Abstract
Lung transplant outcomes remain inferior largely due to mismatching in human leukocyte antigens (HLAs) that leads to chronic rejection and chronic allograft dysfunction. The mismatched donor HLAs can be recognized by the effector T cells or donor-specific HLA antibodies. This review summarizes mechanisms leading to immune responses as a result of HLA mismatching. It specifically focuses on sensitized lung transplant candidates with preformed anti-HLA antibodies, which represent a significant management challenge for physicians. In this review, we describe the diagnostic histocompatibility testing and therapeutic options for managing the sensitized lung transplant patients and discuss how multidisciplinary approach may help to improve lung transplantation outcomes.
Keywords: HLA matching, Lung transplant, Histocompatibility testing, Desensitization
Introduction
Lung transplantation (LTx) is an established therapy for many end-stage pulmonary diseases; however, the post-transplant long-term survival remains modest, mostly due to higher rates of acute and chronic allograft rejection [1]. One of the main reasons for this poor outcome is recognition of the new allograft by the recipient T cells as non-self through the mismatched major histocompatibility complex (MHC) proteins commonly referred to as human leukocyte antigens (HLAs) [2]. Despite contemporary immunosuppressive treatments, LTx is accompanied by graft dysfunction, transplant rejection, and poor overall survival due to T cell activation and humoral alloresponses characterized by the development of donor-specific antibodies (DSAs) against mismatched HLA [3]. Some transplant candidates may have anti-HLA antibodies in their blood even prior to transplantation (pre-transplant HLA sensitization) due to exposure to non-self HLA via blood transfusions, pregnancies, or previous transplants. These pre-formed anti-HLA antibodies represent one of the major immunological barriers to transplantation. The high-titer HLA DSA may cause hyperacute antibody-mediated rejection (AMR) by binding to donor’s HLAs expressed on the endothelium of blood vessels resulting in the activation of the complement cascade with resultant thrombosis and infarction of the graft [4]. Additionally, lower titer antibodies are able to cause rejection via NK cell activation or endothelial cell proliferation [5]. Development of single-antigen bead (SAB) assays using microsphere technology with conjugated purified HLAs provided unmatched specificity and sensitivity of HLA antibody detection. It improved our understanding of the significance of lower titer antibodies in LTx; however, SAB assays are prone to both false negativity and positivity and it is important to use multiple methods to determine the clinical relevance of detected HLA antibodies [6]. This article focuses on the role of HLA mismatch in alloimmune injury, summarizes considerations of contemporary HLA antibody testing methods for pre- and post-transplant management of lung candidates, and discusses approaches for transplanting high immunologic risk candidates. Here, we discuss the importance of close interaction between the HLA laboratory and clinical team to ensure accurate interpretation of HLA data and optimal management of patients before and after organ transplantation.
The HLA system and transplantation
The most recognized genes involved in an alloimmune response are encoding MHC proteins, referred to as HLA in human genome. HLA genes are highly polymorphic with over 15,000 alleles identified to date and the number of alleles keeps increasing [7]. Based on the structure and function, the HLA proteins are classified into HLA class I and class II. Class I HLA includes HLA-A, HLA-B, and HLA-Cw that are expressed on all cells. Class II HLAs include HLA-DR (consist of DRB1 and DRA1 polypeptides), HLA-DQ (consist of DQB1 and DQA1 polypeptides), and HLA-DP (consist of DPB1 and DPA1 polypeptides) that are expressed on antigen-presenting cells (APCs), including B cells, macrophages, dendritic cells, Langerhans cells, and capillary endothelium. In a therapeutic transplant setting, when the donor’s and the recipient’s HLAs are different, the HLA mismatch leads to activation of T cell and B cell (antibody) responses, resulting in graft injury [8]. There is overwhelming evidence of the benefits from HLA matching in LTx including longer graft and patient survival, reduced risk of acute rejection, bronchiolitis obliterans syndrome (BOS), and sensitization in case another transplant is needed [9, 10]. However, due to scarcity of lung allografts, high requirements for organ maintenance, medical urgency, and an expected wide variation in allograft size, it is not considered feasible to distribute lung allografts based on HLA matching between donor and recipient.
In solid organ transplantation (SOT), the “HLA match grade” is determined by counting the number of HLA-A, HLA-B, and HLA-DRB1 mismatches between a donor and recipient with 0–2 antigen values per locus (e.g., 0-antigen mismatch, 1-antigen mismatch, or 2-antigen mismatch) and 0–6 antigens per 3 loci. This narrow approach does not account for mismatches at other loci or allele-specific differences within each HLA antigen (e.g., A*02:01 and A*02:05 are considered matched in SOT), although lessons from bone marrow transplantation show that only completely matched HLA proteins could potentially prevent T cell responses [11].
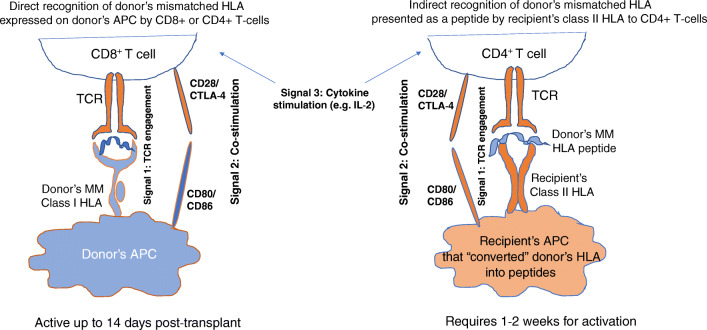
In transplantation, T cells can recognize non-self HLA antigens via the direct pathway (Fig. 1). For several decades, this pathway was considered to dominate transplant rejection due to recipient’s T cell receptor (TCR) directly recognizing mismatched HLA upon migration of donor dendritic cells from the allograft to host secondary lymphoid tissue [8]. In addition, some transplant candidates may demonstrate memory T cell responses to mismatched HLAs because of prior exposure to non-self HLAs or due to developed virus-specific T cell cross-reactive with HLAs [12]. There are two main subclasses of memory cells: central memory T cells (TCM) characterized by the increased potential for proliferation after antigen reencounter and effector memory T cells (TEM) that display rapid effector function (granzyme B and IFN-γ production) [13]. Since TEM cells are trafficking through nonlymphoid tissues, they act as “first responders” at the peripheral site where reinfection/transplantation occurs. The patients with higher levels of CD8 TEM are at higher risk for severe acute rejection in the presence of mismatched HLA [14]. The direct pathway is limited to the early post-transplant period, as donor’s dendritic cell lifespan does not exceed 2–3 weeks [15]. The prevention of direct alloresponse relies on immunosuppressive regimens. In particular, T cell depleting induction therapies that include anti-thymocyte globulins (ATGs) and monoclonal agents such as basiliximab and daclizumab targeting CD25 (IL-2R a-chain) or alemtuzumab targeting CD52 may inhibit memory T cell response and de novo direct pathway immediately after transplantation, thus preventing early acute rejection. Although the use of induction therapy remains controversial in LTx [15], published reports indicate that HLA mismatch may have a greater effect on primary graft dysfunction, acute rejection, and BOS when it is not used [16, 17]. Therefore, many centers choose to tailor it to the individual patient. For example, some centers may withhold induction therapy for older lung transplant recipients (>65 years) or patients who are at higher risk of infection (e.g., Cytomegalovirus (CMV) or Epstein-Barr virus (EBV) serologic status of +donor/−recipient; cystic fibrosis with highly resistant organisms), but use it for highly sensitized patients.
