Abstract
The identification of specific molecular aberrations guides the prognostic stratification and management of grade 2 astrocytomas. Mutations in isocitrate dehydrogenase (IDH) 1 and 2, found in the majority of adult diffuse low-grade glioma (DLGG), seem to relate to a favorable prognosis compared to IDH wild-type (IDH-wt) counterparts. Moreover, the IDH-wt group can develop additional molecular alterations worsening the prognosis, such as epidermal growth factor receptor amplification (EGFR-amp) and mutation of the promoter of telomerase reverse transcriptase (pTERT-mut). This review analyzes the prognostic impact and therapeutic implications of genetic alterations in adult LGG.
Keywords: diffuse low-grade gliomas, prognostic molecular stratification, IDH-wt grade 2 astrocytoma, pTERT mutation, EGFR amplification
1 Introduction
Grade 2 astrocytomas are rare tumors occurring in almost 1 out of 200,000 people per year with a peak incidence of between 30 and 35 years (1–4). Since 2016, neuro-pathologists introduced genetic parameters to differentiate them from oligodendrogliomas (5–8). Due to their slow growth, they determine cortical adaptor mechanisms leading to a functional and morphologic brain reorganization; as a consequence, tumor onset is usually characterized by seizures in the absence of other neurological deficits (9–11). Grade 2 astrocytomas are prognostically differentiated depending on the presence or absence of isocitrate dehydrogenase (IDH) 1 and 2 mutations, which, when occurring, correlate to a favorable outcome. Additionally, the IDH-wt patient’s prognosis worsens in presence of pTERT-mutations (pTERT-mut) and EGFR amplification (EGFR-amp) (12, 13). The treatment of lower-grade astrocytomas is established according to several stratification features (anaplastic gliomas, patients aged over 40 years, and subtotal removal). In presence of at least one of those risk factors, the suggested treatment is multimodal, consisting of post-surgical radiation therapy followed by chemotherapy with either temozolomide or a combination of procarbazine, lomustine, and vincristine (PCV). Due to limited available evidence, the predictive role of the molecular landscape of these tumors is still debated and not fully understood. However, both pTERT-mut and EGFR amplification (EGFR-amp) in IDH-wt tumors seem to identify a subgroup of patients not benefiting from adjuvant therapies (13–15).
Presently, there is no certainty about adequate therapeutic strategies for different molecular subtypes of grade 2 astrocytomas. Thus, in this review, we realize a comprehensive overview of the prognostic role of molecular aberrations and the most effective post-surgical strategies.
2 The Backbone of Diffuse Low-Grade Glioma Molecular Alterations: IDH Mutation
IDH1/2-mut gliomas usually harbor genetic and clinical characteristics conferring them to be a better outcome with respect to their IDH-wt counterpart. IDH mutations, usually localized at the arginine residue (R132 for IDH1, R140, or R172 for IDH2), are somatic heterozygous and missense point mutations producing the oncometabolite d-2-hydroxyglutarate (D-2HG) and promoting the transformation in immortalized human astrocytes (8). Although an early molecular event, IDH mutation is not sufficient to generate gliomas; further molecular alterations are required (16–19). Precisely, when IDH1-mutated, astrocytomas are often associated with TP53 mutation, while oligodendrogliomas present loss of 1p/19q and very rarely TP53 mutation. Therefore, three different molecular pathways can be identified, the first one arising with the mutation of IDH followed by TP53 mutation, which generates grade 2 astrocytomas. The second one involves grade 2 oligodendrogliomas, characterized by IDH mutation, followed by the loss of 1p and 19q. The last pathway includes gliomas without mutations in IDH gene, but with multiple genetic alterations, such as amplification or mutation of EGFR and loss of PTEN gene. This last subgroup of tumors becomes early aggressive glioblastoma (GBM) (20, 21).
3 Beyond IDH Mutation: Molecular-Guided Glioma Classifications
3.1 WHO Classification of Tumors of the Central Nervous System, 2016 Classification (WHO 2016CNS)
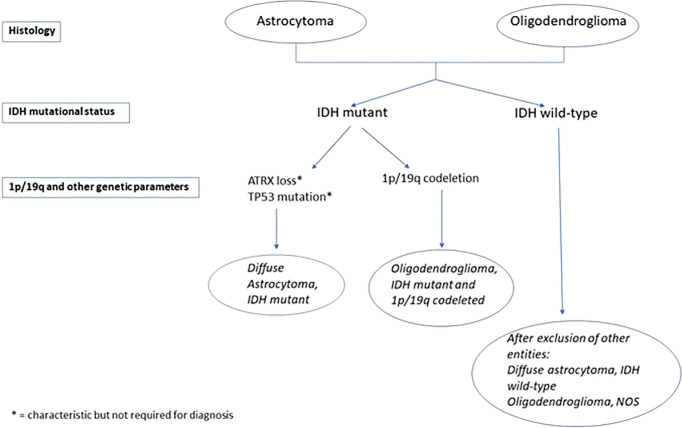
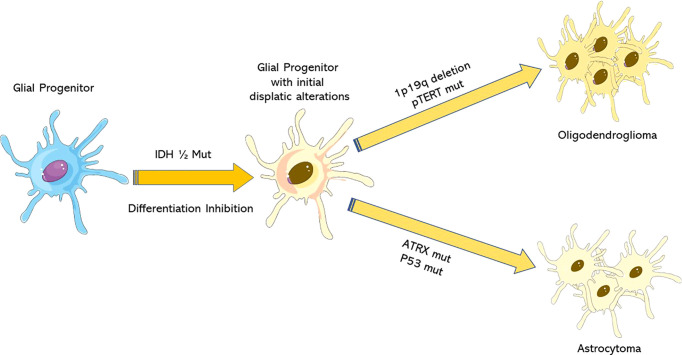
For the first time, WHO2016CNS stratified diffuse low-grade glioma (DLGG) into separate entities according to molecular parameters: IDH-mut, IDH-wt, and not otherwise specified (NOS) categories, as evidenced in Figures 1 and 2 (16–24).
