Abstract
In France, two therapeutic strategies can be offered after fingolimod (FNG) withdrawal to highly active relapsing–remitting multiple sclerosis (RRMS) patients: natalizumab (NTZ) or anti-CD20. We compared the effectiveness of these two strategies as a switch for FNG within the OFSEP database. The primary endpoint was the time to first relapse. Other outcomes were the relapse rates over 3-month periods, time to worsening the EDSS score, proportion of patients with worsened 24-month MRI, time to treatment discontinuation, and incidence rates of serious adverse events. The dynamics of event rates over time were modeled using multidimensional penalized splines, allowing the possibility to model the effects of covariates in a flexible way, considering non-linearity and interactions. A total of 740 patients were included (337 under anti-CD20 and 403 under NTZ). There was no difference between the two treatments regarding the dynamic of the first occurrence of relapse, with a monthly probability of 5.0% at initiation and 1.0% after 6 months. The rate of EDSS worsening increased in both groups until 6 months and then decreased. No difference in the proportion of patients with new T2 lesions at 24 months was observed. After 18 months of follow-up, a greater risk of NTZ discontinuation was found compared to anti-CD20. This study showed no difference between NTZ and anti-CD20 after the FNG switch regarding the clinical and radiological activity. The effect of these treatments was optimal after 6 months and there was more frequent discontinuation of NTZ after 18 months, probably mainly related to JC virus seroconversions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01202-1.
Keywords: Multiple sclerosis, Fingolimod, Natalizumab, Anti-CD20, Therapeutics, Effectiveness, Flexible model
Introduction
The advent of many innovative treatments in the past recent years has profoundly changed the management of relapsing–remitting multiple sclerosis (RRMS). Individualized management relies on the choice of the most appropriate molecules and allows an improvement in the prognosis of patients. However, due to the lack of head-to-head treatment comparison, these choices are sometimes difficult to make.
In Europe, fingolimod (FNG) is indicated for highly active RRMS, either for naive patients who experienced at least two severe relapses (needing the use of corticosteroids) in 1 year or patients with persistent clinical or radiological activity despite a well-conducted first-line treatment. Indeed, several therapeutic trials have demonstrated the superiority of FNG in terms of efficacy compared to first-line treatments [1–3]. However, FNG is sometimes insufficient to prevent the occurrence of relapses or new lesions detectable on magnetic resonance imaging (MRI) scans, thus justifying a therapeutic escalation.
Classically, for patients not responding to FNG, natalizumab (NTZ), available in France since 2007 [4, 5], and anti-CD20 (mainly rituximab in an off-label use from 2014 and ocrelizumab since 2019) are used as a switch therapy. The superiority of NTZ compared to FNG in terms of effectiveness on the occurrence of relapses and progression of disability has been demonstrated in the OFSEP cohort [6] and other international cohorts [7–9]. The superiority of rituximab (and by extension other anti-CD20) over FNG has been demonstrated in more recent observational studies, but often with a low level of evidence [10–12]. These findings have also been reported in therapeutic trials using rituximab, ocrelizumab, or ofatumumab in comparison with first-line or placebo treatments, making these treatments an alternative to NTZ and FNG [13–17]. In addition, the marketing authorization of ocrelizumab in France since 2019 has significantly increased the number of patients administered anti-CD20 drugs. The use of anti-CD20 was justified by the limited use of NTZ due to the occurrence of progressive multifocal leukoencephalopathy (PML) associated with this treatment [18, 19]. However, since 2013, the use of JC (John Cunningham) virus serology and the related index have allowed the development of better therapeutic strategies for the use of NTZ, significantly reducing the occurrence of new PML cases [20].
Today, for a patient treated with FNG and presenting residual disease activity, the choice for disease management is often based on the serology and the anti-JCV antibody index, but other parameters should also be considered (desire of pregnancy, constraints of treatments, duration of treatment, patient willingness).
To date, no study has compared the relative effectiveness of these two therapeutic strategies, particularly regarding the dynamics of the first relapse rate and the rate of EDSS (expanded disability status scale) worsening according to the time since their initiation and the impact of covariates on these dynamics. This innovative approach, developed in cancer studies [21–23], provides essential clinical and therapeutic information over time that cannot be modeled using a conventional propensity score approach. It has recently been used in the MS context by studying the excess mortality rates [24].
Using this approach on data from the OFSEP cohort, the present study aimed to describe and compare the dynamics of disease activity over a 24-month follow-up period in highly active RRMS patients for whom treatment was switched from FNG to either NTZ or anti-CD20.
Patients and Methods
Study Design
This retrospective observational cohort study was based on data from 36 French MS expert centers participating in the French MS database called Observatoire Français de la Sclérose En Plaques (OFSEP) [25]. For each patient, clinical and imaging data were collected during routine follow-up visits, usually once a year, using a dedicated software, the European Database on Multiple Sclerosis “EDMUS” [26], by a neurologist with a special interest in MS. These data were collected retrospectively at the time of the first visit and prospectively thereafter.
Standard Protocol Approvals, Registrations, and Patient Consents
Patients enrolled in the OFSEP study (registered on clinicaltrials.gov [NCT02889965]) provide written informed consent for participation. In accordance with the French legislation, the OFSEP cohort was approved by both the French data protection agency (Commission Nationale de l’ Informatique et des Libertés [CNIL]; authorization request 914066v3) and a French ethical committee (Comité de Protection des Personnes [CPP]: reference 2019-Ă6-51), and the present study was declared compliant to the MR-004 (Méthodologie de reference 004) of the CNIL.
Patients
All adults with RRMS at baseline and having initiated treatment with NTZ or anti-CD20 (ocrelizumab/rituximab) between January 1, 2014 (date from which these treatments were simultaneously available in France) and December 15, 2019 as a switch therapy for FNG were included in the present study. Patients for whom FNG treatment had been discontinued for more than 6 months or for whom no follow-up data was available were excluded. The diagnostic criteria for MS were defined by the 2010 revised MacDonald criteria [27]. The baseline was defined as the initiation date of NTZ or anti-CD20. Hence, patients were followed from baseline until the last clinical evaluation and censored at 24 months.
Definition of Outcomes Measures
The primary outcome was the occurrence of the first relapse. A relapse was defined as the appearance, recurrence, or worsening of neurological signs due to MS immediately preceded by a stable or improved neurological state lasting a least 30 days. Also, the relapse symptoms persisted for at least 24 h, without fever, and were accompanied by objective neurological aggravation different from fatigue alone [28]. Each relapse was documented by the neurologist in EDMUS database along with the change in treatment and the reason for the change.
