Abstract
The use of heparin for anticoagulation has changed the face of cardiac surgery by allowing a bloodless and motionless surgical field throughout the introduction of cardiopulmonary bypass (CPB). However, heparin is a drug with complex pharmacologic properties that can cause significant interpatient differences in terms of responsiveness. Heparin resistance during CPB is a weighty issue due to the catastrophic consequences stemming from inadequate anticoagulation, and the treatment of it necessitates a rationalized stepwise approach due to the multifactorial contributions toward this entity. The widespread use of activated clotting time (ACT) as a measurement of anticoagulation during CPB is examined, as it may be a false indicator of heparin resistance. Heparin resistance also has been repeatedly reported in patients infected with COVID-19, which deserves further exploration in this pandemic era. This review aims to examine the variability in heparin potency, underlying mechanisms, and limitations of using ACT for monitoring, as well as provide a framework towards the current management of heparin resistance.
Key Words: albumin, anticoagulation, antithrombin, activated clotting time, COVID-19
THE USE OF cardiopulmonary bypass (CPB) in cardiac surgery has revolutionized the field and led to tremendous advancements by allowing a bloodless and motionless surgical field.1 The technical challenge of preventing thrombosis within the extracorporeal circuit (ECC) was overcome with the use of heparin2 , 3 and its effective antidote, protamine,4 , 5 which allowed for safe anticoagulation to be established and subsequently reversed at the end of surgery. The adequacy of heparin anticoagulation during CPB commonly is monitored using the activated clotting time (ACT),6 which is a point-of-care test of coagulation developed by Hattersley in 1966.7
However, a decreased responsiveness to heparin, also known as heparin resistance, can occur in some patients during CPB, leading to subtherapeutic ACT levels. Inadequate anticoagulation potentially may result in activation of the coagulation cascade, leading to complications such as consumptive coagulopathy, excessive postoperative bleeding, and thromboembolic phenomenon.8 Unfortunately, the target ACT, which balances the risks of circuit thrombosis and excessive bleeding, is still unclear, resulting in a wide variation of ACT targets, ranging from 400-to-500 seconds, used for initiation and maintenance of CPB in clinical practice.6 This has led to inconsistency in defining heparin resistance, with varying criteria used in the current literature for both the initial bolus dose of heparin and target ACT for initiating CPB.6 , 9
This review aims to examines the variability in heparin potency, underlying mechanisms, limitations of using ACT for monitoring, and the current management of heparin resistance. The impact of the ongoing COVID-19 pandemic on heparin responsiveness also is discussed.
Activation of the Hemostatic System During Cardiac Surgery
The function of the hemostatic system is to limit blood loss during vessel wall endothelial injury by forming a localized platelet-fibrin plug through activation of the coagulation cascade, which can occur via the extrinsic (tissue factor) or intrinsic (contact activation) pathways, though the latter has not been shown to play an important role for in vivo hemostasis.10 These 2 pathways eventually merge when factor X becomes activated and catalyzes the formation of thrombin from prothrombin (factor II) with the help of activated cofactor V. Thrombin, in turn, converts fibrinogen (factor I) to fibrin, which is important for clot stabilization. In addition, thrombin also is essential for the activation of platelets, factors V, VIII, and XI, as well as limiting clot propagation by activating protein C and releasing tissue plasminogen activator and tissue factor pathway inhibitor.11 These enzymatic reactions occur rapidly on the phospholipid surfaces of activated platelets to produce a platelet-fibrin clot, though newer in vivo imaging data have suggested that the activated endothelium adjacent to sites of injury may be an important biologic membrane surface as well.12
During CPB, both intrinsic and extrinsic pathways of the hemostatic system are activated by blood contact with foreign non-endothelial ECC surfaces, release of tissue factor from surgical manipulation, reinfusion of shed blood via cardiotomy suction, and systemic inflammatory responses.13 Thrombin generation has been shown to persist during CPB, with marked and sustained increases in thrombin activation markers, though much of the thrombin generated is in the non-hemostatic form, which gives rise to soluble fibrin.14 Thrombin bound to soluble and circuit-bound fibrin is resistant to antithrombin (AT) inhibition15; hence, adequate thrombin suppression is required to prevent thrombus formation and consumptive coagulopathy within the ECC.
Heparin
Unfractionated heparin (UFH) is the mainstay anticoagulant used for CPB during cardiac surgery today. The advantages of its use include the ease of administration, low cost, effectiveness, short half-life, and rapid antagonism by protamine.16 However, the variability in its pharmacologic properties has led to significant interpatient differences in heparin responsiveness, which have been observed both in vitro and in vivo.17 , 18
Unfractionated heparin is a heterogeneous mixture of naturally occurring, negatively charged, and highly sulfated polysaccharides with low- and high-molecular-weight fractions ranging from 1,000-to50,000 Daltons found in mast-cell granules.16 , 19 Pharmaceutical heparin is isolated and purified from either bovine or porcine mucosal tissues. Heparin exerts its anticoagulant action indirectly by binding to AT, a serine protease inhibitor, which accelerates the latter's irreversible inhibition of thrombin and activated factor X (factor Xa) and, to a lesser extent, activated factors IX, XI, and XII, plasmin, and kallikrein (Fig 1 ).20 However, variability in the response to heparin occurs as only one-third of heparin molecules possess the specific pentasaccharide sequence required for AT binding.21 In addition, AT-mediated thrombin inhibition requires long-chain heparin molecules with a minimum chain length of 18 oligosaccharide units.22 This serves as a template for the binding of AT and thrombin simultaneously, which accelerates thrombin inhibition by up to 4,000-fold.23
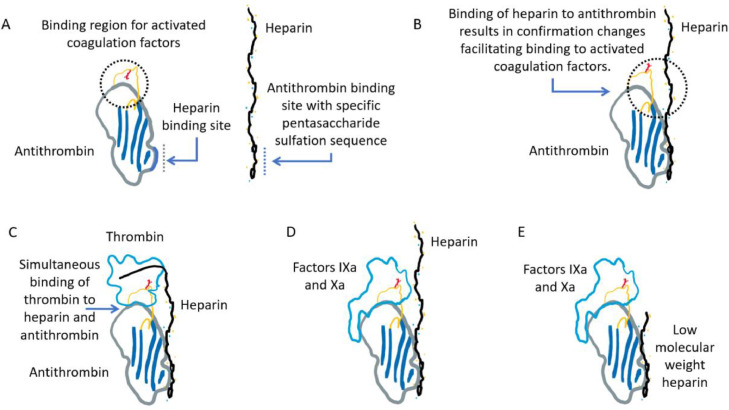
Fig 1.
Interactions among antithrombin (AT), heparin, and activated coagulation factors (thrombin in C and factor IXa or Xa in D and E). (A and B) Binding of AT to a specific pentasaccharide sulfation sequence within the heparin polymer induces a conformational change in the binding sites of AT for activated coagulation factors. (C) Both AT and thrombin bind to the same heparin chain. The ability of heparin to act as a template for AT and thrombin depends on its length and, thus, on its molecular weight. Approximately 18 monosaccharide units are minimal to bridge AT to thrombin. (D) Factors IXa and Xa bind to an allosteric site on the pentasaccharide-activated AT without simultaneous binding to the same heparin chain. The inhibition of factors IXa and Xa can occur with heparin lengths <18 monosaccharide units. (E) The binding and inhibition of factors IXa or Xa by AT in the presence of low-molecular-weight heparin.