Figure 1.
Molecular pathways of IDH-mut lower-grade gliomas development. IDH1/2 mutations are early events in glial progenitors after which these cells acquire additional mutations: ATRX and TP53 mutations in astrocytomas, and 1p/19q co-deletion and pTERT mutations in oligodendrogliomas.
Figure 2.
WHOCNS2016 algorithm for diffuse low-grade glioma (DLGG) diagnosis. A scheme of molecular analysis is needed to formulate a precise diagnosis of DLGG, according to WHOCNS2016.
3.2 cIMPACT-NOW Updates 3 and 5
In 2016, a Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) was created to update neuro-oncologists on the novel insights of the DLGG molecular landscape. In particular, the third and fifth updates focused on adult IDH-wt and IDH-mut diffuse astrocytic gliomas (25–28). The first one concerned a specific subset of IDH-wt diffuse or anaplastic astrocytomas prognostically similar to grade 4 IDH-wt GBM (27, 29–34). Precisely, it was established that at least one of the following alterations is necessary to compare them to a GBM since the lack of IDH mutation is not sufficient to confer a worse prognosis: EGFR-amp, pTERT-mut, the combined chromosome 7 gain, and chromosome 10 loss. These entities rarely occur in grade 2 astrocytoma, belonging mainly to grade 3 (35–40). However, pTERT-mut, when not associated with the abovementioned alterations, may relate to IDH-wt gliomas with favorable prognosis (pleomorphic xanthoastrocytoma, ganglioglioma, and nearly all oligodendrogliomas) (34, 38, 41). The fifth update focused on IDH-mut DLGG prognostic molecular features and identified the following alterations conferring the worse impact: CDKN2A/B homozygous deletion, CDK4 amplification, RB1 mutation or homozygous deletion, PIK3CA or PIK3R1 mutations, PDGFRA amplification, MYCN amplification, global DNA methylation levels, genomic instability, and chromosome 14 loss, as listed in Table 1.
Table 1.
Genetic aberrations conferring an aggressive behavior to grade 2 astrocytomas.
| Molecular alterations with a poor prognostic role in grade 2 astrocytomas | |
|---|---|
| IDH-mut | IDH-wt |
| CDKN2A/B homozygous deletion | EGFR amplification |
| CDK4 amplification | pTERT mutation |
| Chromosome 14 loss | Chromosome 7 gain and chromosome 10 loss |
| G-CIMP-low DNA methylation pattern | |
| PIK3CA mutation | |
| MYCN amplification | |
Several studies evidenced the role of CDKN2A/B homozygous deletion as an independent poor prognostic factor in IDH-mut diffuse astrocytomas (42–44). Further analyses showed that CDK4 amplifications are common among IDH-mut astrocytomas with poor prognosis, and their combination with chromosome 14 loss predicted an even shorter overall survival (OS) (40, 45). Moreover, mutations in PIK3R1 and PIK3CA genes, as well as amplification in MYCN and genomic instability, are associated with a worse outcome (32). In conclusion, IDH-mut lower-grade astrocytomas can be split into 3 different prognostic subgroups. The first one shows the best prognosis (median OS (mOS) greater than 10 years) with no evidence of mitotic activity, histologic anaplasia, microvascular proliferation, necrosis, or CDKN2A/B homozygous deletion (46). The second one, with a shorter life expectancy (nearly 8 years), showed the presence of mitosis and anaplasia, but neither microvascular proliferation nor necrosis nor CDKN2A/B homozygous deletion (47). The third subgroup corresponds to WHO grade 4 gliomas when at least one of the following features is identified: microvascular proliferation, necrosis, or CDKN2A/B homozygous deletion. Moreover, it is currently classified as astrocytoma IDH-mut; grade 4 causes its outcome results to be more favorable compared to GBM IDH-wt one (32–48).
3.3 CNS WHO 2021 Classification, Fifth Edition (WHO CNS5)
WHO CNS Classification’s fifth edition (WHO CNS5), published in 2021, is the most practice-changing one, mainly focusing on the molecular alterations’ role (rather than on the morphological analysis) considered as biomarkers of grading and for further estimating prognosis. According to this classification, GBM diagnosis can be formulated even in presence of a histologically lower-grade glioma, if including one of the following alterations: CDKN2A/B homozygous deletion in IDH-mut astrocytomas, as well as pTERT-mut, EGFR-amp, and +7/−10 copy number changes in IDH-wt diffuse astrocytomas (49). Therefore, WHO CNS5 identifies only 3 types of gliomas: astrocytoma, IDH-mut; oligodendroglioma, IDH-mut and 1p/19q-codeleted; and GBM, IDH-wt.
In other words, the current classification aims to add value to molecular parameters compared to histological findings in defining the tumoral grade. Due to the new 2021 WHO classification way of diagnosing lower-grade gliomas (different from the 2016 WHO still widely used), an Expert Panel of the American Society of Clinical Oncology (ASCO) and Society for Neuro-Oncology (SNO) published guidelines concerning the therapeutic management of diffuse astrocytic and oligodendroglial tumors in adults in order to implement both of them, till the new classification system will be definitively adopted (50).