Five secondary outcomes were considered in the analyses. The first was the relapse rates (RRs) during each 3-month period. The second was the progression of disability, using the EDSS score measured remotely to any relapses (30 days). A progression was considered for an increase of 1.5 points if the EDSS at baseline was equal to 0, 1 point if the baseline EDSS was < 5.0 and > 0, and 0.5 point if the baseline EDSS was ≥ 5.0. The third outcome was the worsening of MRI lesions, indicated by an increase in the number of new T2 lesions compared to baseline MRI or positive contrast enhancement. The fourth was the discontinuation of NTZ or anti-CD20 treatment. The last outcome was the incidence rates (IR) of serious adverse events (SAE).
Statistical Analyses
We used a novel analytical method developed by the biostatistics department of the Hospices Civils de Lyon [21, 22] to model the logarithm of the event rate (ER) by a multidimensional penalized spline function based on tensor product splines. This method allows to model the dynamics of ER (i.e., the evolution of the ER according to the follow-up) and the effects of covariates on these dynamics; this model is flexible, as the effects can be non-linear and/or time-dependent (i.e., non-proportional), meaning that the dynamics of ER may change smoothly with each covariate. Once estimated, the dynamics of ER can be represented graphically according to the follow-up. The ER is a fundamental concept but is not always easily interpretable (because it is a conditional probability per unit of time and can therefore be greater than one). However, when ER is low (< 0.10), it may be easily translated in probability of event per unit of time: for example, a constant rate of 0.05 event per person-month over 1 month corresponds approximately to a probability of event of 5% within the month.
For each outcome, we used this flexible approach in a univariate approach by then introducing in the model the confusing factors at baseline causing both the outcomes (p-value < 0.05) and the treatment allocation (Cohen’s values |d > 0.2). The final model was selected based on a corrected AIC (Akaike information criterion) [29, 30], as a minimal AIC identifies the model that offers an optimal trade-off between the model’s goodness of fit and its parsimony. The studied factors were sex, age, inclusion period, EDSS score ([0.0–1.5], [2.0–3.5], ≥ 4.0), FNG treatment duration, reasons for FNG discontinuation, disease duration, number of relapses within the last year, washout period (< 3 months, [3–6] months), gadolinium-enhancing brain lesions (positive, negative, not available), and JC virus serology (positive, negative, not determined).
Regarding the secondary objective (worsening of MRI lesions), a multivariate logistic regression was used by introducing factors associated with a p-value < 0.2 in the univariate analysis and by providing the adjusted odds ratios (OR). RRs were estimated for the 15 months preceding NTZ or anti-CD20 initiation and during 24-month period post-initiation, and expressed with their 95% confidence interval (CI). They were compared after baseline using negative binomial regression adjusted with the covariates used in the final model of the primary analysis.
The IR of the SAE were defined as the total number of SAE divided by the entire duration of follow-up and the 95% CI were estimated assuming a Poisson distribution. They were estimated separately in two types: cancer and overall SAE (excluding cancer). For cancer types, the exposure period was defined until the last clinical evaluation and for the other types, it was defined until the last clinical evaluation or 180 days after treatment discontinuation, whichever occurred first. This analysis was performed in patients for whom the treatment was switched after 01/01/2017, which corresponds to the date of systematic collection of SAE in the OFSEP cohort.
To corroborate the results with a more conventional statistical method, we used propensity scores by inverse probability of treatment weighting (IPTW) to compare the effectiveness of NTZ and anti-CD20 on the occurrence of the first relapse after baseline in a sensitivity analysis.
The statistical analyses were performed using SAS v9.4 (SAS Inst, Cary, NC, USA) for descriptive analyses and R software version 3.5.0 [31] with the survPen package version 1.0.0 to model the event rates.
Results
Characteristics of the Population
In December 2020, 68,847 patients with MS were included in the OFSEP cohort and 740 patients met the inclusion criteria of the present study at baseline (403 (54.5%) were treated with NTZ, and 337 (45.5%) with anti-CD20, including 59 (17.5%) with ocrelizumab; Fig. 1). The median [interquartile range, IQR] follow-up duration was 22.9 [11.2–39.0] months. The sex ratio (female/male) was 3.0 (553, 74.7% female) and the mean ± standard deviation (SD) age was 37.7 ± 9.9 years at baseline (range: 18.6–71.8; Table 1).
Fig. 1.
Patient selection diagram
Table 1.