Thrombin inhibition also occurs via an AT-independent pathway mediated by heparin cofactor II (HCII), which requires heparin molecules with chain lengths of ≥6 oligosaccharide units, though optimal inhibition of up to 10,000-fold is achieved with chain lengths of 20-to-24 oligosaccharide units.24 The HCII-heparin complex has the added advantage of inhibiting clot-bound thrombin, which can form during CPB and is otherwise resistant to AT-heparin complex inhibition.15 , 25 However, the physiologic importance of HCII as an anticoagulant remains unclear because its deficiency has not been proven to be strongly associated with prothrombotic pathologic states.26
All molecular weight fractions of heparin are able to mediate factor Xa inhibition via AT so long as they possess the specific pentasaccharide sequence, as this reaction does not require the simultaneous binding of factor Xa and AT.16 Heparin also has been shown to mediate the release of tissue factor pathway inhibitors27 and activate fibrinolysis.28
The pharmacokinetic properties of UFH also play a role in influencing its potency. Unfractionated heparin binds to a wide variety of positively charged plasma proteins, including platelet factor 4 (PF4), histidine-rich glycoprotein, lipoproteins, albumin, von Willebrand factor, factor VIII, and fibrinogen, as well as endothelial cells, platelets, and macrophages.29 , 30 In conditions such as thromboembolic disease or sepsis, in which there can be varying concentrations of these heparin-binding proteins, the anticoagulation response to UFH often is unpredictable.31, 32, 33, 34 These nonspecific interactions are facilitated by the long chain lengths (ie, higher molecular weights) of heparin found in UFH, which are present in much lower concentrations in low-molecular-weight heparin (LMWH) preparations, thus explaining the more consistent and predictable pharmacokinetic profile of the latter.35 , 36 Metabolism occurs primarily in the reticuloendothelial system, and ≤50% of UFH is excreted unchanged by the kidneys.37 Elimination occurs through the following 2 mechanisms: a rapid saturable pathway via the reticuloendothelial system, and a slower nonsaturable pathway via the kidneys.37 As a result, the biologic half-life of UFH is dependent on the dose administered, ranging from 30 minutes with an intravenous dose of 25 U/kg, 60 minutes with 100 U/kg, and up to 150 minutes with 400 U/kg.8 , 38 , 39 Hepatic and renal clearance also are reduced during CPB due to hypothermia and decreased organ perfusion, which may further prolong heparin's elimination half-life.40
Activated Clotting Time for Monitoring Coagulation
The ACT is considered to be the gold standard for monitoring anticoagulation during CPB.41 It is a modified Lee and White42 point-of-care test of whole blood coagulation involving platelet phospholipids in the hemostatic process, as opposed to other standard tests of coagulation, which primarily are focused on plasma hemostasis. The target ACT values maintained during CPB are usually between 400 and 480 seconds.6
Measurement of ACT involves the use of a contact activator, such as celite, kaolin, or glass beads, which activates the intrinsic and common pathways of the clotting cascade when exposed to fresh, whole blood by mimicking the negatively charged foreign surfaces of the CPB circuit. The blood sample is collected in a plastic syringe free of anticoagulant, and the test is performed immediately, as contact activation begins in the syringe. After warming the blood sample to 37°C, the ACT measurement device measures the time taken for fibrin clot formation. Therefore, different syringe materials or delays in testing may cause variability in the results. The different ACT measurement devices incorporate different technical methods to detect end-point ACT (eg, mechanical optical, photo-optical, amperometric, or photomechanical technology).43 Depending on the detection method used, the ACT either is derived or obtained in real time. Hence, ACT values obtained from different ACT measurement devices are not interchangeable, as different contact activators, sample volumes, and clot detection methodologies are employed.43 , 44 The advantages of using ACT for monitoring include its cost, simplicity, rapid turnover, fairly linear relationship with heparin concentrations >1 U/mL, and ability to detect the effects of heparin, even at the high concentrations required for cardiac surgeries (2-10 U/mL), which otherwise would render the activated partial thromboplastin time (aPTT) test unclottable.45
However, there are many factors that can influence the ACT during cardiac surgery, in addition to the different technologies employed to derive the ACT (Table 1 ).8 , 16 , 43 , 46 Studies have demonstrated a lack of correlation between ACT values and plasma heparin concentrations during CPB, and postulated that this could be due to the influence of hypothermia and hemodilution effects on the ACT assays.47, 48, 49
Table 1.
Factors Influencing Measurement of Activated Clotting Time
| Patient |
1. Preoperative medications
3. Factor deficiencies
|
| Cardiopulmonary bypass |
| 1. Hypothermia 2. Hemodilution |
| Method of evaluating ACT |
3. Derived or real time ACT |
| Intraoperative anticoagulant |
| 1. Source of heparin (eg, bovine, porcine) 2. Parenteral direct thrombin inhibitors (eg, bivalirudin, argatroban) |
Abbreviations: ACT, activated clotting time; DIVC, disseminated; DVT, deep vein thrombosis; intravascular coagulopathy.
Due to the limitations of using ACT for monitoring, alternative methods have been suggested, such as measuring whole blood heparin concentrations with the Hepcon Hemostasis Management System (Medtronic, Minneapolis, MN), though results regarding its correlation with plasma anti-Xa activity, traditionally considered to be the gold standard for measuring anticoagulation effects of heparin, have been mixed.49 , 50 The Hepcon HMS allows individualized heparin and protamine dosing based on each patient's responsiveness to heparin. At least 2.5 mL of non-heparinized blood are collected from the patient, and 0.4 mL are allocated by the machine to each of the 6 chambers within the heparin-dose response (HDR) cartridge. These 6 chambers contain varying concentrations of heparin—2 with no heparin, 2 with 1.5 U/mL of heparin, and 2 with 2.5 U/mL of heparin. The resulting ACT levels corresponding to the known heparin concentrations are plotted to determine the HDR curve from which a heparin loading dose can be calculated using the patient's estimated blood volume to achieve a target ACT. Heparin assay cartridges subsequently are used to measure circulating heparin concentrations during CPB using an automated heparin protamine titration method, which also can calculate the protamine dose required for heparin neutralization at the end of CPB.44 , 51 High-dose thrombin time (HiTT) is another point-of-care test that is more specific for the effects of heparin because it involves only the final common pathway of the clotting cascade and is unaffected by temperature and hematocrit changes during CPB.52
Heparin Resistance
Due to the widespread use of heparin for anticoagulation during CPB, a frequently encountered problem is that of heparin resistance. It is defined as the inability of an adequate dose of heparin to achieve a desired ACT or a decreased slope on the HDR curve.8 The incidence of heparin resistance is between 4% and 26%, depending on the initial heparin bolus administered and the target ACT level required for initiating CPB.6 , 8 A generally accepted definition is the need for >500 U/kg of heparin to achieve an ACT of 400-to-480 seconds.53 , 54
The HDR curve was described first by Bull et al,55 to account for the interindividual variability seen in heparin responsiveness, and may play a role in the early detection of heparin resistance.56 As previously mentioned, it is generated using a minimum of 2 ACT values (baseline and an ACT response to a known in vivo or in vitro concentration of heparin), though additional ACT values will provide more points for a better-fitted curve.55 Extrapolation of the HDR curve determines the optimal in vivo heparin concentration required to achieve a target ACT value, and the corresponding heparin loading dose can be calculated. Besides observing a decreased slope of the HDR curve, a heparin sensitivity index can be obtained using the slope values from the HDR curve to quantify heparin responsiveness. A heparin sensitivity index <1 s/U/kg usually is indicative of heparin resistance.57
Mechanisms of Heparin Resistance
Underlying causes of heparin resistance can be complex and usually are multifactorial, namely due to the variability of heparin potency on anticoagulation, as well as patient-specific factors (Table 2 ).8 , 9 In addition, there are limitations of using ACT for monitoring, which are not specific for the anticoagulation effects of heparin, thus complicating the clinical picture.
Table 2.
Mechanisms of Heparin Resistance
| Antithrombin Deficiency |
| 1. Congenital 2. Acquired
|
| Non-Antithrombin Mediated |
1. Increased heparin binding to other proteins, cells and non-endothelial surfaces
3. Low albumin concentrations ≤35 g/dL (albumin exhibits heparin-like action) 4. Preoperative relative hypovolemia (dehydration leading to increased concentration of other compatible molecules binding to heparin) 5. Medications (eg, andexanet alfa) |
Abbreviations: CPB, cardiopulmonary bypass; DIVC, disseminated intravascular coagulopathy; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; PE, pulmonary embolism; PF4, platelet factor 4.
