. Add-on effect of honeysuckle in the treatment of coronavirus disease 2019: a systematic review and meta-analysis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan A.Y., Gu S., Alemi S.F. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J Integr Med. 2020;18(5):385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Z., Guo G., Che X., Yang Y., Liu Y., Li L., et al. Efficacy and safety of Lianhuaqingwen for mild or moderate coronavirus disease 2019: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021;100(21):e26059. doi: 10.1097/MD.0000000000026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F.F., Pu L.L., Rong X.X., Liu J.Y., Yang Y., Yang W.N. Efficacy and safety of Chinese herbal decoction combined with Western medicine in treatment of COVID-19: a meta-analysis. Shi Yong Yi Xue Za Zhi. 2021;37(5):564–568. [Chinese with abstract in English] [Google Scholar]
- 27.Li F., Jiang Y., Yue B., Luan L. Use of traditional Chinese medicine as an adjunctive treatment for COVID-19: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100(30):e26641. doi: 10.1097/MD.0000000000026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang S.B., Fang M., Liang C.H., Lan H.D., Shen C., Yan L.J., et al. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: a rapid systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L.L., Duan F., Du Q.T., Cui C.H. A review and meta-analysis of clinical efficacy and safety of integrated medicine on COVID-19. Zhong Yi Lin Chuang Yan Jiu. 2021;13(5):24–30. [Chinese with abstract in English] [Google Scholar]
- 30.Pang W., Liu Z., Li N., Li Y., Yang F., Pang B., et al. Chinese medical drugs for coronavirus disease 2019: a systematic review and meta-analysis. Integr Med Res. 2020;9(3) doi: 10.1016/j.imr.2020.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun C.Y., Sun Y.L., Li X.M. The role of Chinese medicine in COVID-19 pneumonia: a systematic review and meta-analysis. Am J Emerg Med. 2020;38(10):2153–2159. doi: 10.1016/j.ajem.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Xu B., Zhang Y., Duan Y., Gao R., He H., et al. Efficacy and safety of traditional Chinese medicine in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W.B., Liu L.N., Wang Z., Liu Y. Meta-analysis of the efficacy and safety of Lianhua Qingwen combined with Western medicine in the treatment of common patients with new coronary pneumonia. Hainan Yi Xue Yuan Xue Bao. 2020;26(14):1045–1050. [Chinese with abstract in English] [Google Scholar]
- 34.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y.L., Wang J.M., Yang C., Zhuang T.Y., Fu J.P. Effect of Lianhua Qingwen preparation on COVID-19: a meta-analysis. Tianjin Zhong Yi Yao. 2021;38(11):1414–1420. [Chinese with abstract in English] [Google Scholar]
- 36.Yin B., Bi Y.M., Sun L., Huang J.Z., Zhao J., Yao J., et al. Efficacy of integrated traditional Chinese and Western medicine for treating COVID-19: a systematic review and meta-analysis of RCTs. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.622707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng M., Li L., Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019 (COVID-19): meta-analysis of randomized controlled trials. PLoS One. 2020;15(9):e0238828. doi: 10.1371/journal.pone.0238828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar N., Awasthi A., Kumari A., Sood D., Jain P., Singh T., et al. Antitussive noscapine and antiviral drug conjugates as arsenal against COVID-19: a comprehensive chemoinformatics analysis. J Biomol Struct Dyn. 2022;40(1):101–116. doi: 10.1080/07391102.2020.1808072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee S., Kumar N., Sehrawat H., Yadav N., Mishra V. Click triazole as a linker for drug repurposing against SARs-CoV-2: a greener approach in race to find COVID-19 therapeutic. Curr Res Green Sustain Chem. 2021;4 [Google Scholar]
- 40.Kumar N., Admane N., Kumari A., Sood D., Grover S., Prajapati V.K., et al. Cytotoxic T-lymphocyte elicited vaccine against SARS-CoV-2 employing immunoinformatics framework. Sci Rep. 2021;11(1):7653. doi: 10.1038/s41598-021-86986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar N., Sood D., Chandra R. Design and optimization of a subunit vaccine targeting COVID-19 molecular shreds using an immunoinformatics framework. RSC Adv. 2020;10(59):35856–35872. doi: 10.1039/d0ra06849g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence (background document). (2011) [2022-05-10]. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 43.PLoS Medicine Editors. Best practice in systematic reviews: the importance of protocols and registration. PLoS Med 2011;8(2):e1001009. [DOI] [PMC free article] [PubMed]
- 44.Paez A. Grey literature: an important resource in systematic reviews. J Evid Based Med. 2017 doi: 10.1111/jebm.12265. [DOI] [PubMed] [Google Scholar]
- 45.Okagaki L.H., Dean R.A. The influence of funding sources on the scientific method. Mol Plant Pathol. 2016;17(5):651–653. doi: 10.1111/mpp.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiavo J.H. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.