Abstract
The thymocyte selection-related HMG box protein (TOX) subfamily comprises evolutionarily conserved DNA-binding proteins, and is expressed in certain immune cell subsets and plays key roles in the development of CD4+ T cells, innate lymphoid cells (ILCs), T follicular helper (Tfh) cells, and in CD8+ T-cell exhaustion. Although its roles in CD4+ T and natural killer (NK) cells have been extensively studied, recent findings have demonstrated previously unknown roles for TOX in the development of ILCs, Tfh cells, as well as CD8+ T-cell exhaustion; however, the molecular mechanism underlying TOX regulation of these immune cells remains to be elucidated. In this review, we discuss recent studies on the influence of TOX on the development of various immune cells and CD8+ T-cell exhaustion and the roles of specific TOX family members in the immune system. Moreover, this review suggests candidate regulatory targets for cell therapy and immunotherapies.
Keywords: TOX, TOX subfamily, innate lymphoid cells, follicular helper T, CD8+ T-cell exhaustion
Graphical Abstract
Introduction
Thymocyte selection-related HMG box protein (TOX), TOX2, TOX3, and TOX4 are four-related HMG-box proteins with similar HMG-box DNA-binding domain and genomic organization, as well as an N-terminal domain, which has a transactivation activity and approximately 30–40% sequence identity among the TOX subfamily members. In contrast, the C-terminal domain is specific to different family members, which is suggestive of the distinct function of individual proteins [1]. Although they share a structural similarity, the biological roles of various TOX family members vary [1]. For example, the characterized function of TOX involves the development of the adaptive and the innate immune system. In addition, it acts during multiple stages of mammalian corticogenesis and is associated with the occurrence, diagnosis, and classification of cutaneous T-cell lymphoma (CTCL) [2–4]. TOX’s roles in growth regulation, DNA repair, and genomic instability in T-cell acute lymphoblastic leukemia have also been reported [5]. Meanwhile, TOX2, TOX3, and TOX4 are also involved in the natural killer (NK) cell development [6], reproductive organ function [7, 8], cell cycle regulation [9], neuronal cell survival [10], chromatin structural regulation [9, 11], and cancer progression [12–14].
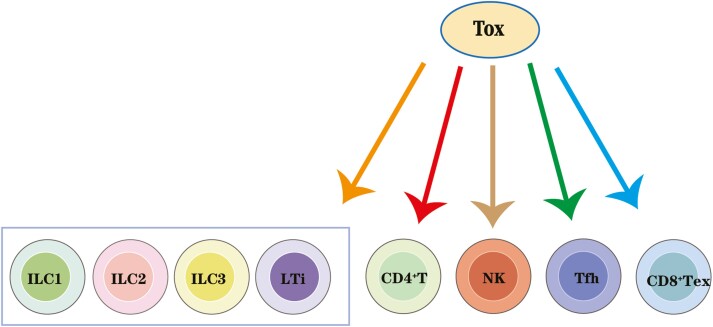
TOX was originally discovered as a thymic transcription factor specifically upregulated in CD4+CD8+ double-positive (DP) thymocytes throughout the differentiation stages during thymic positive selection [15]. As a transcription factor with a decisive role in the development of immune cell subtypes, the roles of TOX in CD4+ T, NK, and lymphoid tissue inducer (LTi) cells have been previously reviewed by Aliahmad et al [16]. TOX regulates transcriptional factor ThPOK and CD4+T cells thereby modulating CD4+ T lineage development, while also affecting that of NK cells by reducing the expression of DNA binding 2 (Id2) and T-bet inhibitors. Furthermore, TOX is essential for LTi cell development [16]. Recent studies have reported novel roles for TOX during the development of innate lymphoid cells (ILCs) and T follicular helper (Tfh) cells, as well as in the regulation of CD8+ T-cell exhaustion [17–21]. The specific immune-related functions of TOX2, 3, and 4 are also of great relevance. TOX2 co-operates with TOX to regulate normal NK cell development [6] and Tfh development [22]. Besides, TOX2 is also a key factor in the transcriptional program of CD8+ T-cell exhaustion downstream of calcineurin-dependent activation of nuclear factor of activated T cells (NFAT) [23]. Conversely, TOX3 influences neuronal [10] and breast cancer [13, 14, 24] cells survival. As a potential T-cell exhaustion marker, TOX3 is further investigated as a prognostic marker and/or therapeutic target against colorectal cancer [25] and lung adenocarcinoma [26]. It has been demonstrated that TOX4 interacts with a phosphatase complex to regulate chromatin structure and cell cycle progression [9, 11]. Furthermore, TOX4 can also modulate cell fate reprogramming [27], thereby switching off immune cells’ identity to induced pluripotent stem cells (iPSCs) and regulating the immune response. In this review, we have summarized the available data on the TOX subfamily members focusing on the specific roles of TOX in the development of immune cells and CD8+ T-cell exhaustion, and the specific function of TOX2, TOX3, and TOX4 across multiple immune processes. Table 1 lists the biological roles of various TOX family members.
Table 1:
The biological roles of various TOX family members.
| Type | Biological role |
|---|---|
| TOX | 1. Regulate the development of CD4+ T cells, innate lymphoid cells (ILCs), and T follicular helper (Tfh) cells2. |
| 2. Regulate CD8+T cell exhaustion. | |
| 3. Regulate mammalian corticogenesis | |
| 4. Mark the occurrence, diagnosis, and classification of cutaneous T-cell lymphoma (CTCL). | |
| 5. Regulate growth, DNA repair, and genomic instability in T-cell acute lymphoblastic leukemia. | |
| TOX2 | 1. Regulate normal NK cell development. |
| 2. Regulate Tfh development. | |
| 3. Regulate CD8+ T cell exhaustion. | |
| TOX3 | 1. Influences neuronal cells survival. |
| 2. Influences breast cancer cells survival. | |
| 3. As a prognostic marker and/or therapeutic target against colorectal cancer and lung adenocarcinoma. | |
| TOX4 | 1. Regulate chromatin structure and cell cycle progression. |
| 2. Modulate cell fate reprogramming. |
The role of TOX in T-cell development
CD4+ T cells play an essential role in immune response via recruiting and controlling the functions of most cells involved in defenses against pathogens, hence better understanding of the intracellular events that lead to CD4+ T-cell development is essential. Here, we review the key roles of TOX in regulating CD4+ T-cell development, as well as the commitment factor ThPOK and its interplay with Runx3 transcriptional regulators, focusing on how transcription factor TOX acts upstream of ThPOK and how TOX acts in thymocytes to promote the emergence of CD4-lineage specific gene expression patterns. Although the exact molecular mechanism of TOX remains to be elucidated, the role of TOX in establishment of gene programs in the thymus is discussed.
The thymus is the primary site of T-cell development, where progenitors from the bone marrow lacking CD4+ and CD8+ coreceptor expression undergo T-cell receptor (TCR) rearrangement to generate CD4+CD8+ double-positive (DP) thymocytes. DP cells undergo selection giving rise to CD4+ or CD8+ single-positive (SP) thymocytes that ultimately emerge into the periphery as naïve T cells exhibiting CD45RA+CCR7+ phenotypes [28]. T-cell development in the thymus involves rigorous selection events. T-cell receptor (TCR) β-selection (herein referred to as β-selection) is a pivotal checkpoint in mammalian T-cell development when immature CD4-CD8- T-cells (thymocytes) express pre-TCR following successful Tcrb gene rearrangement. At this stage, αβ T-cell lineage commitment and allelic exclusion to restrict one β-chain per cell take place and thymocytes undergo a proliferative burst [29]. Besides, the initially generated repertoire of TCR specificities in immature thymocytes is selected upon the interaction between TCRs and self-peptides associated with MHC molecules provided in the thymic cortical microenvironment. Low-affinity TCR interactions with self-peptide MHC complexes transduce signals for the survival of DP thymocytes and their further differentiation into CD4+ CD8- or CD4-CD8+ single-positive (SP) thymocytes. This process, termed positive selection, enriches a potentially useful repertoire of TCR specificities [30]. TOX is transiently upregulated during β-selection and positive selection of developing thymocytes, which are essential processes for thymocytes development [15]. Previous studies have used both transgenic and knockout models to determine the roles of TOX in the thymus. For instance, upregulation of TOX by DP cells is mediated via TCR-mediated calcineurin signaling, linking this critical signaling pathway to nuclear changes during positive selection [31]. Expression of TOX-transgenes in DP cells induced CD8-lineage commitment and the CD4-silencing factor, Runx3 [32, 33] in the absence of positive selection signals, thereby inducing CD4 downregulation and CD8 single-positive (CD8SP) cell formation [31]. However, these CD8SP cells cannot fully mature or leave the thymus, suggesting that TOX alone is not sufficient to replace the TCR signaling during positive selection and that T-cell receptor-mediated signaling can alter the fate of this cell. CD4−CD8- double negative (DN) thymocytes progress through distinct developmental stages; in DN3 blast cells, TOX is upregulated due to β-selection and subsequently downregulated before the DP phase [15]. At the CD4-CD8- DN stage, induced expression of TOX was sufficient to induce upregulation of both CD4 and CD8αβ, but not the cell proliferation associated with progression to the DP stage [31]. In addition, induced TOX expression was also sufficient to upregulate both CD8α and CD8β on DN thymocytes, and changed the methylation status of these loci [31]. This finding suggests that TOX is sufficient to initiate coreceptor changes associated with β-Selection. TOX also induced de-repression of CD4 in DN thymocytes, in a similar fashion observed during deficiency in SWI/SNF-like chromatin-remodeling BAF complex components [34]. Chromatin remodeling in vivo requires HMG-dependent DNA bending, but whether TOX influences chromatin remodeling remains to be determined.
