Abstract
Immunosuppression for lung transplant recipients is a critical part of post-transplant care, to prevent acute and chronic rejection. Treatment protocols consist of induction and maintenance immunotherapy. Induction agents provide an immediate state of immunosuppression following transplantation and over time, and their use has become more commonplace. Several agents are available for clinical use, including anti-thymocyte globulin, alemtuzumab, and basiliximab, the latter being most commonly employed. Each induction agent has unique side effects and caveats to their use, of which we must be aware. Maintenance immunosuppression is initiated following transplant but requires multiple doses prior to reaching therapeutic levels. A calcineurin inhibitor, an anti-metabolite, and a corticosteroid are traditionally used, most commonly tacrolimus, mycophenolate mofetil, and prednisone. Dosing regimens and goal trough levels vary and are tailored to a patient’s clinical status and duration post-transplant. Future clinical studies may be able to assist in determining the optimal induction and maintenance immunosuppression regimens. In the interim, we use cohort and registry data to guide our therapies.
Keywords: Lung transplant, Induction, Immunosuppression
Introduction
For individuals with end-stage lung disease, lung transplantation can increase life expectancy and improve quality of life. Despite improvements in surgical techniques, immunosuppressive regimens, and organ preservation, median survival following a lung transplant is just 6.7 years [1]. Graft survival is associated with numerous recipient and donor characteristics such as recipient age, gender, and underlying diagnosis [1].
Acute rejection is common in the first year following transplant, with over 25% of recipients experiencing at least one episode. US registry data demonstrates chronic rejection occurs in 41.8% by 5 years and remains the leading cause of death after lung transplant [2]. Lifelong immunosuppression is essential to prevent acute rejection and delay chronic rejection.
In the absence of consensus, lung transplant centers develop immunosuppressive protocols based on experience and dogma. Here we explore the evidence for the use of induction and chronic immunosuppression medications.
Induction immunosuppression
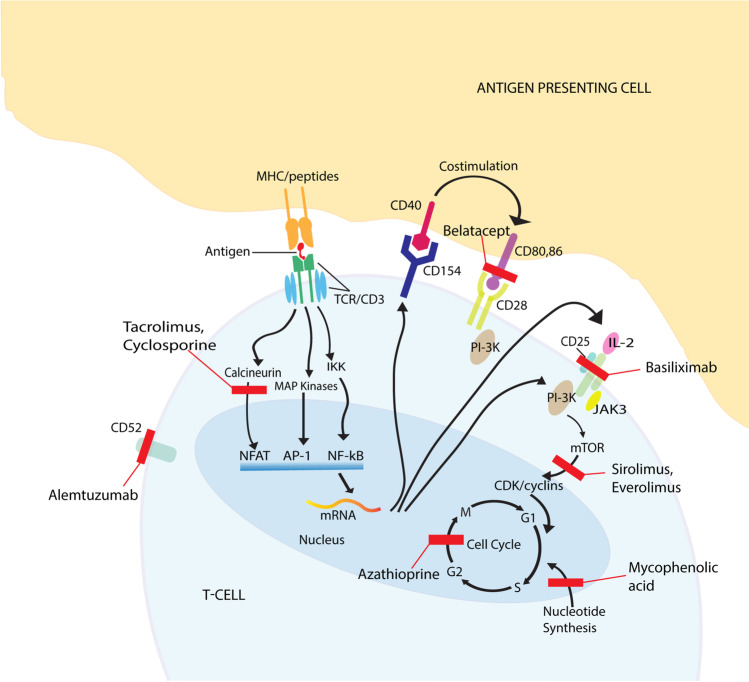
Induction achieves a rapidly therapeutic state of immunosuppression immediately after lung transplantation. A variety of agents can be used as induction agents, including polyclonal and monoclonal T-cell-depleting antibodies and IL-2 receptor antagonists—these agents target T-cells by decreasing their numbers and inhibiting activation or proliferation. Simultaneous initiation of maintenance immunosuppression is usual; however, it often takes time to attain therapeutic levels, and induction can bridge this gap (Fig. 1).
Fig. 1.
Site of action for induction and maintenance immunosuppressants. Red bars indicate the site of binding/pathway blockade caused by each immunosuppressant agent. Notably, most immunosuppression regimens address multiple pathways that inhibit T-cell function. Due to their multiple sites of action, ATG and corticosteroids are not represented in the figure. Created by Adobe Illustrator
Most centers in the USA use induction agents; in 2018, 68.9% of recipients received an IL-2 receptor antagonist and 9.1% a T-cell-depleting agent [2]. In the International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation (ISHLT) registry, the use of induction immunosuppression was reported for over 80% of patients. Over time, the use of IL-2 antagonists has become the most common treatment as the use of polyclonal depleting agents and CD52 monoclonal antibody treatments has decreased [1].
Potential benefits of induction include a reduction in the rate of acute rejection, a reduced risk of acute kidney injury, and a reduced chronic nephrotoxicity due to lower required doses of calcineurin inhibitors and corticosteroids. Evidence also suggests potential for increased freedom from bronchiolitis obliterans syndrome (BOS) and a graft survival benefit [3, 4]. The use of induction may also narrow the differential diagnosis for allograft dysfunction in the acute post-operative period by reducing the likelihood of rejection [5]. Risks of induction include an increased risk of infection (including anastomotic infections [6]) and lymphoproliferative disorders [7]. Based on US data (Scientific Registry of Transplant Recipients (SRTR)), rates of acute rejection were slightly lower (15.7–15.9%) in recipients who received induction than those who did not (18%) [2].
Induction immunosuppression is generally divided into three categories: (1) monoclonal T-cell-depleting agents include alemtuzumab (Campath®), (2) polyclonal T-cell-depleting agents include anti-thymocyte globulin (ATG), and (3) IL-2 receptor antagonists including daclizumab (removed from the market in 2018) and basiliximab (BAS) (Table 1).
Table 1.
Induction agents
| Agent | Mechanism of action | Adverse effects | Cost | Dosing | Additional considerations |
|---|---|---|---|---|---|
| Basiliximab (BAS) (Simulect®) | Monoclonal antibody that binds and blocks IL-2 receptor a-chain on the surface of activated T-cells | Hypersensitivity reactions, including rash, hypotension, cardiac and respiratory failure | $$$ | 20 mg IV POD 0, POD 4 |
Non-T-cell-depleting Not used for rejection |
| Anti-thymocyte globulin (ATG) (Thymoglobulin®, Atgam®) | Polyclonal antibody that activates and depletes T-cells | Cytokine release syndrome1 | $$$–$$$$$ | Variable dosing, up to 1.5 mg/kg daily for a total dose of 5–15 mg/kg body weight |
High doses deplete B-cells and NK cells Can be used for recurrent/refractory acute rejection or chronic lung allograft dysfunction Avoid in high-risk CMV mismatch, due to risk of viremia Requires pre-treatment |
| Alemtuzumab (ALM) (Campath ®) | IgG monoclonal antibody against CD52 | Sustained immunosuppression, cytokine release syndrome1 | $0 | 30 mg IV or SC at time of transplant |
CD52 is found on T-cells, B-cells, monocytes, and macrophages but is not present on lymphoid progenitor cells Post-alemtuzumab autoimmunity, especially thyroiditis, can be seen [57] Avoid in high-risk CMV mismatch Produces sustained immunosuppression with recovery of B cells at 3 months, and 50% of T-cell function at 36 months [9] Requires pre-treatment Consider alternative therapy in older adults (> 60–65 years old) |
1Cytokine release syndrome often manifested by rigors, fever, hypotension, headache, nausea, vomiting, rash, bronchospasm
2Campath only available through limited distribution program www.clinigengroup.com, $ = $2500 USD
Muromonab-CD3, marketed as OKT3®, was an induction agent previously used in solid organ transplant. This mouse monoclonal antibody (mAb) to CD3 frequently resulted in severe cytokine storm reactions and ultimately fell out of favor as other better tolerated agents, ATG and anti-CD52 mAb, became available. OKT3® was removed from the US and European markets in 2010 due to declining sales [8].
