Abstract
Introduction
Exercise-based cardiac rehabilitation (CR) is a beneficial tool for the secondary prevention of cardiovascular diseases with, however, low participation rates. Telerehabilitation, intergrading mobile technologies and wireless sensors may advance the cardiac patients’ adherence. This study will investigate the efficacy, efficiency, safety and cost-effectiveness of a telerehabilitation programme based on objective exercise telemonitoring and evaluation of cardiorespiratory fitness.
Methods and analysis
A supervised, parallel-group, single-blind randomised controlled trial will be conducted. A total of 124 patients with coronary disease will be randomised in a 1:1 ratio into two groups: intervention telerehabilitation group (TELE-CR) (n=62) and control centre-based cardiac rehabilitation group (CB-CR) (n=62). Participants will receive a 12-week exercise-based rehabilitation programme, remotely monitored for the TELE-CR group and standard supervised for the CB-CR group. All participants will perform aerobic training at 70% of their maximal heart rate, as obtained from cardiopulmonary exercise testing (CPET) for 20 min plus 20 min for strengthening and balance training, three times per week. The primary outcomes will be the assessment of cardiorespiratory fitness, expressed as peak oxygen uptake assessed by the CPET test and the 6 min walk test. Secondary outcomes will be the physical activity, the safety of the exercise intervention (number of adverse events that may occur during the exercise), the quality of life, the training adherence, the anxiety and depression levels, the nicotine dependence and cost-effectiveness. Assessments will be held at baseline, end of intervention (12 weeks) and follow-up (36 weeks).
Ethics and dissemination
The study protocol has been reviewed and approved by the Ethics Committee of the University of Thessaly (1108/1-12-2021) and by the Ethics Committee of the General University Hospital of Larissa (3780/31-01-2022). The results of this study will be disseminated through manuscript publications and conference presentations.
Trial registration number
Keywords: telemedicine, rehabilitation medicine, cardiology
Strengths and limitations of this study.
Telerehabilitation as an alternative tool to contemporary centre/community-based cardiac rehabilitation.
Intergrading real-time supervision and group-based exercise sessions in cardiac telerehabilitation.
Objective monitoring and evaluation of physical activity and exercise intensity in cardiac rehabilitation interventions.
Inability, by study design, to blind participants to treatment allocation.
Possible selection bias, since only low and moderate cardiac risk patients will be recruited.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of morbidity and premature mortality globally.1–5 Coronary artery diseases (CAD) account for the largest proportion of CVD mortality.6 A systematic review by the Global Burden of Disease (GBD) reveals an increase of 11.8% in the mortality rates due to ischaemic CVDs in Greece6 with cardiac risk factors7 being very common among Greek patients and without signs of future decline.8 The increased rates of CVDs put additional pressure on the healthcare systems, especially under the ongoing austerity climate across Europe.
Cardiac rehabilitation (CR) can play a key role as a multidisciplinary secondary intervention aiming to the reduction of CVDs’ risk factors, the adoption of healthy lifestyle behaviours and the minimisation of disability among patients with CVD.9 Recent guidelines on CVD prevention recommend a multifaceted approach addressing exercise training, dietary counselling, smoking cessation, risk factor modification and psychosocial support.10–12 A number of meta-analyses confirm the effectiveness of exercise training in reducing cardiovascular mortality, morbidity, rehospitalisation rates,13 14 physical inactivity and all CVD risk factors including blood pressure, blood lipid profile, glucose metabolism and weight status.15 16 Despite global recommendations, patients’ participation in CR programmes is low mainly due to insufficient medical referral, travel distance, low self-efficacy, perceived body image and lack of time.17–19 Moreover, during the COVID-19 pandemic, new barriers have arisen, such as the suspension of centre-based CR and in-person sessions, travelling and circulation restrictions.20 21 Thus, the need to avoid the downgrading of CR is imperative.22
Rapid development in information and communication technologies may help overcome the barriers to CR.23–25 Telerehabilitation is proposed as a feasible,26 27 safe and cost-effective intervention,28 29 leading to long-term improvement of CVD risk factors, reduced healthcare costs and increased CR participation adherence.30 Recent systematic reviews advocate to the use of telehealth interventions as an adjunct to CR31–33 for the continuance of CR through pandemic circumstances.20 21
A recent review proclaims the integration of remote technologies and wearable sensors in the telerehabilitation, mentioning though the need for further investigation.34 Another systematic review indicates that software-enabled systems reduce timing-related barriers to patients’ participation.32 The feasibility and safety of cardiac telerehabilitation need further investigation since most relevant studies are not addressing safety matters and a formal cost-effectiveness analysis.32 34
Our study focuses on the objective recording and monitoring of the exercise implementation and physical activity (PA) through the use of wearable sensors (heart rate (HR) monitors, accelerometers). Based on thorough literature review, it is the first study to integrate real-time supervision (use of videoconference platforms) and a group-based design for home exercising (up to five participants).