TOX is required to establish CD4+ T-cell lineage gene programs and CD4+ T-cell lineage development. In germline TOX-deficient (TKO) mice, studies have found that the development of CD4+ T-cells was significantly damaged in the absence of TOX [35]. In germline TKO, the positive selection phase of developing thymocytes is not affected, while CD69, CD5, GATA3 are upregulated, CD4 and CD8 are downregulated, and DP phenotype (CD4+CD8+, double-positive DP) is converted to DD (CD4LOCD8LO, double dull DD) phenotype due to TCR signaling. In addition, the developmental progression of the DD phenotype to the CD4+CD8LO SP stage was strongly inhibited, which is significant since the CD4+CD8LO SP stage is the fate-decided key node for the development and differentiation of thymocytes into CD4+ T and CD8+ T. Therefore, CD4+ T development was blocked; the effects of this developmental disorder involve all CD4+ T-lineages, such as normal CD4+ T, natural killer T cells (NKT), and Treg. Under normal circumstances, many or most CD8+ T cells may also develop from the CD4+CD8LO SP stage via a “coreceptor reversal” pathway as has been proposed. Thus, the CD4+ CD8LO thymocytes transitional population is also important for CD8+ T-cell development [36]. But, some CD8SP that bear some class I MHC specificities may skip the CD4+CD8LO SP stage to directly develop from cells in the DD stage. Therefore, TKO has little effect on the development of CD8+ T cells. CD8SP thymocyte development in germline TKO mice is widely distributed among the spleen and can be activated to exert CD8+ T function [35]. Taken together, these results demonstrate that the developmental phase of CD4+CD8LO is the critical node of fate determination of the differentiation of thymocytes into CD4+ T or CD8+ T, which is required for the development of all CD4+ T cells, but not indispensable for the development of all CD8+ T cells [35, 37].
Runx3 and ThPOK (encoded by the Zbtb7b gene) are key nuclear factors for CD8 and CD4 T cell fate in the thymus, respectively, at least in part due to their ability to antagonize the expression of one another [38, 39]. However, CD4+ T cells develop in mice that lack both ThPOK and Runx3 activity, suggesting that the primary role of ThPOK in CD4+ T-lineage development is to inhibit that of CD8. There are additional complementary pathways that also induce CD4+T lineage specification [40]. The way in which CD4+ T-lineage gene program is established during positive selection in the thymus remains to be elucidated.
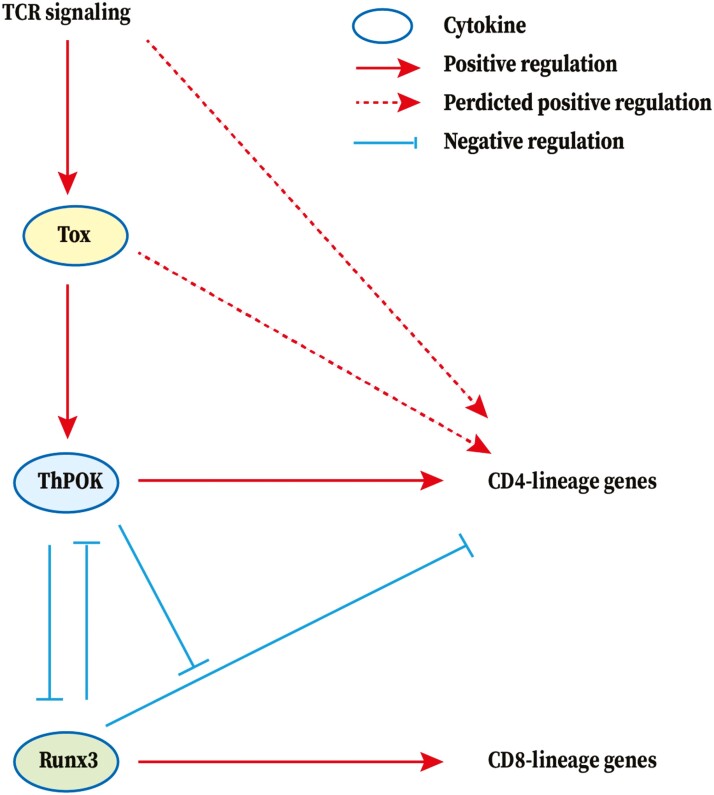
A small amount of “lineage-confused” T cells in the spleen of older germline TKO mice accumulate to express CD4 and CD8, and express low levels of ThPOK, suggesting that TOX acts as an upstream regulator of ThPOK in ThPOK and Runx3 expression [35, 41]. Besides, similar cells have also been reported in mice expressing a hypomorphic ThPOK allele [42]. However, the expression of a ThPOK transgene (ThPOK-Tg) in germline TKO mice did not rescue the TKO phenotype [41]. Furthermore, in the thymus, ThPOK-Tg/germline TKO mice contain a population of post-selected CD4-low SP cells, suggesting that TOX may regulate CD4+ T itself [41]. Furthermore, CD4+ T cells in the spleens of ThPOK-Tg/germlineTKO mice are weakly expressed by FOXO1, and when activated, they express low levels of the CD4+ T lineage marker, CD40L, which further indicates that the CD4+ T lineage gene program cannot be normalized in germline TOX-deficient mice. Consistent with the role of TOX as an upstream regulator of ThPOK, ThPOK-Tg/germline TKO cells failed to upregulate endogenous ThPOK locus despite transgene-encoded protein expression [41]. These findings demonstrate that TOX is essential for the development of the CD4+ T cell lineage gene program, which is not only due to its effect on ThPOK expression. However, the mechanism underlying TOX involvement in the development of CD4+ T cells remains to be elucidated. The speculative TOX-related regulatory network in T cell development was shown in Fig. 1.
Figure 1:
TOX-related regulatory network in T-cell development. TOX is required in the development of T-cell development. CD4-lineage genes are shown in blue and CD8-lineage genes are shown in red. The arrow represents a positive (stimulatory) connection and the ‘−’ represents a negative (inhibitory) connection. The solid line represents a link based on genetic evidence, mostly whether this linkage is through direct or indirect binding of transcription factors to target genes remains to be determined. The dashed line indicates an association inferred from the literature, but where genetic support is absent or indirect. Runx3 and ThPOK are key nuclear factors for CD8 and CD4 T cell fate in the thymus, respectively, partly due to their ability to antagonize the expression of one another. Runx3 inhibits CD4 expression, which is antagonized by ThPOK. In the upstream, T-cell receptor-mediated signaling in DP thymocytes promotes the calcineurin-dependent up-regulation of TOX to further alter the fate of T cells. But, TOX alone is not sufficient to replace the TCR signaling during positive selection. Then, TOX acts as an upstream regulator of ThPOK in ThPOK and Runx3 expression to regulate the T-cell lineage commitment but is not entirely dependent on ThPOK.
The roles of TOX in innate lymphoid cell (ILC) development
The establishment of ILC lineage and its essential roles in innate immunity, tissue stereotyping, and metabolism recently have been reported. This highlights the need to better understand the intracellular events that lead to ILC development. Here, we review the key role of TOX in regulating ILC development. Although the transcriptional pathways that lead to ILC-lineage specification, including the exact molecular mechanism of action of TOX, remain to be elucidated, we summarize the expression and functions of TOX and relative transcription factors in ILCs and propose a complex transcriptional regulatory network for the lineage commitment of ILCs. There are three identified ILC groups: group 1 cells (ILC1 and NK cells), which depend on T-BET and/or EOMES, produce interferon-γ (IFN-γ) [43, 44], and promote immunity against intracellular pathogens [45, 46]; group 2 cells (ILC2), which depend on GATA-3 and RORα, produce type 2 cytokines, such as IL-5/13, and amphiregulin [47–50], and promote tissue repair and anti-helminth immunity [51]; group 3 cells (ILC3 and LTi cells), which depend on the RORγt and produce IL-17/22 [52, 53]. NK cells are the most frequent type of ILCs and have been extensively studied. ILC1, ILC2, and ILC3 are referred to as non-cytotoxic ILCs, a newly defined cell type specializing in rapidly secreting cytokines and chemokines to resist infection and promote the repair of the mucosal barrier [54]. The functions of ILC1, ILC2, ILC3, and NK cells correspond to those of Th1, Th2, Th17, and cytotoxic CD8+ T cells, respectively [55].