Alemtuzumab
Alemtuzumab is an IgG monoclonal antibody against CD52 approved for chronic lymphocytic leukemia. Although it is not Food and Drug Administration (FDA)-approved for use in solid organ transplant, alemtuzumab has been clinically used and studied in induction and chronic lung allograft rejection [9, 10]. CD52 is a glycoprotein found on most immune cells (T, B, NK cells, monocytes, and macrophages). Use of alemtuzumab leads to lysis of these cells and sustained T and B cell depletion of more than 50% at 6 months [9] that can persist for more than a year for lymphocytes [11]. It is only available directly through the manufacturer [12], and is administered post-operatively as a single injection of 30 mg. Vital signs should be monitored given the risk of hypersensitivity reactions in up to 92% of patients [11].
Current literature shows limited support for the use of alemtuzumab in induction. No difference in acute rejection, or lymphocytic bronchiolitis was seen in prospective cohort investigations [13]; however, retrospective cohorts have shown higher survival with alemtuzumab than in those patients with no induction and greater freedom from acute rejection [14, 15]. The use of alemtuzumab in transplant recipients over the age of 60 has been associated with an increased risk of mortality and graft loss in kidney transplantation [16]. For this reason, its use is often avoided in lung transplant recipients over the age of 60–65 years. Additionally, there is some concern for an increased risk of malignancy in older adults; however, this is not currently supported by published data in solid organ transplant. Registry data from the Scientific Registry of Transplant Recipients/Organ Procurement and Transplantation Network (SRTR/OPTN) and ISHLT database investigations appear to support the use of induction over none, with evidence of improved survival [17–19] (see Tables 2 and 3).
Table 2.
Selected cohort investigations of induction agents
| Agent (s) | Author/year | N | Intervention/type of trial | Exclusion | Outcomes |
|---|---|---|---|---|---|
| Randomized control trials | |||||
| ATG | Hartwig et al. 2008 [22] |
44 Group 1: ATG, n = 22 Group 2: No induction, n = 22 |
Randomized, open-label prospective trial Randomized 1:1 |
Age < 18, D + /R − CMV mismatch |
No difference in survival, acute rejection, or infectious complications Reduction in BOS at 5 years post-transplant and increase in incidence of malignancy (not statistically significant) |
| ATG | Palmer et al. 1999 [23] |
44 Group 1: ATG, n = 22 Group 2: No induction, n = 22 |
Randomized controlled trial, partially blinded (only the pathologist was blinded to treatment and patient and investigators not blinded) Randomized 1:1 |
Age < 18, D + /R − CMV mismatch |
Significant reduction in the number of patients who experienced episodes of acute rejection in the ATG group (23%) compared with no induction (55%, p = 0.03) Reduction in the incidence of BOS with ATG induction (20%) compared to the control (38%), not statistically significant (p = 0.43) Non-significant increase in CMV infection rates with ATG induction compared to control (p = 0.23) No difference in 30-day, 1- or 2- year survival between ATG induction and no induction |
| ATG | Snell et al. 2014 [58] |
223 Group 1: ATG low dose (5 mg/kg), n = 61 Group 2: ATG high dose (9 mg/kg), n = 82 Group 3: Placebo, n = 78 |
Multicenter, double-blind, randomized parallel group design Randomized 1:1:1 Interim analysis showed group 1 dosing to be inefficacious and enrollment stopped for group 1 |
Age < 18 Clinical instability, re-transplant, HIV, pulmonary infection See article supplement for full list |
No difference in survival, graft loss, or acute rejection at 12 months Higher rate of death/graft loss in the first 3 months after transplant in high-dose ATG group (p = 0.003), but not a significant difference between groups at 1 year Treatment-related and severe adverse events were higher in ATG groups (p = 0.002) Malignancy and infection were more common in ATG groups, but not with statistical significance CMV mismatches were higher in the low-dose ATG group |
| ATG, ALM | Jaksch et al. 2014 [9] |
60 Group 1: ALM, n = 30 Group 2: ATG, n = 30 |
Open-label, controlled, randomized trial Randomized 1:1 |
None specified |
No significant difference in survival at 1 and 2 years (p = 0.1) or BOS (p = 0.19). No difference in CMV, bacterial or aspergillus infections Fewer episodes of ≥ A2 rejection in the first year, in the ALM group. (p = 0.019) More frequent episodes of leukopenia seen in the ALM group (p = 0.01) |
| Prospective cohort | |||||
| ALM | van Loenhout et al. 2010 [13] |
40 Cohort 1: alemtuzumab, n = 20 Cohort 2: control, n = 20 |
Prospective experimental, retrospective control groups Intervention: ALM within 12 h of transplant |
None specified |
No significant difference in survival between the two groups (p = 0.52) No differences in acute rejection, high-grade rejection (A3), or lymphocytic bronchiolitis (p = 0.32, 0.44, 1.00) No difference in bacteria-positive BAL cultures, viral, or fungal isolates (p = 0.26, p = 0.87) ALM group trended toward lower lymphocyte counts at all intervals (p = 0.29) |
| Retrospective cohort | |||||
| BAS and ATG | Clinckart et al. 2009 [29] |
37 Cohort 1: ATG n = 16 Cohort 2: BAS (n = 21) |
Retrospective cohort | None specified |
BAS with better safety profile; higher platelet count (p = 0.046) Similar number of rejection episodes Survival improved by 20% in BAS group (p = 0.03) in 2-year follow-up |
| BAS and ATG | Hachem et al. 2005 [31] |
157 Cohort 1: BAS, n = 82 Cohort 2: ATG, n = 75 |
Retrospective cohort |
Use of tacrolimus, n = 18, < 100-day survival, n = 6 No surveillance biopsies, n = 6 Missing data, n = 5 |
No difference in CMV viremia between groups Non-significant difference in survival favoring ATG (p = 0.066) Higher grade rejection (> = A2) was more common in the BAS group, 60% vs. 38% in the ATG group (p = 0.04) Development of BOS stage 1 was more common in those who received BAS than those who received ATG (p = 0.036) No CMV mismatches in ATG group, 33 (40%) in BAS group. Median 2-year follow-up |
| Alemtuzumab, ATG, DZM | Shyu et al. 2011 [14] |
336 Cohort 1: alemtuzumab, n = 127 Cohort 2: ATG, n = 43 Cohort 3: DZM, n = 73 Cohort 4: No induction, n = 93 |
Retrospective cohort | Age < 18 |
ATG patients had longer length of hospital stay at their transplant admission (p < 0.05) and more readmissions during the first year post-transplant than other patient groups (p < 0.01) Alemtuzumab induction was associated with higher survival than the no induction (59% vs. 47%, p = .011) and daclizumab groups (59% vs. 44%, p = . 045). Alemtuzumab also showed the lowest percentage of death from graft failure (13.5%) Alemtuzumab also showed a greater freedom from acute rejection by 5 years post-transplant than the other groups (p < 0.01) Alemtuzumab maintained the highest rate of freedom from BOS at 5 years (86%, p = 0.003) There was no significant difference across the study groups in PTLD incidence |
| ALM, BAS | Whited et al. 2015 [15] |
89 Cohort 1: BAS, n = 44 Cohort 2: alemtuzumab, n = 45 |
Retrospective cohort Alemtuzumab administered subcutaneously (to limit infusion-related reactions) |
Age < 18, re-transplant, multi-organ transplant, transfer of care to another center within the first year ALM is not administered to EBV-negative recipients |
There was no significant difference between infectious outcomes and mortality at 6 months The average biopsy score (total grade A + B divided by the number of biopsies) was significantly lower in the ALM group than the BAS group (p < 0.0001) There were fewer episodes of rejection (A2 rejection) with ALM than BAS (p < 0.0001) Stepwise logistical regression showed induction with BAS to be a significant risk factor for an A2 or greater rejection (p = 0.0006) |
Table 3.