The primary aims of this study are to compare the effects between telerehabilitation and regular outpatient CR methods, related to cardiorespiratory fitness (CRF) and functional capacity, while possible effects in PA, training adherence, health-related quality of life (HRQoL), anxiety and depression levels, safety, nicotine dependence and cost-effectiveness are considered as secondary aims.
The hypothesis of the study will be that the telerehabilitation intervention will have at least the same efficiency with the regular, centre-based rehabilitation and that it will be as safe as and even more cost-effective than the centre-based rehabilitation intervention.
Methods
Study design
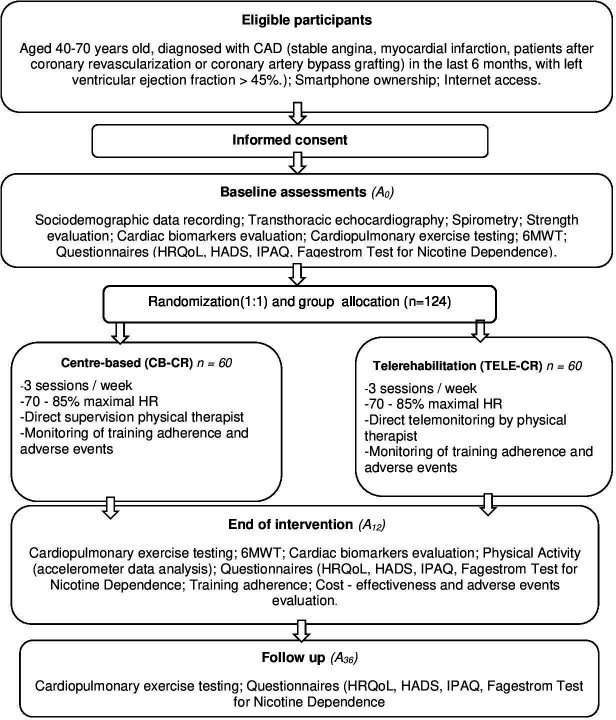
A supervised, parallel-group, single-blind randomised controlled trial with 6 months of follow-up will be employed. The study includes patients with CAD, enrolled in a telerehabilitation group (TELE-CR), and a control centre-based group undertaking regular outpatient CR (CB-CR) for comparison reasons. Three assessments will take place at baseline (A0), end of intervention (A12) and follow-up at 36 weeks (A36). A Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in figure 1.
Figure 1.
Flow chart of the study design. CAD, coronary artery disease; CR, cardiac rehabilitation; HADS, Hospital Anxiety and Depression Scale; HR, heart rate; HRQoL, health-related quality of life; IPAQ, International Physical Activity Questionnaire; 6MWT, 6 min walk test.
The study protocol complies with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement guidelines35 and the intervention procedures are described according to the CONSORT-EHEALTH checklist.36 We used the SPIRIT checklist when writing our report.35 The trial is registered at ClinicalTrials.gov with registration number NCT05019157.
Table 1 presents a summary of the study schedule and assessments.
Table 1.
Summary of study schedule
| Enrolment | Baseline (A0) | End of intervention (A12) | Follow-up (A36) | |
| Eligibility screening | X | |||
| Informed consent | X | |||
| Randomisation | X | |||
| Allocation | X | |||
| Interventions | ||||
| Centre-based CR |

|
|||
| Telerehabilitation |

|
|||
| Assessments | ||||
| Demographic characteristics | X | |||
| Spirometry, TTE | X | |||
| Strength evaluation test | X | |||
| Cardiac biomarkers (BNP, NT-proBNP, troponins, creatine kinase) | X | X | ||
| CPET–6MWT | X | X | X | |
| HRQoL | X | X | X | |
| HADS | X | X | X | |
| IPAQ | X | X | X | |
| FTND | X | X | X | |
| Cost analysis | X | |||
| Training adherence | X | |||
BNP, B-type natriuretic peptide; CPET, cardiopulmonary exercise testing; CR, cardiac rehabilitation; FTND, Fagerstrom Test for Nicotine Dependence; HADS, Hospital Anxiety and Depression Scale; HRQoL, health-related quality of life; IPAQ, International Physical Activity Questionnaire; 6MWT, 6 min walk test; NT-proBNP, N-terminal pro-brain natriuretic peptide; TTE, transthoracic echocardiography.