Antithrombin Deficiency
Antithrombin deficiency long has been purported as the main cause of heparin resistance because heparin exerts its effects indirectly by catalyzing the anticoagulation activity of endogenous AT. Normal AT activity in adults ranges from 80% to 120%, and AT deficiency commonly is defined as AT activity <80%.58
Antithrombin deficiency can be either acquired or congenital. Several disease states or their treatments can contribute to acquired AT deficiency either by reduced synthesis or increased consumption. These conditions include heparin treatment, liver disease, malnutrition, nephrotic syndrome, sepsis, acute disseminated intravascular coagulopathy, asparaginase use in patients with acute leukemia, and the use of mechanical support devices, (eg, intra-aortic balloon pump, CPB, and extracorporeal membrane oxygenation [ECMO]).8 , 9 , 13 , 53 , 59 , 60 The ECC devices cause thrombin formation and AT depletion through the exposure of blood to ECC foreign surfaces and ischemic endothelium of the heart, inadequate heparinization, direct reinfusion of shed blood from cardiotomy suction, and exposure to non-heparinized blood.13 The mechanisms of heparin resistance are detailed in Table 2.
The preoperative use of heparin (24-48 hours) is thought to contribute to heparin resistance by depleting AT through the clearance of thrombin-AT complexes via the reticuloendothelial system,61 though this is seen mostly with UFH compared with LMWH preparations.8 , 53 , 54 , 62 This results in diminished AT reserves and subsequently reduced efficacy for heparinization prior to CPB.63 Despite this, several studies have shown that HDR is independent of AT concentrations,63, 64, 65, 66 and not all of the patients who had an inadequate ACT demonstrated low AT activity, questioning the validity of using ACT in this group of patients. A more suitable measure of anticoagulation, such as HiTT or anti-Xa levels, might be more appropriate in such circumstances.67 , 68
Non-Antithrombin–Mediated Mechanisms
As previously mentioned, heparin binds to a multitude of positively-charged plasma proteins and cellular components due to its strong negative charge. It also binds to a variety of cells, such as endothelial cells, platelets, and macrophages. In addition, heparin binds to the ECC, contributing to its reduced bioavailability.9 , 28 , 30
High platelet counts of ≥300,000 cells/mm3 have been found to increase heparin resistance.54 , 57 , 69 A possible explanation for this could be due to the activation of PF4, which is a strong inhibitor of heparin that is released by activated platelets.70 Heparin also displaces PF4 from its endothelial binding to heparinoids, increasing the plasma concentrations of PF4 and providing an additional mechanism for heparin resistance.57
Low albumin concentrations of ≤35 g/dL have been demonstrated to increase heparin resistance.54 Albumin possesses some structural similarity to heparin and seems to exert a heparin-like action. The highly negatively charged albumin molecule potentially could bind to positively charged groups on AT.71 Hence, hypoalbuminemia may act as a surrogate indicator for heparin resistance.
Relative hypovolemia also has been proposed as a possible contributor to heparin resistance.54 This could be due to the vasoconstriction from dehydration, concentrating plasma proteins and acute phase reactants per unit volume of blood, thereby increasing the chances of heparin binding to other compatible molecules instead of AT. Therefore, a larger dose of heparin would be required to overcome the competitive binding.
Several studies have shown increased factor VIII concentrations in patients with apparent heparin resistance.72, 73, 74, 75 Factor VIII is an acute-phase reactant and is increased in inflammatory states. Increased factor VIII activity augments the propagation phase of clotting and has been shown to reduce HDR.76 However, most of these studies measured heparin resistance with aPTT instead of ACT. An interesting point to note would be that, in such situations, anti-Xa activity was not influenced by increased levels of factor VIII, even though aPTT was shortened. Hence, aPTT underestimated the effects of anticoagulation with UFH, and anti-Xa activity would be a more accurate depiction of adequate heparinization in this scenario.74 However, the question now lies in whether increased factor VIII levels potentially could be thrombogenic and if therapeutic anti-Xa levels could lead to a false sense of security with regard to adequate anticoagulation. There currently is no clear answer to this question, and further research may be warranted.
A potential new contributor toward heparin resistance would be andexanet alfa, which is a recombinant coagulation factor Xa decoy protein recently introduced for the emergency reversal of anti-Xa-targeted direct oral anticoagulants.77 Clinical experience with andexanet alfa is limited for elective cardiac surgeries, but several case reports have documented difficulties with intraoperative heparinization after its administration.78, 79, 80 An abstract presented at the International Society on Thrombosis and Hemostasis suggested that andexanet alfa produces heparin resistance during CPB due to its binding to UFH-AT complexes and extreme doses of UFH and, sometimes, AT supplementation may be necessary to achieve adequate anticoagulation.81
Management of Heparin Resistance
The management of heparin resistance during CPB follows 4 general pathways. The first is to administer additional heparin to achieve the desired ACT. Secondly, fresh frozen plasma (FFP) is administered to provide additional AT. Thirdly, AT is supplemented via AT concentrate, and the last option is to accept the subtherapeutic ACT and commence CPB without any additional treatment.6 , 8 , 82, 83, 84
The additional dosing of heparin to counter heparin resistance is a commonly used practice for cardiothoracic anesthesiologists.8 Heparin usually is administered at 300-to-500 U/kg in an attempt to achieve an ACT of 400-to-480 seconds. In fact, the survey conducted by Sniecinski et al. showed that >30% of respondents (largest proportion) would administer additional heparin ≤600 U/kg, and a small percentage of respondents would administer >800 U/kg to achieve the desired ACT before commencing any alternative therapy.6 Although it is the simplest treatment for heparin resistance, high doses of heparin come with adverse consequences. For one, high doses of heparin may not necessarily prevent fibrin formation during CPB for patients with heparin resistance.85 The risks of heparin rebound and postoperative bleeding are increased, as higher concentrations of heparin also bind non-specifically to multiple plasma proteins—this provides a reservoir of heparin that dissociates over time and, in turn, binds with AT even after the reversal with protamine and clearance of heparin-protamine complexes.86 There also appears to be a ceiling dose with regard to anticoagulation for heparin, as blood concentrations of >4.1 U/mL (corresponding to 300 U/kg body weight) failed to increase ACT in an in vitro study conducted by Levy et al.87
Using FFP was one of the earliest treatments found to neutralize heparin resistance.88 , 89 Fresh frozen plasma contains approximately 1 IU of AT per mL; hence, usually, 500 mL (2 units) of FFP are given to provide 500 IU of AT.58 Despite its long history of use, there is a paucity of evidence on the treatment of heparin resistance with FFP. A systematic review by Spiess in 2008 showed that the administration of FFP may resolve heparin resistance only in some patients, and comes with added safety concerns (eg, transmission of viral infections, volume overload, and the risk of transfusion-related lung injury).90 In centers where FFP is not immediately available, a delay to the commencement of CPB is expected due to the need for thawing and transportation of FFP to the operating room.90 A best evidence topic review by Beattie et al. in 2014 concluded that FFP might not restore ACT to target values despite adequate heparinization in patients with heparin resistance,91 possibly because 500 mL of FFP were inadequate to restore heparin responsiveness, and much larger volumes were required.82 Thus far, no studies have demonstrated FFP to be beneficial in improving clinical outcomes for postoperative bleeding. Only 1 study in patients requiring ECMO support showed that early treatment with FFP in heparin-resistant patients improved survival substantially, though this result was not statistically significant, likely due to the small sample size.92 Considering the body of evidence, the recommendation is mostly for the supplementation with AT concentrate instead of FFP should it be available.82 , 84 , 90 , 91
Antithrombin concentrate has been approved for use by the United States Food and Drug Administration since the 1980s. It is currently the treatment of choice for heparin resistance in relation to AT deficiency, and its use is highly recommended to reduce plasma transfusions in patients with AT-mediated heparin resistance immediately before CPB (Class I, Level A evidence).93 Although the causes of reduction in plasma AT concentration mostly are acquired (in up to 23% of cardiac surgical patients) following preoperative heparin therapy, sepsis, and circulating blood through the ECC,57 , 90 , 94 a deficiency in AT also can be inherited in an autosomal dominant pattern, with a prevalence of 1:500 to 1:5000.95 There are 2 forms of AT concentrates available for use—purified human and recombinant forms. The human concentrate (hAT) is harvested from donated pools of plasma, which then undergoes a complex multistep extraction and purification process that includes pathogen testing before rendering it safe for use. Recombinant AT (rAT) is produced from transgenic goats and procured from milk.83 Hence, rAT should not be given to patients with a known sensitivity to goat milk proteins.8 Both forms of AT concentrates are similar and exhibit equivalent activity for in vitro thrombin and factor Xa inhibition assays. This is despite a 4-fold increase in heparin-binding affinity in rAT, as well as a shorter half-life (10.49 ± 7.19 hours for rAT compared with 56.8-68 hours for hAT), thus necessitating an infusion if rAT is to be used for extended periods.95 Antithrombin concentrate usually is dosed at 500-to-1000 IU (equivalent to 1-2 vials of hAT).58 , 83 , 96 , 97 Patnik et al. recommended a formula to estimate the initial dose of AT: AT dose (IU) = (desired minus current AT level as % of normal level) X weight (kg) divided by 1.4.95 However, this formula requires a laboratory AT level that may not be timely enough before CPB. In another study by Stammers et al, the average dose of AT concentrate required for the treatment of heparin resistance was found to be 1,029.0 ± 164.5 IU or 14.1 ± 3.4 IU/kg when normalized to body weight.83 Each vial is reconstituted with water for injection to 10 mL of solution, which obviates the need for the large volumes required to replenish plasma AT levels seen with plasma transfusions. . Although studies have shown that AT concentrate does not necessarily improve postoperative outcomes of bleeding as compared to treatment with FFP or no treatment at all,58 , 96, 97, 98 the majority of the evidence suggests that AT concentrate is a safe and effective method of restoring an adequate ACT for CPB.58 , 82 , 83 , 85 , 87 , 90 , 91 , 93 , 96 , 97 One prohibitive factor would be its cost, which can be 8 times that of 2 units of FFP,91 and another would be its lack of availability in certain institutions.6 In the circumstances in which cost is a concern, the authors' recommendation would be to administer 1 vial (500 IU) first, followed by a second vial should the repeat ACT be inadequate for CPB.