Registry studies for induction agents
| Agent (s) | Author/year | N | Intervention/type of trial | Exclusion | Outcomes |
|---|---|---|---|---|---|
| BAS, ALM, ALG, ATG | Duffy et al. 2015 [17] | 3405 |
Retrospective cohort review of the UNOS database, COPD transplant recipients Cohort 1: induced (52%) with basiliximab, alemtuzumab, thymoglobulin, ALG, or ATG, n = 1761 Cohort 2: no induction (48%), n = 1644 |
Transplanting diagnosis other than COPD, re-transplantation, other than deceased donor; transplant year prior to 5/1/2005 or after 6/26/2014; age < 18 years; induction with daclizumab or OKT3; use of more than one induction agent, patient, or graft survival < 1 day |
Induced patients observed a survival benefit compared to no induction. HR 0.793 (95% CI = 0.693, 0.909; p = 0.001) No differences were noted in death due to infection or acute rejection Induced patients had a delay in BOS onset compared no induction (SHR = 0.801, p = 0.003) |
| BAS, ALM, ALG, ATG | Kirkby et al. 2015 [18] | 1721 |
Retrospective cohort review of the UNOS and SRTR database Cohort 1: induced (46%) basiliximab, alemtuzumab, thymoglobulin, ALG, or ATG, n = 792 Cohort 2: no induction (54%), n = 929 |
Transplanting diagnosis other than CF, re-transplantation, other than deceased donor; transplant year before 1/1/2001 or after 7/6/2011; age < 6 or > 55 years; induction with daclizumab or OKT3; use of more than one induction agent, patient, or graft survival < 1 day | Recipients who underwent induction had a longer group median survival of 93.8 months compared to no induction 61.8 months (p < 0.001) |
| BAS, ATG | Hachem et al. 2008 [24] | 3970 |
Retrospective cohort review of the ISHLT Registry Cohort 1: no induction (56.6%), n = 2249 Cohort 2: IL-2 RA-BAS, daclizumab (28.3%), n = 1124 Cohort 3: ATG (15%), n = 597 |
Re-transplantation; transplant year < 1/1/2000 or > 3/31/2004; age < 18 years; death within 14 days of transplantation; use of multiple induction agents; no known immunosuppression; use of multiple calcineurin inhibitors or anti-metabolites during the initial hospitalization; induction with OKT 3; patients requiring inotropic support prior to transplantation |
The IL-2 RA cohort observed a graft survival benefit over the ATG and no induction groups (64%; 60%; 57%; log rank p = 0.0067) Single and bilateral recipients who received IL-2 RA had a significant survival advantage compared with no induction (RR = 0.82, p = 0.007) ATG offered a survival advantage over the no induction group in bilateral recipients (RR = 0.78; p = 0.043); but not in single lung recipients (RR = 1.06; p = 0.58) Fewer early post-transplant infections in the no induction group 38% compared to those who received an IL-RA (45%) or ATG (43%), p < 0.0005 Patients who did not receive induction were more likely to be treated for rejection early after transplant (22%) compared to those who received IL-2 RA (15%) or ATG (17%); p < 0.005 |
| BAS, ALM, ALG, ATG | Whitson et al. 2014 [19] | 12,858 |
Retrospective cohort review of the UNOS/SRTR database Cohort 1: induction (44%) with BAS, alemtuzumab, thymoglobulin, ALG, or ATG, n = 5713 Cohort 2: no induction (56%), n = 7145 |
Re-transplantation, other than deceased donor; transplant year before 1/1/2001 or after 12/21/2012; age < 18 years; induction with daclizumab or OKT3; use of more than one induction agent, patient, or graft survival < 1 day |
Recipients who underwent induction had a reduced risk of death (p < 0.0001) Lower risk of death for patients receiving BAS compared to no induction (HR = 0.822; p < 0.0001) or ALG/ATG (HR = 0.89; p = 0.0223) Median survival: induced patients 71.3 months; no induction 63.2 months No significant difference found in the incidence of post-transplant dialysis |
Anti-thymocyte globulin
ATG is derived from horses (ATGAM®) or rabbits (Thymoglobulin®) immunized with human thymocytes. It functionally inactivates and depletes T-cells by blocking multiple T-cell clusters of differentiation factors and human leukocyte antigens. When ATG binds to the T-cell receptor, this can cause activation and cellular apoptosis, leading to a cytokine release syndrome (e.g., fevers, chills, hypotension, pulmonary edema). Due to these commonly observed side effects, ATG requires pre-treatment with acetaminophen, diphenhydramine, and methylprednisolone and close monitoring of vital signs should occur during the infusion [12]. Multiple dosing regimens have been described and are often institution-specific. Medication-related toxicities include thrombocytopenia and leukopenia; therapy should be reduced if they occur [20] (see Table 1).
Trials have produced conflicting evidence supporting the use of ATG as induction therapy for lung transplant. Several randomized controlled trials, comparing ATG to other induction agents, showed no difference in acute rejection, bronchiolitis obliterans (BOS), or survival [5, 21]. However, when comparing ATG to no induction, use of ATG demonstrates a 20–32% reduction in BOS [22, 23]. Special consideration must be observed for cytomegalovirus (CMV) mismatches (donor-positive, recipient negative status) as induction may increase the risk of viremia and early infection [24], and as a consequence, this therapy is often avoided in these patients. An increased frequency of readmissions following transplant has also been observed in patients induced with ATG [14].
Basiliximab
Basiliximab (BAS) is a chimeric (murine/human) monoclonal antibody directed against the IL-2 alpha chain, on the surface of T-cells (CD25). It prevents binding of IL-2, the signal for T-cell proliferation, but does not cause T-cell depletion. As a non-depleting agent, BAS’s role in lung transplantation is isolated to induction therapy. No pre-treatment medications are required, but adverse effects including anaphylactoid symptoms can occur [12, 25, 26]. Daclizumab, an agent similar to BAS, was voluntarily withdrawn from the market in 2018 due to multiple meningoencephalitis reports [27]. Daclizumab has not been included explicitly in our literature review, as it is not available for clinical use and noted adverse effects.
In several retrospective cohorts, IL-2 agents (daclizumab and BAS) appeared to reduce the frequency of acute rejection, increase the time to first rejection episode, and increase survival duration [28, 29]. In addition, the 2019 ISHLT registry found 50% of all lung transplant recipients received induction therapy with an IL-2 receptor antagonist. While direct comparator studies have failed to find survival benefit with BAS over other agents [15, 30, 31], analysis of a large cohort registry data has consistently demonstrated favorable effects of IL-2 receptor antagonist induction therapy [18, 19]. In one of the most extensive UNOS registry reviews, BAS was found to reduce the risk of mortality over no induction (HR = 0.822; p < 0.0001) and induction with ATG (HR = 0.89; p = 0.02) [19]. This finding supported an earlier cohort review of the ISHLT registry [24] that found basiliximab offered a graft survival benefit over no induction and ATG. The heterogeneity of the patient populations and the retrospective nature of registry studies makes the appropriate application of this information challenging, but BAS use in induction has continued to grow over the last 20 years (Tables 2 and 3).