4 Clinical-Therapeutic Implications of Grade 2 Astrocytomas Molecular Landscapes
4.1 IDH-mut Grade 2 Astrocytomas
4.1.1 Clinical and Radiological Features
Approximately 80% of newly diagnosed astrocytomas harbor IDH mutations. Survival differences between IDH-mut and IDH-wt LGG (mOS 10 vs. 2.1 years) led to separate them since WHOCNS2016 (8). Clinical and radiological peculiarities help discern between the abovementioned entities; for instance, epilepsy is frequently evidenced in IDH-mut DLGG due to the biological activity of D2HG, which is the product of the mutant enzyme. IDH1-mut cells expose neurons to D2HG, which structurally resembles glutamate, and disrupt the balance between inhibition and excitation, leading to seizures (51). Radiologically, MR spectroscopy might discriminate between IDH-mut and IDH-wt astrocytomas by quantifying 2-HG, detected only in IDH-mut gliomas (8). Moreover, IDH-mut astrocytomas display a characteristic T2 fluid-attenuated inversion recovery (T2-FLAIR) mismatch sign, defined as the presence of complete/near-complete hyperintense signals on a T2-weighted image, and a relatively hypointense signal on FLAIR, except for a hyperintense peripheral rim, with a specificity rate ranging at 100% (52).
4.1.2 Role of Surgery
The need for tumor specimens for molecular analysis has further increased the indications for neurosurgery. Its indications and extent of resection (EOR) depend on tumor-specific factors; observation is usually reserved in the presence of comorbidities contraindicating surgical approach, and biopsy in the case of tumors located in delicate areas or the presence of disseminated, multicentric, and/or bulky gliomas (53, 54). Indeed, resection needs to be maximally radical to reach an oncological efficacy, with EOR positively influencing both progression-free survival (PFS) and OS. EOR can be quantified and identified as biopsy, subtotal resection (STR), gross total resection (GTR), and supratotal resection (SuTR) (55). GTR seems to be more feasible for IDH-mut low-grade astrocytomas due to their less infiltrative behavior, conferring longer survival and benefits in terms of seizure control (56).
4.1.3 Adjuvant Therapies
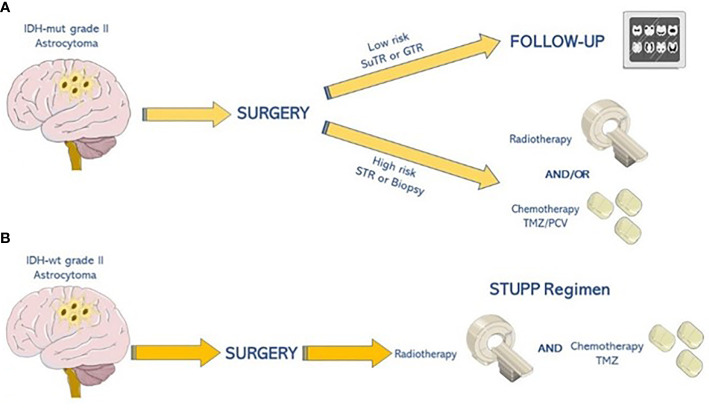
As shown in Figure 3, post-surgical strategies have been established by analyzing the long-term results of several phases III clinical trials, which started back in the 1990s by the European Organisation for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG). Patients with IDH-mut lower-grade astrocytomas are divided into two categories (low versus high risk) based on the EOR and/or unfavorable prognostic factors (i.e., age older than 40 years, presence of neurological deficits, and uncontrolled seizures). Low-risk patients undergo radiological follow-up without any adjuvant treatment. On the contrary, high-risk patients are directed to adjuvant treatments, such as radiotherapy (RT) alone (50–54 Gray in 1.8 Gray/fraction), chemotherapy alone (with temozolomide or PCV), or a combination of the two approaches (57). In particular, the EORTC 22845 trial compared postoperative RT to RT performed at the time of disease progression in adults with grade 2 gliomas. A significantly better PFS was evidenced in the first group of patients. Conversely, there was no significant difference in terms of OS (58). Moreover, the RTOG 9802 study evaluated RT alone versus RT followed by PCV in high-risk low-grade gliomas. Both PFS and OS were significantly improved in the combination arm, with a gain of 5.5 years on survival (13.3 versus 7.8 years) (59). Finally, the EORTC 22033 trial showed no significant differences in terms of PFS in upfront RT versus dose-dense temozolomide (75 mg/m2 daily on a 21/28 days scheme) in high-risk, grade 2 gliomas. Data on OS are not yet available. Further results concerning OS will help clarify the adequate adjuvant therapy for IDH-mut grade 2 astrocytomas (60).
Figure 3.
Therapeutic algorithm for grade 2 astrocytoma. (A) Patients with IDH-mut grade 2 astrocytomas are divided into two categories (low-risk versus highrisk). Low-risk patients should undergo radiological follow-up, while high-risk ones are eligible for adjuvant therapies, such as radiotherapy (50–54 Gray in 1.8 Gray/fraction) followed by chemotherapy alone with temozolomide (TMZ) or procarbazine, lomustine, and vincristine (PCV). (B) Patients with IDH-wt astrocytomas undergo concomitant and adjuvant regimens based on radio-chemotherapy with temozolomide as STUPP regimen.