The roles of TOX in the development from CLPs to ILC1, ILC2, and ILC3
Similar to T and B cells, ILCs are developed from common lymphoid progenitors (CLPs) [54]. During this process, CLPs first develop into the committed ILC precursors, CXCR6+αLP cells, which give rise to all ILC lineages, and further develop into ID2+ common helper-like innate lymphoid precursors (CHILP) and NK precursors (NKp) that ultimately develop into NK cells; subsequently, ID2+ CHILP develops into LTi and PLZF+ ILC progenitors (ILCPs), which can develop to ILC1, ILC2 and ILC3 [17, 56, 57]. Various studies attempted to determine the regulatory mechanisms underlying the commitment of CLPs to different ILCs lineages [57, 58]. The influence of TOX during this process has been determined, TOX is expressed in CLPs, CXCR6+ αLP cells, ID2+CHILP, PLZF+ILCP, and mature ILC types [17, 56, 57]. In germline Tox−/− mice, the number of CLPs is normal, yet that of CXCR6+ αLP cells, ID2+CHILP, and PLZF+ILCP is reduced and mature ILCs are almost not produced [17, 56, 57]. Therefore, the development of CLPs to common ILC progenitor cells and mature ILC lineages requires TOX. However, the specific mechanism by which TOX is involved in this process remains unknown. Given such a complex top-down differentiation program, and that TOX affects the development of CXCR6+αLP cells, which in turn are capable of generating NK, PLZF+ILCP, and LTi cells, some earlier developmental defects may be reflected during the mature stage of NK cells, LTi cells, and ILCPs cells. This makes it difficult to distinguish the specific role of TOX in these cells; therefore, models of conditional knockout are essential to further study the role and associated mechanisms of TOX.
TOX was downregulated in the CLPs of germline Nfil3−/−mouse, suggesting that NFIL3 may regulate TOX expression in CLPs [59]. Further, studies have shown that the development of CLPs required the presence of the transcription factor, NFIL3, which is the key mediator in the production of ID2+ common helper-like innate lymphoid precursors and promyelocytic leukemia zinc finger (PLZF)+ ILCP. NFIL3 also influences the development of a specific bone marrow precursor cell population (CXCR6+ cells in the αLP population) [57, 60]. Among them, CXCR6+αLP cells include committed ILC precursors that have been shown to differentiate into all major ILC lineages both in vitro and in vivo [57]. Further, the frequency and the absolute number of all ILCs types were reduced in germline Nfil3−/− mice [57, 61], suggesting that NFIL3 is required for all ILCs development. This indicates that NFIL3 is essential for the whole process of differentiation from CLPs to the ILC lineages. However, since ILC progenitors and all ILC lineages were reduced in germline NFIL3−/− mice, other pathways, besides NFIL3, may also be involved in the whole differentiation process from CLPs to the ILC lineages. Further, studies have reported that reduced TOX expression can lead to extensive ILC deficiency in germline Nfil3−/− mice and induced TOX expression in germline Nfil3−/− bone marrow progenitors can mitigate several ILC developmental defects [57]. A chromatin immunoprecipitation (ChIP) assay with an NFIL3-specific antibody has demonstrated that NFIL3 directly binds to the Tox promoter [62], which is enhanced by overexpression of NFIL3 [57]. Finally, NFIL3 activated Tox promoter activity as previously illustrated using a luciferase reporter assay [57]. Therefore, NFIL3 activates Tox expression by directly binding to its promoter, through which it drives the development of ILCs. The NFIL3-TOX transcription factor cascade significantly influences ILC lineage differentiation [57]. Ectopic Id2 expression in germline Nfil3-null precursors allowed germline Nfil3−/− progenitors to re-acquire their potential to differentiate into ILC1, ILC2, and ILC3 in vivo, thereby rescuing impaired ILC lineage development [60]. Ectopic Id2 expression also allowed germline Nfil3−/− CLP to develop into PLZF+ILCP, in vitro. Besides, NFIL3 exerts its function by bounding the Id2 locus close to the Id2 promoter to directly regulate Id2 which is required for the development of all ILCs in the CHILP [63]. Therefore, NFIL3 can directly regulate Id2 expression in ILC progenitors and orchestrates ILC progenitor emergence from CLPs. Moreover, TOX can modulate the activity of Id2, a known driver of cytotoxic T cell (CTL) differentiation [64]. Therefore, during the development of ILC1, ILC2, and ILC3, NFIL3 can function through the NFIL3-TOX-Id2 axis or bypass TOX to directly function through the NFIL3-Id2 axis. This may contribute to the development of a small proportion of ILC in germline TOX-deficient mice. Notably, PLZF+ ILCP develops into all ILCs lineages, except for NK and LTi cells [56], indicating that these two cell types follow a distinct developmental pathway compared with other ILC populations. Given their similarity in transcription factor expression and cytokine secretion profiles, ILC1, ILC2, and ILC3 have been considered as the innate phenocopy of T helper cells, i.e., Th1, Th2, and Th17 cells, respectively. PLZF+ILCP can differentiate into ILC1, ILC2, and ILC3 in innate immunity, and CD4+T can differentiate into Th1, Th2, and Th17 in adaptive immunity. Therefore, PLZF+ILCP may possibly be the counterpart of naive CD4+T in innate immunity. Whether the role of TOX in these two counterparts is consistent remains to be determined. During the development of CD4+T, TOX functions by regulating ThPOK or directly acting on CD4+ T cells. The lack of TOX inhibits the CD4+ T lineages specification program, which illustrated TOX’s influence, but its specific role remains to be identified. During the development of PLZF+ILCP, the NFIL3-TOX axis regulates Id2, and so does NFIL3, directly; in addition, there are other pathways, besides NFIL3, which participate in the regulation of the development of PLZF+ILCP. Hence, the development regulation of PLZF+ILCP is complicated.
The roles of TOX in NK cell development
While both NKp and invariant natural killer (iNK) subsets are present (with a trend towards a decrease in iNK), an approximately 40-fold reduction of mature natural killer (mNK) cells was observed in the spleens of germline Tox−/− mice. Consistently, in the bone marrow of germline Tox−/− mice, the frequency of mNK cells was reduced by approximately 50-fold compared to the wild-type. Besides, low amounts of Tox mRNA were detected in bone marrow NKp, while both iNK and mNK from bone marrow highly expressed Tox. The upregulation of Tox during the iNK and mNK stages is consistent with the observed block in NK cell development in the germline absence of TOX [59], which suggests that the inhibition of NK cell development is attributed to a cell-intrinsic defect. Thus, the development of NK cells is impaired due to a cell-intrinsic TOX defection in the TOX germline knockout mouse model. Furthermore, NKp transition to the iNK phase was blocked, and NK-mediated cytotoxicity was reduced in germline TOX-deficient mice [59]. However, NK cells that escape this developmental obstacle remained to have some compromised effector functions in the germline absence of TOX [59]. These data suggest that TOX is important but not essential in regulating the development of NK cells. NK development also depends on Id2 [65–67]. In the thymus of ThPOK-Tg/germline TKO mice, Id2 expression was reduced in residual NK cells. But, reinfusion of Id2 in germline TKO mice could not fully restore the developmental process from bone marrow precursor cells to NK cells [59]. This suggests that Id2 is not the only factor downstream of TOX that affects the development of NK cells. Moreover, Nfil3 is required for commitment to the NK lineage and promotes NK development in a cell-intrinsic manner by directly regulating the expression of the downstream transcription factor Id2. Id2 can rescue NK production from germline Nfil3−/− progenitors, suggesting that they act downstream of Nfil3. Nfil3 binds directly to the regulatory regions of Id2, thereby promoting transcription [68, 69]. In the development of NK cells, whether TOX can also function through the NFIL3-TOX-Id2 axis, as in the development of non-cytotoxic ILC, remains to be determined. Consistent with findings reported in mice models, TOX also regulates human NK cell differentiation. Indeed, conditional knockdown of TOX in differentiating cells decreased NK cell population. In addition, over-expression of TOX enhanced the differentiation of NK cells to mNK cells with effector functions. Moreover, TOX influenced the expression of T-bet during NK cell development. Overall, these findings suggest that TOX is required for NK cell differentiation and affects the expression of T-bet which plays a critical role in NK differentiation and maturation [70].