Clinical and sociodemographic characteristics of the study population at baseline
| Total | NTZ | Anti-CD20 | |d| Cohen* | ||||
|---|---|---|---|---|---|---|---|
| Variables | % | N | % | N | % | ||
| Total | 740 | 403 | 54.5 | 337 | 45.5 | ||
| Sex | |||||||
| Male | 187 | 25.3 | 92 | 22.8 | 95 | 28.2 | 0.123 |
| Female | 553 | 74.7 | 311 | 77.2 | 242 | 71.8 | |
| Age at baseline | |||||||
| Mean ± SD (range) |
37.7 ± 9.88 (18.6–71.8) |
36.8 ± 9.56 (19.1–71.8) |
38.8 ± 10.14 (18.6–68.2) |
0.203 | |||
| [18; 30[ | 187 | 25.3 | 112 | 27.8 | 75 | 22.3 | 0.259 |
| [30; 40[ | 268 | 36.2 | 150 | 37.2 | 118 | 35.0 | |
| [40; 50[ | 185 | 25.0 | 102 | 25.3 | 83 | 24.6 | |
| [50; 60[ | 89 | 12.0 | 34 | 8.4 | 55 | 16.3 | |
| ≥ 60 | 11 | 1.5 | 5 | 1.2 | 6 | 1.8 | |
| Inclusion period | 0.977 | ||||||
| [2014–2015] | 189 | 25.5 | 169 | 41.9 | 20 | 5.9 | |
| [2016–2017] | 321 | 43.4 | 156 | 38.7 | 165 | 49.0 | |
| [2018–2019] | 230 | 31.1 | 78 | 19.4 | 152 | 45.1 | |
| EDSS (± 3 months) | |||||||
| [0.0; 1.5] | 132 | 17.8 | 82 | 20.4 | 50 | 14.8 | 0.218 |
| [2.0; 3.5] | 189 | 25.5 | 110 | 27.3 | 79 | 23.4 | |
| ≥ 4.0 | 237 | 32.0 | 113 | 28.0 | 124 | 36.8 | |
| Not available | 182 | 24.6 | 98 | 24.3 | 84 | 24.9 | |
| FNG treatment duration (months) | |||||||
| Mean ± SD (range) |
24.6 ± 18.60 (0.03–110.4) |
22.5 ± 16.63 (0.03–71.9) |
27.2 ± 20.43 (0.03–110.4) |
0.248 | |||
| [0; 12] | 222 | 30.0 | 134 | 33.2 | 88 | 26.1 | 0.159 |
| ]12; 24] | 202 | 27.3 | 107 | 26.6 | 95 | 28.2 | |
| > 24 | 316 | 42.7 | 162 | 40.2 | 154 | 45.7 | |
| Reasons for FNG treatment discontinuation | |||||||
| SAE | 22 | 3.0 | 10 | 2.5 | 12 | 3.6 | 0.063 |
| Ineffectiveness | 549 | 74.2 | 278 | 69.0 | 271 | 80.4 | 0.265 |
| Intolerance (general, local, and/or biological) | 106 | 14.3 | 65 | 16.1 | 41 | 12.2 | 0.114 |
| Pregnancy (desire) | 29 | 3.9 | 28 | 7.0 | 1 | 0.3 | 0.362 |
| Scheduled discontinuation | 22 | 3.0 | 13 | 3.2 | 9 | 2.7 | 0.033 |
| Patient’s convenience | 26 | 3.5 | 19 | 4.7 | 7 | 2.1 | 0.146 |
| Others | 11 | 1.5 | 6 | 1.5 | 5 | 1.5 | 0.001 |
| Unknown | 12 | 1.6 | 4 | 1.0 | 8 | 2.4 | 0.108 |
| Disease duration (years) | |||||||
| Mean ± SD (range) |
10.2 ± 6.65 (0.3–38.7) |
9.4 ± 6.19 (0.3–32.9) |
11.2 ± 7.05 (0.9–38.7) |
0.264 | |||
| [0; 5] | 173 | 23.4 | 101 | 25.1 | 72 | 21.4 | 0.234 |
| ]5; 10] | 253 | 34.2 | 152 | 37.7 | 101 | 30.0 | |
| > 10 | 314 | 42.4 | 150 | 37.2 | 164 | 48.6 | |
| Number of relapses within the year before inclusion | 0.029 | ||||||
| 0 | 229 | 31.0 | 123 | 30.5 | 106 | 31.5 | |
| 1 | 313 | 42.3 | 170 | 42.2 | 143 | 42.4 | |
| ≥ 2 | 198 | 26.7 | 110 | 27.3 | 88 | 26.1 | |
| Washout period (months) | |||||||
| < 3 | 593 | 80.1 | 330 | 81.9 | 263 | 78.0 | 0.096 |
| [3; 6] | 147 | 19.9 | 73 | 18.1 | 74 | 22.0 | |
| Brain MRI | 0.004 | ||||||
| Yes | 524 | 70.8 | 285 | 70.7 | 239 | 70.9 | |
| No | 216 | 29.2 | 118 | 29.3 | 98 | 29.1 | |
| If brain MRI, | |||||||
| Gadolinium-enhancing brain lesions | 524 | 285 | 239 | 0.055 | |||
| Positive | 269 | 51.3 | 142 | 49.8 | 127 | 53.1 | |
| Negative | 221 | 42.2 | 121 | 42.5 | 100 | 41.8 | |
| Not available | 34 | 6.5 | 22 | 7.7 | 12 | 5.0 | |
|
If brain MRI, Number of T2 brain lesions |
524 | 285 | 239 | 0.046 | |||
| [1; 9] | 16 | 3.1 | 8 | 2.8 | 8 | 3.3 | |
| ≥ 9 | 404 | 77.1 | 222 | 77.9 | 182 | 76.2 | |
| Not available | 104 | 19.8 | 55 | 19.3 | 49 | 20.5 | |
| JC virus status | |||||||
| Positive | 365 | 49.3 | 136 | 33.8 | 229 | 68.0 | 0.831 |
| Negative | 172 | 23.2 | 144 | 35.7 | 28 | 8.3 | |
| Not done/not available | 203 | 27.4 | 123 | 30.5 | 80 | 23.7 | |
| JCV index | 537 | 280 | 257 | ||||
| Negative | 172 | 32.0 | 144 | 51.4 | 28 | 10.9 | 1.135 |
| [0; 0.9] | 77 | 14.3 | 48 | 17.1 | 29 | 11.3 | |
| ]0.9; 1.5] | 49 | 9.1 | 21 | 7.5 | 28 | 10.9 | |
| > 1.5 | 176 | 32.8 | 47 | 16.8 | 129 | 50.2 | |
| Not available | 63 | 11.7 | 20 | 7.1 | 43 | 16.7 | |
*Standardized mean or proportion difference (Cohen’s d values): a value ≤ 0.2 is considered acceptable, > 0.2 and ≤ 0.5 is considered as a moderate difference, > 0.5 and ≤ 0.8 is considered as significant differences, and > 0.8 as a major difference
FNG, fingolimod; JCV, JC virus; MRI, magnetic resonance imaging; NTZ, natalizumab; SD, standard deviation
At baseline, compared to the 403 NTZ-treated patients, the 337 anti-CD20-treated patients were older, displayed a more critical disability (EDDS score), had a longer disease duration, and were treated with FNG for a longer duration (Cohen’s values |d|> 0.20). However, the two groups of patients were similar in terms of clinical and radiological activity (number of relapses, reasons for FNG discontinuation, number of T2 brain lesions, gadolinium-enhancing brain lesions), as well as regarding the duration of the washout period between FNG discontinuation and the following treatment (this period lasted less than 3 months for 330 (81.9%) patients from the NTZ group and 263 (78.0%) patients from the anti-CD20 group; Table 1).
It should be noted that before 2016, NTZ was a more frequent treatment choice compared to anti-CD20, but the proportion of patients switching from FNG to anti-CD20 dramatically increased after this date with the advent of ocrelizumab (for example, during the [2018–2019] period, 78 (19.4%) patients were included in the study in the NTZ group and 152 (45.1%) in the anti-CD20 group, |d|= 0.977). Another strong determinant for the treatment choice was the serological status for the JC virus, as expected. Indeed, 229 (68.0%) patients under anti-CD20 were seropositive for the JC virus while 136 (33.8%) in patients treated with NTZ were (|d|= 0.831; Table 1).