The last option of accepting a lower ACT for CPB is seldom executed due to the fear of catastrophic consequences from inadequate anticoagulation. Clinical practice guidelines generally recommend for the ACT to be kept >480 seconds so as to provide a margin of safety.41 , 99 The concept of a minimal ACT goes back to 1975 when Bull et al. demonstrated that CPB could be established safely at an ACT of 300 seconds, with no formation of small clots in the ECC, even at the conclusion of bypass.18 This then was challenged by Young et al. in 1978, who demonstrated that the minimal ACT to avoid fibrin monomer formation during CPB was ≥400 seconds.100 However, more recent studies have recognized that an ACT as low as 250 seconds has been accepted with no adverse consequences, especially if employing heparin-coated circuits or modern, minimally invasive ECC systems.101, 102, 103, 104 The studies that employed a fixed-dose regimen of heparin instead of pursuing a minimal ACT showed no increase in adverse outcomes.105, 106, 107 In particular, Metz and Keats administered a single dose of heparin (300 U/kg) and commenced CPB without additional treatment, even when the ACT was <400 seconds. There were no reports of clot formation in the CPB circuits nor an increase in postoperative bleeding.107 Shore-Lesserson et al. also demonstrated that adequate levels of anticoagulation are achieved despite a lower than threshold ACT when using HiTT as an alternative monitor of anticoagulation.67 Therefore, accepting a lower ACT for bypass in “heparin-resistant” patients might be a viable option should conventional treatments fail.
Alternatives for Anticoagulation
Direct thrombin inhibitors (DTI), such as bivalirudin and argatroban, have been established as substitutes for heparin in patients who have contraindications to heparin or protamine (eg, hypersensitivity reactions or heparin-induced thrombocytopenia).41 , 99 Bivalirudin is a recombinant hirudin analog, and argatroban is a synthetic L-arginine derivative.108 These agents directly inhibit thrombin without requiring AT, and may be alternative treatments for heparin resistance. However, the use of bivalirudin requires an avoidance of the stagnation of blood in the CPB circuit to avoid thrombosis—this requires prior modification of the ECC to allow for shunting and continuous flow of cardiotomy blood between the arterial inflow and venous circuit at the end of CPB. Bivalirudin has a relatively short half-life of 25 minutes in patients with normal renal function, which accounts for 20% of its elimination, and the remaining 80% is eliminated via proteolysis by thrombin and blood proteases.108 , 109 In areas of stasis or areas isolated from the circuit, bivalirudin levels may be depleted due to metabolism by thrombin.110 Although the decrease in bivalirudin via this mechanism may not be relevant, as bivalirudin is a reversible inhibitor of thrombin,109 slow thrombin accumulation in a stagnant reservoir eventually may result in thrombosis, rendering the cardiotomy blood unsafe for transfusion. Also, both bivalirudin and argatroban have no specific reversal agents available. Thus, the routine use of DTI is restricted to a skilled anesthesiologist-perfusion-surgical team.99
Nafamostat mesilate has emerged as another possible alternative for heparin. It is a short-acting synthetic protease inhibitor, and prolongs ACT by directly inhibiting thrombin, as well as other activated coagulation factors. It also inactivates fibrinolysis and platelet aggregation, as well as suppresses blood-foreign surface reaction by blocking activated factor XII.111, 112, 113 When administered intravenously, together with low-to-normal doses of heparin (not exceeding 500 U/kg) in cases of heparin resistance, nafamostat mesilate successfully overcame heparin resistance during the conduct of CPB without increasing the risk of perioperative ischemic stroke or death.113 , 114
Treatment Algorithm
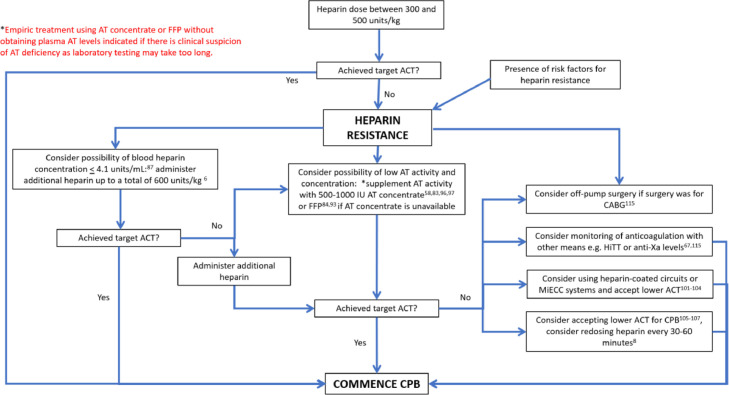
When faced with heparin resistance, it always is prudent to take a stepwise approach toward treatment instead of administering drugs and products without understanding the mechanism behind it. An easy algorithm to follow is the one adapted from Finley and Greenberg's review paper in 2013 (Fig 2 ).8
Fig 2.
Algorithm for treatment of heparin resistance. Flow chart adapted from Finley and Greenberg.8 ACT, activated clotting time; AT, antithrombin; CABG, coronary bypass graft surgery; CPB, cardiopulmonary bypass; FFP, fresh frozen plasma; HiTT, high-dose thrombin time; MiECC, minimally invasive extracorporeal circulation.
The first step would be to obtain a detailed preoperative history on whether heparin was administered or if the patient has any risk factors for acquired AT deficiency. Should a deficiency be suspected, plasma AT concentrations can be obtained, although it is not essential. Normal plasma concentrations of AT range from 112-to-140 μg/mL. Due to the presence of interlaboratory variations, most laboratories express the AT antigen and activity levels in terms of percentages, with a normal range between 80% and 120%, where 100% of AT corresponds to 1 unit of AT in 1 mL of reference plasma.95 If the AT activity levels are low, prebypass supplementation is possible with AT concentrate starting at 500-to-1,000 IU or with FFP should AT concentrate be unavailable,84 , 93 bearing in mind that a large volume of FFP beyond 500-to-1,000 mL may be required.82 In institutions that do not offer tests for plasma AT activity levels or where it is not practical to wait for laboratory determination of AT activity levels before the commencement of CPB, empiric treatment with AT concentrate is justified should there be a clinical suspicion of AT deficiency without laboratory testing.