Maintenance immunosuppression
Lifelong immunosuppression after lung transplant is essential to prevent acute rejection, postpone chronic rejection, and improve survival. Three-drug maintenance immunosuppression is the standard of care after lung transplant and typically includes a calcineurin inhibitor (CNI) (tacrolimus or cyclosporine), an anti-metabolite (mycophenolate or azathioprine), and a corticosteroid (prednisone). According to the 2019 International Thoracic Organ Transplant Registry of the ISHLT, tacrolimus (Tac), mycophenolate, and prednisone were the leading maintenance immunosuppressants 1 year after transplant, with approximately 62% of patients from 2005 to 2018 prescribed this regimen [1]. Given the delicate checks and balances post-transplantation, the goal of maintenance immunosuppression is to not only minimize immune-mediated allograft injury but also to minimize medication-related side effects.
Calcineurin inhibitors
Prior to the advent of CNIs, survival following lung transplant was measured in weeks rather than years. Cyclosporine A (CsA) was the first CNI approved by the US Food and Drug Administration (FDA) in 1983 with Tac following in 1997. Today, CNIs are the cornerstone of conventional maintenance immunosuppression for all solid organ transplants.
CsA is a lipophilic peptide that binds with high affinity for cyclophilin in T-lymphocytes while Tac binds to intracellular FKBP-12. Both form a complex that prevents transcription of interleukin-2, ultimately reducing T-lymphocyte proliferation [32]. Non-modified CsA (Sandimmune®) has poor and variable oral absorption which led to the development of modified CsA (Neoral®, Gengraf®) with enhanced bioavailability and a more desirable pharmacokinetic profile [33].
Like CsA, Tac has poor and variable absorption but is 10–100 times more potent [34]. Sublingual administration of Tac, which increases bioavailability by approximately 100% [35], can be utilized in cases where variable absorption prevents consistent serum levels. Once-daily extended-release formulations were approved by the FDA in 2013 (Astagraf XL®) and 2015 (Envarsus XR®). Benefits of extended-release Tac formulations include lower peak levels leading to reduced adverse effects and the potential for better medication adherence due to once-daily dosing. Target immunosuppression levels for Tac and CsA vary based on patient characteristics and the time following transplant. Generally, higher C0 and C2 targets are reserved for the early post-transplant period and levels can be reduced as time passes to minimize nephrotoxicity and infection risk. When setting goals, clinicians should take into account patient characteristics such as time since transplant, past episodes of rejection, and infection history.
Significant drug interactions may occur with CsA and Tac due to their metabolism via the hepatic cytochrome P450 enzymes. CsA is also a moderate inhibitor of CYP3A4 which may cause additional interactions. Adverse effects associated with Tac and CsA can be found summarized in Table 4.
Table 4.
Maintenance immunosuppression post-lung transplantation
| Immunosuppressive agents | Mechanism of action | Adverse effects | Cost | Dosing | Additional considerations |
|---|---|---|---|---|---|
| Calcineurin inhibitors | |||||
| Tacrolimus (Tac) (Prograf®, Envarsus®, Astagraf®) | Prevents nuclear translocation of NF-AT and cytokine gene transcription, blocking the production of IL-2 and inhibiting T-cell activation and proliferation by binding to FKBP-12 |
Alopecia (Tac), neurotoxicity (Tac > CsA), hyperglycemia (Tac > CsA) Nephrotoxicity (Tac = CsA), hyperkalemia (Tac = CsA) hypomagnesemia (Tac = CsA), hyperuricemia (Tac = CsA) |
$–$$$ |
Available in oral capsules, solution, intravenous (IV). Sublingual administration possible Therapeutic monitoring with C0¹ = 5–15 ng/mL [58] |
Once daily extended-release Envarsus® dose conversion varies based on ethnicity |
| Cyclosporine (CsA) (Neoral®, Gengraf®, Sandimmune®) | Prevents nuclear translocation of NF-AT and cytokine gene transcription, blocking the production of IL-2 and inhibiting T-cell activation and proliferation by binding to FKBP-12 by binding to cyclophilin | Gingival hyperplasia (CsA), hirsutism (CsA), hypertension (CsA > Tac), hyperlipidemia (CsA > Tac) | $–$$ |
Available in oral capsules, oral solution, IV Therapeutic monitoring with C01 = 100–450 ng/mL, C22 = 800–1400 ng/mL [58] |
Modified CsA (Neoral®, Gengraf®) is not interchangeable with non-modified CsA (Sandimmune®) |
| Anti-metabolites | |||||
|
Mycophenolate mofetil (MMF) (Cellcept©) Mycophenolate sodium (MPS) (Myfortic©) |
Converted to mycophenolic acid which targets inosine monophosphate dehydrogenase, the rate-limiting enzyme in the synthesis of guanosine nucleotides, essential for DNA synthesis | Gastrointestinal side effects (diarrhea, nausea, vomiting, abdominal pain), bone marrow suppression |
$–$$$ $ |
MMF available in oral capsule, tablet, suspension, and IV MPS available as oral tablet MMF 1–1.5 g twice daily [36, 41] MPS up to 1.08 g twice daily [59]. Decrease for adverse effects |
Due to teratogenicity, risk evaluation and mitigation strategy (REMS) program’s participation is required |
| Azathioprine (AZA) (Imuran©) | Metabolized to 6-MP and then converted to 6-thiouric acid, 6-methyl-MP, and 6-thioguanine which are incorporated into replicating DNA and halt replication | Bone marrow suppression, hepatotoxicity | $–$$$ |
Available in oral tablet and IV Dosing based on body weight with typical starting dose of 2 mg/kg daily [23, 43] |
Polymorphism in TPMT causing low or absent levels can lead to increased myelosuppression |
| mTOR inhibitors | |||||
|
Sirolimus (SIR) (Rapamune ®), Everolimus (Zortress®) |
Prevents T-cell proliferation by binding to mammalian target of rapamycin (mTOR) which is downstream of IL-2 in T-cell activation pathways. This causes the T-cell to arrest in the late G1 phase and prevents DNA and protein synthesis | Impaired wound healing, thrombocytopenia, leukopenia, anemia, hyperlipidemia, peripheral edema, proteinuria, pneumonitis, venous thromboembolism, hemolytic uremic syndrome/ thrombotic microangiopathy, infection |
$–$$$ $$ |
SIR target trough levels are 10 to 15 ng/mL Lower trough levels of 5 to 10 ng/mL are targeted when used in combination with CNIs Everolimus is administered twice daily with a target trough level of 5 to 15 ng/mL [59] |
Sirolimus has a 60-h half-life. Underdosing or overdosing can occur if frequent dose-adjustments are made based on non-steady-state levels Co-administration with CsA can cause increased levels of both drugs |
| Corticosteroids | |||||
| Prednisone, methylprednisolone | Decreases T-cell proliferation and macrophage activation, inhibits cytokine production, and alters lymphocyte migration | Hyperglycemia, hypertension, hyperlipidemia, insomnia, osteoporosis, weight gain, fluid retention, hirsutism, Cushing’s syndrome, menstrual irregularities, growth retardation, GI disturbances, cataracts, impaired wound healing, infection | $ | 500–1000 mg given prior to perfusion of the allograft at the time of transplant. Gradually tapered to prednisone 5–10 mg daily for maintenance therapy |
Chronic low-dose (5 to 10 mg) prednisone is continued lifelong in lung transplant recipients Used for Induction, maintenance and treatment of rejection |
| CD 80/86 co-stimulation blocker | |||||
| Belatacept (Nulojix®) | Co-stimulation blocker blocks T-cell activation by binding to CD 80 and 86 on the antigen-presenting cells | Fever, hypertension, headache, cough, anemia, leukopenia, nausea, vomiting, diarrhea, constipation, peripheral edema, PTLD, PML, infection | $$$ |
Intravenous administration Induction dose differs if used de novo vs. converted from CNI Standard maintenance dose is 5 mg/kg every 4 weeks |
Contraindicated in transplant recipients who are EBV-seronegative or with unknown EBV serostatus |
$ = < $25, $$ = $25.01–100.99, $$$ = $101–1000, $$$$ = > $1000
1C0 levels: trough
2C2 levels: 2 h post-dose
To date, evidence has not demonstrated that there is a significant difference in post-transplant survival between Tac and CsA [36, 37]. However, Tac appears to be superior to CsA with regard to acute rejection, incidence of BOS, and chronic lung allograft dysfunction (CLAD) [38, 39]. Generally, agent selection is dictated by patient tolerance of one mediation over the other as some adverse effects (i.e., PRES) are agent-specific rather than class-specific and re-introduction of a CNI following a wash-out period is often attempted (see Table 5).