Fig. 1.
Activation of alloreactive T cells via direct and indirect pathways. Optimal T cell activation requires 3 signals, including TCR engagement by donor’s mismatched (MM) class I HLA antigens expressed on antigen-presenting cells (APC) for direct pathway and donor’s MM HLA peptide presented by recipient’s class II HLA via indirect pathway (signal 1), co-stimulation (signal 2), and cytokine stimulation (signal 3). Direct pathway terminates shortly after transplantation, while indirect pathway requires at least 1–2 weeks for activation, but remains active throughout the life of the allograft and can mediate late graft damage by contributing to humoral aloimmunity
The indirect pathway of allorecognition, in which CD4 T cells recognize mismatched HLA in the form of alloantigen-derived peptide on self HLA class II molecules (Fig. 1), has emerged as a potent inducer of allograft rejection via antibody-mediated and CD8 T cell–mediated rejection [18]. Since this pathway is operational throughout the life of the graft, this may explain a greater benefit from HLA-DRB1 matching observed in some studies [16, 19].
Mismatched donor HLAs may also serve as targets for the HLA DSA, which play a role in AMR [20]. HLA antibodies are produced by plasma cells that differentiated from naïve B cells expressing immunoglobulin receptors that received help from activated CD4 cells. Immunoglobulins recognize the specific antigenic portions of intact class I and class II HLA proteins called an epitope, at the center of which there is an eplet [5, 6]. DSAs are classified into preformed (pre-existing) DSA (pDSA) and de novo DSA (dnDSA). The development of dnDSA post-transplant is strongly associated with the mismatched epitope load and modifications in the immunosuppressive regimen, non-adherence, and under-immunosuppression. The lower mismatched eplet loads are protective against chronic lung allograft dysfunction (CLAD) [21]. However, in the absence of HLA matching, the optimization of tacrolimus immunosuppression might be an important factor in the prevention of dnDSA development, since the maturation of naive B cells into high-affinity antibody-producing plasma cells requires T follicular helper cell signaling [22]. In contrast to dnDSA, the presence of preformed anti-HLA antibodies likely indicates the existence of long-lived plasma cells specific for previously encountered non-self HLA epitopes. Since HLA epitopes/eplets are shared among numerous HLA proteins, sensitizing events can induce antibodies to many seemingly “non-seen” HLA alleles. Early DSA (eDSA) are not detected prior to transplant, but increase rapidly after post-transplant due to differentiation of memory B cells into antibody-secreting cells and are associated with early AMR [23]. The presence of preformed HLA antibodies is one of the key factors limiting transplantation in lung transplant candidates. It is now common practice in LTx to consider a donor as unacceptable if the transplant candidate has preformed anti-HLA antibodies specific to that donor.
Histocompatibility testing
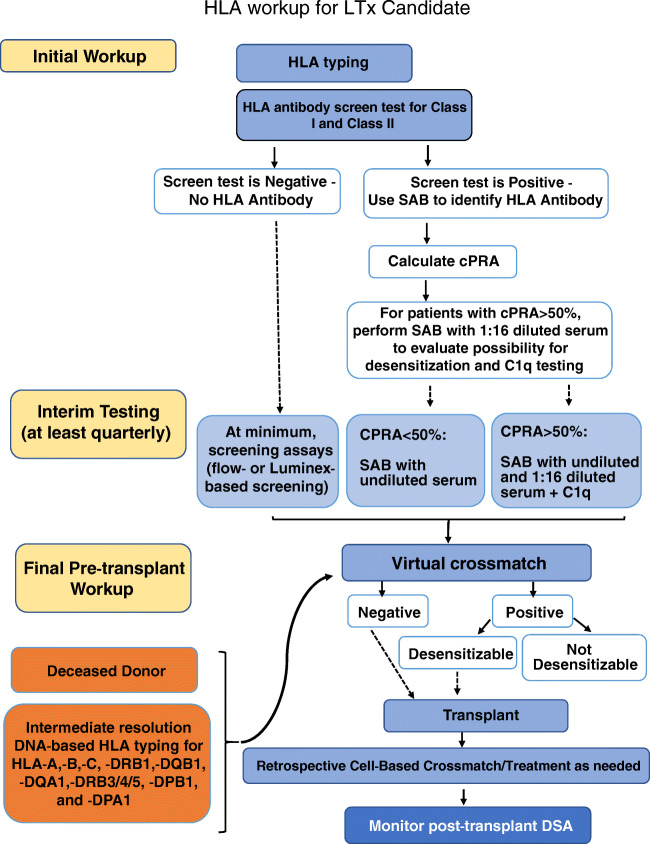
Immunologic assessment, determining donor-recipient compatibility, involves multiple techniques for assessing the risk of rejection against a specific donor. The current practice relies on the characterization of anti-HLA antibodies in a patient’s serum and molecular typing of donor HLA antigens, to determine if a patient has antibodies to a specific donor’s HLA. In general, HLA workup for the transplant candidate can be divided into three phases: initial workup, interim testing, and final pre-transplant workup (Fig. 2).
Fig. 2.