For patients with normal AT activity levels, the dose of heparin can be up to 600 U/kg. If the ACT still remains persistently low, heparin concentrations can be obtained prior to increasing the dosage, as whole blood concentrations of >4.1 U/mL seem to have limited effects on ACT.87 Furthermore, increased doses of heparin also are associated with heparin rebound and postoperative bleeding.
In patients in whom both AT activity levels and heparin concentrations are adequate, the options would be to accept a lower ACT for CPB or to consider monitoring anticoagulation with other means, such as HiTT or anti-Xa levels.115 Cardiopulmonary bypass also can be initiated with lower than targeted ACT on a fixed-dose heparin regimen (ie, redosing heparin every 30-60 minutes).8 Additional heparin can be administered as well, though, as previously mentioned, there is no evidence for heparin concentrations >4.1 units/mL in improving anticoagulation.87 Alternatively, if the surgery was coronary artery bypass graft surgery, the surgeons can be engaged in a discussion as to whether the surgery can be performed without CPB as an off-pump procedure.115 Supplementation of AT concentrate to provide supraphysiologic activity levels of AT is an option to increase the ACT. However, if AT concentrations already are sufficient, the mechanism of AT deficiency is unlikely to be AT-mediated, and supplementation would be not only pricey but also ineffive.8 , 76 , 116
Finally, alternative anticoagulation, such as DTIs or nafamostat mesilate, may have a role to play in the management of heparin resistance, but the pros and cons should be weighed carefully before embarking on such a route.
Heparin Resistance in the COVID-19 Era
After SARS-CoV-2 first emerged in Wuhan city, Hubei province, China, in December 2019,117 anecdotal case reports and eventually observational studies have demonstrated a higher incidence of thromboembolic events in critically ill patients infected with COVID-19 despite anticoagulation.118 , 119 The underlying hyperinflammatory and hypercoagulopathic processes provoked by the COVID-19 infection120 , 121 place these patients at increased thrombotic risk, and possibly contribute to the development of heparin resistance both during CPB and in the intensive care unit.122, 123, 124 A retrospective cohort study conducted in 15 critically ill patients with COVID-19 infection receiving therapeutic anticoagulation demonstrated heparin resistance in 80% of patients on UFH and suboptimal peak anti-Xa activity in 100% of patients on LMWH, which did not correlate with either factor VIII, fibrinogen, or AT levels.123 Similarly, an observational study of patients with COVID-19 on continuous renal replacement therapy and ECMO noted that all of these patients conformed to the definition of heparin resistance, with no association with either AT, factor VIII, fibrinogen, thrombocytes, C-reactive protein, or ferritin.125
Much is not yet known about the underlying mechanisms of heparin resistance in COVID-19, and one postulated theory is that the increased concentrations of acute-phase proteins produced during the inflammatory process bind to heparin, resulting in a much lower plasma heparin concentration available for binding to AT and thrombin. In addition, elevated concentrations of factor VIII, fibrinogen, antiphospholipid antibodies, and von Willebrand factor, which commonly occur in inflammatory states and endothelial dysfunction, may contribute to heparin resistance as well.126 Further research is, therefore, needed in this area to explore the underlying pathogenesis and determine the optimal management of anticoagulation in this high-risk patient group.
Conclusions
Heparin resistance is a complex multifactorial issue that is related to both intrinsic as well as iatrogenic factors. Apart from understanding the pharmacology of heparin, clinicians need to examine the patient's medical history, and history of drug use, as well as remain cognizant of the limitations of ACT in determining adequacy of anticoagulation for CPB before embarking on a treatment plan. Accepting a lower ACT for CPB or considering off-pump surgery also may be viable alternatives should the conventional treatment strategies fail, especially if heparin-coated circuits or minimally invasive ECC systems are used for CPB. Moreover, the COVID-19 pandemic has presented a new group of patients who are at a higher risk of heparin resistance, thus compelling the need for further research to provide optimal care for this at-risk population.
Conflict of Interest
None.
References
- 1.Stoney WS. Evolution of cardiopulmonary bypass. Circulation. 2009;119:2844–2853. doi: 10.1161/CIRCULATIONAHA.108.830174. [DOI] [PubMed] [Google Scholar]
- 2.McLean J. The thromboplastic action of cephalin. Am J Physiol. 1916;41:250–257. [Google Scholar]
- 3.Howell WH, Holt E. Two new factors in blood coagulation: Heparin and pro-antithrombin. Am J Physiol. 1918;47:328–341. [Google Scholar]
- 4.Chargaff E, Olson K. Studies on the action of heparin and other anticoagulation. The influence of protamine on the anticoagulant effect in vivo. J Biol Chem. 1937;122:153–167. [Google Scholar]
- 5.Jorpes E, Edman P, Thaning T. Neutralisation of action of heparin by protamine. Lancet. 1939;2:975–976. [Google Scholar]
- 6.Sniecinski RM, Bennett-Guerrero E, Shore-Lesserson L. Anticoagulation management and heparin resistance during cardiopulmonary bypass: A survey of Society of Cardiovascular Anesthesiologists members. Anesth Analg. 2019;129:e41–e44. doi: 10.1213/ANE.0000000000003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattersley P. Activated coagulation time of whole blood. JAMA. 1966;196:150–154. [PubMed] [Google Scholar]
- 8.Finley A, Greenberg C. Heparin sensitivity and resistance: Management during cardiopulmonary bypass. Anesth Analg. 2013;116:1210–1222. doi: 10.1213/ANE.0b013e31827e4e62. [DOI] [PubMed] [Google Scholar]
- 9.Levy JH, Connors JM. Heparin resistance - clinical perspectives and management strategies. N Engl J Med. 2021;385:826–832. doi: 10.1056/NEJMra2104091. [DOI] [PubMed] [Google Scholar]
- 10.Hawiger J. Formation and regulation of platelet and fibrin hemostatic plug. Hum Pathol. 1987;18:111–122. doi: 10.1016/s0046-8177(87)80330-1. [DOI] [PubMed] [Google Scholar]
- 11.Crawley JT, Zanardelli S, Chion CK, et al. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5:S95–101. doi: 10.1111/j.1538-7836.2007.02500.x. [DOI] [PubMed] [Google Scholar]
- 12.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood. 2014;124:1705–1714. doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–1333. doi: 10.1213/ANE.0b013e3182354b7e. [DOI] [PubMed] [Google Scholar]
- 14.Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood. 2003;101:4355–4362. doi: 10.1182/blood-2002-08-2400. [DOI] [PubMed] [Google Scholar]
- 15.Weitz JI, Hudoba M, Massel D, et al. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Despotis GJ, Gravlee G, Filos K, et al. Anticoagulation monitoring during cardiac surgery: A review of current and emerging techniques. Anesthesiology. 1999;91:1122–1151. doi: 10.1097/00000542-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsson TD, Wolfram KM. Intersubject variability in the anticoagulant response to heparin in vitro. Eur J Clin Pharmacol. 1982;21:491–497. doi: 10.1007/BF00542044. [DOI] [PubMed] [Google Scholar]