Table 5.
Summary of evidence for maintenance immunosuppression post-lung transplantation
| Agent(s) | Author/year | N | Type of trial/intervention | Exclusion criteria | Outcomes |
|---|---|---|---|---|---|
| Tac, CsA | Treede et al. 2012 [38] | 265 |
Open-label, multicenter, prospective, randomized superiority investigation Randomized 1:1 Tac (doses adjusted to target trough levels of 10–15 ng/mL for the first 3 months after transplantation and 8–12 ng/mL thereafter) or CsA (target trough levels 200–300 ng/mL for the first 3 months after transplantation and 150–200 ng/mL thereafter) in combination with MMF and corticosteroids and stratified for diagnosis of cystic fibrosis (CF) |
Need for immunosuppressive regimen other than study medication or received additional organ transplantations See full article for additional criteria |
Cumulative incidence of BOS grade > 1 at 3 years was 11.6% (Tac) vs. 21.3% (CsA) (p = 0.037) CsA was a risk factor for BOS HR 1.97 (p = 0.039) Three-year cumulative incidence of acute rejection was not significant between Tac and CsA One- and 3-year survival rates were not significant between Tac and CsA Cumulative infection rates and new-onset renal failure were not significant |
| Tac, CsA | Hachem et al. 2007 [39] | 90 |
Open-label, randomized, controlled trial Treatment: study drug initiated within 72 h of transplant. Dose adjusted to achieve target troughs Other immunosuppression: AZA and corticosteroids |
Post-operative renal failure, primary graft failure, or seizures |
Recipients randomized to CsA were significantly more likely to develop composite outcome *of a cumulative acute rejection A score1 of > 3, a cumulative lymphocytic bronchitis B score2 > 4, or the development of BOS stage3 0-p than those randomized to Tac (p = 0.002) Trend to a higher incidence of diabetes among those in Tac No difference in graft survival or other significant complications: infections, hypertension, CKD or malignancy |
| Tac, CsA | Zuckermann et al. 2003 [36] | 74 |
Prospective, multicenter, open randomized trial CsA and Tac started immediately after transplantation with target levels adjusted per protocol Other immunosuppression: MMF and corticosteroids |
Switching from one study drug to the other or undergoing re-transplant |
No significant difference between survival, acute rejection, number of treated rejection episodes, new-onset diabetes mellitus, or infections Higher incidence of hypertension (60% vs. 11%, p = 0.03) in CsA group |
| Tac, CsA | Treede et al. 2001 [37] | 50 |
Prospective, open, randomized, two-center trial Tac or CsA was initiated immediately after surgery with target whole-blood trough levels 12–15 ng/mL or 250–300 ng/mL, respectively Other immunosuppression: MMF and corticosteroids |
None specified |
Treated rejection episodes per 100-patient days significantly lower in the Tac group (0.225 vs. 0.426, p = .05) Six- and 12-month survival, freedom from acute rejection, and incidence of infections were not significantly different |
| MMF, AZA | McNeil et al. 2006 [43] | 320 |
Prospective, randomized, open-label, multicenter study Patients randomized in 1:1 ratio to receive MMF or AZA within 7 days of lung transplant Other immunosuppression: CsA and corticosteroids administered according to local protocol |
< 18 or > 65 years of age; not receiving first lung transplant (single, bilateral, or heart–lung), not able to take MMF or AZA orally within 7 days of procedure |
No differences in rejection, survival, or time to BOS at 1 and 3 years More patients withdrew from AZA treatment than from MMF (59.6% vs. 46.5%, p = 0.02) |
| MMF, AZA | Zuckermann et al. 1999 [42] | 38 |
Single-center pilot study comparing MMF to AZA initiated within 24 h of transplant Induction and maintenance immunosuppression (CsA and corticosteroids) were administered according to local protocol |
None specified |
No significant difference in 6-month survival MMF group had fewer rejection episodes (p < 0.01) and more patients who were completely free of rejection at 6 months AZA group trended toward more bacterial infections than MMF group but was not statistically significant |
| Sirolimus (SIR) | Bhorade et al. 2010 [47] | 181 |
Multicenter, open-label, prospective, randomized controlled trial Patients randomized 1:1 to continue AZA or switch to SIR 90 days post-transplant Maintenance immunosuppression: Tac and corticosteroids |
Previous organ transplant, leukopenia, thrombocytopenia, hypercholesterolemia, uncontrolled infection, airway dehiscence or necrosis, declining pulmonary function |
No significant difference in the incidence of acute cellular rejection SIR group had lower incidence of CMV than AZA group at 1 year (p < 0.001) Higher incidence of adverse events was associated with SIR (64%) compared to AZA (49%), resulting in early discontinuation of therapy |
| Everolimus (RAD) | Snell et al. 2006 [48] | 213 |
Multicenter, prospective, randomized, double-blind study Randomly assigned to receive everolimus or AZA Maintenance immunosuppression: CsA, corticosteroids |
Patients with histological evidence of bronchiolitis obliterans |
Incidence of efficacy failure (decline in FEV1 > 15%, graft loss, death, or loss to follow-up) at 12 months was significantly lower in the everolimus group vs. AZA group (p = 0.046) RAD group experienced more adverse events resulting in early discontinuation |
| Everolimus | Glanville et al. 2015 [60] | 202 |
Prospective, randomized, open-label, multicenter, superiority study Patients randomized 1:1 to receive MPS or delayed onset everolimus (RAD) Maintenance immunosuppression: CsA and corticosteroids |
None specified |
Incidence of BOS within 3 years post-transplant between the two groups was not significantly different Three-year survival between two groups was not significantly different A significantly higher number of patients needed to be switched from RAD (36.9%) compared to MPS (16.25%) p < 0.01 |
| Belatacept | Iasella et al. 2018 [56] | 11 |
Single-center, retrospective, case-series of patients converted from CNIs to belatacept Induction immunosuppression: alemtuzumab or basiliximab Maintenance immunosuppression: MMF and prednisone |
None specified |
Acute cellular rejection before and after conversion to belatacept was not significantly different Mean eGFR was significantly higher post-belatacept conversion: CNIs: 32.53 mL/min vs. belatacept: 45.26 mL/min, p = 0.04 2 out of 11 patients progressed to CLAD |
| Belatacept | Timofte et al. 2016 [55] | 8 |
Retrospective, chart review of patients converted to belatacept Maintenance immunosuppression: anti-metabolite (MMF or AZA) and corticosteroids |
None specified |
After conversion to belatacept, mean GFR increased from 24 to 36 mL/min/1.73 m2 at 6 months, p = 0.01 Serial FEV1 measurements remained stable at 1, 3, and 6 months respectively post-conversion to belatacept One patient developed mild acute cellular rejection which responded to 3 doses of IV methylprednisolone |