Pre-transplant HLA workup for a lung transplant candidate consists of initial evaluation, interim testing, and final workup at the time of transplant. Post-transplant HLA workup consists of donor-specific antibody (DSA) testing
Initial evaluation and interim HLA antibody testing prior to transplant
One of the most important tests in SOT is a panel reactive antibody (PRA) test, which helps to identify antibodies against HLAs and minimize the risk of AMR. Classic PRA test is a complement-dependent cytotoxicity (CDC-PRA) assay in which recipient’s serum is mixed with a panel of T cells from various blood donors in the presence of complement to identify high-titer anti-HLA antibodies that cause T cell death. The percent PRA is calculated based on the percentage of positive reactions (dead cells) out of the total number of reactions (e.g., if recipient serum is tested against 50 different donors and produces 5 positive reactions, the percent PRA is 10%). Patients with preformed DSAs identified by CDC-PRA may experience hyperacute or accelerated acute AMR. To reduce the risk of AMR, these antigens that produce a positive reaction should be avoided (unacceptable antigens (UAs)), resulting in limited access to transplantation for patients with high PRA. For patients with PRA of 20–80%, there is a 50% chance to be transplanted compared to non-sensitized patients, while there is only 5% chance to be transplanted for patients with PRA greater than 80%. The CDC-PRA test provided relevant clinical results; however, the results of this test were variable, insensitive, and nonspecific. Currently, anti-HLA antibody detection is performed via HLA-specific solid-phase assay and the percent of calculated PRA (cPRA) is determined. The percent cPRA is calculated based on the population frequency of antigens that should be avoided (e.g., if a patient has an antibody against HLA-A2 which is expressed by 48% of population, then cPRA is 48%). Similar to PRA, cPRA reflects the percent of incompatible donors.
Some centers choose to perform HLA antibody screening test first and proceed with antibody “identification” test only if the screening test is positive. Others directly perform antibody screening and identification for class I and class II antigens using SAB assay. Both approaches have their limitations: although screening tests are rather sensitive, they do not always detect weak antibodies; on another hand, SAB is prone to detecting antibodies against cryptic epitopes that have no clinical relevance and to under-detecting weak antibodies against shared epitopes. It has been proposed that antibody testing should be performed using multiple platforms for accurate antibody characterization [6].
Once a patient is deemed an acceptable candidate for transplantation and listed in the United Network for Organ Sharing (UNOS)-developed online database system, called UNetSM, the HLA antibody testing is repeated on a regular basis, usually 3–12 times per year depending on regional regulations and patient sensitization status (Fig. 2). This allows to obtain the most comprehensive immunologic profile, since even transient antibodies in sensitized patients may represent potential for future immunologic memory and affect risk assessment. If the candidate experiences a new sensitizing event, the development of new antibodies may take at least 2 weeks and the antibody testing may need to be performed on more frequent basis until the antibody profile is stable. Recent reports showed that patients may become more sensitized as a result of bridging patients to thoracic transplantation with mechanical devices, such as extracorporeal membrane oxygenation (ECMO) or ventricular assist devices (VADs), which are commonly accompanied by blood transfusions [24]. It is beneficial to monitor HLA PRAs using Luminex assay on at least bi-weekly basis while patients are on ECMO.
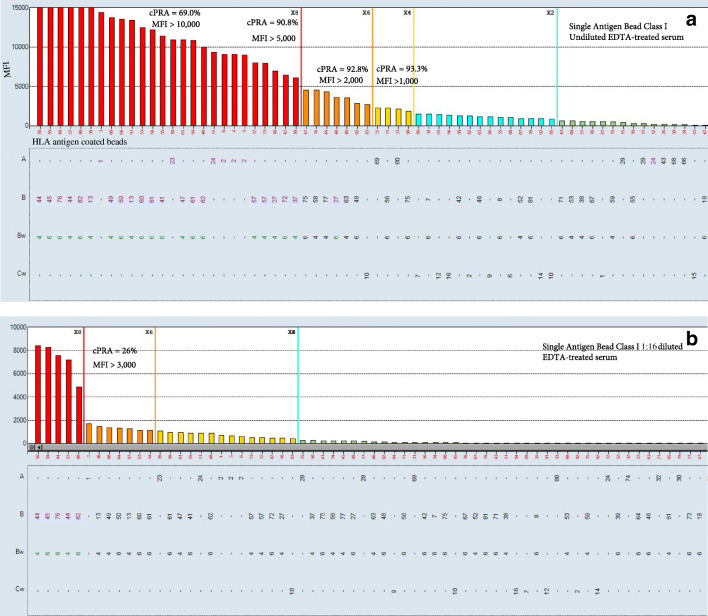
The specificity of single antigen antibody test allows determining which antigens should be avoided (UAs) and estimate a percent of cPRA, which provides an estimate of the incompatible donor pool (the percentage of donors to whom a recipient will have DSA). Lung transplant candidates with increased cPRA are at higher risk of death while waiting for the transplant due to a limited donor pool [25]. It is important to keep in mind that cPRA depends on how UAs are defined (Fig. 3A). As soon as UAs are listed in UNetSM, the donors expressing those antigens will not be matched to the recipient; therefore, careful approach is needed to determine which antigens must be avoided and which can be evaluated additionally at the time of transplant. Since not all HLA antibodies pose the same risk to the graft, it may be beneficial to adopt a combination of various assays, including mean fluorescent intensities (MFIs), complement-fixing abilities, and serial dilution studies to stratify HLA antibodies according to the potential risk [26]. Usually, positive CDC crossmatch (XM) due to anti-HLA DSA signifies a high risk of hyperacute rejection. It is desirable to avoid CDC-positive XM, so C1q-positive antibodies and antibodies with MFI values >7500 MFI in 1:16 diluted serum have to be avoided [27]. However, positive flow cytometry XM (FCXM) in the presence of negative CDC-XM is a gray area and open for interpretation. Positive FCXM in the presence of DSA indicates an increased risk for acute rejection; however, it also indicates that there is sufficient time to prepare for treatment if/as needed. For highly sensitized patients, it may be the only option to be transplanted. However, if it is desirable to avoid positive FCXM, then antibodies with MFI >3000 must be listed as UA [6, 28]. Usually, DSA with MFI values <2000 (except for antibodies against shared epitopes) do not result in positive FCXM. Nevertheless, any detectable DSA in a sensitized patient may increase the risk of AMR post-transplant due to activation of memory B cell response.
Fig. 3.
cPRA value may vary based on the definition of unacceptable antigens. A cPRA can be calculated using different MFI cut-off values. B cPRA can be determined based on 1:16 serum dilution. As a result, only antibodies that are predicted not to respond to peri-operative treatment are listed as unacceptable antigens (UAs). Antibodies that decrease by 75% in 1:16 diluted serum and have MFI of less than 3000 in 1:16 diluted serum are predicted to respond to peri-operative therapeutic plasma exchange
Since “UNet-reported cPRA” does not include all antibodies present in patient’s serum, it may be helpful if “total cPRA” is provided. Total cPRA includes all weak antibodies (Fig. 3A) and provides transplant clinical team with a realistic expectation of finding a donor without DSA. In addition, if sensitized transplant candidates have antibodies against DQA1, DPA1, and DPB1, it is advisable to use Canadian cPRA calculator to estimate a likelihood of finding a compatible donor [29].
