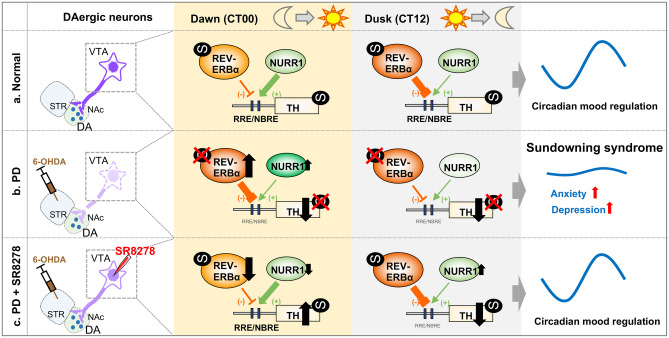
Fig. 5.
Mood regulation and crosstalk of REV-ERBα and NURR1 in PD and SR8278-treated PD model. (a) In the normal DAergic state, the circadian nuclear receptor REV-ERBα repressed the TH gene transcription via competition with NURR1, another nuclear receptor on the same cis-element (R/N sites), inducing rhythmic TH expression [13]. Circadian oscillation of TH expression in VTA neurons resulted in the daily variation of mood-like behaviors. (b) In the PD model induced by 6-OHDA-lesion, transcription level of Rev-erbα and Nurr1 in the VTA DAergic neurons were altered at dawn, thereby inducing disappearance of rhythmic Rev-erbα transcription and disturbance of consistent Nurr1 transcription. Furthermore, 6-OHDA lesion induced atypical binding activity of REV-ERBα and NURR1 to R/N sites of TH promoter. REV-ERBα binding activity to R/N sites was increased at dawn but decreased at dusk. NURR1 binding activity was decreased at dawn without an alternation at dusk. In PD model, depression- and anxiety-like behaviors were exhibited at specific time, dawn, which time corresponds to dusk in diurnal human, characterizing the sundowning syndrome. (c) SR8278 microinjection completely restored rhythmic mood-related behaviors in 6-OHDA-lesioned mice at dawn, exhibiting the antidepressant and the anxiolytic effects in a time-dependent manner. Transcription levels of Rev-erbα and Nurr1 in the VTA DAergic neurons and binding activities of REV-ERBα and NURR1 to R/N sites were recovered by SR8278 microinjection at dawn. TH protein levels of VTA were also elevated by SR8278 microinjection at dawn. Although the binding activities of REV-ERBα and NURR1 were restored at dusk, TH expression in VTA was not recovered by SR8278 at dusk. It is noteworthy that the competitive actions of REV-ERBα and NURR1 are essential in regulating the circadian TH gene expression and mood regulation