1Summation of all A scores for each subject (grade 0 to 4, obtained at 90, 180, 365 days and at the end of the study period to give cumulative scores, e.g., a patient with A2 at 90 days, A2 at 180 days, and A3 at 365 days post-transplant would have a cumulative score of A7 at 365 days)
2The sum of all B scores for each subject excluding B scored in the setting of a confirmed bacterial or viral respiratory tract infection (grade 0 to 4, at 90, 180, 365 days and the end of the study period to give a cumulative score)
3According to the ISHLT definition (FEV1 81 to 90% of baseline and/or FEF25–75 < 75% of baseline)
Anti-metabolites
Azathioprine (AZA) was FDA-approved in 1968 and is one of the oldest medications used for solid organ transplant. It is metabolized to 6-mercaptopurine (6-MP) before being converted to several compounds that are incorporated into replicating DNA. These compounds stop DNA replication and block the de novo pathway of purine synthesis, thereby preventing T-cell and B-cell proliferation [32]. AZA is associated with adverse effects that can be found summarized in Table 4. A common polymorphism of thiopurine S-methyltransferase (TPMT) exists, resulting in low or absent enzyme activity that can cause enhanced myelosuppression [40], and this should be assessed in patients prior to medication initiation. The most significant drug interaction to keep in mind with AZA is its use with xanthine oxidase inhibitors, as the combination can cause significant bone marrow suppression.
Mycophenolate mofetil (MMF) and mycophenolate sodium (MPS) were FDA-approved in 2000 and 2004, respectively. They are metabolized to the active form, mycophenolic acid, which targets inosine monophosphate dehydrogenase, the enzyme responsible for B- and T-lymphocyte DNA production [32]. Common side effects can be seen in Table 4. Mycophenolate administration should be separated from antacids containing magnesium and aluminum hydroxides, cholestyramine, and sevelamer as these can decrease its efficacy [41].
Selected studies comparing MMF to AZA can be found in Table 5. While there was no statistical significance in most of the endpoints of the selected studies, there was a slight benefit favoring MMF compared to AZA in terms of acute rejection and tolerability. Zuckermann et al. [42] showed that study subjects taking MMF had fewer episodes of rejection (p < 0.01) and more patients who were completely free of rejection at 6 months [42]. In a prospective, randomized, open-label, multicenter study by McNeil et al., more patients withdrew from AZA therapy than MMF therapy (p = 0.02) [43].
Corticosteroids
Steroids, mainly prednisone and methylprednisolone, play an important role in maintenance immunosuppression and treatment of active rejection. They exert both anti-inflammatory and immunosuppressive effects. Steroid receptors are expressed ubiquitously and suppress multiple inflammatory genes which decreases T-cell proliferation, decreases macrophage activation, inhibits cytokine production, and alters lymphocyte migration [32]. To minimize adverse events associated with steroids, there is interest in steroid withdrawal in solid organ transplant, with renal transplant having the most data for steroid reduction and withdrawal. However, this strategy is not recommended in thoracic transplant due to significant risk of graft loss and limited available data [44]. Most lung transplant centers continue lifelong corticosteroids with all immunosuppression regimens. The goal is to maintain the lowest possible dose that provides stable and optimal lung function while minimizing drug-related adverse effects.
mTOR inhibitors
The two main drugs in this class are sirolimus and everolimus. They have a similar molecular structure to CNIs and bind to the same cytosolic immunophilin FKBP12; however, they prevent T-cell proliferation through a unique mechanism of action. The sirolimus-FKBP12 complex binds to the mammalian target of rapamycin (mTOR) which is downstream of IL-2 in T-cell activation pathways. This causes the T-cell to arrest in the late G1 phase and prevents DNA and protein synthesis [32]. mTOR inhibitors are a substrate of CYP3A4 and are subject to drug-drug interactions. They should be used cautiously in conjunction with CsA, as co-administration can result in increased levels of both drugs and significant nephrotoxicity. This interaction is not apparent with tacrolimus [32].
The potential benefits of using mTOR inhibitors (mTORi) include mitigating the adverse events of CNIs and anti-metabolites, including decreasing CNI target levels, which may reduce nephrotoxicity, and decrease the risk of cancers in high-risk patients [45, 46]. Despite potential benefits, mTORi have high rates of adverse events and are discontinued in two-thirds of lung transplant patients for this reason [47, 48]. mTORi are associated with an increased risk of infections, proteinuria, venous thromboembolism, and pulmonary pathologies such as interstitial pneumonitis, alveolar hemorrhage, and pulmonary vasculitis [49–51]. As potent anti-fibroproliferative agents, mTORi can impair wound healing and contribute to airway dehiscence if initiated perioperatively. As such, clinicians should avoid mTORi in the first 3 months following transplant, or until an airway exam demonstrates complete healing. Sirolimus and everolimus should also be discontinued 4 weeks prior to major surgery and resumed 2–4 weeks afterward [52]. Due to high incidences of adverse events, this drug class is usually initiated only in cases of intolerance to CNIs or anti-metabolites.
Belatacept
Belatacept is a novel immunosuppressant that exerts its activity on the co-stimulatory pathway by binding to CD80/86 on antigen-presenting cells and blocks CD28-mediated T-cell stimulation, thereby inhibiting proliferation and cytokine production [53, 54]. Belatacept is contraindicated in EBV-seronegative or unknown serostatus patients due to significantly higher rates of post-transplant lymphoproliferative disorder (PTLD). It also has a higher risk of infections, malignancies, and progressive multifocal leukoencephalopathy, compared to traditional immunosuppression with CsA [53].
It has been studied and is FDA-approved in kidney transplantation as a CNI alternative and should always be used in combination with MMF and steroids due to higher rates of acute cellular rejection [54]. The primary benefit of using belatacept is its renal preservation properties in patients with CNI-induced nephrotoxicity. There is limited data for its use in lung transplantation and more data is needed to evaluate the safety profile and associated risks in this patient population [55, 56].
Modifications to maintenance immunosuppression
While it may be difficult to provide absolute recommendations on how best to adjust maintenance immunosuppression in certain clinical situations, there is a general consensus to the management of common clinical scenarios after lung transplant. These heuristics vary by clinical practice in each transplant program, but generally include reduction in immunosuppression in the setting of leukopenia, infection, PTLD, and malignancy (see Table 6).
Table 6.