It has been shown that candidates with a cPRA value of 50.1–75% were 25% less likely to undergo lung transplant and 44% more likely to die on the waitlist, and candidates with a cPRA value of 75.1–100% were 52% less likely to undergo lung transplant and 92% more likely to die on the waitlist [25]. Therefore, for patients with cPRA>50%, the possibility of desensitization needs to be considered and discussed with the clinical team. Since currently there are no guidelines for desensitization, we will discuss various approaches and present our center strategy to desensitization in the following text.
Final pre-transplant HLA workup
Historically, a physical CDC-XM was a prerequisite for SOT due to its ability to prevent hyperacute rejection. To further reduce the incidence of rejection episodes early post-transplant, FCXM was employed to detect low titer DSA. In part, this practice was predicated on the fact that the characterization of recipient’s HLA antibodies and donor’s HLA typing was incomplete. However, prospective physical crossmatching, whether CDC-XM or FCXM, is not always feasible in lung recipients due to the duration for which potential grafts can be preserved. Therefore, for a long time, there were no requirements for prospective crossmatching in LTx and often patients were treated based on the results of retrospective crossmatching. With the implementation of solid-phase assays into clinical practice, virtual crossmatch (VXM) can be performed in which the donor’s HLAs are compared with the patient’s alloantibody profile to identify any DSA that were not deemed unacceptable [30] (Fig. 2). It has been shown that pre-transplant allosensitization does not adversely affect outcomes after LTx when the potentially reactive donor HLA proteins are avoided using a VXM [31].
When VXM is reported as “negative,” it means that a recipient does not have any detectable DSA against the donor and an immunologic risk for AMR early post-transplant is low even in sensitized patients [26]. When VXM is reported as positive, it usually means that there is a DSA present. However, there are no clear guidelines on how to recognize a clinically significant DSA based on SAB results. A physical XM is performed following VXM immediately after transplantation. A positive FCXM is associated with a significantly higher risk of early rejection and it often requires complex immunosuppression, including peri-operative plasma exchange, high dose of intravenous immunoglobulin (IVIG), and or rituximab in addition to induction therapy and maintenance immunosuppressive drugs. A negative FCXM in the presence of DSA may not require any treatment but requires close DSA monitoring post-transplant. However, since the physical cell–based XM is usually performed after organ is already accepted, the transplant programs do rely on the solid-phase assay to provide information for decision-making.
DSA testing post-transplant
The presence of DSA post-transplant increases risk for rejection. In the presence of pDSA or if memory B cell response is suspected (sensitized patients), it is important to test patients on weekly basis for the first month and then monthly up to 3 months. This allows for timely detection of pDSA and eDSA using SAB assay. For patients transplanted without DSA with negative XM, the routine monitoring may be performed monthly or even quarterly. Considering that class II HLA mismatching, especially at DQB1 and DQA1, is a major determinant of DSA development especially in the presence of non-adherence or less potent immunosuppression, monthly DSA monitoring may be warranted for patients with higher load of mismatched epitopes for DRB1 and DQB1/DQA1 and/or patients on sub-optimal immunosuppressive regimen post-transplant [32].
Since not all DSAs are associated with adverse outcomes, caution must be taken before treating an antibody result in isolation. For a patient with graft dysfunction in the presence of DSA and, if/when all other causes were ruled out, a DSA treatment may be initiated. Although there are no defined guidelines, the treatment usually includes therapeutic plasma exchange (TPE) with IVIG with or without rituximab or TPE/bortezomib/IVIG with or without rituximab. We have recently shown that serial dilutions may guide treatment selection and that successful treatment of DSA improves patient survival [33].
Desensitization in lung recipients
The prime goal of desensitization is to increase access to transplantation through expansion of the donor organ pool. Existing therapies are directed at key components of the humoral immune response with newer biologically based regimens able to target plasma cells as the source of antibody production, as well as complement activation that has a central role in antibody-mediated injury. Despite the emergence of early promising results for these agents, no approach has demonstrated significant and sustainable reductions in HLA antibody pre-transplant, and the ideal desensitization strategy remains elusive. There are two main approaches to desensitization: (1) waitlist desensitization to achieve negative donor–recipient XM and (2) peri-operative desensitization when suitable donor with “acceptable” DSA is identified by VXM and risk of DSA is mitigated post-transplant. We will discuss several routine therapies utilized in desensitization as well as the clinical aspects that may help the transplant team better determine the risks and benefits of desensitization approaches for the individual patient. In addition, we will discuss emerging approaches in measuring the efficacy of desensitization procedures that currently relies on HLA antibody detection.
Desensitization treatments
TPE removes circulating antibodies by replacing a patient’s blood plasma. Five sessions of TPE remove approximately 80% of immunoglobulin G (IgG) or reduce HLA antibody titers by 3–4 logs [34, 35]. However, TPE in the absence of immune suppression is prone to antibody rebound [36]. For this reason, additional immune suppression is required [36]. Plasmapheresis requires the insertion of a central venous catheter potentially increasing the risk of an infection in a patient who may be immunocompromised. Additional potential complications include coagulopathies, hypotension, metabolic derangements, and increase risk of infection.
High-dose IVIG is a blood product derived from thousands of healthy pooled donors and is a part of most desensitization regimens [37]. The therapy is typically well-tolerated as long as physicians are aware of the additional intravenous volume associated with infusion. Previously described risks of acute renal injury from IVIG were most likely the result of sucrose-containing products causing an osmotic nephropathy, but newer formulations do not contain sucrose. In addition, the immunologists on the transplant team should be aware of patients receiving IVIG and assist with the timing of cPRA testing since IVIG has been reported to result in false-positive HLA antibody tests [38].
To our knowledge, there is no published data to support using IVIG alone as a desensitization method in lung transplant; however, that approach is occasionally used in patients who may be deemed too high risk for more aggressive strategies that include plasmapheresis and additional immune suppression. The mechanisms by which IVIG impacts circulating antibodies are complex and not fully understood. The primary mechanisms that have been described include T cell proliferation inhibition, inhibition of cytokine synthesis, and the inhibition of complement pathways [39]. IVIG also contains neutralizing anti–B cell–activating factor [40]. Since the half-life of IVIG is 25–32 days, we typically continue IVIG infusions every 2 weeks in sensitized patients awaiting transplant.