Although TOX is an essential regulator of NK cell differentiation in mice, little is known regarding the roles of the other TOX family members in NK cell development. As recently discovered, TOX2 co-operates with or complements TOX function within the immune system. TOX2 is expressed in mNK cells and is upregulated during the differentiation of CD34+ cells derived from human umbilical cord blood in vitro. Downregulating the TOX2 gene blocks the transition between early developmental stages of NK cells, while upregulating TOX2 enhanced the transition of umbilical cord blood CD34+ cells into mNK cells [6]. Subsequently, the expression of TBX21 (encoding T-BET) is directly upregulated by TOX2. T-BET overexpression can also rescue the TOX2 knockdown phenotype. Considering the essential function of T-BET in NK cell differentiation, TOX2 regulates normal NK cell development by acting upstream of TBX21 [6]. The function of TOX2 is complementary to that of TOX in human NK cell differentiation.
The roles of TOX in LTi cell development
Since LTi cells share a similar developmental process with NK cells, including reliance on transcription factors Id2 and Ikaros, it is worth addressing whether the development of LTi cells depends on TOX. Both fetal and adult LTi cells normally express Tox. It has been previously demonstrated that all peripheral lymph nodes were largely absent in germline Tox-deficient mice [59]. Besides, further studies reported that the structures of lymph nodes were intact in mice with conditional Tox−/− on T cells [59], suggesting that the deficiency of lymph nodes was not due to a lack of TOX in T cells. Previous results also showed that in the Tox germline knockout mouse model, the development of LTi cells, which significantly influences the integration of lymphoid tissue organogenesis [71–73], was blocked. The quantity of LTi cells was reduced by over 10 folds, leading to developmental abnormalities in lymphoid tissues and organs [59]. Therefore, TOX is essential for the development of LTi cells and can drastically affect the development of lymphatic tissues and organs. Despite the lack of lymph nodes, a small number of LTi-like cells can be identified in adult germline Tox−/− mice [59]. Therefore, in addition to TOX, there are other pathways that affect the development of LTi, which warrant further investigations. Adult LTi-like cells have other functions in the immune system, including the production of IL-17 and IL-22 [74]. Therefore, determining whether the lack of TOX also impacts these functions has important implications.
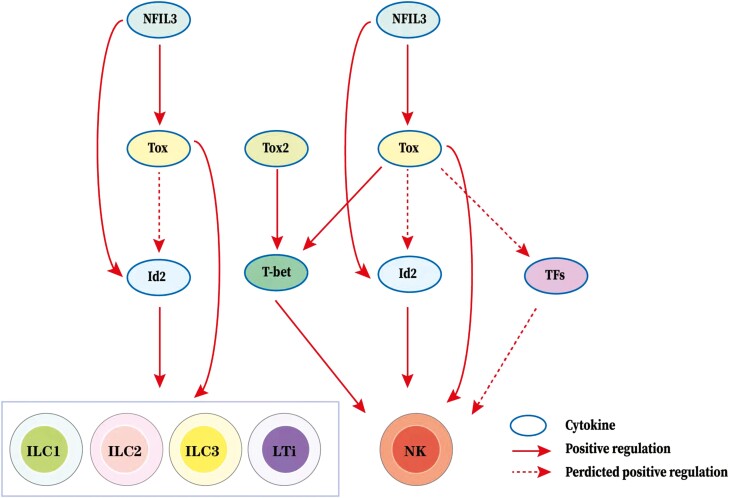
The development of mature cells requires coordinated expression of gene regulatory networks that promote differentiation of precursors, while simultaneously inhibiting alternate cell fates. Many nuclear factors, including transcription factors, cofactors, and chromatin modifiers, play essential roles in ILC development. TOX inhibits the activities of Id2 and Notch but enhances the activity of TCF1 in CTLs [64]. Regulation of TOX by GATA3 was also observed in CTCL [2]. Further evidence showed that the transcriptional regulators Id2, TOX, Nfil3, TCF1, and GATA-3 were expressed and active throughout the entire ILC development process [75]. Collectively, these results indicate that TOX regulates ILC development probably through a complex transcriptional network with factors, including Id2, Nfil3, TCF1, GATA-3, and Notch, rather than a single signaling pathway. TOX-related regulatory network in ILC development is shown in Fig. 2.
Figure 2:
TOX-related regulatory network in ILC development. The arrow represents a positive (stimulatory) connection. The solid line represents a link based on genetic evidence, mostly whether this linkage is through direct or indirect binding of transcription factors to target genes remains to be determined. The dashed line indicates an association inferred from the literature, but where genetic support is absent or indirect. TOX is required in the development of non-cytotoxic ILCs and LTi cells. NFIL3 may function through the NFIL3-TOX-Id2 axis or bypass TOX to directly function through NFIL3-Id2 axis in the development process. In addition, NFIL3 may also bypass Id2 to function only through NFIL3-TOX axis in this process. Besides, TOX is also important in the development of NK cells. Similarly, NFIL3 may also function through the NFIL3-TOX-Id2 axis, NFIL3-Id2 axis, or NFIL3-TOX axis in the development process. But, TOX and TOX2 can affect the development process by regulating T-bet expression, moreover, TOX can also affect the development process by regulating other TFs expression.
The role of TOX in follicular helper T (Tfh) cell development
Follicular helper T (Tfh) cells are a specialized T-cell subset with critical roles in supporting B-cell-mediated humoral immune responses [76, 77]. Tfh cells are classified into Tfh1, Tfh2, and Tfh17 cells, which produce respective cytokines, namely IFN‐γ, IL-4, and IL-17, through which they regulate different aspects of humoral immunity [78]. Determining the underlying molecular mechanisms of Tfh cell differentiation regulation would have a significant implication for human health. Here, we review the key roles of TOX in regulating Tfh cell development, summarize the expression and functions of TOX and relative transcription factors in Tfh cells, and propose a complex transcriptional regulatory network for the lineage commitment of Tfh cells.
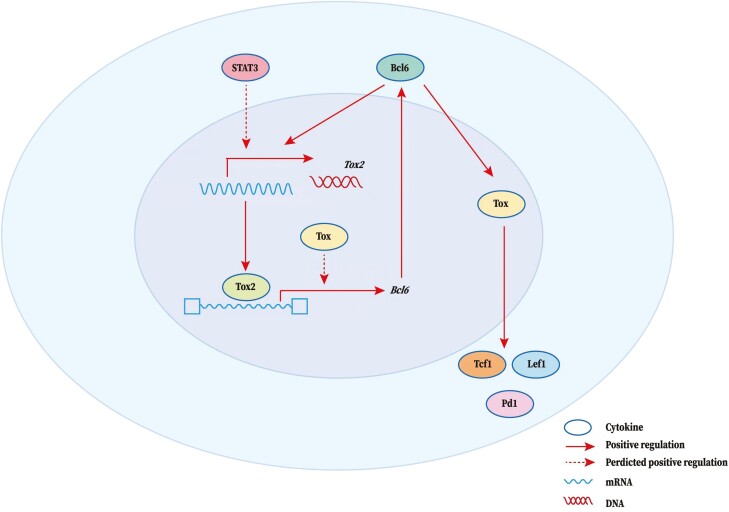
Tfh cells are identified by a high expression of programmed cell death receptor-1 (PD-1), chemokine receptor CXCR5, and when activated, inducible T-cell co-stimulator (ICOS), and associated with the production of their signature cytokine, the IL-21 [76, 79]. Tfh cell development is a complex and tightly controlled process regulated by a complicated network of transcription factors. This starts during T-cell priming by dendritic cells (DCs) and continues through the first T: B cognate interaction at the T-B junction. Further changes occur when Tfh cells enter follicles and differentiate into mature GC Tfh cells. At each of these stages, developing Tfh cells are influenced by changing cytokine, chemokine, and cellular environments [80]. BCL6 is the key transcription factor required for Tfh cell development [81–83]. Besides, achaete-scute complex-like 2 (ASCL2, a basic helix-loop-helix transcription factor required for promoting CXCR5 expression) [84], and c-MAF (an ICOS-inducing transcription factor, which can induce the production of IL-21) [85, 86] are also involved in regulating Tfh cell development. However, it is important to further elucidate the mechanism underlying the induction of BCL6 gene expression and BCL6-dependent function during Tfh cell commitment and whether TOX or related family members can regulate BCL6. Recently, Wu et al. demonstrated that ectopic TOX expression in T cells increased Tfh cell numbers, while TOX reduction impaired Tfh cell responses [18]. Therefore, TOX functions as a central transcription regulator in Tfh cells. The elevated expression of TOX in Tfh cells is driven by BCL6 in both mice and humans [18]. In turn, TOX can promote the expression of multiple molecules including TCF1, lymphoid enhancer-binding factor 1 (LEF1), and PD-1 that play critical roles in Tfh cell differentiation and function [18]. The authors provided a link between TOX and Tfh cell differentiation, function, and the maintenance of optimal Tfh cell responses for humoral immunity and showed that Tfh cells are coordinately controlled by multiple transcription factors. Besides, another TOX subfamily member TOX2 is highly expressed in Tfh cells and is regulated by STAT3 and BCL6 [22]. Genome-wide ChIP-seq results showed that TOX2-bound loci were associated with Tfh cell differentiation and function, involving BCL6. Using ATAC-seq, the direct binding of TOX2 was also found to increase the chromatin accessibility at these sites. Accordingly, induced ectopic TOX2 expression can drive BCL6 expression and Tfh development. Germline Tox2-/- mice exhibit defective Tfh differentiation. Removal of both TOX2 and TOX also blocked Tfh differentiation [22]. Thus, the TOX2-BCL6 axis constructs a transcriptional feed-forward loop that facilitates the Tfh program. Combined with downstream TOX signaling, the TOX2-BCL6 axis constitutes a regulatory cascade of Tfh development. Both TOX and TOX2 affect Tfh development, but whether they play redundant or independent roles remains to be explored.