Primary Outcome: Occurrence of a First Relapse over a 24-Month Period
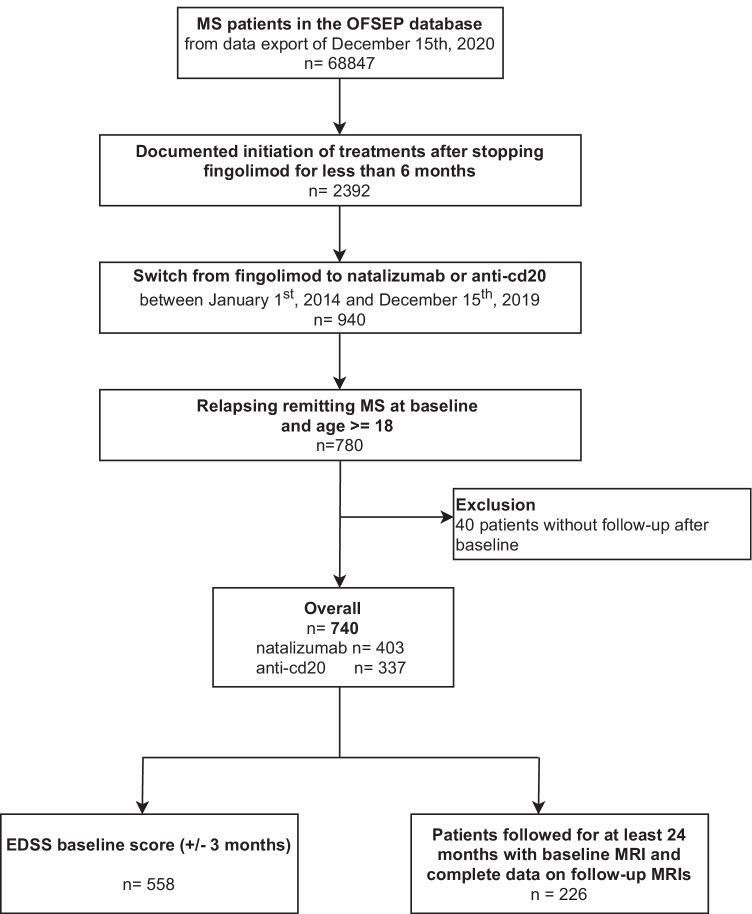
The best model selection including flexible effects on the variable treatment is shown in Appendix 1A. There was no treatment effect and the dynamic of the first relapse rate was comparable between NTZ and anti-CD20 patients. There was a strong decrease in the first relapse rate after treatment initiation and until 6 months of follow-up from 5.2 to 1.3 relapses per 100-person-month, meaning in other words that the probability of a first relapse occurring within 1 month after initiation was 5.2%, while it was 1.3% at 6 months. After 6 months, the first relapse rates were closed to around 1 relapse per 100 person-month (Fig. 2A). The cumulative effect of these rates, represented by the cumulative probability [95% CI] of a first relapse, was 30.8% [27.1; 34.9] at 24 months for the two groups (Fig. 2B). The associations between the factors at baseline and the outcome are presented in Table 2 for significant covariates of the Table 1 causing the treatment allocation. The covariates retained for the multivariate analysis were the EDSS, the duration of FNG treatment, and the year of FNG treatment initiation. The model-building strategy is provided in Appendix 1B. The final model fitted by forcing the variable treatment with a simple proportional effect as shown in Table 3. There was no treatment effect between NTZ and anti-CD20 after adjustment on covariates (hazard ratio (HR) [95% CI] of anti-CD20 versus NTZ: 1.04 [0.74; 1.46]; p = 0.820).
Fig. 2.
First relapse occurrence rates (A) and cumulative probabilities (B) with their 95% confidence interval according to the time since treatment initiation obtained from modeling in the overall population
Table 2.
Unadjusted hazard ratio of confounding factors associated with the occurrence of a first relapse
| Unadjusted HR | 95% CI | p value | |
|---|---|---|---|
| Age | 0.310 | ||
| [18; 30[ | 1 | ||
| [30; 40[ | 1.30 | [0.88; 1.92] | 0.188 |
| [40; 50[ | 1.18 | [0.77; 1.82] | 0.436 |
| [50; 60[ | 1.37 | [0.82; 2.28] | 0.225 |
| ≥ 60 | 0.34 | [0.05; 2.45] | 0.282 |
| Inclusion period | 0.002 | ||
| [2014–2015] | 1 | ||
| [2016–2017] | 0.59 | [0.42; 0.81] | 0.001 |
| [2018–2019] | 0.57 | [0.37; 0.87] | 0.009 |
| EDSS | 0.049 | ||
| [0.0; 1.5] | 1 | ||
| [2.0; 3.5] | 1.50 | [0.89; 2.53] | 0.130 |
| ≥ 4.0 | 1.91 | [1.16; 3.14] | 0.011 |
| Not available | 1.40 | [0.82; 2.38] | 0.217 |
| Duration FNG (months) | 0.98 | [0.97; 0.99] | 0.002 |
| Discontinuation of FNG treatment related to ineffectiveness | 0.299 | ||
| No | 1 | ||
| Yes | 0.84 | [0.61; 1.16] | 0.293 |
| MS duration (years) | 0.99 | [0.98; 1.02] | 0.883 |
| Washout period duration (months) | 0.848 | ||
| < 3 | 1 | ||
| [3; 6] | 0.96 | [0.66; 1.40] | 0.849 |
CI, confidence interval; EDSS, expanded disability status scale; FNG, fingolimod; HR, hazard ratio; MS, multiple sclerosis
Table 3.
Adjusted hazard ratio for covariates with proportional and linear effects associated with the occurrence of first relapse
| Adjusted HR | 95% CI | p value | |
|---|---|---|---|
| Group | |||
| NTZ | 1 | 0.820 | |
| Anti-CD20 | 1.04 | [0.74; 1.46] | |
| Year of treatment initiation | 0.89 | [0.78; 1.01] | 0.081 |
| EDSS | |||
| [0.0; 1.5] | 1 | ||
| [2.0; 3.5] | 1.35 | [0.79; 2.30] | 0.271 |
| ≥ 4.0 | 1.71 | [1.02; 2.87] | 0.041 |
| Not available | 1.29 | [0.75; 2.21] | 0.360 |
Abbreviations: CI, confidence interval; EDSS, expanded disability status scale; HR, hazard ratio; NTZ, natalizumab
Sensitivity Analyses
In a causal approach, we estimated the marginal treatment effect using the propensity scores with the IPTW method. As in the primary analysis, the studied factors were those causing both the outcomes and the treatment allocation, i.e., the inclusion period, the EDSS, and the FNG treatment duration. The confounder-adjusted [95% CI] percentage of patients experiencing at least one relapse within the 24-month post-FNG initiation was 29.7% [25.3; 37.4] for the NTZ group and 30.0% [21.8; 38.8] for the anti-CD20 group. The corresponding HR [95% CI] for patients treated with anti-CD20 versus NTZ was 0.99 [0.59; 1.30]; p = 0.937 (Fig. S1).