Clinical considerations in the modification of immunosuppression
| Clinical scenario | Management of immunosuppression | Notes |
|---|---|---|
| Leukopenia | • Decrease or hold anti-metabolite | • May depend on severity of leukopenia or if neutropenia is involved |
| Infection |
• Decrease C0 target levels of CNI and/or • Decrease or hold anti-metabolite |
• May depend on severity of infection and if patient is in the intensive care unit or on vasoactive medications or respiratory support |
| Chronic kidney disease |
• Decrease C0 target levels of CNI and/or • Addition of mTOR inhibitor or • Conversion of CNI to belatacept |
|
| Acute or chronic liver injury |
• Decrease CNI dose • Decrease mTOR inhibitor dose (if applicable) |
|
| PTLD | • Decrease C0 target levels of CNI | • Avoid conversion to belatacept |
| Malignancy |
• Stop anti-metabolite • Decrease C0 target levels of CNI |
Discussion
Despite concerns for deep levels of immunosuppression leading to risks of infection and malignancy, the use of induction immunosuppression is increasingly common. Though cohort investigations show variable outcomes in head-to-head and comparative control trials, registry studies consistently support the use of induction with evidence of improved survival, fewer episodes of acute rejection, and delayed onset of CLAD. Overall, IL-2 antagonists are well tolerated and appear to be the safest option in terms of adverse effects with evidence in registry and cohort investigations supporting their use.
Long-term triple-drug immunosuppression is the standard of care for post-lung transplant recipients with Tac, MMF, and low-dose prednisone is the most commonly prescribed combination. Deviations from a three-drug regimen are generally the result of patient intolerance to medications and the need to mitigate undesired side effects. Protocols vary widely across lung transplant centers, but agent selection should be individualized for each patient based on time since transplant, rejection and infection history, side effect profile, and potential drug-drug interactions. With careful selection and vigilant monitoring, these high-risk medications can be used safely to improve both the length and quality of life for our patients.
Acknowledgements
The authors thank Kristina Bailey for her editing assistance.
Author contribution
B. Small began the idea for the article. B. Small; J. Au; H. Brink; and I. Shah completed the literature search and drafted the work. H. Strah created the figure and critically revised the work.
Funding
Nil.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–55. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 annual data report: lung. Am J Transplant. 2020. 10.1111/ajt.15677. [DOI] [PubMed]
- 3.Bennett D, Fossi A, Marchetti L, et al. Postoperative acute kidney injury in lung transplant recipients. Interact Cardiovasc Thorac Surg. 2019;28:929–35. doi: 10.1093/icvts/ivy355. [DOI] [PubMed] [Google Scholar]
- 4.Hayes D, Jr, Black SM, Tobias JD, et al. Influence of human leukocyte antigen mismatching on bronchiolitis obliterans syndrome in lung transplantation. J Heart Lung Transplant. 2016;35:186–94. doi: 10.1016/j.healun.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Brock MV, Borja MC, Ferber L, et al. Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti-thymocyte globulin, and daclizumab. J Heart Lung Transplant. 2001;20:1282–90. doi: 10.1016/s1053-2498(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 6.Hadjiliadis D, Howell DN, Davis RD, et al. Anastomotic infections in lung transplant recipients. Ann Transplant. 2000;5:13–9. [PubMed] [Google Scholar]
- 7.Zaffiri L, Long A, Neely ML, Cherikh WS, Chambers DC, Snyder LD. Incidence and outcome of post-transplant lymphoproliferative disorders in lung transplant patients: Analysis of ISHLT Registry. J Heart LungTransplant. 2020;39:1089–99. doi: 10.1016/j.healun.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet SC. Induction therapy in lung transplantation. Transpl Int. 2013;26:696–703. 10.1111/tri.12115. [DOI] [PubMed]
- 9.Jaksch P, Ankersmit J, Scheed A, et al. Alemtuzumab in lung transplantation: an open-label, randomized, prospective single center study. Am J Transplant. 2014;14:1839–45. doi: 10.1111/ajt.12824. [DOI] [PubMed] [Google Scholar]
- 10.Trindade AJ, Thaniyavarn T, Townsend K, et al. Alemtuzumab as a therapy for Chronic lung allograft dysfunction in lung transplant recipients with short telomeres. Front Immunol. 2020;11:1063. doi: 10.3389/fimmu.2020.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campath® (Alemtuzumab) [package insert].Richmond, CA: Berlex Laboratories; 2001.
- 12.Korom S, Boehler A, Weder W. Immunosuppressive therapy in lung transplantation: state of the art. Eur J Cardiothorac Surg. 2009;35:1045–55. doi: 10.1016/j.ejcts.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 13.van Loenhout KCJ, Groves SC, Galazka M, et al. Early outcomes using alemtuzumab induction in lung transplantation. Interact Cardiovasc Thorac Surg. 2010;10:190–4. doi: 10.1510/icvts.2009.213892. [DOI] [PubMed] [Google Scholar]
- 14.Shyu S, Dew MA, Pilewski JM, et al. Five-year outcomes with alemtuzumab induction after lung transplantation. J Heart Lung Transplant. 2011;30:743–54. doi: 10.1016/j.healun.2011.01.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whited LK, Latran MJ, Hashmi ZA, et al. Evaluation of Alemtuzumab versus Basiliximab induction: a retrospective Cohort study in lung transplant recipients. Transplantation. 2015;99:2190–5. doi: 10.1097/tp.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 16.Hurst FP, Altieri M, Nee R, Agodoa LY, Abbott KC, Jindal RM. Poor outcomes in elderly kidney transplant recipients receiving alemtuzumab induction. Am J Nephrol. 2011;34:534–41. doi: 10.1159/000334092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy JS, Jr, Tumin D, Pope-Harman A, Whitson BA, Higgins RSD, Hayes D., Jr Induction therapy for lung transplantation in COPD: analysis of the UNOS registry. COPD. 2016;13:647–52. doi: 10.3109/15412555.2015.1127340. [DOI] [PubMed] [Google Scholar]
- 18.Kirkby S, Whitson BA, Wehr AM, Lehman AM, Higgins RS, Hayes D Jr. Survival benefit of induction immunosuppression in cystic fibrosis lung transplant recipients. J Cystic Fibros. 2015;14:104–10. 10.1016/j.jcf.2014.05.010. [DOI] [PubMed]
- 19.Whitson BA, Lehman A, Wehr A, et al. To induce or not to induce: a 21st century evaluation of lung transplant immunosuppression’s effect on survival. Clin Transplant. 2014;28:450–61. 10.1111/ctr.12339. [DOI] [PubMed]
- 20.Thymoglobulin ® (anti-thymocyte globulin (rabbit)) [package insert].Cambridge, MA: Genzyme Corporation; 2017.
- 21.Mullen JC, Oreopoulos A, Lien DC, et al. A randomized, controlled trial of daclizumab vs anti-thymocyte globulin induction for lung transplantation. J Heart Lung Transplant. 2007;26:504–10. doi: 10.1016/j.healun.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Hartwig MG, Snyder LD, Appel JZ, 3rd, et al. Rabbit anti-thymocyte globulin induction therapy does not prolong survival after lung transplantation. J Heart Lung Transplant. 2008;27:547–53. doi: 10.1016/j.healun.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Palmer SM, Baz MA, Sanders L, et al. Results of a randomized, prospective, multicenter trial of mycophenolate mofetil versus azathioprine in the prevention of acute lung allograft rejection. Transplantation. 2001;71:1772–6. doi: 10.1097/00007890-200106270-00012. [DOI] [PubMed] [Google Scholar]
- 24.Hachem RR, Edwards LB, Yusen RD, Chakinala MM, Alexander Patterson G, Trulock EP. The impact of induction on survival after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. Clin Transplant. 2008;22:603–8. doi: 10.1111/j.1399-0012.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 25.Simulect ® (Basiliximab)) [package insert]. Hanover, New Jersey: Novartis Pharaceutical Corporation; 2003.