The role of TOX in regulating the development of multiple immune cell groups has been previously identified [64]. However, TOX regulation of peripheral effector cells is rarely studied. Therefore, more analysis, such as gene expression profiling and/or ChIP-seq analysis, of TOX-related genes are essential to obtain detailed mechanisms underlying the TOX-mediated regulation of Tfh cells. TOX-related regulatory network in Tfh cell development is shown in Fig. 3.
Figure 3:
TOX-related regulatory network in Tfh cell development. TOX functions as a key transcription regulator in Tfh cells. The arrow represents a positive (stimulatory) connection. The solid line represents a link based on genetic evidence, mostly whether this linkage is through direct or indirect binding of transcription factors to target genes remains to be determined. A dashed line indicates an association inferred from the literature, but where genetic support is absent or indirect. BCL6 can promote TOX expression to further promote the expression of multiple molecules including TCF1, lymphoid enhancer-binding factor 1 (LEF1), and PD-1 which play critical roles in Tfh cell differentiation and function. In addition, Tox2 is highly expressed in Tfh cells and regulated by Bcl6 and STAT3. Tox2 directly binds to Tfh-associated genes (especially BCL6), promoting chromatin accessibility, to further promote the expression of these genes. TOX may also play a facilitating role in this process. Thus, the TOX2-BCL6 axis constructs a transcriptional feed-forward loop that facilitates the Tfh program combined with downstream TOX signaling.
The role of TOX in CD8+ T-cell exhaustion
CD8+ T cell exhaustion is an abnormal state of T-cell function along with persistent antigens and prolonged T-cell receptor (TCR) stimulation, as observed during chronic antigen infection, or more recently in response to tumors. Exhausted CD8+ T (Tex) cells overexpress inhibitory receptors while retaining some effector function, leading to pathogen–host “stalemate” [87]. Adjusting Tex can affect pathogen-host “stalemate” thereby more effectively treating chronic infection and cancer, but the exact regulatory targets remain to be determined. Here, we review the key roles of TOX in regulating CD8+ T cell exhaustion, summarize the expression and functions of TOX and relative transcription factors in Tex cells, and propose a complex transcriptional regulatory network for the lineage commitment of Tex cells.
Transcriptional and epigenetic evidence of TOX regulating CD8+ T cell exhaustion
According to previous studies, NFAT [88] and NFAT-driven TCR-responsive nuclear receptor subfamily 4 group A (NR4A) [23, 89–91], are key mediators in the upregulation of inhibitory receptors expression as well as the maintenance of long-term exhausted T cell survival. These observations suggest that chronic TCR-calcium-calcineurin signaling is a core mechanistic driver of exhaustion. Recently, TOX has been identified as another NFAT-driven TCR-responsive transcription factor in mouse exhausted CD8+T cells [23]. Based on single-cell transcriptomes and epigenetic profiles of CD8+T cells that respond to acute and chronic infections in the mouse model, TOX is developmentally unnecessary for effector T (Teff) and memory T (Tmem) cells, but is essential for exhaustion, since mouse Tex cells are not observed in the germline absence of TOX [19, 21, 92]. However, the extent of TOX analogous roles in humans remains to be determined. TOX and TCF1 shape the processes of exhaustion and memory differentiation among subpopulations of human CD8+ T cells. Under homeostatic conditions, effector memory CD8+ T cells primarily expressed TOX, whereas naive and early-differentiated memory CD8+ T cells primarily expressed TCF1. Cytolytic gene and protein expression signatures among human CD8+T cells were also defined by the expression of TOX. During ongoing viral replication, dysfunctional HIV-specific CD8+ T cells commonly express TOX, which is clustered with various activation markers and inhibitory receptors, and rarely express TCF1 [93]. Therefore, TOX plays a key role in the normal functioning of human CD8+ T cells (especially effector memory CD8+ T cells) and the exhaustion of CD8+ T cells. TOX-regulated exhaustion of CD8+ T cells may be an adaptive mechanism of CD8+ T cells in ongoing viral replication environments. In humans, TOX is expressed by most circulating effector memory CD8+ T cell subsets specific for chronic viruses and not exclusively linked to exhaustion, suggesting the limitations of TOX as a predictive target for disease. Besides, TOX is a universal regulator of human memory CD8+ T cells specific for chronic viruses [93]. These findings suggest that TOX may play a role in addition to developmental effects in human effector T (Teff) and memory T (Tmem) cells; therefore, further studies are warranted. Kim et al. reported that TOX is the major regulatory factor for T cell exhaustion based on single-cell transcriptomes and epigenetic profiles of CD8+ T cells that respond to different types of human cancers [94]. Besides, in mice, higher TOX transcriptional activity is associated with a higher abundance of active histone marks in Tex cells compared to memory precursor cells [95]. TOX contributes to the antiviral CD8+ T cells durability and is required for Tex cells programming [95]. Thus, Tex responding to chronic viral infection and cancers in mouse model and humans requires unique transcriptional and epigenetic programs associated with the transcription factor TOX.
Transcriptional evidence of TOX regulating CD8+ T cell exhaustion
The mechanism underlying TOX-regulated CD8+ T cell exhaustion remains largely unknown. At the transcriptional level, TOX is induced by continuous antigen stimulation of TCR during chronic viral infection. Conditional removing the DNA-binding domain of TOX makes T-cell polyfunctional. These T cells initially show enhanced effector function along with severe immunopathology, followed by a significant decline in numbers [20], which may be due to the overstimulation of T cells and activation-induced cell death in settings of chronic antigen stimulation in chronic viral infection. Thus, TOX is positively correlated with the exhausted phenotype and longevity in chronic viral infection. Further, TOX is strongly expressed in malfunctioning tumor-specific T (TST) cells. Ectopic TOX expression in effector T cells in vitro induces a transcriptional program associated with T cell exhaustion (upregulated genes for inhibitory receptors, high chromatin accessibility, and high expression of transcription factors such as Tcf7, Lef1, and Id3). Conversely, conditional knockout of Tox in TST cells in tumors abrogated the exhaustion program. Despite their normal, ‘non-exhausted’ immunophenotype, conditional Tox−/− TST cells remained dysfunctional, which suggests that the regulation of expression of inhibitory receptors is uncoupled from the loss of effector function [19]. Therefore, TOX can also be identified as a major regulator of TST cell differentiation. Notably, although conditional Tox−/− CD8+ T cells differentiated normally to effector and memory states in response to acute infection, conditional Tox−/− TST cells failed to persist in tumors [19]. Thus, TOX-induced exhaustion may prevent the overstimulation of T cells and activation-induced cell death in settings of chronic antigen stimulation such as cancer. The exact signaling network of TOX function in T cell exhaustion remains unknown. One study suggested that TOX, TOX2, and NR4A are targets of the NFAT [23]. Besides, TOX and TOX2 are strongly induced in CD8+CAR+PD-1highTIM3high (“exhausted”) tumor-infiltrating lymphocytes (CAR TILs) in a CAR T cell model. Compared with TOX or TOX2 knockout and wild-type CAR TILs, both TOX and TOX2 knockout (Tox DKO) more effectively inhibited tumor growth and improved the survival of tumor-bearing mice. Similar to NR4A-deficient CAR TILs, Tox DKO CAR TILs showed higher cytokine expression, less inhibitory receptors expression, and more accessibility to regions containing abundant binding motifs of transcription factors activation-associated NF-κB and basic region-leucine zipper (b-ZIP), which are associated with T cell activation and effector function [23]. The above evidence shows that TOX, TOX2, and NR4A are key factors in the transcriptional program of CD8+T cell exhaustion downstream of NFAT. The study also provides evidence for positive regulation of NR4A by TOX and of TOX by NR4A [23], and suggests that disruption of TOX, TOX2, and NR4A expression or activity could be promising strategies for cancer immunotherapy. However, as this study was conducted in tumor-responsive Tex, further validation in chronic viral-responsive Tex is warranted. TOX is induced by calcineurin and NFAT2 and participates in the feed-forward loop. In this loop, TOX becomes calcineurin-independent and maintained within this loop in all Tex cells [21]. Therefore, the strong expression of TOX leads to Tex commitment by transforming continuous stimulation into a specific Tex cell transcriptional program. VEGF-A also induced the expression of TOX in T cells of a colorectal cancer mouse model to drive the exhaustion-specific transcription program [92]. Further validation of Tex response to other cancer models and the chronic virus is also required. In a mouse HCC model and HCC patient-derived xenograft mouse model, TOX exerts a role in regulating the antitumor effect of CD8+ T cells in hepatocellular carcinoma [96]. Mechanically, TOX binds PD-1 in the cytoplasm to reduce PD-1 degradation and promote PD-1 translocation to the cell surface in CD8+ T cells, thus maintaining high PD-1 expression at the cell surface, ultimately causing CD8+ T-cell exhaustion in hepatocellular carcinoma. Meanwhile, high expression of TOX in peripheral CD8+ T cells correlated with poorer anti-PD-1 responses and prognosis [96]. Therefore, downregulating TOX expression improves the antitumor function of CD8+T cells, which shows the synergetic role of anti-PD-1 therapy, highlighting a promising strategy for the enhancement of cancer immunotherapy. Consistently, in human tumors, the expression of TOX increases with the exhaustion of CD8+ T cells. Additionally, TOX positively regulated the expression of PD-1, TIM-3, TIGIT, and CTLA-4 in the human tumor-infiltrating (TI) CD8+T cells. This suggests that TOX is a key transcription factor that promotes T cell exhaustion by inducing IC molecules in human cancers. Finally, the expression levels of TOX in the TI T cells could predict the overall survival and response to anti-PD-1 therapy in human melanoma and NSCLC [94]. These results suggest that TOX levels can be used for patient stratification during anti-cancer treatment, including immunotherapy, and that TOX can be targeted in the background of immune checkpoint inhibitor (ICI) therapy.