Rituximab, a chimeric monoclonal antibody against CD20, is frequently utilized in desensitization strategies. It causes B cell destruction via complement activation, apoptosis signaling, and cell-mediated cytotoxicity [41]. For pre-transplant desensitization, rituximab is used in combination with IVIG or TPE/IVIG. The immunology team needs to be aware of rituximab administration since it results in false-positive B cell XM even 9 months after infusion. The most common adverse events have been found to be infusion reactions with life-threatening anaphylaxis being very rare [42]. Cytopenias, especially lymphopenias, may occur but this seems to be more common when given in combination with additional agents prone to myelosuppression [42]. Despite this risk as well as rituximab’s effect on B cells, the limited dosing of rituximab for the purposes of desensitization and in the absence of being in a combination of additional potent immune suppressants, the risk of significant infection associated with rituximab appears to be low. However, the risk of hepatitis B reactivation is well documented [43], and patients should be screened prior to infusion initiation. In our practice, patients with any combination of abnormal hepatitis B markers receive entecavir upon initiating rituximab and continue the antiviral post-transplant for at least 1 year. Additional adverse effects associated with rituximab such as cardiac disorders are probably more frequently encountered when rituximab is given in combination with additional agents as well [42].
Bortezomib has been included in desensitization strategies in combination with other modalities. The drug causes plasma cell apoptosis by inhibiting the 26s proteasome [44]. It rapidly distributes into tissues, and proteasome inhibition returns to baseline within 72–96 h [44]. Bortezomib is used in combination with plasmapheresis [45]. This strategy can also be employed for refractory AMR. The data on the use of bortezomib for desensitization is generally favorable but is limited to small-unblinded trials that are not specific to lung transplant. Since pulmonary patients are particularly sensitive to pulmonary edema and may not tolerate worsening of underlying hypoxemia, care should be taken to monitor closely for bortezomib-induced capillary leak or congestive heart failure [46]. In addition, bortezomib-induced thrombocytopenia may be particularly relevant in the sensitized patient, since concomitant plasmapheresis may result in coagulopathies and bleeding [47]. In severe cases, a recipient’s transplant window may be missed if thrombocytopenia precludes the operation due to the risk of bleeding or if transfusions are needed, which may potentially increase cPRA.
Tocilizumab, a humanized monoclonal antibody directed against interleukin-6, has been found to have encouraging but limited data in sensitized renal transplant patients and is an interesting consideration in sensitized lung transplants. Tocilizumab has been most thoroughly studied in rheumatoid arthritis and has been shown to cause a decline in memory B cell subsets, prevents B cell differentiation, and decreases B cell hyperactivity [48]. As with all of these treatment modalities, the literature regarding tocilizumab is limited and focused primarily on sensitized renal transplants. Vo et al. completed a phase I/II pilot study of 10 sensitized potential renal transplant patients who failed to respond adequately to plasmapheresis + IVIG + rituximab, of which 5 patients were transplanted without AMR [49]. These results highlight the need for further study, but this approach may be reasonable to consider with potential lung transplant recipients who have refractory sensitization. Tocilizumab is generally well tolerated with infection being the most common adverse effect. However, the rate of serious infections is low and comparable to rates seen with methotrexate [50]. Another rare complication is anaphylaxis. In addition, mild neutropenia has been reported and should be managed appropriately [50].
IgG endopeptidase is another exciting option for hopeful lung transplant recipients who are highly sensitized. This novel medication has been studied in highly sensitized renal transplant patients [51]. The therapy is recombinant cysteine protease of Streptococcus pyogenes produced in Escherichia that cleaves IgG into F(ab′)2 and Fc fragments. The Fc portion is crucial for complement binding and plays a key role in antibody-mediated rejection. This approach differs from other desensitization strategies in that it is not administered until the day of transplant with an incompatible graft usually 4–6 h prior to induction. At 1 h after the treatment is given, near-complete inhibition of C1q binding has been shown [51]. At 6 h, all levels of anti-HLA antibodies were significantly reduced [51]. At the time of this paper, there is an active phase II trial to evaluate the effectiveness of this therapy in patients who are on the waiting list for kidney transplants and have already undergone unsuccessful desensitization or in whom effective desensitization will be highly unlikely.
Table 1 summarizes the mechanism of action, dosing, and recommended testing for monitoring the response to treatment for most commonly used immunomodulatory therapeutics.
Table 1.
Commonly used immunetherapeutics for desensitization and AMR treatment
| Immunotherapy | Mechanism of action | Dosing | Application | Monitoring response to treatment |
|---|---|---|---|---|
| TPE | The removal of anti-HLA antibodies from the blood. Other possible mechanisms include changes in lymphocyte proliferation, B and T cell numbers and activation, increased T suppressor function, and alteration in T-helper cell type 1/2 (Th1/Th2) ratio. | 5 TPE sessions for DSA with a titer≤1:256 | Pre-operative desensitization Peri-operative desensitization. Acute AMR treatment | SAB (MFI and/or titers) before and after each session |
| IVIg | The mechanisms include immunomodulatory and anti-inflammatory effects via interaction between the Fc portion of infused IgG with the Fcγ receptors on the surface of target cells or with the variable regions of antibodies in the preparation. | 1 g/kg bi-weekly or 2 g/kg monthly for several months, depending on antibody titer | Pre-operative desensitization Preventive AMR treatment or low-level DSA | SAB (MFI and/or titers) 3 weeks post-IVIG treatment |
| Rituximab | Targeting B cells | 375 mg/m2 × body surface area |
Pre-operative desensitization in combination with IVIG. AMR treatment |
SAB (MFI and/or titers) 3 weeks post-rituximab treatment |
| Bortezomib | Inhibiting immunoproteasome and IgG production | 1.3 mg/m2/dose × 3–4 doses commonly combined with TPE | Pre-operative desensitization peri-operative desensitization and acute AMR treatment | SAB (MFI and/or titers) 24 h after treatment; when combined with TPE before and after TPE session |
| Eculizumab | Blocking complement protein C5 and preventing the generation of the terminal complement complex C5b-9 | 1200 mg IV over 1 h then 900 mg IV over 1 h weekly × 3 doses or more per clinical response | Peri-operative desensitization, AMR treatment | It is not clear whether Eculizumab affects antibody levels. Weekly SAB testing is recommended. |
Considerations for multi-modality desensitization treatment
There are only a handful of studies on pre-transplant desensitization in lung recipients. Aguilar et al. reported the use of IVIG and rituximab in 11 sensitized patients [52]. Due to a perceived lack of efficacy, mycophenolate mofetil (MMF) and TPE were added. The cPRA decreased from 97 to 82% after desensitization. Only 3 out of 11 patients were transplanted because DSA were successfully depleted with desensitization. Three other patients received an offer with no DSA before desensitization. Snyder et al. reported outcomes of desensitization protocol using TPE, solumedrol, bortezomib, and rituximab given in combination over 19 days followed by IVIG [53]. There were no significant changes in PRA or cPRA changes, and 9 of 18 candidates subsequently had a transplant. The authors concluded that an aggressive multi-modal desensitization protocol does not significantly reduce pre-transplant HLA antibodies in a broadly sensitized lung transplant candidate cohort. However, it has been shown that the response to desensitization may need to be measured in titers not MFI or cPRA in undiluted serum. The success rate ranged from 30 to 50% in these studies, and the similar outcomes were reported in desensitization for other solid organs, with slightly better response from TPE/bortezomib vs rituximab/IVIG protocols [45, 54].