To assess the robustness of the primary analysis, we only considered patients who discontinued FNG for ineffectiveness, i.e., patients who experienced a relapse or displayed an MRI activity in the year before FNG discontinuation, or patients who discontinued their treatment for ineffectiveness reported by their neurologist. A total of 634 patients (85.7% of the whole population) were included in this sensitivity analysis. The same methodology as for the primary analysis was used, and precisely the same results were found, i.e., there was no treatment effect on the occurrence of a first relapse within 24 months (data not shown).
Secondary Outcomes
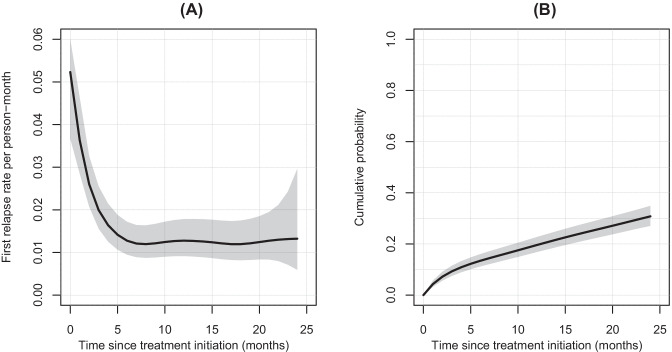
To determine the evolution of the relapse activity after FNG discontinuation, the RRs during each 3-month period within the 15 months preceding NTZ or anti-CD20 treatment initiation and within the 24-month period post-initiation were determined. We observed a sharp increase of the RR in the last 6 months of FNG use compared to the previous period, peaking at 0.092, 95% CI: [0.080; 0.106]. Up to 6 months after initiation of NTZ or anti-CD20, a dramatic decrease in the RR was observed without difference between the two treatments and then remained relatively stable. Taken together, these results show that over each 3-month period the RRs were comparable between the two treatments after adjustment on the EDSS, the year of treatment initiation, and the FNG treatment duration (Fig. 3).
Fig. 3.
Relapse rates per 3-month interval by patient group pre- and post-natalizumab or anti-CD20 initiation with their 95% confidence interval. Abbreviations: FNG, fingolimod; NTZ, natalizumab
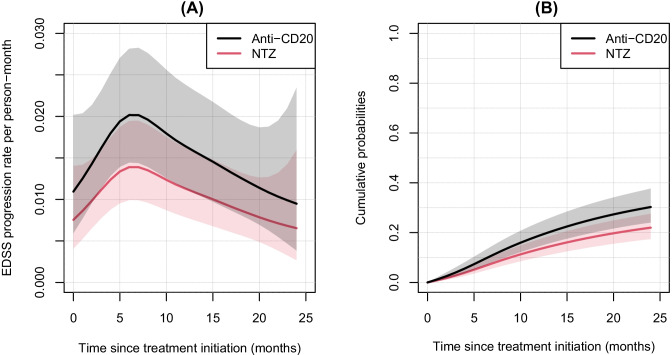
Regarding the EDSS outcome, for the 558 patients for whom an EDSS score at baseline was available, the best model was the one considering a proportional effect of the treatment variable. The dynamic of the rate of EDSS worsening is reported in Fig. 4 and the associated HR [95% CI] was 1.45 [1.00; 2.10]; p = 0.049, meaning that the rate of EDSS worsening was increased by about half in the anti-CD20 group compared to the NTZ group. We observed an increase from treatment initiation up to 6 months of follow-up from 0.7 to 1.4 per 100 person-month for NTZ and from 1.1 to 2.0 per 100 person-month for anti-CD20. Then, we observed a gradual decrease of up to 24 months for the two groups. Applying the model-building strategy in a multivariate way on the factors causing both the treatment allocation (Tables S1) and the outcomes (Table S2), we did not find any difference between NTZ and anti-CD20 groups after adjustment on age, year of treatment initiation, and FNG treatment duration (HR [95% CI] of anti-CD20 versus NTZ = 1.13 [0.75; 1.70]; p = 0.553; Table 4).
Fig. 4.
EDSS (expanded disability status scale) progression rates (A) and cumulative probabilities (B) with their 95% confidence interval according to the time since treatment initiation obtained from modeling in the natalizumab and anti-CD20 groups. Abbreviations: NTZ, natalizumab
Table 4.
Adjusted hazard ratio adjusted for covariates with proportional and linear effects associated with the EDSS progression
| Adjusted HR | 95% CI | p value | |
|---|---|---|---|
| Group | |||
| NTZ | 1 | ||
| Anti-CD20 | 1.13 | [0.75; 1.70] | 0.553 |
| Age | 1.02 | [0.99; 1.04] | 0.069 |
| Year of inclusion | 1.17 | [1.00; 1.37] | 0.045 |
| Washout period | 1.13 | [0.99; 1.30] | 0.072 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NTZ, natalizumab
The factors associated with the worsening of MRI lesions at 24 months are provided by the univariate analysis in Table S3 for the 226 patients for whom MRI data were available. There was no association between the treatment and the MRI activity after adjusting for confounding factors in a multivariate logistic regression including age at treatment initiation, inclusion period, and FNG discontinuation for patient’s convenience (odds ratio (OR) of anti-CD20 versus NTZ = 1.79; 95% CI [0.92; 3.47]; p = 0.085; Table 5).
Table 5.