4.2 IDH-wt, Morphologically Grade 2, Astrocytomas
4.2.1 Genomic Landscape and Risk Assessment
IDH-wt grade 2 astrocytomas (WHO 2016) usually harbor GBM’s molecular alterations, leading to consider them as an immature GBM still missing microvascular proliferation and necrosis (36, 61–63). However, the outcome of IDH-wt astrocytomas is strictly related to several genetic features such as K27M mutation of histone 3 family 3A (H3F3A; H3-K27M); V600E mutation of B-rapidly accelerated fibrosarcoma (BRAF); pTERT-mut; EGFR-amp; and chromosome 7 gain, and chromosome 10 loss (64–67). Three retrospective studies evaluated IDH-wt grade 3 astrocytomas, and only one focused on grade 2 astrocytomas (68, 69). The first one collected 718 LGG including 166 IDH-wt gliomas. EGFR-amp, BRAF, and H3F3A mutations were observed in a mutually exclusive pattern in 13.8%, 6.9%, and 9.5% of patients, respectively. pTERT mutations were evidenced in 26.8% of specimens. Patients younger than 45 years of age with grade 2 oligodendroglioma showed the most favorable prognosis. On the contrary, older patients with anaplastic gliomas who underwent STR, with EGFR-amp and H3F3A mutation, experienced shorter survival. Furthermore, gliomas were divided into “molecularly” low- and high-grade, based on the absence or presence of EGFR, H3F3A, or pTERT gene alterations; the first group showed a mOS improvement of about 6 months. Notably, the most favorable outcome was evidenced in molecularly low-grade gliomas with MYB amplification (68). The second study, published by Wijnenga and colleagues, confirmed the detection of pTERT-mut or chromosome 7 gain and chromosome 10 loss status as poor prognostic factors (39). The third retrospective study explored molecular alterations in 160 IDH-wt gliomas divided into 120 anaplastic and 40 grade 2 astrocytomas. The authors identified four molecularly driven subgroups: 78% considered conventional GBM due to the same molecular aberrations, the second group (9% of patients) showed a mutation in H3F3A gene, and the third one (8%) shared the methylation profile with GBM-H3-K27 mutated. Finally, the last group (5%) showed a molecular profile similar to the large cellular GBM (68). The latter study, published in 2019, evaluated 35 patients with IDH-wt lower-grade astrocytomas confirming the negative prognostic role of chromosome 7 gain and chromosome 10 loss and pTERT-mut. mOS was shorter in patients with at least one of these negative prognostic factors (18.5 vs. 54.5 months) (68). Recently, literature data highlighted that the presence of pTERT-mut alone could not be enough to call molecular GBM a grade 2 IDH-wt glioma (69).
4.2.2 Role of Surgery
EOR prognostic impact in IDH-wt grade 2 astrocytomas is still being debated because randomized controlled trials are still missing. However, a systematic review published in 2020 considered maximal resection with preservation of eloquent brain areas an essential treatment strategy in terms of survival benefits (70). Moreover, Poulen et al. performed a retrospective analysis of 31 patients receiving a high rate of maximal resection (nearly 95%) not followed by any postoperative adjuvant treatment. In their population, 5 patients underwent STR dying rapidly (3.5 years from diagnosis), while all patients undergoing GTR are still alive after a 5-year follow-up. So GTR may improve outcomes even in IDH-wt patients. On the contrary, a retrospective single-center study from Patel et al. found no association between EOR and outcome in 25 IDH-wt grade 2 astrocytomas out of 172 LGG patients (71).
4.2.3 Adjuvant Therapies
There is still a paucity of evidence about post-surgical strategies in this setting. Treatment should be decided considering patient-specified prognostic factors such as age, Karnofsky Performance Status (KPS), molecular profile, clinical and radiological course, and MGMT promoter methylation status. Usually, due to expected poor prognosis, concomitant RT and temozolomide-based chemotherapy are often carried out even if never formally evaluated within randomized controlled trials. In particular, RT sensitivity has never been established and might be lower due to wild-type IDH enzymes’ protective effect through the maintenance of NADPH levels counteracting apoptosis (72). EORTC 22033 and 26033 studies suggest the Stupp regimen as the most adequate treatment, due to the aggressiveness of the majority of IDH-wt grade 2 astrocytomas (WHO 2016) (60).
4.2.4 Outcome
There is huge variability in terms of survival among IDH-wt, morphologically grade 2, astrocytomas. A recently published meta-analysis examined data from 3,204 patients including 556 IDH-wt astrocytomas. In the entire cohort, the OS varied from a minimum of 87 to a maximum of 218 months (mean 118 months) for IDH-mut patients. On the contrary, IDH-wt astrocytomas showed shorter survival (from 9 to 120, mean 59 months). To date, the molecular profile resulted in a strong independent prognostic factor in the univariate analysis. EOR’s higher rate improved prognosis, while adjuvant therapies did not seem to impact the outcome in the IDH-wt population (39–43).