An alternative was explored by Tinckam et al., where the potential impact of DSA was mitigated peri-operatively with TPE/IVIG and ATG leading to equivalent rejection and graft survival outcomes [55]. This approach has gained interest, especially now when the availability of DSA titer information and response to dilutions can guide per-operative TPE-based treatment protocols [34, 35]. Specifically, DSA with titers less than 1:256 could be effectively decreased with 5 TPE sessions. We found that a reduction in MFI at 1:16 dilution can predict the effectiveness of TPE. Therefore, for sensitized patients, we list UA only if corresponding antibodies have MFI values greater than 3000 in 1:16 diluted serum (Fig. 3B) [34].
While both desensitization approaches result in a similar AMR rate and require prevention of memory B cell response and inhibition of plasma cell activity early post-transplant [56], the advantage of peri-operative desensitization is in initiating treatment only when needed. When waitlist sensitization is performed, less than 50% of patients proceed to transplant. The limitation of peri-operative sensitization is that it is not effective against DSA with a titer greater than 1:256 (or > 3000 MFI at 1:16). Therefore, careful antibody characterization using serum dilutions is a key for peri-operative desensitization.
When considering the degree of acceptable immunologic risk between a lung transplant candidate and a potential donor, in addition to immunologic variables, a multitude of non-immunologic characteristics relating to the recipient’s likelihood of survival while awaiting transplant should be considered. These considerations center around a wide range of factors such as the expected trajectory of the recipient’s underlying disease, limitations in the donor pool, and operative limitations relating to the recipient’s chest cavity and frailty. Assessing the recipient’s trajectory of disease is the most important aspect when determining the degree of acceptable immunologic risk to take. Patients with high lung allocation score (LAS) have an increased risk of waitlist mortality, and LAS has been shown to be a better predictor of waitlist mortality when compared to clinical judgment, based on a large retrospective study of three Canadian lung transplant centers showing hazard ratio for waitlist mortality of 1.06 per unit LAS [57]. Therefore, a transplant team would likely be more willing to accept increased immunologic risks for a patient with a high LAS as a means to hopefully reduce the recipient’s risk of waitlist mortality. In addition, it is reasonable to consider patients on ECMO with either venovenous (VV) or venoarterial (VA) configuration as candidates for accepting increased immunologic risk as their window for transplant is likely narrow [58]. Such patients would have a very high LAS status by default; however, patients with high cPRAs may lose potential opportunities, especially if their cPRA keeps increasing while on ECMO. Another consideration for patients being bridged with ECMO is that the potential complications of ECMO such as infection or coagulopathy may result in precluding a patient from transplant [58]. For this reason, the transplant team may consider accepting additional immunologic risks as a means of transplanting a patient in a timelier manner who is ECMO dependent.
Disease-specific physiologic markers can also be helpful when determining if accepting increased immunologic risk is appropriate in relation to a patient’s risk of waitlist mortality. For example, patients with pulmonary fibrosis who experience a more rapid decline in forced vital capacity (FVC) have been found to be at increased risk of mortality than similar patients with stable FVC predicted [59]. Therefore, a transplant team may be more inclined to accept increased immunologic risk in a patient with high cPRA and rapidly decreasing FVC alone. Of course, a reduction in FVC would be a less reliable predictor of mortality in patients with concomitant emphysema. In such cases of combined pulmonary fibrosis and emphysema, the pulmonary team should be cognizant of other established predictors of mortality such as a reduction in diffusion or the development of pulmonary hypertension [59]. Patients who developed pulmonary hypertension had a 1-year survival of only 60% despite relatively preserved predicted FVC and 6-min walk distances [59]. In such patients who also have elevated cPRAs, accepting increased immunologic risk may be necessary.
The transplant team may also consider accepting a higher level of immunologic risk in situations in which the recipient’s potential donor pool is reduced for other reasons, including patients who require a double lung transplant as opposed to a single lung transplant due to a history of severe pulmonary hypertension or suppurative pulmonary infections with bronchiectasis. In addition, some patients will be limited to either the right or left hemithorax for transplant if there is a history of mycetomas, prior spontaneous pneumothorax with or without a pleurodesis, or severely asymmetric pulmonary perfusion. Anecdotally, our program is also more likely to consider accepting an elevated immunologic risk in patients with short stature and small chest cavities as these patients often have fewer potential donors.
At our institution, we conduct a weekly multidisciplinary meeting to discuss both pre-transplant and post-transplant patients with elevated immunologic risk. The team consists of immunologists, pulmonologists, surgeons, pharmacists, transplant coordinators, and nurses. We have found this approach to be beneficial for patients as well as for our team. From an immunology standpoint, we have found that by evaluating a recipient’s HLAs in more detail with serial dilutions and C1q, the team may accept crossing antigens that were previously viewed as incompatible. When waitlist desensitization is warranted, our most common approach is IVIG + rituximab with or without plasmapheresis; however, just like other centers reported, we experienced limited success with such desensitization. On another hand, we successfully used peri-operative desensitization based on serial dilutions combined with careful post-transplant monitoring for DSA and graft function [34]. The post-transplant immune suppression is usually the same for patients at increased immunologic risk, aside from being more inclined to aim for slightly higher doses of MMF, if tolerated. In general, the choice of alemtuzumab or basiliximab for induction is driven by other protocolized factors such as age, CMV status, and history of cancer and would typically not be affected by a patient’s immunologic risk based on our assessment of the risks and benefits of altering these protocols. Since tacrolimus is the drug of choice for every patient in our institution, there is no difference in the management of the calcineurin inhibitor.
A multidisciplinary approach has also helped give the immunology team additional clinical context, they may have otherwise not been aware of when recommending potential desensitization strategies. For example, the risk of coagulopathy and hemorrhage just prior to transplant would have different implications for a patient more prone to adhesions of the native lung at increased risk of post-operative bleeding such as patients with pleuro-parenchymal fibroelastosis, prior pleurodesis, and some patients with sarcoidosis. In addition, we have found that the presence of pharmacists as well as the transplant coordinators and nurses have helped to streamline the implementation of many of these desensitization strategies in the outpatient setting. This approach also enables a seamless transition to monitor these patients in the outpatient setting post-transplant and allows for more structured and regular monitoring of DSAs and the management of antibody-mediated rejection, if needed.