- 18.Bull BS, Korpman RA, Huse WM, et al. Heparin therapy during extracorporeal circulation. I. Problems inherent in existing heparin protocols. J Thorac Cardiovasc Surg. 1975;69:674–684. [PubMed] [Google Scholar]
- 19.Humphries DE, Wong GW, Friend DS, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 20.Danishefsky I, Zweben A, Slomiany BL. Human antithrombin III. Carbohydrate components and associated glycolipid. J Biol Chem. 1978;253:32–37. [PubMed] [Google Scholar]
- 21.Choay J, Petitou M, Lormeau JC, et al. Structure-activity relationship in heparin: A synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983;116:492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- 22.Bray B, Lane DA, Freyssinet JM, et al. Anti-thrombin activities of heparin. Effect of saccharide chain length on thrombin inhibition by heparin cofactor II and by antithrombin. Biochem J. 1989;262:225–232. doi: 10.1042/bj2620225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan RE, Oosta GM, Gardner WT, et al. The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin. J Biol Chem. 1980;255:10081–10090. [PubMed] [Google Scholar]
- 24.Tollefsen DM. Insight into the mechanism of action of heparin cofactor II. Thromb Haemost. 1995;74:1209–1214. [PubMed] [Google Scholar]
- 25.Bendayan P, Boccalon H, Dupouy D, et al. Dermatan sulfate is a more potent inhibitor of clot-bound thrombin than unfractionated and low molecular weight heparins. Thromb Haemost. 1994;71:576–580. [PubMed] [Google Scholar]
- 26.Corral J, Aznar J, Gonzalez-Conejero R, et al. Homozygous deficiency of heparin cofactor II: Relevance of P17 glutamate residue in serpins, relationship with conformational diseases, and role in thrombosis. Circulation. 2004;110:1303–1307. doi: 10.1161/01.CIR.0000140763.51679.D9. [DOI] [PubMed] [Google Scholar]
- 27.Abildgaard U. Heparin/low molecular weight heparin and tissue factor pathway inhibitor. Haemostasis. 1993;23:S103–S106. doi: 10.1159/000216918. [DOI] [PubMed] [Google Scholar]
- 28.Upchurch GR, Valeri CR, Khuri SF, et al. Effect of heparin on fibrinolytic activity and platelet function in vivo. Am J Physiol. 1996;271:H528–H534. doi: 10.1152/ajpheart.1996.271.2.H528. [DOI] [PubMed] [Google Scholar]
- 29.Lane DA, Pejler G, Flynn AM, et al. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor 4. J Biol Chem. 1986;261:3980–3986. [PubMed] [Google Scholar]
- 30.Weiss RJ, Esko JD, Tor Y. Targeting heparin and heparan sulfate protein interactions. Org Biomol Chem. 2017;15:5656–5668. doi: 10.1039/c7ob01058c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosmi B, Fredenburgh JC, Rischke J, et al. Effect of nonspecific binding to plasma proteins on the antithrombin activities of unfractionated heparin, low-molecular-weight heparin, and dermatan sulfate. Circulation. 1997;95:118–124. doi: 10.1161/01.cir.95.1.118. [DOI] [PubMed] [Google Scholar]
- 32.Manson L, Weitz JI, Podor TJ, et al. The variable anticoagulant response to unfractionated heparin in vivo reflects binding to plasma proteins rather than clearance. J Lab Clin Med. 1997;130:649–655. doi: 10.1016/s0022-2143(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 33.Young E, Prins M, Levine MN, et al. Heparin binding to plasma proteins, an important mechanism for heparin resistance. Thromb Haemost. 1992;67:639–643. [PubMed] [Google Scholar]
- 34.Hage A, Louzada M, Kiaii B. Sepsis-induced heparin resistance during extracorporeal membrane oxygenation. CMAJ. 2019;191:E283–E285. doi: 10.1503/cmaj.181061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:S188–S203. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- 36.Young E, Wells P, Holloway S, et al. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost. 1994;71:300–304. [PubMed] [Google Scholar]
- 37.Boneu B, Caranobe C, Sie P. Pharmacokinetics of heparin and low molecular weight heparin. Baillieres Clin Haematol. 1990;3:531–544. doi: 10.1016/s0950-3536(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 38.Olsson P, Lagergren H, Ek S. The elimination from plasma of intravenous heparin: An experimental study on dogs and humans. Acta Med Scand. 1963;173:619–630. doi: 10.1111/j.0954-6820.1963.tb17446.x. [DOI] [PubMed] [Google Scholar]
- 39.Bjornsson TD, Wolfram KM, Kitchell BB. Heparin kinetics determined by three assay methods. Clin Pharmacol Ther. 1982;31:104–113. doi: 10.1038/clpt.1982.16. [DOI] [PubMed] [Google Scholar]
- 40.Buylaert WA, Herregods LL, Mortier EP, et al. Cardiopulmonary bypass and the pharmacokinetics of drugs. An update. Clin Pharmacokinet. 1989;17:10–26. doi: 10.2165/00003088-198917010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Shore-Lesserson L, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105:650–662. doi: 10.1016/j.athoracsur.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 42.Lee RI, White PD. A clinical study of the coagulation time of blood. Amer J Med Sci. 1913;145:495–503. [Google Scholar]
- 43.Li H, Serrick C, Rao V, et al. A comparative analysis of four activated clotting time measurement devices in cardiac surgery with cardiopulmonary bypass. Perfusion. 2021;36:610–619. doi: 10.1177/0267659120949351. [DOI] [PubMed] [Google Scholar]
- 44.Prisco D, Paniccia R. Point-of-care testing of hemostasis in cardiac surgery. Thromb J. 2003;1:1. doi: 10.1186/1477-9560-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry DJ, Fitzmaurice DA, Kitchen S, et al. Point-of-care testing in haemostasis. Br J Haematol. 2010;150:501–514. doi: 10.1111/j.1365-2141.2010.08223.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang JS, Lin CY, Hung WT, et al. Monitoring of heparin-induced anticoagulation with kaolin-activated clotting time in cardiac surgical patients treated with aprotinin. Anesthesiology. 1992;77:1080–1084. doi: 10.1097/00000542-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Culliford AT, Gitel NS, Starr N, et al. Lack of correlation between activated clotting time and plasma heparin level during cardiopulmonary bypass. Ann Surg. 1981;193:105–111. doi: 10.1097/00000658-198101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ottesen S, Stormorken H, Hatteland K. The value of activated coagulation time in monitoring heparin therapy during extracorporeal circulation. Scand J Thorac Cardiovasc Surg. 1984;18:123–128. doi: 10.3109/14017438409102391. [DOI] [PubMed] [Google Scholar]
- 49.Despotis GJ, Summerfield AL, Joist JH, et al. Comparison of activated coagulation time and whole blood heparin measurements with laboratory plasma anti-Xa heparin concentration in patients having cardiac operations. J Thorac Cardiovasc Surg. 1994;108:1076–1082. [PubMed] [Google Scholar]
- 50.Hardy JF, Bélisle S, Robitaille D, et al. Measurement of heparin concentration in whole blood with the Hepcon/HMS device does not agree with laboratory determination of plasma heparin concentration using a chromogenic substrate for activated factor X. J Thorac Cardiovasc Surg. 1996;112:154–161. doi: 10.1016/s0022-5223(96)70191-5. [DOI] [PubMed] [Google Scholar]