Epigenetic evidence of TOX regulating CD8+ T cell exhaustion
The epigenetic role of TOX in CD8+ T cell exhaustion has attracted significant attention, although the exact mechanism is not fully understood. Epigenetically, TEX is a distinct immune subset, with a unique chromatin landscape compared to TEFF and TMEM [97, 98]; whether TOX regulated this epigenetic commitment of TEX remains to be determined. In the conditional absence of TOX in T cells, increases in chromatin accessibility are associated with terminal Teff differentiation, suggesting that TOX represses the accessibility of genes involved in Teff. In contrast, loci with reduced chromatin accessibility included genes associated with TMEM and TEX progenitors. Indeed, loci with significantly reduced accessibility in conditional TOX−/− cells were highly enriched for TEX-specific sites, whereas sites with increased accessibility were enriched in TEFF-specific sites [21]. Consistently, conditional TOX−/− TST cells in tumors abrogated the exhaustion epigenetical program, and the chromatin of inhibitory receptors remained largely inaccessible [19]. The mechanism may be attributed to TOX epigenetic regulation of genes for immune checkpoint receptors by controlling distal cis-regulatory elements [92]. Besides, Tox DKO CAR TILs display increased accessibility of chromatin regions enriched for motifs that bind nuclear factor κB (NF-κB) and basic region leucine zipper transcription factors, which are classically associated with T cell activation and effector function [23]. Therefore, TOX represses terminal TEFF-specific epigenetic events while initiating key TEX-specific epigenetic changes. Besides, proteins involved in chromatin organization and remodeling, RNA processing and translation, and DNA replication were identified as TOX binding partners. Network analysis identified the HBO1 complex, which is involved in histone H4 and H3 acetylation, as a major set of TOX-bound proteins. TOX also bound proteins involved in repressive epigenetic events, indicating interactions with proteins involved in both the closing and opening of chromatin [21]. In T cell exhaustion programs during chronic viral infection, TOX is also involved in epigenetic remodeling and stable fixation of the dysfunctional phenotype [20]. Thus, TOX can bind and likely recruit diverse sets of chromatin remodeling proteins. We also suggest that TOX modulates epigenetic accessibility and indirectly impacts gene expression by altering the transcription factors network and their targets in TEX [21]. By comparing epigenetic profiles of CD8+T cells responding to acute and chronic viral infections, studies have reported a co-expression gene module containing Tox that exhibited higher transcriptional activity associated with more abundant active histone marks in Tex than memory precursors [95]. These data identify TOX as a critical TEX-programming epigenetic coordinator. Moreover, these observations have implications for the ontogeny of TEX and therapeutic opportunities.
NK cells, in innate immunity, and CD8+ T cells, their counterparts in adaptive immunity, become exhausted and dysfunctional during long-term antigenic stimulation [19, 20, 99, 100]. CD8+ T exhaustion is primarily mediated by TOX-related pathways, while TOX regulates the development of NK cells through the NFIL3-TOX-Id2 Axis. Therefore, NK cell exhaustion might be mediated by TOX-related pathways. NK cell exhaustion has been reported to be mediated by TIGIT, PD-1, tim-3, and other immune checkpoint molecules [101–103], while in CD8+ T cell exhaustion, TOX upregulates these molecules. Therefore, NK cell exhaustion may also be mediated by TOX; this requires further research.
The potential of TOX as a therapeutic target
The TOX-driven transcriptional program in CD8+ T cells prolongs T cell response and attenuates immunopathology. In detail, TOX adjusts CD8+ T cell to differentiate toward exhaustion, but not effector, state to induce a persistent response accompanied by limited immunopathology. Combining in vitro, ex vivo, and in vivo data from mouse models, Kim et al. demonstrated that combined blockade of PD-1 and VEGF-A restored the antitumor functions of Tex cells, which enhanced control of microsatellite-stable colorectal cancer tumors [92]. Exhausted CD8+ T cells not only produce a long-term immune response to chronic infection and cancer but also respond effectively to immune checkpoint blockade [20]. As previously mentioned, in human tumors, TOX prompts CD8+ T cell exhaustion by upregulating IC molecules, which indicates that TOX suppression could enhance the efficacy of immune checkpoint inhibitors (ICIs). Further, the expression of TOX in TI T cells could be used to stratify patients during anti-PD-1 immunotherapy [94]. Thus, Tex cells could be potential clinical targets for checkpoint blockade and other immunotherapies. Notably, TOX-driven exhaustion program can also reduce the overstimulation and activation-induced T cell death under conditions of chronic antigen stimulation, which are often observed in cancer [19]. Taken together, TOX drives CD8+ T cells to differentiate in the most suitable direction with maximal therapeutic benefit, which assists other immune cells and helps the host to mitigate the stress associated with persistent antigen exposure (such as immuno-oncology and chronic inflammation) [104]. TOX-related regulatory network in CD8+ T cell exhaustion is shown in Fig. 4.
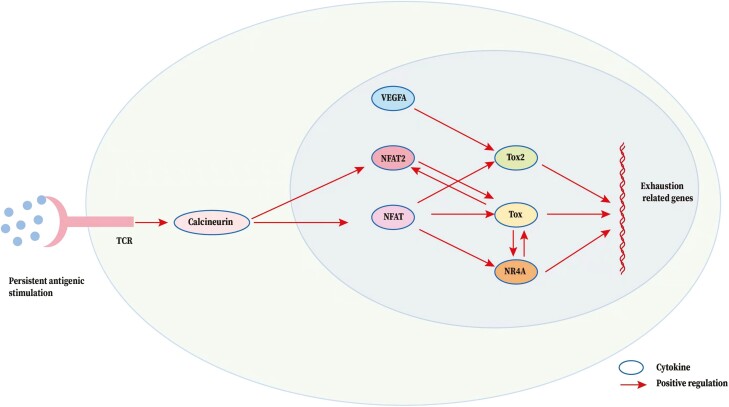
Figure 4:
TOX-related regulatory network in CD8+T cell exhaustion. The arrow represents a positive (stimulatory) connection. The solid line represents a link based on genetic evidence, mostly whether this linkage is through direct or indirect binding of transcription factors to target genes remains to be determined. Persistent antigens activated T-cell receptor (TCR) triggers calcineurin and NFAT signaling. NFAT is a core mechanistic driver of CD8+ T cell exhaustion. TOX, TOX2, and NR4A are targets of the NFAT and they are also key factors in the transcriptional program of CD8+ T cell exhaustion downstream of NFAT. There also exists a feed-forward loop between TOX and NR4A. Besides, TOX is necessarily and sufficiently induced by NFAT2 and participates in the feed-forward loop. In this loop, TOX becomes calcineurin-independent and maintained within this loop in all Tex cells. Of note, VEGF-A also induced the expression of TOX to drive the exhaustion-specific transcription program.