Conclusion
HLA mismatching between the transplant candidate and the donor is one of the major reasons for post-transplant rejection, including both cell-mediated and AMR. HLA antibody testing is a readily available diagnostic test that could be used for pre- and post-transplant immunologic risk assessment and for guiding desensitization treatments for sensitized patients, who are clearly disadvantaged by a limited donor pool and have a higher likelihood of dying on a waitlist. Currently, desensitization requires careful consideration in a multidisciplinary setting and the team should weigh the risks and benefits of the desensitization modality itself as well as clinical factors that may impact the patient’s likelihood of waitlist mortality, while awaiting a suitable donor. Many regimens including TPE in combination with IVIG, bortezomib, and rituximab show some promise, but desensitizing patients with preformed anti-HLA antibodies remains controversial. In addition, DSA treatment in the context of AMR after transplantation also requires a multidisciplinary approach. Further understanding of the clinical significance of DSA detected by SAB technology as well as collaborative studies and multicenter trials will be key in further advancing LTx knowledge and improving outcomes.
Funding
None.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
Authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olga Timofeeva, Email: Olga.a.timofeeva@gunet.georgetown.edu.
James Brown, Email: James.Brown@tuhs.temple.edu.
References
- 1.Opelz G, Döhler B, Ruhenstroth A, et al. The collaborative transplant study registry. Transplant Rev (Orlando). 2013;27:43–5. [DOI] [PubMed]
- 2.Marino J, Paster J, Benichou G. Allorecognition by T Lymphocytes and allograft rejection. Front Immunol. 2016;7:582. doi: 10.3389/fimmu.2016.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verleden SE, Vanaudenaerde BM, Emonds M-P, et al. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. Eur Respir J. 2017;50:1701248. doi: 10.1183/13993003.01248-2017. [DOI] [PubMed] [Google Scholar]
- 4.Courtwright AM, Cao S, Wood I, et al. Clinical outcomes of lung transplantation in the presence of donor-specific antibodies. Ann Am Thorac Soc. 2019;16:1131–1137. doi: 10.1513/AnnalsATS.201812-869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19:33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brann SH, Geier SS. Timofeeva OA, Shigemura N, Cordova F, Toyoda Y. Perioperative care for lung transplant recipients: a multidisciplinary approach. Perioperative Care for Organ Transplant Recipient, ed. A. Vitin. 2019, London, United Kindom: IntechOpen 147.
- 7.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SGE. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–D431. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001;1:97–102. doi: 10.1034/j.1600-6143.2001.10201.x. [DOI] [PubMed] [Google Scholar]
- 9.Hosenpud JD, Edwards EB, Lin HM, Daily OP. Influence of HLA matching on thoracic transplant outcomes. An analysis from the UNOS/ISHLT Thoracic Registry. Circulation. 1996;94:170–174. doi: 10.1161/01.CIR.94.2.170. [DOI] [PubMed] [Google Scholar]
- 10.Todd JL, Neely ML, Kopetskie H, et al. Risk factors for acute rejection in the first year after lung transplant. a multicenter study. Am J Respir Crit Care Med. 2020;202:576–585. doi: 10.1164/rccm.201910-1915OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard CA, Fernandez-Vina MA, Appelbaum FR, et al. Recommendations for donor human leukocyte antigen assessment and matching for allogeneic stem cell transplantation: consensus opinion of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). Biol Blood Marrow Transplant. 2015;21:4–7. [DOI] [PMC free article] [PubMed]
- 12.van der Zwan A, van der Meer-Prins EMW, van Miert PPMC, van den Heuvel H, Anholts JDH, Roelen DL, Claas FHJ, Heidt S. Cross-reactivity of virus-specific CD8+ T cells against allogeneic HLA-C: possible implications for pregnancy outcome. Front Immunol. 2018;9:2880. doi: 10.3389/fimmu.2018.02880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature.1999;401:708–12. [DOI] [PubMed]
- 14.Irure J, Ballesteros MA, Iturbe D, et al. CD8 T effector memory T cells as predictive biomarker of severe acute rejection in lung transplantation. Transplantation. 2018;102:S682.
- 15.Wijesinha M, Hirshon JM, Terrin M, et al. Survival associated with sirolimus plus tacrolimus maintenance without induction therapy compared with standard immunosuppression after lung transplant. JAMA Netw Open.2019; 2:e1910297. [DOI] [PMC free article] [PubMed]
- 16. Bando K, Paradis IL,Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg.1995;110:4–13. [DOI] [PubMed]
- 17.Zhang J, Liu D, Zhang C, et al. The value of high-resolution HLA in the perioperative period of non-sensitized lung transplant recipients. Ann Transl Med.2020;8:37. [DOI] [PMC free article] [PubMed]
- 18.Gokmen MR, Lombardi G, Lechler RI. The importance of the indirect pathway of allorecognition in clinical transplantation. Curr Opin Immunol. 2008;20(5):568–574. doi: 10.1016/j.coi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Iwaki Y, Yoshida Y, Griffith B. The HLA matching effect in lung transplantation. Transplantation. 1993;56(6):1528–1529. doi: 10.1097/00007890-199312000-00047. [DOI] [PubMed] [Google Scholar]
- 20.Terasaki P, Mizutani K. Antibody mediated rejection: update 2006. Clin J Am Soc Nephrol. 2006;1:400–3. [DOI] [PubMed]
- 21.Walton DC, Hiho SJ, Cantwell LS, et al. HLA Matching at the eplet level protects against chronic lung allograft dysfunction. Am J Transplant.2016;16:2695–703. [DOI] [PubMed]
- 22.Heidt S, Roelen DL, Eijsink C, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol.2010;159:199–207. [DOI] [PMC free article] [PubMed]
- 23.Tambur AR, Campbell P, Claas FH, et al. Sensitization in transplantation: assessment of risk (STAR) 2017 Working Group Meeting Report. Am J Transplant.2018;18:1604–1614. [DOI] [PubMed]
- 24.Hayes D, Preston TJ, Kirkby S, Nicol KK,et al.Human leukocyte antigen sensitization in lung transplant candidates supported by extracorporeal membrane oxygenation. Am J Respir Crit Care Med.2013;188:627–8. [DOI] [PubMed]