5 General Conclusions and Future Perspectives
Despite new evidence on the prognostic role of molecular features, there is no certainty about adequate therapeutic strategies, due to the lack of both perspective data, as well as technical limits of many oncological centers not performing an entire molecular analysis. According to the available studies, the IDH-mut subgroup achieves a demonstrable survival benefit by adjuvant chemoradiotherapy, as opposed to the IDH-wt counterpart (mOS 22 vs. 120 months) (13, 34, 42, 46, 49). Moreover, targeted therapy might also become a new potential treatment strategy. Indeed, encouraging results from basket trials showed clinically meaningful efficacy in gliomas harboring BRAF V600E mutation or NTRK 1/2/3 fusions, although missing enough data about grade 2 astrocytoma (73, 74). Currently, as previously mentioned, since these data derive from retrospective cohorts of patients treated according to clinicians’ choice, American Society of Clinical Oncology (ASCO)–SNO guidelines were found to be really helpful and affordable for the therapeutic management of diffuse astrocytic and oligodendroglial tumors in adults (50). In conclusion, while waiting for highly awaited new perspective trials, a multidisciplinary tumor board including an expert neuro-oncologist is strictly needed to plan a proper post-surgical therapeutic strategy for grade 2 astrocytomas, especially if IDH-wt.
Author Contributions
VI conceived the analysis and wrote the paper. GT, PDS, and LS designed the figures and tables. MT and CP revised the paper and performed the English editing.
Funding
This work was supported by the Apulia Region "Oncogenomic" Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the Pandora for Research, ONLUS Association for Oncology.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. “CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015”. Neuro-Oncology (2018) vol. 20:iv1–iv86. doi: 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mortazavi SMJ, Mortazavi SAR, Paknahad M. Cancers of the Brain and CNS: Global Patterns and Trends in Incidence. J BioMed Phys Eng (2018) 8(1):151–2. [PMC free article] [PubMed] [Google Scholar]
- 3. Rice T, Lachance DH, Molinaro AM, Eckel-Passow JE, Walsh KM, Barnholtz-Sloan J, et al. Understanding Inherited Genetic Risk of Adult Glioma - a Review. Neuro-Oncol Pract (2016) 3:10–6. doi: 10.1093/nop/npv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenkins RB, Xiao Y, Sicotte H, Decker PA, Kollmeyer TM, Hansen HM, et al. A Low-Frequency Variant at 8q24.21 Is Strongly Associated With Risk of Oligodendroglial Tumors and Astrocytomas With IDH1 or IDH2 Mutation. Nat Genet (2012) 10:1122–5. doi: 10.1038/ng.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ. Interobserver Variation of the Histopathological Diagnosis in Clinical Trials on Glioma: A Clinician’s Perspective. Acta Neuropathol (2010) 120:297–304. doi: 10.1038/ng.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, et al. Farewell to Oligoastrocytoma: In Situ Molecular Genetics Favor Classification as Either Oligodendroglioma or Astrocytoma. Acta Neuropat (2014) 128:551–9. doi: 10.1007/s00401-014-1326-7. Heim. [DOI] [PubMed] [Google Scholar]
- 7. Jenkins RB, Xiao Y, Sicotte H, Decker PA, Kollmeyer TM, Hansen HM, et al. A Low-Frequency Variant at 8q24.21 Is Strongly Associated With Risk of Oligodendroglial Tumors and Astrocytomas With IDH1 or IDH2 Mutation”. Nat Genet (2012) 44:1122–5. doi: 10.1038/ng.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiff D, Van den Bent M, Vogelbaum MA, Wick W, Miller CR, Taphoorn M. Recent Developments and Future Directions in Adult Lower-Grade Gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) Consensus. Neuro-Oncol (2019) 21:837–53. doi: 10.1093/neuonc/noz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smits A, Asgeir SJ. Clinical Presentation, Natural History, and Prognosis of Diffuse Low-Grade Gliomas. Neurosurg Clin North America (2019) 30:35–42. doi: 10.1016/j.nec.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 10. de Groot M, Reijneveld JC, Aronica E, Heimans JJ. Epilepsy in Patients With a Brain Tumour: Focal Epilepsy Requires Focused Treatment. Brain: J Neurol (2012) 135:1002–16. doi: 10.1093/brain/awr310 [DOI] [PubMed] [Google Scholar]
- 11. Murphy ES, Leyrer CM, Parsons M, Suh JH, Chao ST, Yu JS. Risk Factors for Malignant Transformation of Low-Grade Glioma. Int J Rad Oncol Biol Phys (2018) 100:965–71. doi: 10.1016/j.ijrobp.2017.12.258 [DOI] [PubMed] [Google Scholar]