Concluding remarks
TOX exerts various functions during the development of immune cell types such as CD4+ T cells, ILCs, and Tfh cells and participates in CD8+ T cell exhaustion. At present, different roles have been described for TOX within these processes, but an overall TOX activity map has not been constructed. During T cell development, the expression of TOX is indirectly regulated by the upstream TCR signal through the TCR-mediated calcineurin signal; subsequently, TOX regulates the mutually exclusive expression balance of ThPOK and Runx3 by affecting ThPOK expression downstream of TOX, which imposes the lineage fate of CD4+T cells and CD8+ T cells, respectively [38, 39, 105, 106]. Therefore, in addition to the essential role of TOX during CD4+ T cell development, it also significantly influences CD8+ T lineage by adjusting the mutually exclusive expression balance of ThPOK and Runx3, which warrants further investigation. ILC differentiation involves a cascade of NFIL3-TOX transcription factors, which influence the development of CLPs into all ILC lineage types [17, 57, 59]. Furthermore, TOX can affect ILC development by regulating Id2 [16, 65, 75], the Notch signaling pathway, and other cascades [17]. Various cell subtypes follow their respective development paths through distinct downstream signaling [56, 75]. Thus, adjusting these differentiation pathways to induce specific functional ILC subgroups is a direction for future research. TOX regulation of the development of ILCs during the intestinal inflammatory response and immune response to tumors, pathogens, or self-antigens is also a topic for future studies.
Furthermore, investigating the TOX-mediated control of Tfh cell responses, through gene expression profiling and/or ChIP-seq analysis of TOX-related genes in Tfh cells, will elucidate the underlying mechanism, which has a significant health implication.
TOX can promote and maintain the exhaustion of CD8+ T cells by upregulating IC molecules [94] and regulating transcription through epigenetic modifications, but the underlying mechanism remains unknown [21]. Further studies are warranted to determine the cellular signaling pathways in which TOX participates to drive the exhausted T cell phenotype, and how to reverse the exhaustion-associated changes in chromatin and transcription by regulating TOX in exhausted cells.
Additionally, TOX is a potential therapeutic target for the indirect regulation of CTL differentiation and sensitivity to immune checkpoints in autoimmunity, which warrants further investigations [64]. Future research on TOX should focus on these roles to discover wider applications of TOX. Based on previous findings for TOX, the roles of other subfamily members such as TOX2, TOX3, and TOX4 in immune cell development and differentiation also require further research.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Glossary
Abbreviations
- BCL6
B-cell lymphoma 6
- CLP
common lymphoid progenitor
- CTL
cytotoxic T-cell
- ID2
inhibitor of DNA binding 2
- IFN-γ
interferon-γ
- ILC
innate lymphoid cells
- LTi
lymphoid tissue inducer
- NFAT
nuclear factor of activated T cells
- NK
natural killer cells
- NR4A
nuclear receptor subfamily 4 group A.
- PD-1
programmed cell death receptor-1
- PLZF
promyelotic leukemia zinc finger
- TCF1
T-cell factor 1
- TCR
T-cell receptor
- Teff
effector T cells
- Tex
exhausted CD8+ cells
- Tfh
Follicular T helper
- Tmem
memory T cells
- TOX
thymocyte selection-related HMG box protein
- TST
tumor-specific T cells
Funding
This work was supported by grants from the National Natural Science Foundation of China (81671592) and the Science and Technology Department of Jilin Province (20190201140JC).
Conflicts of Interest
The authors report no conflicts of interest in this work.
Author contributions
All authors contributed equally to the manuscript.
Data availability
Not applicable. No data were generated in the making of this article.
References
- 1. O’Flaherty E, Kaye J.. TOX defines a conserved subfamily of HMG-box proteins. BMC Genomics 2003, 4(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGirt LY, Degesys CA, Johnson VE, Zic JA, Zwerner JP, Eischen CM.. TOX expression and role in CTCL. J Eur Acad Dermatol Venereol 2016, 30, 1497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Litvinov IV, Tetzlaff MT, Thibault P, Gangar P, Moreau L, Watters AK, et al. Gene expression analysis in Cutaneous T-Cell Lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunology 2017, 6, e1306618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lefrançois P, Xie P, Wang L, Tetzlaff MT, Moreau L, Watters AK, et al. Gene expression profiling and immune cell-type deconvolution highlight robust disease progression and survival markers in multiple cohorts of CTCL patients. Oncoimmunology 2018, 7, e1467856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lobbardi R, Pinder J, Martinez-Pastor B, Theodorou M, Blackburn JS, Abraham BJ, et al. TOX regulates growth, DNA repair, and genomic instability in T-cell acute lymphoblastic leukemia. Cancer Discov 2017, 7, 1336–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vong QP, Leung W-H, Houston J, Li Y, Rooney B, Holladay M, et al. TOX2 regulates human natural killer cell development by controlling T-BET expression. Blood 2014, 124, 3905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pau CT, Mosbruger T, Saxena R, Welt CK.. Phenotype and tissue expression as a function of genetic risk in polycystic ovary syndrome. PLoS One 2017, 12, e0168870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hartanti MD, Rosario R, Hummitzsch K, Bastian NA, Hatzirodos N, Bonner WM, et al. Could perturbed fetal development of the ovary contribute to the development of polycystic ovary syndrome in later life?. PLoS One 2020, 15, e0229351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J-H, You J, Dobrota E, Skalnik DG.. Identification and characterization of a novel human PP1 phosphatase complex. J Biol Chem 2010, 285, 24466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dittmer S, Kovacs Z, Yuan SH, Siszler G, Kögl M, Summer H, et al. TOX3 is a neuronal survival factor that induces transcription depending on the presence of CITED1 or phosphorylated CREB in the transcriptionally active complex. J Cell Sci 2011, 124, 252–60. [DOI] [PubMed] [Google Scholar]
- 11. Bounaix Morand du Puch C, Barbier E, Kraut A, Couté Y, Fuchs J, Buhot A, et al. TOX4 and its binding partners recognize DNA adducts generated by platinum anticancer drugs. Arch Biochem Biophys 2011, 507, 296–303. [DOI] [PubMed] [Google Scholar]
- 12. Tessema M, Yingling CM, Grimes MJ, Thomas CL, Liu Y, Leng S, et al. Differential epigenetic regulation of TOX subfamily high mobility group box genes in lung and breast cancers. PLoS One 2012, 7, e34850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 2007, 39, 865–9. [DOI] [PubMed] [Google Scholar]
- 14. Seksenyan A, Kadavallore A, Walts AE, de la Torre B, Berel D, Strom SP, et al. TOX3 is expressed in mammary ER(+) epithelial cells and regulates ER target genes in luminal breast cancer. BMC Cancer 2015, 15, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilkinson B, Chen J YF, Han P, Rufner KM, Goularte OD, Kaye J.. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat Immunol 2002, 3, 272–80. [DOI] [PubMed] [Google Scholar]
- 16. Aliahmad P, Seksenyan A, Kaye J.. The many roles of TOX in the immune system. Curr Opin Immunol 2012, 24, 173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seehus CR, Aliahmad P, de la Torre B, Iliev ID, Spurka L, Funari VA, et al. The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor. Nat Immunol 2015, 16, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu C-J, Cho S, Huang H-Y, Lu C-H, Russ J, Cruz LO, et al. MiR-23~27~24-mediated control of humoral immunity reveals a TOX-driven regulatory circuit in follicular helper T cell differentiation. Sci Adv 2019, 5, eaaw1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 2019, 571, 270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 2019, 571, 265–9. [DOI] [PubMed] [Google Scholar]
- 21. Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8 T cell exhaustion. Nature 2019, 571, 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu W, Zhao X, Wang X, Feng H, Gou M, Jin W, et al. The transcription factor Tox2 drives T follicular helper cell development via regulating chromatin accessibility. Immunity 2019, 51, 826–839.e5. [DOI] [PubMed] [Google Scholar]
- 23. Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8 T cell exhaustion. Proc Natl Acad Sci USA 2019, 116, 12410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Easton DF, Pooley KA, Dunning AM, Pharoah PDP, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007, 447, 1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saleh R, Taha RZ, Toor SM, Sasidharan Nair V, Murshed K, Khawar M, et al. Expression of immune checkpoints and T cell exhaustion markers in early and advanced stages of colorectal cancer. Cancer Immunol Immunother 2020, 69, 1989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng D, Lin H, Cui J, Liang W.. TOX3 is a favorable prognostic indicator and potential immunomodulatory factor in lung adenocarcinoma. Oncol Lett 2019, 18, 4144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vanheer L, Song J, De Geest N, Janiszewski A, Talon I, Provenzano C, et al. Tox4 modulates cell fate reprogramming. J Cell Sci 2019, 132, jcs232223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar BV, Connors TJ, Farber DL.. Human T cell development, localization, and function throughout life. Immunity 2018, 48, 202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dutta A, Zhao B, Love PE.. New insights into TCR β-selection. Trends Immunol 2021, 42, 735–50. [DOI] [PubMed] [Google Scholar]