Patient population and eligibility
Patients will be recruited prior to their hospital discharge from the Cardiology Department of the General University Hospital of Larissa in Greece and will be screened for eligibility by the medical staff (cardiologists and medical physicians) of the corresponding hospital, according to the criteria shown in box 1. Through risk stratification and pre-exercise assessment, only low and moderate cardiac risk patients will be included in the study groups (table 2). Risk stratification and pre-exercise procedures will be implemented by cardiologists and an exercise physiologist, trained in the cardiopulmonary exercise testing (CPET), from the corresponding hospital. Those who consent to participation will be screened for eligibility and will be provided with a trial information sheet (explanation of the study design and scopes, participants’ responsibilities, confidentiality of the collected data) and a consent form to be signed. Basic sociodemographic data including sex, age, weight, height, cardiac diagnosis, pharmacological treatment, educational level, place of residence and profession will also be collected.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Adults >18 years old.
Diagnosed with coronary artery disease (CAD) (stable angina, myocardial infarction, patients after coronary revascularisation or coronary artery bypass grafting) in the last 6 months, with left ventricular ejection fraction >45%.
Current outpatients, stable for at least 4 weeks prior to the intervention enrolment.
Able to perform physical exercise.
Able to speak, read and write Greek.
Possession of a mobile phone/smartphone.
Internet access at home.
Exclusion criteria
Severe ventricular arrhythmia, with functional or prognostic significance or exercise-induced myocardial ischaemia as assessed by cardiopulmonary exercise testing (CPET) at baseline.
Heart failure.
Comorbidity precluding exercise training (eg, orthopaedic, neurological or cognitive conditions).
Unstable angina.
Uncontrolled atrial or ventricular arrhythmia.
Acute pulmonary embolism.
Acute myocarditis or pericardial effusion.
Uncontrolled diabetes mellitus (type I, II).
Severe obstructive respiratory disease (forced expiratory volume in 1 s (FEV1) <50%).
Table 2.
Cardiac risk stratification
| Low risk | Moderate risk | High risk |
|
|
|
BP, blood pressure; METs, metabolic equivalents.
Randomisation and blinding
A total of 124 eligible patients will be randomised via a computerised randomisation system in a 1:1 ratio (adjusted for age and gender) into two groups: CB-CR and TELE-CR. The allocation will be hidden until the completion of the baseline assessment in sequentially numbered, sealed, opaque envelopes. Due to the nature of the intervention, both the participants and the hospital’s staff supervising the exercise programmes are unable to be blinded to the treatment allocation. The researchers, responsible for all study assessments, will be blinded to the intervention allocation. Primary investigators will be unaware of the randomisation allocation until the completion of the intervention and the collection of all study data.
Patient and public involvement
There was no patient or public involvement in the design of this study (setting of the research question or the outcome measures). The patients will not be asked to take part either in the interpretation or the writing procedures of the results of this study.
Interventions
Individually determined CR programmes will be implemented in both study groups based on the participants’ referral diagnosis, physical fitness level and expected training goals. All participants will undertake a 12-week, exercise-based CR programme, including three training sessions of 60 min/week. Exercise will be prescribed individually, according to the results of the baseline CPET and to the frequency, intensity, time (duration) and type of exercise model.37 Participants will exercise with an intensity of 70% of their maximal heart rate, as assessed during baseline CPET.10 Each exercise circuit will consist of 20 structured stations for aerobic, strength and balance training of 2 min duration/station (tables 3 and 4). Aerobic progression will go through the duration, followed by an increase of 5%–10%/week in the exercise intensity.38 39 Progression in the resistance training involves primarily the achievement of the desired volume (number of training sets), followed by the gradual increase in intensity (amount of load lifted) and adaptations in density (rest periods).38 40 Resistance training will follow a low-pace rest/recovery to work/contraction ratio of 2:1.37 Participants in the CB-CR group will use free weights or machines for their resistance training, while participants in the TELE-CR group will exercise using their body weight or resistance bands. At the completion of the intervention, the effectiveness of the training programme will be assessed and patients will be encouraged to maintain a physically active lifestyle. However, no specific exercise prescription or face-to-face feedback will be provided until the follow-up assessment.