- 25.Aversa M, Benvenuto L, Kim H, Shah L, Robbins H, Stanifer BP, D’Ovidio F, Vasilescu ERR, Sonett J, Arcasoy SM. Effect of calculated panel reactive antibody value on waitlist outcomes for lung transplant candidates. Ann Transplant. 2019;24:383–392. doi: 10.12659/AOT.915769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinsmoen NL, Patel J, Mirocha J, et al.Optimizing transplantation of sensitized heart candidates using 4 antibody detection assays to prioritize the assignment of unacceptable antigens. J Heart Lung Transplant.2016;35:165–72. [DOI] [PubMed]
- 27.Zeevi A, Lunz J, Feingold B,et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. [DOI] [PMC free article] [PubMed]
- 28.Baranwal AK, Bhat DK, Goswami S,et al.Comparative analysis of Luminex-based donor-specific antibody mean fluorescence intensity values with complement-dependent cytotoxicity & flow crossmatch results in live donor renal transplantation. Indian J Med Res.2017;145:222–228. [DOI] [PMC free article] [PubMed]
- 29.Tinckam KJ, Liwski R, Pochinco D, et al. cPRA increases with DQA, DPA, and DPB unacceptable antigens in the Canadian cPRA calculator. Am J Transplant. 2015;15:3194–201. [DOI] [PMC free article] [PubMed]
- 30.Morris AB, Sullivan HC, Krummey SM, Gebel HM, Bray RA. Out with the old, in with the new: Virtual versus physical crossmatching in the modern era. HLA.2019;94:471–481. [DOI] [PMC free article] [PubMed]
- 31.Tambur AR, Lavee J. Incorporating human leukocyte antibody results into clinical practice. J Heart Lung Transplant.2016;35:851–6. [DOI] [PubMed]
- 32.Konvalinka A,Tinckam K.Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol.2015;26:1489–502. [DOI] [PMC free article] [PubMed]
- 33.Timofeeva OA, Choe J, Alsammak M, et al. Guiding therapeutic plasma exchange for antibody-mediated rejection treatment in lung transplant recipients - a retrospective study. Transpl Int. 2021;34:700–8. [DOI] [PubMed]
- 34.Timofeeva OA, Alvarez R, Pelberg J, Yoon E, Alsammak M, Geier SS, Ruggia-Check C, Hassler J, Hoosain J, Brisco MA, Afari-Armah N, Rakita V, Brann S, Keshavamurthy S, Gomez-Abraham J, Minakata K, Toyoda Y, Hamad E. Serum dilutions as a predictive biomarker for peri-operative desensitization: an exploratory approach to transplanting sensitized heart candidates. Transpl Immunol. 2020;60:101274. doi: 10.1016/j.trim.2020.101274. [DOI] [PubMed] [Google Scholar]
- 35.Pinelli DF, Zachary AA, Friedewald JJ, et al. Prognostic tools to assess candidacy for and efficacy of antibody-removal therapy. Am J Transplant. 2019;19:381–390. [DOI] [PubMed]
- 36.Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol.2014;164:342–51. [DOI] [PubMed]
- 37.Hartung HP.Advances in the understanding of the mechanism of action of IVIg. J Neurol.2008;255:3–6. [DOI] [PubMed]
- 38.Ravindranath MH, Terasaki PI, Pham T, Jucaud V, Kawakita S. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles. Blood.2013;121:2013–28. [DOI] [PubMed]
- 39.Garces JC, Giusti S, Staffeld-Coit C, Bohorquez H , Cohen AJ, Loss GE. Antibody-mediated rejection: A review. Ochsner J.2017;17:46–55. [PMC free article] [PubMed]
- 40.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol.2017;29:491–498. [DOI] [PubMed]
- 41.Regazzi MB, Iacona I, Avanzini MA,et al. Pharmacokinetic behavior of rituximab: a study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit.2005;27:785–92 [DOI] [PubMed]
- 42.Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS. Clinical review: serious adverse events associated with the use of rituximab - a critical care perspective. Crit Care. 2012;16:231. [DOI] [PMC free article] [PubMed]
- 43.Seto WK. Hepatitis B virus reactivation during immunosuppressive therapy: Appropriate risk stratification. World J Hepatol.2015;7:825–30. [DOI] [PMC free article] [PubMed]
- 44.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park). 2004;18:14–21. [PubMed]
- 45.Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant.2011;30:1320–6 [DOI] [PubMed]
- 46.Hsiao S-C, Wang M-C, Chang H, Pei S-N. Recurrent capillary leak syndrome following bortezomib therapy in a patient with relapsed myeloma. Ann Pharmacother.2010;44:587–9. [DOI] [PubMed]
- 47.Murai K, Kowata S, Shimoyama T, et al. Bortezomib induces thrombocytopenia by the inhibition of proplatelet formation of megakaryocytes. Eur J Haematol.2014;93:290–6. [DOI] [PubMed]
- 48.Roll P, Muhammad K, Schumann M,et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum.2011;63:1255–64. [DOI] [PubMed]
- 49.Vo AA, Choi J, Kim I, et al. A phase I/II trial of the Interleukin-6 receptor-specific humanized monoclonal (Tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation.2015;99:2356–63. [DOI] [PubMed]
- 50.Scott LJ. Tocilizumab: A review in rheumatoid arthritis. Drugs.2017;77:1865–1879. [DOI] [PMC free article] [PubMed]
- 51.Jordan SC, Lorant T, Choi J,et al. IgG Endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med.2017;377:442–453. [DOI] [PubMed]
- 52.Aguilar PR, Witt CA, Bemiss BC, et al. Desensitization therapy before lung transplantation. J Heart Lung Transplant. 2016;35:S237.
- 53.Snyder LD, Gray AL, Reynolds JM,et al. Antibody desensitization therapy in highly sensitized lung transplant candidates. Am J Transplant. 2014;14:849–56. [DOI] [PMC free article] [PubMed]
- 54.Marfo K, Ling M, Bao Y,et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation.2012; 94:345–51. [DOI] [PubMed]
- 55.Tinckam KJ, Keshavjee S, Chaparro C,et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant.2015;15:417–26. [DOI] [PubMed]
- 56.Chih S, Patel J. Desensitization strategies in adult heart transplantation-Will persistence pay off? J Heart Lung Transplant. 2016;35:962–72. [DOI] [PubMed]
- 57.Hirji A, Zhao H, Ospina MB,et al. Clinical judgment versus lung allocation score in predicting lung transplant waitlist mortality. Clin Transplant.2020;34:e13870. [DOI] [PubMed]
- 58.Inci I, Klinzing S, Schneiter D, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation: an institutional experience and literature review. Transplantation.2015;99:1667–71. [DOI] [PubMed]
- 59.Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105–11. [DOI] [PubMed]