- 51.Ural K, Owen C. Pro: The Hepcon HMS should be used instead of traditional activated clotting time (ACT) to dose heparin and protamine for cardiac surgery requiring cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30:1727–1729. doi: 10.1053/j.jvca.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang JS, Lin CY, Karp RB. Comparison of high-dose thrombin time with activated clotting time for monitoring of anticoagulant effects of heparin in cardiac surgical patients. Anesth Analg. 1994;79:9–13. doi: 10.1213/00000539-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Staples MH, Dunton RF, Karlson KJ, et al. Heparin resistance after preoperative heparin therapy or intraaortic balloon pumping. Ann Thorac Surg. 1994;57:1211–1216. doi: 10.1016/0003-4975(94)91359-5. [DOI] [PubMed] [Google Scholar]
- 54.Chan T, Hwang NC, Lim CH. A statistical analysis of factors predisposing patients to heparin resistance. Perfusion. 2006;21:99–103. doi: 10.1191/0267659106pf855oa. [DOI] [PubMed] [Google Scholar]
- 55.Bull BS, Huse WM, Brauer FS, et al. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–689. [PubMed] [Google Scholar]
- 56.Ichikawa J, Mori T, Kodaka M, et al. Changes in heparin dose response slope during cardiac surgery: Possible result in inaccuracy in predicting heparin bolus dose requirement to achieve target ACT. Perfusion. 2017;32:474–480. doi: 10.1177/0267659117692661. [DOI] [PubMed] [Google Scholar]
- 57.Ranucci M, Isgrò G, Cazzaniga A, et al. Different patterns of heparin resistance: Therapeutic implications. Perfusion. 2002;17:199–204. doi: 10.1191/0267659102pf562oa. [DOI] [PubMed] [Google Scholar]
- 58.Lemmer JH, Jr, Despotis GJ. Antithrombin III concentrate to treat heparin resistance in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2002;123:213–217. doi: 10.1067/mtc.2002.119060. [DOI] [PubMed] [Google Scholar]
- 59.Bucur SZ, Levy JH, Despotis GJ, et al. Uses of antithrombin III concentrate in congenital and acquired deficiency states. Transfusion. 1998;38:481–498. doi: 10.1046/j.1537-2995.1998.38598297219.x. [DOI] [PubMed] [Google Scholar]
- 60.Wang TF, Makar RS, Antic D, et al. Management of hemostatic complications in acute leukemia: Guidance from the SSC of the ISTH. J Thromb Haemost. 2020;18:3174–3183. doi: 10.1111/jth.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthai WH, Jr, Kurnik PB, Groh WC, et al. Antithrombin activity during the period of percutaneous coronary revascularization: relation to heparin use, thrombotic complications and restenosis. J Am Coll Cardiol. 1999;33:1248–1256. doi: 10.1016/s0735-1097(98)00696-2. [DOI] [PubMed] [Google Scholar]
- 62.Hansen JB, Sandset PM, Huseby KR, et al. Differential effect of unfractionated heparin and low molecular weight heparin on intravascular tissue factor pathway inhibitor: Evidence for a difference in antithrombotic action. Br J Haematol. 1998;101:638–646. doi: 10.1046/j.1365-2141.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 63.Linden MD, Schneider M, Baker S, et al. Decreased concentration of antithrombin after preoperative therapeutic heparin does not cause heparin resistance during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2004;18:131–135. doi: 10.1053/j.jvca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 64.Garvin S, Fitzgerald D, Muehlschlegel JD, et al. Heparin dose response is independent of preoperative antithrombin activity in patients undergoing coronary artery bypass graft surgery using low heparin concentrations. Anesth Analg. 2010;111:856–861. doi: 10.1213/ANE.0b013e3181ce1ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esposito RA, Culliford AT, Colvin SB, et al. Heparin resistance during cardiopulmonary bypass. The role of heparin pretreatment. J Thorac Cardiovasc Surg. 1983;85:346–353. [PubMed] [Google Scholar]
- 66.Nicholson SC, Keeling DM, Sinclair ME, et al. Heparin pretreatment does not alter heparin requirements during cardiopulmonary bypass. Br J Anaesth. 2001;87:844–847. doi: 10.1093/bja/87.6.844. [DOI] [PubMed] [Google Scholar]
- 67.Shore-Lesserson L, Manspeizer HE, Bolastig M, et al. Anticoagulation for cardiac surgery in patients receiving preoperative heparin: Use of the high-dose thrombin time. Anesth Analg. 2000;90:813–818. doi: 10.1097/00000539-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Falter F, MacDonald S, Matthews C, et al. Evaluation of point-of-care ACT coagulometers and anti-Xa activity during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2020;34:2921–2927. doi: 10.1053/j.jvca.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 69.Gayoso JM. 5-year incidence of thrombocytosis and the effect on heparin dose response and heparin requirements. J Extra Corpor Technol. 1999;31:184–190. [PubMed] [Google Scholar]
- 70.Kaplan KL, Owen J. Plasma levels of platelet secretory proteins. Crit Rev Oncol Hematol. 1986;5:235–255. doi: 10.1016/s1040-8428(86)80040-3. [DOI] [PubMed] [Google Scholar]
- 71.Pulimood TB, Park GR. Debate: Albumin administration should be avoided in the critically ill. Crit Care. 2000;4:151–155. doi: 10.1186/cc688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumano N, Ikeda S, Arimori Y, et al. Heparin resistance associated with elevated factor VIII. Masui. 2008;57:471–473. [PubMed] [Google Scholar]
- 73.Thota R, Ganti AK, Subbiah S. Apparent heparin resistance in a patient with infective endocarditis secondary to elevated factor VIII levels. J Thromb Thrombolysis. 2012;34:132–134. doi: 10.1007/s11239-012-0692-z. [DOI] [PubMed] [Google Scholar]
- 74.Mitsuguro M, Okamoto A, Shironouchi Y, et al. Effects of factor VIII levels on the APTT and anti-Xa activity under a therapeutic dose of heparin. Int J Hematol. 2015;101:119–125. doi: 10.1007/s12185-014-1702-z. [DOI] [PubMed] [Google Scholar]
- 75.Connors JM, Levy JH. Covid-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2340. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Despotis GJ, Levine V, Joist JH, et al. Antithrombin III during cardiac surgery: Effect on response of activated clotting time to heparin and relationship to markers of hemostatic activation. Anesth Analg. 1997;85:498–506. doi: 10.1097/00000539-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Sartori M, Cosmi B. Andexanet alfa to reverse the anticoagulant activity of factor Xa inhibitors: A review of design, development and potential place in therapy. J Thromb Thrombolysis. 2018;45:345–352. doi: 10.1007/s11239-018-1617-2. [DOI] [PubMed] [Google Scholar]
- 78.Watson CJ, Zettervall SL, Hall MM, et al. Difficult intraoperative heparinization following andexanet alfa administration. Clin Pract Cases Emerg Med. 2019;3:390–394. doi: 10.5811/cpcem.2019.9.43650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eche IM, Elsamadisi P, Wex N, et al. Intraoperative unfractionated heparin unresponsiveness during endovascular repair of a ruptured abdominal aortic aneurysm following administration of andexanet alfa for the reversal of rivaroxaban. Pharmacotherapy. 2019;39:861–865. doi: 10.1002/phar.2306. [DOI] [PubMed] [Google Scholar]
- 80.Apostel HJCL, Winckers K, Bidar E, et al. Successful antithrombin administration in andexanet alfa-associated heparin resistance. J Cardiothorac Vasc Anesth. 2021;35:904–907. doi: 10.1053/j.jvca.2020.10.042. [DOI] [PubMed] [Google Scholar]
- 81.Thalji N, Chabata C, Patel P, et al. Characterization of andexanet alfa-associated heparin resistance: Implications for management [abstract]. Available at: https://abstracts.isth.org/abstract/characterization-of-andexanet-alfa-associated-heparin-resistance-implications-for-management/. Accessed June 10, 2022.