Adjusted odds ratio for covariates associated with the occurrence of new T2 lesion or the presence of Gadolinium enhancing lesion
| N = 226 | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| Group | |||
| NTZ | 1 | ||
| Anti-CD20 | 1.79 | [0.92; 3.47] | 0.085 |
| Age | 0.97 | [0.95; 1.00] | 0.072 |
| Inclusion period | |||
| [2014–2015] | 1 | ||
| [2016–2017] | 1.32 | [0.72; 2.42] | 0.367 |
| Discontinuation of FNG treatment related to patient’s convenience | |||
| No | 1 | ||
| Yes | 4.27 | [1.05; 17.27] | 0.042 |
CI, confidence interval; FNG, fingolimod; NTZ, natalizumab; OR, odds ratio
The Rate and Reasons for Treatment Withdrawal After Baseline Differed Between the Two Groups
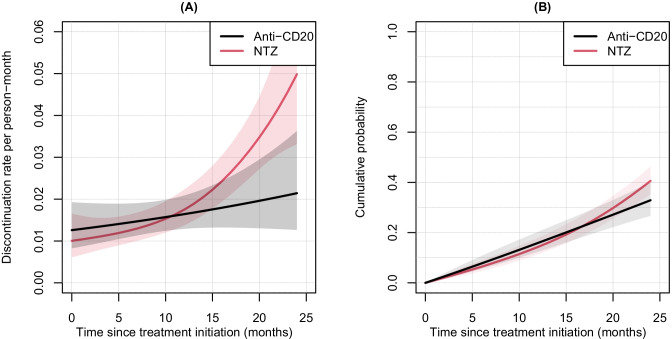
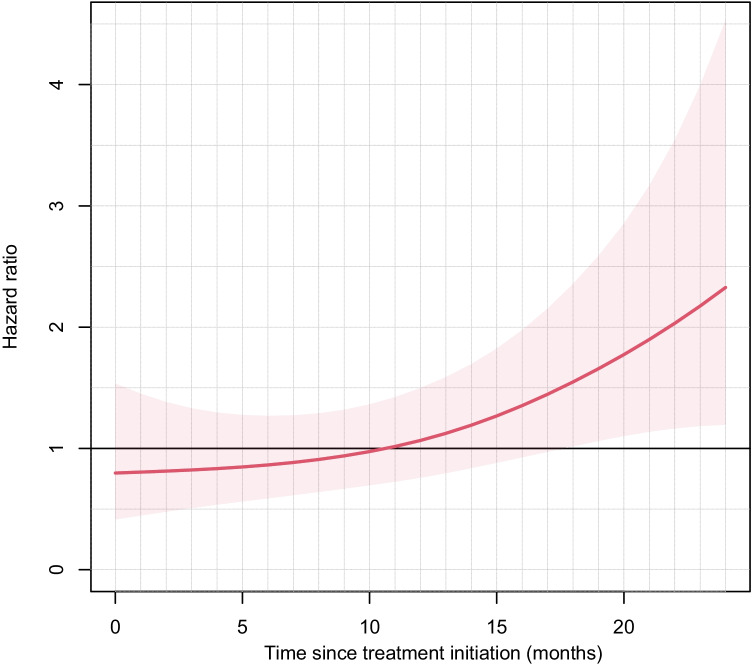
The best model on the variable treatment was the one with a main effect of treatment with interaction with time. The treatment had a non-proportional effect, i.e. it changed over the follow-up and the dynamic of the rate of treatment withdrawal was different between NTZ and anti-CD20 patients (Fig. 5A). Along the 24-month follow-up, there was a steady slight increase in the anti-CD20 group from 1.2 to 2 withdrawals per 100 person-months, while a sharp increase in the rate of NTZ discontinuation was observed from 12 months of follow-up onwards to reach 5 withdrawals per 100 person-months at 24 months. Even if the dynamics of the rates of treatment discontinuation were different between the two treatment groups towards the end of the follow-up, we nevertheless observed similar cumulative probabilities of treatments discontinuation between NTZ and anti-CD20 drugs with a slightly higher probability for NTZ at 24 months: 40.6%, 95% CI [35.3; 46.4] for NTZ vs 32.9%, 95% CI [26.7; 40.1] for anti-CD20 (Fig. 5B). Only the disease duration was considered in the multivariate analysis because associated with the outcome but also causing the treatment allocation (Table S4). The model-building strategy made it possible to consider a linear and proportional effect of the disease duration as well as a main effect on treatment with an interaction with time. The HRs of NTZ versus anti-CD20 changing over time are presented in Fig. 6. During the first 18 months of follow-up, there was no significant difference between NTZ and anti-CD20, whereas at 19 months, the risk was increased by 59.2% for the NTZ group compared to the anti-CD20 group (HR = 1.59; 95% CI [1.02; 2.49]). This risk was multiplied by more than 2 at 24 months (HR = 2.23; 95% CI: [1.15; 4.35]). A 1-year increase of disease duration decreased the risk of discontinuation by 3.0% (95% CI: [1.0; 5.0]; p = 0.025).
Fig. 5.
Treatment discontinuation rates (A) and cumulative probabilities (B) with their 95% confidence interval according to the time since treatment initiation obtained from modeling in the natalizumab and anti-CD20 groups. Abbreviations: NTZ, natalizumab
Fig. 6.
Group-adjusted hazard ratios (natalizumab vs anti-CD20) over time associated with treatment discontinuation with 95% confidence interval
To summarize, on the one hand, the longer the follow-up, the higher the risk of NTZ discontinuation compared to anti-CD20, but on the other hand, the longer the disease duration, the lower the risk of treatment discontinuation.
To understand the previous results, we considered the reason for treatment withdrawal for ineffectiveness or intolerance as an event. Based on a model-building strategy as previously described, there was no difference between the two groups (data not shown). Thus, we suspected that the difference observed between the two groups could be due to the stratification risk for PML. Indeed, among the 178 patients who discontinued their NTZ treatment, the JCV serology could be determined for 107 (60.1%), and 79 (73.8%) were positive, which is in accordance with this hypothesis.
Taken together, this result suggests that the main reason for NTZ discontinuation, and thereby the difference observed between NTZ and anti-CD20 groups for treatment discontinuation, was mainly driven by the seropositivity to the JC virus.
The IR of the SAE for each treatment group of the 403 patients is provided in Table 6. There was only one cancer case in each group (one breast cancer in the NTZ group and one squamous cancer in the anti-CD20 group) and there were 7 and 10 other SAEs reported in the NTZ and anti-CD20 groups, respectively. For cancers, this corresponded to an IR of 0.49 per 100 person-years (95% CI: [0.03; 8.03]) for the NTZ group and of 0.35 per 100 person-years (95% CI: [0.01; 9.60]) for the anti-CD20 group. It was 3.73 per 100 person-years for the other SAEs for both groups. Thus, there was no significant difference between the treatment groups in terms of the IR of SAE.