Table 3.
CB-CR exercise programme
| Warm-up | |
| Cycling or mild treadmill walking | 5 min |
Stretching activities
|
5 min |
| Main training part | |
| Aerobic training | |
|
Exercise with an intensity of 70% of the patients’ maximal heart rate (HRmax) at a level of 12/20–14/20 of Borg scale |
| Strengthening training | |
|
12 repetitions 1 set/exercise, starting at 30% and 70% of one-repetition maximum (1RM) for the upper body and lower body, respectively Increase gradually to 70% and 80% of 1RM for the upper body and lower body, respectively |
| Balance training | |
|
Starting with the patient’s own body weight. Later add unstable surfaces. |
| Duration 40 min 1RM: the maximum weight a patient can lift in one complete repetition for a given exercise in a controlled way through a full range of motion with good posture | |
| Cool-down | |
|
10 min |
CB-CR, centre-based cardiac rehabilitation group.
Table 4.
TELE-CR exercise programme
| Warm-up | |
| Marching on the spot | 5 min |
Stretching activities
|
5 min |
| Main training part | |
| Aerobic training | |
|
10 repetitions 2 sets/exercise |
| Strengthening training | |
|
12 repetitions 1 set/exercise, starting at 30% and 70% of one-repetition maximum (1RM) for the upper body and lower body, respectively Increase gradually to 70% and 80% of 1RM for the upper body and lower body, respectively |
| Balance training | |
|
Starting with the patient’s own body weight Later add unstable surfaces |
| Duration 40 min 1RM: the maximum weight a patient can lift in one complete repetition for a given exercise in a controlled way through a full range of motion with good posture | |
| Cool-down | |
|
10 min |
TELE-CR, telerehabilitation group.
Blood samplings will be taken in all assessment endpoints from all study participants to assess any effects of the intervention on the cardiac biomarkers’ blood concentration (B-type natriuretic peptide, N-terminal pro-brain natriuretic peptide, troponins, creatine kinase).
All patients will be receiving educational and informational videoconference sessions regarding issues of upright exercising, PA, diet/nutritional and smoking cessation counselling (based on recent guidelines)10 and psychosocial support (via psychotherapy) on stress and anxiety management. Consultation sessions may include a family member or a friend, especially for elderly patients.41 Communication strategies such as motivational interviewing, during telephone calls or videoconferences, will be integrated, as they appear to be useful in helping to promote patients’ adherence and avoid incidents of early dropouts. Motivational interviewing will be based on the open-ended questions, affirmation, reflective listening and summarising principle that helps patients to present their perceptions and clinicians to summarise.
Any adverse effects that may occur during the intervention period will be reported for safety monitoring and future data interpretation analysis.42 Adverse effects are defined as all-cause mortality, hospitalisation for CVD or serious atrial or ventricular arrhythmia, musculoskeletal problems (muscle, tendon or joint problems) or other diseases preventing exercise participation. Constant supervision of the CB-CR exercise programme and the existence of a defibrillator will ensure the participants’ safety, while for the TELE-CR group, real-time exercise telemonitoring via videoconference platforms and exercise training within the prescribed HR zone will ensure safety. Box 2 summarises the indications for dropping out training sessions.
Box 2. Indications for dropping out training sessions.
Exercise-induced angina.
Fatigue, shortness of breath, dizziness, sweating, cyanosis, headache.
Orthostatic hypotension, drop in SBP >20 mm Hg during exercise.
SBP ≥220 mm Hg, DBP ≥110 mm Hg.
HR drop (>10 bpm) during exercise.
Ventricular tachycardia (>120 bpm).
When participant reaches the intensity limit of the exercise.
Participants who might withdraw from the study will consent for follow-up assessment of at least the primary outcomes and will be willing to continue with assessments for other outcomes, if they wish to.
Auditing of the study is planned to be performed by periodic in-person visits of the trial investigator at the hospital facilities or via telephone conducts with the leading physiotherapist of the corresponding hospital.
Any substantive protocol amendments will be reviewed by the institutional review boards/research ethics committees (IRBs/RECs) of the University of Thessaly and will be communicated to all relevant stakeholders (RECs/IRBs, trial registries).