- 82.Avidan MS, Levy JH, van Aken H, et al. Recombinant human antithrombin III restores heparin responsiveness and decreases activation of coagulation in heparin-resistant patients during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130:107–113. doi: 10.1016/j.jtcvs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 83.Stammers AH, Francis SG, Miller R, et al. Application of goal-directed therapy for the use of concentrated antithrombin for heparin resistance during cardiac surgery. Perfusion. 2021;36:171–182. doi: 10.1177/0267659120926089. [DOI] [PubMed] [Google Scholar]
- 84.O'Carroll-Kuehn BU, Meeran H. Management of coagulation during cardiopulmonary bypass. BJA Educ. 2007;7:195–198. [Google Scholar]
- 85.Koster A, Fischer T, Gruendel M. Management of heparin resistance during cardiopulmonary bypass: The effect of five different anticoagulation strategies on hemostatic activation. J Cardiothorac Vasc Anesth. 2003;17:171–175. doi: 10.1053/jcan.2003.42. [DOI] [PubMed] [Google Scholar]
- 86.Galeone A, Rotunno C, Guida P, et al. Monitoring incomplete heparin reversal and heparin rebound after cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27:853–858. doi: 10.1053/j.jvca.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 87.Levy JH, Montes F, Szlam F, et al. The in vitro effects of antithrombin III on the activated coagulation time in patients on heparin therapy. Anesth Analg. 2000;90:1076–1079. doi: 10.1097/00000539-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Soloway HB, Christiansen TW. Heparin anticoagulation during cardiopulmonary bypass in an antithrombin-III deficient patient. Implications relative to the etiology of heparin rebound. Am J Clin Pathol. 1980;73:723–725. doi: 10.1093/ajcp/73.5.723. [DOI] [PubMed] [Google Scholar]
- 89.Sabbagh AH, Chung GK, Shuttleworth P, et al. Fresh frozen plasma: A solution to heparin resistance during cardiopulmonary bypass. Ann Thorac Surg. 1984;37:466–468. doi: 10.1016/s0003-4975(10)61132-0. [DOI] [PubMed] [Google Scholar]
- 90.Spiess BD. Treating heparin resistance with antithrombin or fresh frozen plasma. Ann Thorac Surg. 2008;85:2153–2160. doi: 10.1016/j.athoracsur.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 91.Beattie GW, Jeffrey RR. Is there evidence that fresh frozen plasma is superior to antithrombin administration to treat heparin resistance in cardiac surgery? Interact Cardiovasc Thorac Surg. 2014;18:117–120. doi: 10.1093/icvts/ivt327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khazi FM, Elhoufi AM, AbdelAziz TA, et al. Fresh frozen plasma: A solution to heparin resistance during extracorporeal membrane oxygenation (ECMO) Egypt J Crit Care Med. 2018;6:79–86. [Google Scholar]
- 93.Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. J Cardiothorac Vasc Anesth. 2021;35:2569–2591. doi: 10.1053/j.jvca.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Na S, Shim JK, Chun DH, et al. Stabilized infective endocarditis and altered heparin responsiveness during cardiopulmonary bypass. World J Surg. 2009;33:1862–1867. doi: 10.1007/s00268-009-0107-2. [DOI] [PubMed] [Google Scholar]
- 95.Patnaik MM, Moll S. Inherited antithrombin deficiency: A review. Haemophilia. 2008;14:1229–1239. doi: 10.1111/j.1365-2516.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 96.Lund PE, Wassbäck G, Thomas O, et al. Comparison of two infusion rates of antithrombin concentrate in cardiopulmonary bypass surgery. Perfusion. 2010;25:305–312. doi: 10.1177/0267659110377677. [DOI] [PubMed] [Google Scholar]
- 97.Williams MR, D'Ambra AB, Beck JR, et al. A randomized trial of antithrombin concentrate for treatment of heparin resistance. Ann Thorac Surg. 2000;70:873–877. doi: 10.1016/s0003-4975(00)01550-2. [DOI] [PubMed] [Google Scholar]
- 98.Avidan MS, Levy JH, Scholz J, et al. A phase III, double-blind, placebo-controlled, multicenter study on the efficacy of recombinant human antithrombin in heparin-resistant patients scheduled to undergo cardiac surgery necessitating cardiopulmonary bypass. Anesthesiology. 2005;102:276–284. doi: 10.1097/00000542-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Kunst G, Milojevic M, Authors/Task Force Members 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Br J Anaesth. 2019;123:713–757. doi: 10.1016/j.bja.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 100.Young JA, Kisker CT, Doty DB. Adequate anticoagulation during cardiopulmonary bypass determined by activated clotting time and the appearance of fibrin monomer. Ann Thorac Surg. 1978;26:231–240. doi: 10.1016/s0003-4975(10)63676-4. [DOI] [PubMed] [Google Scholar]
- 101.Øvrum E, Tangen G, Tølløfsrud S, et al. Heparinized cardiopulmonary bypass circuits and low systemic anticoagulation: An analysis of nearly 6000 patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141:1145–1149. doi: 10.1016/j.jtcvs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 102.Øvrum E, Tangen G, Oystese R, et al. Comparison of two heparin-coated extracorporeal circuits with reduced systemic anticoagulation in routine coronary artery bypass operations. J Thorac Cardiovasc Surg. 2001;121:324–330. doi: 10.1067/mtc.2001.111205. [DOI] [PubMed] [Google Scholar]
- 103.Mahmood S, Bilal H, Zaman M, et al. Is a fully heparin-bonded cardiopulmonary bypass circuit superior to a standard cardiopulmonary bypass circuit? Interact Cardiovasc Thorac Surg. 2012;14:406–414. doi: 10.1093/icvts/ivr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bauer A, Hausmann H, Schaarschmidt J, et al. Is 300 aeconds ACT safe and efficient during MiECC procedures? Thorac Cardiovasc Surg. 2019;67:191–202. doi: 10.1055/s-0037-1609019. [DOI] [PubMed] [Google Scholar]
- 105.Gravlee GP, Haddon WS, Rothberger HK, et al. Heparin dosing and monitoring for cardiopulmonary bypass. A comparison of techniques with measurement of subclinical plasma coagulation. J Thorac Cardiovasc Surg. 1990;99:518–527. [PubMed] [Google Scholar]
- 106.Neema PK, Sinha PK, Rathod RC. Activated clotting time during cardiopulmonary bypass: Is repetition necessary during open heart surgery? Asian Cardiovasc Thorac Ann. 2004;12:47–52. doi: 10.1177/021849230401200112. [DOI] [PubMed] [Google Scholar]
- 107.Metz S, Keats AS. Low activated coagulation time during cardiopulmonary bypass does not increase postoperative bleeding. Ann Thorac Surg. 1990;49:440–444. doi: 10.1016/0003-4975(90)90251-z. [DOI] [PubMed] [Google Scholar]
- 108.Koster A, Faraoni D, Levy JH. Argatroban and bivalirudin for perioperative anticoagulation in cardiac surgery. Anesthesiology. 2018;128:390–400. doi: 10.1097/ALN.0000000000001976. [DOI] [PubMed] [Google Scholar]
- 109.McNair E, Marcoux JA, Bally C, et al. Bivalirudin as an adjunctive anticoagulant to heparin in the treatment of heparin resistance during cardiopulmonary bypass-assisted cardiac surgery. Perfusion. 2016;31:189–199. doi: 10.1177/0267659115583525. [DOI] [PubMed] [Google Scholar]
- 110.Dyke CM, Smedira NG, Koster A, et al. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The EVOLUTION-ON study. J Thorac Cardiovasc Surg. 2006;131:533–539. doi: 10.1016/j.jtcvs.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 111.Usui A, Hiroura M, Kawamura M, et al. Nafamostat mesilate reduces blood-foreign surface reactions similar to biocompatible materials. Ann Thorac Surg. 1996;62:1404–1411. doi: 10.1016/0003-4975(96)00634-0. [DOI] [PubMed] [Google Scholar]
- 112.Kaminishi Y, Hiramatsu Y, Watanabe Y, et al. Effects of nafamostat mesilate and minimal-dose aprotinin on blood-foreign surface interactions in cardiopulmonary bypass. Ann Thorac Surg. 2004;77:644–650. doi: 10.1016/S0003-4975(03)01513-3. [DOI] [PubMed] [Google Scholar]
- 113.Kikura M, Tanaka K, Hiraiwa T, et al. Nafamostat mesilate, as a treatment for heparin resistance, is not associated with perioperative ischemic stroke in patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2012;26:239–244. doi: 10.1053/j.jvca.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 114.Sakamoto T, Kano H, Miyahara S, et al. Efficacy of nafamostat mesilate as anticoagulation during cardiopulmonary bypass for early surgery in patients with active infective endocarditis complicated by stroke. J Heart Valve Dis. 2014;23:744–751. [PubMed] [Google Scholar]
- 115.Edwards JK, Sniecinski RM, Scott KJ. Non-antithrombin-mediated heparin resistance during cardiac surgery: Two case reports. A A Pract. 2019;13:211–214. doi: 10.1213/XAA.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 116.Slaughter TF, Mark JB, El-Moalem H, et al. Hemostatic effects of antithrombin III supplementation during cardiac surgery: Results of a prospective randomized investigation. Blood Coagul Fibrinolysis. 2001;12:25–31. doi: 10.1097/00001721-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montandrau O, Arana H, Ehooman F, et al. Surgical revascularization with cardiopulmonary bypass on a patient with severe COVID-19. Semin Cardiothorac Vasc Anesth. 2021;25:46–50. doi: 10.1177/1089253220966515. [DOI] [PubMed] [Google Scholar]
- 123.White D, MacDonald S, Bull T, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50:287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beun R, Kusadasi N, Sikma M, et al. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:S19–520. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Streng AS, Delnoij TSR, Mulder MMG, et al. Monitoring of unfractionated heparin in severe COVID-19: An observational study of patients on CRRT and ECMO. TH Open. 2020;4:e365–e375. doi: 10.1055/s-0040-1719083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]