- 26.Enderby C, Keller CA. An overview of immunosuppressionin solid organ transplantation. Am J Manag Care. 2015;21:s12–23. [PubMed] [Google Scholar]
- 27.Cohan SL, Lucassen EB, Romba MC, Linch SN. Daclizumab: mechanisms of action, therapeutic efficacy, adverse events and its uncovering the potential role of innate immune system recruitment as a treatment strategy for relapsing multiple sclerosis. Biomedicines. 2019;7:18. doi: 10.3390/biomedicines7010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ailawadi G, Smith PW, Oka T, et al. Effects of induction immunosuppression regimen on acute rejection, bronchiolitis obliterans, and survival after lung transplantation. J Thorac Cardiovasc Surg. 2008;135:594–602. doi: 10.1016/j.jtcvs.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Clinckart F, Bulpa P, Jamart J, Eucher P, Delaunois L, Evrard P. Basiliximab as an alternative to antithymocyte globulin for early immunosuppression in lung transplantation. Transplant Proc. 2009;41:607–9. doi: 10.1016/j.transproceed.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 30.Slebos D-J, Kauffman HF, Koëter GH, Verschuuren EA, Bij W, Postma DS. Airway cellular response to two different immunosuppressive regimens in lung transplant recipients. Clin Transplant. 2005;19:243–9. 10.1111/j.1399-0012.2005.00330.x. [DOI] [PubMed]
- 31.Hachem RR, Chakinala MM, Yusen RD, et al. A comparison of basiliximab and anti-thymocyte globulin as induction agents after lung transplantation. J Heart Lung Transplant. 2005;24:1320–6. doi: 10.1016/j.healun.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AL, Watson CJE, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed]
- 33.Neoral ® [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2009.
- 34.Prograf ® [package insert]Deerfield, IL: Astellas Pharma US Inc, 2012.
- 35.Bishop M. Clinical Chemistry: Principles, Procedures, Correlations: Lippincott Williams &Wilkins; 2004.
- 36.Zuckermann A, Reichenspurner H, Birsan T, et al. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a 2-center prospective randomized trial. J Thorac Cardiovasc Surg. 2003;125:891–900. doi: 10.1067/mtc.2003.71. [DOI] [PubMed] [Google Scholar]
- 37.Treede H, Klepetko W, Reichenspurner H, et al. Tacrolimus versus cyclosporine after lung transplantation: a prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. J Heart Lung Transplant. 2001;20:511–7. doi: 10.1016/s1053-2498(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 38.Treede H, Glanville AR, Klepetko W, et al. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trialin lung transplantation. J Heart Lung Transplant. 2012;31:797–804. doi: 10.1016/j.healun.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant. 2007;26:1012–8. 10.1016/j.healun.2007.07.027. [DOI] [PubMed]
- 40.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–54. 10.1038/clpt.1989.119. [DOI] [PubMed]
- 41.Cellcept ® [package insert]. Nutley, NJ: Roche Laboratories Inc, 2009.
- 42.Zuckermann A, Klepetko W, Birsan T, et al. Comparison between mycophenolate mofetil- and azathioprine-based immunosuppressions in clinical lung transplantation. J Heart Lung Transplant. 1999;18:432–40. doi: 10.1016/s1053-2498(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 43.McNeil K, Glanville AR, Wahlers T, et al. Comparison of mycophenolate mofetil and azathioprine for prevention of bronchiolitis obliterans syndrome in de novo lung transplant recipients. Transplantation. 2006;81:998–1003. doi: 10.1097/01.tp.0000202755.33883.61. [DOI] [PubMed] [Google Scholar]
- 44.Borro JM, Solé A, De la Torre M, Pastor A, Tarazona V. Steroid withdrawal in lung transplant recipients. Transplant Proc. 2005;37:3991–3. 10.1016/j.transproceed.2005.09.190. [DOI] [PubMed]
- 45.Karia PS, Azzi JR, Heher EC, Hills VM, Schmults CD. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533–40. 10.1001/jamadermatol.2015.5548. [DOI] [PubMed]
- 46.Parada MT, Alba A, Sepúlveda C, Melo J. Long-term use of everolimus in lung transplant patients. Transplant Proc. 2011;43:2313–5. 10.1016/j.transproceed.2011.06.010. [DOI] [PubMed]
- 47.Bhorade S, Ahya VN, Baz MA, et al. Comparison of sirolimus with azathioprine in a tacrolimus-based immunosuppressive regimen in lung transplantation. Am J Respir Crit Care Med. 2011;183:379–87. doi: 10.1164/rccm.201005-0775OC. [DOI] [PubMed] [Google Scholar]
- 48.Snell GI, Valentine VG, Vitulo P, et al. Everolimus versus azathioprine in maintenance lung transplant recipients: an international, randomized, double-blind clinical trial. Am J Transplant. 2006;6:169–77. doi: 10.1111/j.1600-6143.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 49.Ahya VN, McShane PJ, Baz MA, et al. Increased risk of venous thromboembolism with a sirolimus-based immunosuppression regimen in lung transplantation. J Heart Lung Transplant. 2011;30:175–81. doi: 10.1016/j.healun.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Chhajed PN, Dickenmann M, Bubendorf L, Mayr M, Steiger J, Tamm M. Patterns of pulmonary complications associated with sirolimus. Respiration. 2006;73:367–74. 10.1159/000087945. [DOI] [PubMed]
- 51.Gullestad L, Iversen M, Mortensen S-A, et al. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89:864–72. doi: 10.1097/TP.0b013e3181cbac2d. [DOI] [PubMed] [Google Scholar]
- 52.King-Biggs MB, Dunitz JM, Park SJ, Kay Savik S, Hertz MI. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation. 2003;75:1437–43. 10.1097/01.TP.0000064083.02120.2C. [DOI] [PubMed]
- 53.Nulojix (belatacept) [package insert].Princeton, NJ: Bristol-Myers Squibb Company; 2014.
- 54.Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 55.Timofte I, Terrin M, Barr E, et al. Belatacept for renal rescue in lung transplant patients. Transpl Int. 2016;29:453–63. doi: 10.1111/tri.12731. [DOI] [PubMed] [Google Scholar]
- 56.Iasella CJ, Winstead RJ, Moore CA, et al. Maintenance belatacept-based immunosuppression in lung transplantation recipients who failed calcineurin inhibitors. Transplantation. 2018;102:171–7. doi: 10.1097/TP.0000000000001873. [DOI] [PubMed] [Google Scholar]
- 57.Weaver TA, Kirk AD. Alemtuzumab. Transplantation. 2007;84:1545–7. doi: 10.1097/01.tp.0000296680.75175.67. [DOI] [PubMed] [Google Scholar]
- 58.Snell GI, Westall GP, Levvey BJ, et al. A randomized, double-blind, placebo-controlled, multicenter study of rabbit ATG in the prophylaxis of acute rejection in lung transplantation. Am J Transplant. 2014;14:1191–8. doi: 10.1111/ajt.12663. [DOI] [PubMed] [Google Scholar]
- 59.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039–53. doi: 10.3978/j.issn.2072-1439.2014.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glanville AR, Aboyoun C, Klepetko W, et al. Three-year results of an investigator-driven multicenter, international, randomized open-label de novo trial to prevent BOS after lung transplantation. J Heart Lung Transplant. 2015;34:16–25. doi: 10.1016/j.healun.2014.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.