Table 6.
Incidence rate of serious adverse events by treatment group in patients who switched from fingolimod after 01/01/2017
| Overall population | NTZ | Anti-CD20 | |
|---|---|---|---|
| (N = 403) | (N = 153) | (N = 250) | |
| Event: Overall cancer | |||
| N events (incident) | 2 | 1 | 1 |
| Follow-up duration | 487 | 202 | 285 |
| IR [95% CI] per 100 person-years | 0.41 [0.02; 8.74] | 0.49 [0.03; 8.03] | 0.35 [0.01; 9.60] |
| Event: Others SAE (without cancer) | |||
| N events (incident) | 17 | 7 | 10 |
| Follow-up duration | 456 | 188 | 268 |
| IR [95% CI] per 100 person-years | 3.73 [1.35; 10.29] | 3.73 [1.35; 10.28] | 3.73 [1.35; 10.30] |
CI, confidence interval; IR, incidence rate; NTZ, natalizumab; SAE, serious adverse event
Discussion
This French observational study including 740 patients with a residual disease activity despite a highly active treatment found no difference between NTZ and anti-CD20 as a switch therapy for FNG in terms of effectiveness and tolerance. A sharp decrease in the rate of relapses was observed up to 6 months of follow-up, and from 18 months of treatment, the risk of treatment discontinuation was more significant in the NTZ group.
To our knowledge, there is no study yet comparing these two treatments in this specific subset of patients using the dynamics of event rates in such a detailed manner. As expected, at baseline, when the treatments (NTZ or anti-DC20) were initiated, the patients from the two groups differed in their clinical and demographic characteristics, as this study is based on real-life data and is not randomized. Patients treated with anti-CD20 were older and had a longer disease duration than those treated with NTZ, suggesting that neurologists oriented patients towards anti-CD20 when they were at higher risk of developing a progressive form of the disease. However, the essential factor instructing the therapeutic choice was the JCV serological status, as most patients treated with NTZ were seronegative for the JC virus. On the contrary, most were seropositive in the group treated with anti-CD20.
We used an original statistical approach that appears to be more relevant for clinicians than the simple comparison of two groups for an outcome, even after adjustment. Indeed, this is the only approach assessing the dynamics of relapse rate (i.e., the change in the rate of occurrence of the first relapse as a function of time), which corresponds to the dynamics of the risk experienced by the patients still at risk over time. Moreover, the statistical models were adjusted for the different confounders associated with the different outcomes measured in the study, allowing the comparability of the treatments and limiting their indication bias, which were also limited by the inclusion criteria for the study period (2014–2019).
By using this approach, we studied different outcomes during 24-month post-treatment initiation: two were related to the clinical activity (relapse and disability progression), one was related to the activity observed on MRI (new-T2 lesions and/or gadolinium enhancement), and the reasons for treatment withdrawal (ineffectiveness or intolerance/adverse events). Clinical and radiological outcomes were not significantly different between the two groups of patients, as the same dynamics of relapse rate for both treatments were observed, suggesting that both therapeutic strategies are similarly effective to control the disease progression. Using this approach, we were able to show a substantial decrease in the relapse rate immediately after treatment initiation and up to 6 months. The same was observed for EDSS worsening with a delayed reduction (beyond 6 months). The findings also showed also more frequent treatment withdrawals for NTZ than for anti-CD20 after 18 months of treatment, mainly driven by seropositivity or seroconversion to the JC virus.
To date, very few studies have compared the effectiveness of anti-CD20 versus NTZ, and none in this particular setting of switch therapy for FNG. Two Swedish studies based on multicentric cohorts [11, 12] have found results consistent with ours, as a similar effectiveness of rituximab compared to NTZ in relay to first-line treatments was reported. However, these studies were limited by the small number of patients included. In a more recent study from Vollmer et al. based on a single-center analysis of 451 NTZ- and 182 rituximab-treated patients, again no difference in terms of effectiveness was observed between the two treatments at 2 years [32]. Regarding treatment discontinuation, both Swedish studies have observed an increased discontinuation of NTZ compared to rituximab that was not confirmed in the US study. However, the latter was biased by insurance limitations, as attested by the authors. Our results are consistent with those from the Swedish registry, with an increased rate of NTZ discontinuation after the 18th month of treatment. The JCV status or conversion well recapitulated this. No difference was observed between the two treatments regarding the reason of discontinuation related to ineffectiveness or adverse events. A limitation of these studies is that they are only based on comparison with rituximab, while ocrelizumab is now the primary anti-CD20 treatment prescribed in the context of RRMS. This is a strength of our study that includes nearly 20% of these patients. Regarding ocrelizumab in comparison to other treatments in MS, only two network meta-analyses are available. Both have shown that ocrelizumab had a superior efficacy compared to first-line treatments and to FNG [33, 34]. It should be noted that the therapeutic choices for these patients with highly active MS appear to be more limited in France than in other countries, as cladribine and alemtuzumab are not available on the study period.
However, our study also suffers from other limitations related to its observational nature. Even though the cohort of patients included in the present study is the bigger one published to date, the number of patients in each group remains relatively small. We used adjustments to provide information on the comparability of the two groups, but we cannot exclude that some confounders were not taken into account. However, our results are in accordance with other previous studies, even though they did not include similar patients, asserting the confidence of our results and conclusions. Regarding the primary outcome, in our definition, the relapse was defined by the clinical evaluation of the treating neurologist. It is possible that there is a bias, with an over-evaluation of relapses, but this definition is the reflection of a current practice. As the definition is the same for all patients, there is no reason why this phenomenon should affect the choice of the new treatment. Another limitation comes from the fact that for disability progression, we used a one-time EDSS assessment only.
Finally, our cohort-based study found no significant difference between NTZ and anti-CD20 as a switch therapy for FNG in terms of clinical and radiological activity of the disease, as well as in terms of tolerance, but only an increased rate of NTZ discontinuation after 18 months of use probably related to JC virus seropositivity/conversion. An optimal effect of both treatments was observed 6 months after initiation. This result supports the therapeutic strategy of switching to one or the other of the molecules for patients with residual disease activity under treatment with FNG.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and neurologists for their participation in the OFSEP project by contributing and facilitating access to clinical data, as well as the research assistants in all participating centers who contributed to the data collection, and Dr. Hélène Boyer (DRS, Hospices Civils de Lyon, France) for help in manuscript preparation.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This study was supported by the Association ANTARES and OFSEP, which is supported by a grant provided by the French State and handled by the “Agence Nationale de la Recherche,” within the framework of the “Investments for the Future” program, under the reference ANR-10-COHO-002.