- 12. Tesileanu C, Dirven L, Wijnenga M, Koekkoek J, Vincent A, Dubbink HJ. Survival of Diffuse Astrocytic Glioma, IDH1/2 Wildtype, With Molecular Features of Glioblastoma, WHO Grade IV: A Confirmation of the cIMPACT-NOW Criteria”. Neuro-Oncol (2020) 22:515–23. doi: 10.1093/neuonc/noz200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang ZY, Chan AK, Ding XJ, Qin ZY, Hong CS, Chen LC, et al. TERT Promoter Mutations Contribute to IDH Mutations in Predicting Differential Responses to Adjuvant Therapies in WHO Grade II and III Diffuse Gliomas. Oncotarg (2015) 6:24871–83. doi: 10.18632/oncotarget.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M. Interim Results From the CATNON Trial (EORTC Study 26053-22054) of Treatment With Concurrent and Adjuvant Temozolomide for 1p/19q non-Co-Deleted Anaplastic Glioma: A Phase 3, Randomised, Open-Label Intergroup Study. Lancet (London England) (2017) 390:1645–53. doi: 10.1016/S0140-6736(17)31442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gittleman H, Sloan AE, Barnholtz-Sloan JS. An Independently Validated Survival Nomogram for Lower-Grade Glioma. Neuro Oncol (2020) 22(5):665–74. doi: 10.1093/neuonc/noz191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 17. Radaelli E, Ceruti R, Patton V, Russo M, Degrassi A, Croci V, et al. Immunohistopathological and Neuroimaging Characterization of Murine Orthotopic Xenograft Models of Glioblastoma Multiforme Recapitulating the Most Salient Features of Human Disease. Histol Histopathol (2009) 24:879–91. doi: 10.14670/HH-24.879 [DOI] [PubMed] [Google Scholar]
- 18. Joo KM, Kim J, Jin J, Kim M, Seol HJ, Muradov J, et al. Patient-Specific Orthotopic Glioblastoma Xenograft Models Recapitulate the Histopathology and Biology of Human Glioblastomas In Situ . Cell Rep (2013) 3:260–73. doi: 10.1016/j.celrep.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 19. Zeng W, Tang Z, Li Y, Yin G, Liu Z, Gao J, et al. Patient-Derived Xenografts of Different Grade Gliomas Retain the Heterogeneous Histological and Genetic Features of Human Gliomas. Cancer Cell Int (2020) 20:1. doi: 10.1186/s12935-019-1086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W. IDH1 and IDH2 Mutations in Gliomas. New Eng J Med (2009) 360:765–73. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. David N. The Next Step in Brain Tumor Classification: Let Us Now Praise Famous Men”… or Molecules? Acta Neuropathol (2012) 124:761–2. doi: 10.1007/s00401-012-1067-4. Louis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, et al. International Society Of Neuropathology-Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading. Brain Pathol (2014) 24:429–35. doi: 10.1111/bpa.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohgaki H. Kleihues P Population-Based Studies on Incidence, Survival Rates, and Genetic Alterations in Astrocytic and Oligodendroglial Gliomas. J Neuropath Exp Neurol (2005) 64:479–89. doi: 10.1093/jnen/64.6.479 [DOI] [PubMed] [Google Scholar]
- 24. Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, et al. IDH Mutation Status and Role of WHO Grade and Mitotic Index in Overall Survival in Grade II-III Diffuse Gliomas. Acta Neuropathol (2015) 129:585–96. doi: 10.1007/s00401-015-1398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis DN, Aldape K, Brat DJ, Capper D, Ellison DW, Hawkins C, et al. Announcing cIMPACT-NOW: The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy. Acta Neuropathol (2017) 133:1–3. doi: 10.1007/s00401-016-1646-x [DOI] [PubMed] [Google Scholar]
- 26. Komori T. Updating the Grading Criteria for Adult Diffuse Gliomas: Beyond the WHO2016CNS Classification. Brain Tumor Pathol (2020) 37(1):1–4. doi: 10.1007/s10014-020-00358-y [DOI] [PubMed] [Google Scholar]
- 27. Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. cIMPACT-NOW Update 3: Recommended Diagnostic Criteria for “Diffuse Astrocytic Glioma, IDH-Wildtype, With Molecular Features of Glioblastoma, WHO Grade IV”. Acta Neuropathol (2018) 136:805–10. doi: 10.1007/s00401-018-1913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN. Giannini, C.et al. cIMPACT-NOW Update 5: Recommended Grading Criteria and Terminologies for IDH-Mutant Astrocytomas. Acta Neuropathol (2020) 139:603–8. doi: 10.1007/s00401-020-02127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Eng J Med (2015) 372:2481–98. doi: 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med (2015) 372:2499–508. doi: 10.1056/NEJMoa1407279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients With IDH1 Wild Type Anaplastic Astrocytomas Exhibit Worse Prognosis Than IDH1-Mutated Glioblastomas, and IDH1 Mutation Status Accounts for the Unfavorable Prognostic Effect of Higher Age: Implications for Classification of Gliomas. Acta Neuropathol (2010) 120:707–18. doi: 10.1007/s00401-010-0781 [DOI] [PubMed] [Google Scholar]
- 32. Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M, et al. Molecular Classification of Diffuse Cerebral WHO Grade II/III Gliomas Using Genome- and Transcriptome-Wide Profiling Improves Stratification of Prognostically Distinct Patient Groups. Acta Neuropathol (2015) 129:679–93. doi: 10.1007/s00401-015-1409-0 [DOI] [PubMed] [Google Scholar]
- 33. Aibaidula A, Chan AK, Shi Z, Li Y, Zhang R, Yang R, et al. Adult IDH Wild-Type Lower-Grade Gliomas Should Be Further Stratified. Neuro Oncol (2017) 19:1327–37. doi: 10.1093/neuonc/nox078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, et al. Prognostic Relevance of Genetic Alterations in Diffuse Lower-Grade Gliomas. Neuro Oncol (2018) 20:66–77. doi: 10.1093/neuonc/nox132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirose Y, Sasaki H, Abe M, Hattori N, Adachi K, Nishiyama Y, et al. Subgrouping of Gliomas Based on Genetic Profiles. Brain Tumor Pathol (2013) 30:203–8. doi: 10.1007/s10014-013-0148-y [DOI] [PubMed] [Google Scholar]