- 30. Klein L, Kyewski B, Allen PM, Hogquist KA.. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 2014, 14, 377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aliahmad P, O’Flaherty E, Han P, Goularte OD, Wilkinson B, Satake M, et al. TOX provides a link between calcineurin activation and CD8 lineage commitment. J Exp Med 2004, 199, 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohu K, Sato T, Ohno S-I, Hayashi K, Uchino R, Abe N, et al. Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol 2005, 174, 2627–36. [DOI] [PubMed] [Google Scholar]
- 33. Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci USA 2003, 100, 7731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, et al. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 2002, 418, 195–9. [DOI] [PubMed] [Google Scholar]
- 35. Aliahmad P, Kaye J.. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med 2008, 205, 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brugnera E, Bhandoola A, Cibotti R, Yu Q, Guinter TI, Yamashita Y, et al. Coreceptor reversal in the thymus: signaled CD4 + 8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity 2000, 13, 59–71. [DOI] [PubMed] [Google Scholar]
- 37. Correia-Neves M, Mathis D, Benoist C.. A molecular chart of thymocyte positive selection. Eur J Immunol 2001, 31, 2583–92. [DOI] [PubMed] [Google Scholar]
- 38. Egawa T, Taniuchi I.. Antagonistic interplay between ThPOK and Runx in lineage choice of thymocytes. Blood Cells Mol Dis 2009, 43, 27–9. [DOI] [PubMed] [Google Scholar]
- 39. Egawa T. Runx and ThPOK: a balancing act to regulate thymocyte lineage commitment. J Cell Biochem 2009, 107, 1037–45. [DOI] [PubMed] [Google Scholar]
- 40. Egawa T, Littman DR.. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol 2008, 9, 1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aliahmad P, Kadavallore A, de la Torre B, Kappes D, Kaye J.. TOX is required for development of the CD4 T cell lineage gene program. J Immunol 2011, 187, 5931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol 2008, 9, 1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012, 36, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 2013, 38, 769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yokoyama W M, Kim S, French AR.. The dynamic life of natural killer cells. Annu Rev Immunol. 2004, 22, 405–29. [DOI] [PubMed] [Google Scholar]
- 46. Klose C SN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–56. [DOI] [PubMed] [Google Scholar]
- 47. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010, 463, 540–4. [DOI] [PubMed] [Google Scholar]
- 48. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011, 12, 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoyler T, Klose CSN, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012, 37, 634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monticelli LA, Sonnenberg GF, Artis D.. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol 2012, 24, 284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 2009, 10, 75–82. [DOI] [PubMed] [Google Scholar]
- 53. Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–70. [DOI] [PubMed] [Google Scholar]
- 54. Diefenbach A, Colonna M, Koyasu S.. Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41, 354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–66. [DOI] [PubMed] [Google Scholar]
- 56. Constantinides MG, McDonald BD, Verhoef PA, Bendelac A.. A committed precursor to innate lymphoid cells. Nature 2014, 508, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife 2014, 3, e04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seehus C, Kaye J.. Differentiation of murine innate lymphoid cells from common lymphoid progenitor cells. Bio Protoc 2016, 6, e1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aliahmad P, de la Torre B, Kaye J.. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol 2010, 11, 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep 2015, 10, 2043–54. [DOI] [PubMed] [Google Scholar]
- 61. Geiger TL, Abt MC, Gasteiger G, Firth MA, O’Connor MH, Geary CD, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med 2014, 211, 1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science 2013, 342, 727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol. 2011, 12, 949–58. [DOI] [PubMed] [Google Scholar]
- 64. Page N, Klimek B, De Roo M, Steinbach K, Soldati H, Lemeille S, et al. Expression of the DNA-binding factor TOX promotes the encephalitogenic potential of microbe-induced autoreactive CD8 T cells. Immunity 2018, 48, 937–950.e8. doi: 10.1016/j.immuni.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 1999, 397, 702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 66. Boos MD, Yokota Y, Eberl G, Kee BL.. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med 2007, 204, 1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ikawa T, Fujimoto S, Kawamoto H, Katsura Y, Yokota Y.. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci USA 2001, 98, 5164–9. doi: 10.1073/pnas.091537598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Male V, Nisoli I, Kostrzewski T, Allan D SJ, Carlyle JR, Lord GM, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med 2014, 211, 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med 2009, 206, 2977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yun S, Lee SH, Yoon S-R, Kim MS, Piao Z-H, Myung P-K, et al. TOX regulates the differentiation of human natural killer cells from hematopoietic stem cells in vitro. Immunol Lett 2011, 136, 29–36. [DOI] [PubMed] [Google Scholar]
- 71. Eberl G, Marmon S, Sunshine M-J, Rennert PD, Choi Y, Littman DR.. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 2004, 5, 64–73. [DOI] [PubMed] [Google Scholar]
- 72. Cupedo T, Vondenhoff MFR, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol 2004, 173, 2968–75. [DOI] [PubMed] [Google Scholar]
- 73. Mebius RE, Rennert P, Weissman IL.. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997, 7, 493–504. [DOI] [PubMed] [Google Scholar]
- 74. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med 2009, 206, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhong C, Zhu J.. Transcriptional regulatory network for the development of innate lymphoid cells. Mediators Inflamm 2015, 2015, 264502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011, 29, 621–63. [DOI] [PubMed] [Google Scholar]
- 77. Vinuesa CG, Linterman MA, Yu D, MacLennan ICM.. Follicular helper T cells. Annu Rev Immunol 2016, 34, 335–68. [DOI] [PubMed] [Google Scholar]
- 78. Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 2010, 143, 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–68. [DOI] [PubMed] [Google Scholar]
- 84. Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 2014, 507, 513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 2009, 10, 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Spolski R, Leonard WJ.. IL-21 and T follicular helper cells. Int Immunol 2010, 22, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McLane LM, Abdel-Hakeem MS, Wherry EJ.. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019, 37, 457–95. [DOI] [PubMed] [Google Scholar]
- 88. Martinez GJ, Pereira RM, Äijö T, Kim EY, Marangoni F, Pipkin ME, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 2015, 42, 265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen J, López-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567, 530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, et al. Transcription factor IRF4 promotes CD8 T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity 2017, 47, 1129–41. [DOI] [PubMed] [Google Scholar]
- 91. Li J, He Y, Hao J, Ni L, Dong C.. High levels of Eomes promote exhaustion of anti-tumor CD8 T cells. Front Immunol 2018, 9, 2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol 2019, 4, eaay0555. [DOI] [PubMed] [Google Scholar]
- 93. Sekine T, Perez-Potti A, Nguyen S, Gorin J-B, Wu VH, Gostick E, et al. TOX is expressed by exhausted and polyfunctional human effector memory CD8 T cells. Sci Immunol 2020, 5, eaba7918. [DOI] [PubMed] [Google Scholar]
- 94. Kim K, Park S, Park SY, Kim G, Park SM, Cho J-W, et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med 2020, 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yao C, Sun H-W, Lacey NE, Ji Y, Moseman EA, Shih H-Y, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8 T cell persistence in chronic infection. Nat Immunol 2019, 20, 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang X, He Q, Shen H, Xia A, Tian W, Yu W, et al. TOX promotes the exhaustion of antitumor CD8 T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol 2019, 71, 731–41. doi: 10.1016/j.jhep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 97. Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang C, Wang X-M, Li S-R, Twelkmeyer T, Wang W-H, Zhang S-Y, et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat Commun 2019, 10, 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Merino A, Zhang B, Dougherty P, Luo X, Wang J, Blazar BR, et al. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J Clin Invest 2019, 129, 3770–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018, 19, 723–32. [DOI] [PubMed] [Google Scholar]
- 102. Yin M, Di G, Bian M.. Dysfunction of natural killer cells mediated by PD-1 and Tim-3 pathway in anaplastic thyroid cancer. Int Immunopharmacol 2018, 64, 3–9. [DOI] [PubMed] [Google Scholar]
- 103. Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL.. PD-L1 mediates dysfunction in activated PD-1 NK cells in head and neck cancer patients. Cancer Immunol Res 2018, 6, 1548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. . Mann TH, Kaech SM.. Tick-TOX, it’s time for T cell exhaustion. Nat Immunol. 2019;20(9):1092–4. [DOI] [PubMed] [Google Scholar]
- 105. Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D.. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol 2013, 14, 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wildt KF, Sun G, Grueter B, Fischer M, Zamisch M, Ehlers M, et al. The transcription factor Zbtb7b promotes CD4 expression by antagonizing Runx-mediated activation of the CD4 silencer. J Immunol 2007, 179, 4405–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. No data were generated in the making of this article.