Data Availability
Data supporting the findings of this study are available upon a motivated request to the OFSEP coordinator. They will be evaluated by the study coordinator and the OFSEP scientific committee for approval, according to the OFSEP bylaws and access to data procedures.
Footnotes
Statistical analyses were performed by Fabien Rollot, OFSEP
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fabien Rollot, Email: Fabien.rollot@chu-lyon.fr.
David-Axel Laplaud, Email: david.laplaud@univ-nantes.fr.
References
- 1.Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387‑401. [DOI] [PubMed]
- 2.Cohen JA, Barkhof F, Comi G, Hartung H-P, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402‑15. [DOI] [PubMed]
- 3.Kappos L, O’Connor P, Radue E-W, Polman C, Hohlfeld R, Selmaj K, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84(15):1582‑91. [DOI] [PMC free article] [PubMed]
- 4.Miller DH, Soon D, Fernando KT, MacManus DG, Barker GJ, Yousry TA, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390‑401. [DOI] [PubMed]
- 5.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899‑910. [DOI] [PubMed]
- 6.Barbin L, Rousseau C, Jousset N, Casey R, Debouverie M, Vukusic S, et al. Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology. 2016;86(8):771‑8. [DOI] [PMC free article] [PubMed]
- 7.Gajofatto A, Bianchi MR, Deotto L, Benedetti MD. Are natalizumab and fingolimod analogous second-line options for the treatment of relapsing-remitting multiple sclerosis? A clinical practice observational study. Eur Neurol. 2014;72(3–4):173–180. doi: 10.1159/000361044. [DOI] [PubMed] [Google Scholar]
- 8.Kalincik T, Horakova D, Spelman T, Jokubaitis V, Trojano M, Lugaresi A, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 2015;77(3):425–435. doi: 10.1002/ana.24339. [DOI] [PubMed] [Google Scholar]
- 9.Andersen JB, Sharmin S, Lefort M, Koch-Henriksen N, Sellebjerg F, Sørensen PS, et al. The effectiveness of natalizumab vs fingolimod-a comparison of international registry studies. Mult Scler Relat Disord. 2021;53:103012. [DOI] [PubMed]
- 10.Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Björck A, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 11.Boremalm M, Juto A, Axelsson M, Novakova L, Frisell T, Svenningsson A, et al. Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol. 2019;26(8):1060–1067. doi: 10.1111/ene.13936. [DOI] [PubMed] [Google Scholar]
- 12.Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320‑7. [DOI] [PMC free article] [PubMed]
- 13.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676‑88. [DOI] [PubMed]
- 14.Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 15.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221‑34. [DOI] [PubMed]
- 16.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209‑20. [DOI] [PubMed]
- 17.Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain J Neurol. 2015;138(Pt 11):3275–3286. doi: 10.1093/brain/awv260. [DOI] [PubMed] [Google Scholar]
- 18.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369‑74. [DOI] [PubMed]
- 19.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375‑81. [DOI] [PubMed]
- 20.Vukusic S, Rollot F, Casey R, Pique J, Marignier R, Mathey G, et al. Progressive multifocal leukoencephalopathy incidence and risk stratification among natalizumab users in France. JAMA Neurol. 2020;77(1):94‑102. [DOI] [PMC free article] [PubMed]
- 21.Remontet L, Uhry Z, Bossard N, Iwaz J, Belot A, Danieli C, et al. Flexible and structured survival model for a simultaneous estimation of non-linear and non-proportional effects and complex interactions between continuous variables: performance of this multidimensional penalized spline approach in net survival trend analysis. Stat Methods Med Res août. 2019;28(8):2368–2384. doi: 10.1177/0962280218779408. [DOI] [PubMed] [Google Scholar]
- 22.Fauvernier M, Roche L, Uhry Z, Tron L, Bossard N, Remontet L. Multidimensional penalized hazard model with continuous covariates: applications for studying trends and social inequalities in cancer survival. J R Stat Soc Ser C. Epub 2019.
- 23.Fauvernier M, Remontet L, Uhry Z, Bossard N, Roche L. survPen: an R package for hazard and excess hazard modelling with multidimensional penalized splines. J Open Source Softw. 2019;4:1434. doi: 10.21105/joss.01434. [DOI] [Google Scholar]
- 24.Rollot F, Fauvernier M, Uhry Z, Vukusic S, Bossard N, Remontet L, et al. Effects of age and disease duration on excess mortality in patients with multiple sclerosis from a French nationwide cohort. Neurology. 2021;97(4):e403‑13. [DOI] [PubMed]
- 25.Vukusic S, Casey R, Rollot F, Brochet B, Pelletier J, Laplaud D-A, et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler Houndmills Basingstoke Engl janv. 2020;26(1):118–122. doi: 10.1177/1352458518815602. [DOI] [PubMed] [Google Scholar]
- 26.Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55(8):671–676. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, Mcdowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann NY Acad Sci. 1965;122:552‑68. [DOI] [PubMed]
- 29.Akaike H. A new look at the statistical model identification. In: Parzen E, Tanabe K, Kitagawa G, editors. Sel Pap Hirotugu Akaike [online]. New York, NY: Springer New York; 1998. p. 215–222. Accessed at: 10.1007/978-1-4612-1694-0_16. Accessed 20 Sept 2018.
- 30.Wood S. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC; 2006. [Google Scholar]
- 31.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Austria, Vienne; 2019. Accessed at: http://www.R-project.org/.
- 32.Vollmer BL, Nair K, Sillau S, Corboy JR, Vollmer T, Alvarez E. Rituximab versus natalizumab, fingolimod, and dimethyl fumarate in multiple sclerosis treatment. Ann Clin Transl Neurol. 2020;7(9):1466–1476. doi: 10.1002/acn3.51111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCool R, Wilson K, Arber M, Fleetwood K, Toupin S, Thom H, et al. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:55–61. doi: 10.1016/j.msard.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Hu F, Zhang Y, Li K. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 2020;267(12):3489–3498. doi: 10.1007/s00415-019-09395-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available upon a motivated request to the OFSEP coordinator. They will be evaluated by the study coordinator and the OFSEP scientific committee for approval, according to the OFSEP bylaws and access to data procedures.