- 36. Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, et al. Adult IDH Wild-Type Astrocytomas Biologically and Clinically Resolve Into Other Tumor Entities. Acta Neuropathol (2015) 130:407–17. doi: 10.1007/s00401-015-1454-8 [DOI] [PubMed] [Google Scholar]
- 37. Stichel D, Ebrahimi A, Reuss D, Schrimpf D, Ono T, Shirahata M, et al. Distribution of EGFR Amplification, Combined 7gain and 10 Loss, and TERT Promoter Mutation in Brain Tumors and Their Potential for the Reclassification of IDHwt Astro-Cytoma to Glioblastoma. Acta Neuropathol (2018) 136:793–803. doi: 10.1007/s00401-018-1905-0 [DOI] [PubMed] [Google Scholar]
- 38. Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell (2016) 164:550–63. doi: 10.1016/j.cell.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wijnenga MMJ, Dubbink HJ, French PJ, Synhaeve NE, Dinjens WNM, Atmodimedjo PN, et al. Molecular and Clinical Heterogeneity of Adult Diffuse Low-Grade IDH Wild-Type Gliomas: Assessment of TERT Promoter Mutation and Chromosome 7 and 10 Copy Number Status Allows Superior Prognostic Stratification. Acta Neuropathol (2017) 134:957–9. doi: 10.1007/s00401-017-1781-z [DOI] [PubMed] [Google Scholar]
- 40. Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, Jones DT, et al. Distribution of TERT Promoter Mutations in Pediatric and Adult Tumors of the Nervous System. Acta Neuropathol (2013) 126:907–915. doi: 10.1007/s0041-013-1195-5 [DOI] [PubMed] [Google Scholar]
- 41. Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT Promoter Mutations in Human Cancers. Nat Commun (2013) 4:2185. doi: 10.1038/ncomms3185 [DOI] [PubMed] [Google Scholar]
- 42. Appay R, Dehais C, Maurage C-A, Alentorn A, Carpentier C, Colin C, et al. CDKN2A Homozygous Deletion Is a Strong Adverse Prognosis Factor in Diffuse Malignant IDH-Mutant Gliomas. Neuro Oncol (2019) 21:1519–28. doi: 10.1093/neuonc/noz124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korshunov A, Casalini B, Chavez L, Hielscher T, Sill M, Ryzhova M, et al. Integrated Molecular Characterization of IDHmutant Glioblastomas. Neuropathol Appl Neurobiol (2019) 45:108–18. doi: 10.1111/nan.1252 [DOI] [PubMed] [Google Scholar]
- 44. Cimino PJ. Holland EC Targeted Copy Number Analysis Outperforms Histological Grading in Predicting Patient Survival for WHO Grade II/III IDH-Mutant Astrocytomas. Neuro Oncol (2019) 21:819–21. doi: 10.1093/neuonc/noz052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, et al. Novel, Improved Grading System(s) for IDH-Mutant Astrocytic Gliomas. Acta Neuropathol (2018) 136:153–66. doi: 10.1007/s00401-018-1849-4 [DOI] [PubMed] [Google Scholar]
- 46. Weller M, van denBent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat Rev Clin Oncol (2021) 18(3):170–86. doi: 10.1038/s41571-020-00447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branden D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol (2021), 23(8):1231–51. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wen PY, Packer RJ. The 2021 WHO Classification of Tumors of the Central Nervous System: Clinical Implications. Neuro Oncol (2021) 23(8):1215–7. doi: 10.1093/neuonc/noab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fujimoto K, Arita H, Satomi K, Yamasaki K, Matsushita Y, Nakamura T, et al. TERT Promoter Mutation Status Is Necessary and Sufficient to Diagnose IDH-Wildtype Diffuse Astrocytic Glioma With Molecular Features of Glioblastoma. Acta Neuropathol (2021) 142(2):323–38. doi: 10.1007/s00401-021-02337-9 [DOI] [PubMed] [Google Scholar]
- 50. Chen H, Judkins J, Thomas C, Wu M, Khoury L, Benjamin CG, et al. Mutant IDH1 and Seizures in Patients With Glioma. Neurology (2017) 88:1805–13. doi: 10.1212/WNL.00000000003911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lasarge CL, Danzer SC. Mechanisms Regulating Neuronal Excitability and Seizure Development Following mTOR Pathway Hyperactivation. Front Mol Neurosci (2014) 7:18. doi: 10.3389/fnmol.2014.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deguchi S, Oishi T, Mitsuya K, Kakuda Y, Masahiro E, Takashi S, et al. Clinicopathological Analysis of T2-FLAIR Mismatch Sign in Lower-Grade Gliomas. Sci Rep (2020) 10(1):10113. doi: 10.1038/s41598-020-67244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Picca A, Berzero G, Sanson M. Current Therapeutic Approaches to Diffuse Grade II and III Gliomas. Ther Adv Neurol Disord (2018) 11:1756285617752039. doi: 10.1177/1756285617752039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Den Bent MJ, Bromberg JE, Buckner J. Low-Grade and Anaplastic Oligodendroglioma. Handb Clin Neurol (2016) 134:361–80. doi: 10.1016/B978-0-12-802997-8.00022-0 [DOI] [PubMed] [Google Scholar]
- 55. Pouratian N, Schiff D. Management of Low Grade Glioma. Curr Neurol Neurosci Rep (2010) 10:224–31. doi: 10.1007/s11910-010-0105-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berger MS, Hervey-Jumper S, Wick W. Astrocytic Gliomas WHO Grades II and III. Handb Clin Neurol (2016) 134:345–60. doi: 10.1016/B978-0-12-802997-8.00021-9 [DOI] [PubMed] [Google Scholar]
- 57. Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, et al. Prognostic Factors for Survival in Adult Patients With Cerebral Low-Grade Glioma. J Clin Oncol (2002) 20:2076–208458. doi: 10.1200/JCO.2002.08.121 [DOI] [PubMed] [Google Scholar]
- 58. van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council (2005). Long-Term Efficacy of Early Versus Delayed Radiotherapy for Low-Grade Astrocytoma and Oligodendroglioma in Adults: The EORTC 22845 Randomized Trial. Lancet (2005) 366:985–90. doi: 10.1016/S0140-6736(05)67070-5 [DOI] [PubMed] [Google Scholar]
- 59. Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation Plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med (2016) 374(14):1344–55. doi: 10.1056/NEJMoa1500925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K. Temozolomide Chemotherapy Versus Radiotherapy in High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol (2016) 17:1521–32. doi: 10.1016/S1470-2045(16)30313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Di Carlo DT, Duffau H, Cagnazzo F, Benedetto N, Morganti R, Perrini P. IDH Wild-Type WHO Grade II Diffuse Low-Grade Gliomas. A Heterogeneous Family With Different Outcomes. Systematic Review and Meta-Analysis. Neurosurg Rev (2020) 43:383–95. doi: 10.1007/s10143-018-0996-3 [DOI] [PubMed] [Google Scholar]
- 62. Deguchi S, Oishi T, Mitsuya K, Kakuda Y, Masahiro E, Takashi S, et al. Absence of IDH Mutation Identifies a Novel Radiologic and Molecular Subtype of WHO Grade II Gliomas With Dismal Prognosis. Acta Neuropathol (2010) 120:719–29. doi: 10.1007/s00401-010-0777-8 [DOI] [PubMed] [Google Scholar]
- 63. Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelshe C, et al. And IDH1-R132H Immunohistochemistry With Subsequent Copy Number Analysis and IDH Sequencing as a Basis for an “Integrated” Diagnostic Approach for Adult Astrocytoma, Oligodendroglioma and Glioblastoma. Acta Neuropathol (2015) 129:133–46. doi: 10.1007/s00401-014-1370-3 [DOI] [PubMed] [Google Scholar]
- 64. Chan AK, Yao Y, Zhang Z, Chung NY, Liu JS, Li KK, et al. TERT Promoter Mutations Contribute to Subset Prognostication of Lower-Grade Gliomas. Mod Pathol (2015) 28:177–86. doi: 10.1038/modpathol.2014.94 [DOI] [PubMed] [Google Scholar]
- 65. Rudà R, Bruno F, Ius T, Silvani A, Minniti G, Pace A, et al. IDH Wild-Type Grade 2 Diffuse Astrocytomas: Prognostic Factors and Impact of Treatments Within Molecular Subgroups. Neuro Oncol (2021) 24:809–20. doi: 10.1093/neuonc/noab239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan AK, Yao Y, Zhang Z, Shi Z, Chen L, Chung NY, et al. Combination Genetic Signature Stratifies Lower-Grade Gliomas Better Than Histological Grade. Oncotarget (2015) 6:20885–901. doi: 10.18632/oncotarget.4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dahiya S, Emnett RJ, Haydon DH, Leonard JR, Phillips JJ, Perry A, et al. BRAF-V600E Mutation in Pediatric and Adult Glioblastoma. Neuro Oncol (2014) 16:318–9. doi: 10.1093/neuonc/not146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuwahara K, Ohba S, Nakae S, Hattori N, Pareira ES, Yamada S, et al. Clinical, Histopathological, and Molecular Analyses of IDH-Wild-Type WHO Grade II-III Gliomas to Establish Genetic Predictors of Poor Prognosis. Brain Tumor Pathol (2019) 36:135–43. doi: 10.1007/s10014-019-00348-9 [DOI] [PubMed] [Google Scholar]
- 69. Giannini C, Giangaspero F. TERT Promoter Mutation: Is It Enough to Call a WHO Grade II Astrocytoma IDH Wild-Type Glioblastoma? Neuro Oncol (2021) 23(6):865–6. doi: 10.1093/neuonc/noab052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Patel SH, Bansal AG, Young EB, Batchala PP, Patrie JT, Lopes MB, et al. Extent of Surgical Resection in Lower-Grade Gliomas: Differential Impact Based on Molecular Subtype. AJNR Am J Neuroradiol (2019) 40:1149–55. doi: 10.3174/ajnr.A6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Poulen G, Gozé C, Rigau V, Duffau H. Huge Heterogeneity in Survival in a Subset of Adult Patients With Resected, Wild-Type Isocitrate Dehydrogenase Status, WHO Grade II Astrocytomas. J Neurosurg (2018) 1:1–10. doi: 10.3171/2017.10.JNS171825 [DOI] [PubMed] [Google Scholar]
- 72. Pellerino A, Bruno F, Internò V, Rudà R, Soffietti R. Current Clinical Management of Elderly Patients With Glioma. Expert Rev Anticancer Ther (2020) 20:1037–48. doi: 10.1080/14737140.2020.1828867 [DOI] [PubMed] [Google Scholar]
- 73. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos F, et al. Dabrafenib Plus Trametinib in Patients With BRAFV600E-Mutant Low-Grade and High-Grade Glioma (ROAR): A Multicentre, Open-Label, Single-Arm, Phase 2, Basket Trial. Lancet Oncol (2022) 23:53–64. doi: 10.1016/S1470-2045(21)00578-7 [DOI] [PubMed] [Google Scholar]
- 74. Doz F, van Tilburg CM, Geoerger B, Højgaard M, Øra I, Boni V, et al. Efficacy and Safety of Larotrectinib in TRK Fusion-Positive Primary Central Nervous System Tumors. Neuro Oncol (2021), noab274. doi: 10.1093/neuonc/noab274 [DOI] [PMC free article] [PubMed] [Google Scholar]