CB-CR group
Participants will attend a supervised, individually tailored, exercise-based CR programme at the hospital’s facilities. CB-CR participants will be instructed to wear an accelerometer during the entire study period. Due to the accelerometer’s storage capacity of 30 days, recorded data will be uploaded with a USB connection and stored in the hospital server in an encrypted way on a monthly basis. Total training attendance rate will be documented by the hospital’s staff
TELE-CR group
Participants in the TELE-CR group will undertake three training sessions (or more if needed) in the hospital’s outpatient clinic for familiarisation with the use of the wearable sensors, the uploading of the training data to the web application (Polar Flow) and the exercising within their individually determined exercise intensity.
Following the training period, TELE-CR participants will be lent a Polar H10 chest strap that records HR data and a sports wristwatch (Polar M430, Kempele, Finland) and will proceed with the telerehabilitation programme at their homes. Both wearable sensors are validated and reliable tools, allowing effective assessment of exercise intensity43 and will be used only during the exercise training sessions. The sports wristwatch will display continuous HR reading from the Polar H10 chest strap, enabling patients to exercise within their prescribed HR zone and exercise data (duration, training mode, PA tracking). Participants in the TELE-CR group will be exercising in groups of up to maximum five participants in each session. Real-time supervision of this group-based exercise session by a specialised physiotherapist will be implemented via videoconference web platforms or applications. At the end of every training session, patients will upload training data to the web platform (Polar Flow) via Bluetooth or USB connection. Each patient will have his/her username and login account and can check his/her training data graphically and correlate it to his/her personal goals. CR-specialised staff from the corresponding hospital will have access to all patients’ accounts so as to monitor successful data uploading, assess the collected data and provide them with training feedback once a week via telephone video calls. Uploaded data will be further backed up to an external hard drive to be processed and evaluated by the trial investigator after the completion of the intervention.
Additionally, all patients will be lent a triaxial accelerometer (ActiGraph wGT3X) that they will wear around their waist during the 12-week intervention period. Patients should visit the hospital’s outpatient clinic on a monthly basis to upload the recorded data to a secure PC application in an encrypted manner. Training adherence will be monitored by the specialised physiotherapist supervising the telerehabilitation exercise sessions.
Outcome measures
Primary outcome
The primary outcome will be the assessment of the CRF, at baseline, the completion of the intervention (A12) and follow-up (A36) in all study groups (CB-CR, TELE-CR).
Secondary outcomes
Secondary outcomes will be the PA level, safety, HRQoL, training adherence, depression and anxiety levels, nicotine dependence and cost-effectiveness. PA, HRQoL, nicotine dependence and psychosocial well-being will be measured and assessed at baseline, end of intervention (A12) and follow-up (A36). Training adherence and cost evaluation will be assessed at the completion of the intervention (A12).
Measurements
Cardiorespiratory fitness
CRF will be assessed in all study groups by peak oxygen uptake (peak VO2), determined by CPET and a 6 min walk test at the corresponding hospital at baseline, at the completion of the intervention (A12) and follow-up (A36). CPET will be set according to recommendations of European Society of Cardiology (ESC) and the American Heart Association.44 45 The test will be performed on a cycle ergometer using an individual ramp protocol aiming at total test duration of 8–12 min. Patients will be instructed to maintain a pedalling frequency of 60–70 rounds/min. A 12-lead ECG and blood pressure will be recorded continuously during the test. Peak VO2 will be defined as the average value during the last 30 s of exercise. Patients will be encouraged to exercise until they reach respiratory exchange ratio (RER) ≥1.10. If a participant fails to achieve an RER ≥1.10, he/she will be excluded from the study. A cardiologist will be present while testing to deal with any emergencies that may arise.
Physical activity
Daily PA will be measured via a triaxial accelerometer (ActiGraph wGT3X) at baseline and at the completion of the intervention (A12). The ActiGraph will be worn continuously and has been previously identified as a reliable PA tool46 47 validated in healthy and cardiac patients.48 49
The self-reported PA will be assessed at all three assessment endpoints by the offline International Physical Activity Questionnaire adopted in the Greek language that presents acceptable reliability and high repeatability values.50
Cost-effectiveness
The cost-effectiveness analysis will be performed using the assessment of quality-adjusted life-years (QALYs) at baseline (A0) and end of intervention (A12). Patients will complete the EuroQol-5 Dimension questionnaire individually51 and their final scores will be converted into QALYs. The cardiovascular readmission costs (as derived from the invoices from the hospital’s financial department), the cardiologist follow-up visits and the diagnostic tests will constitute the healthcare costs. The CB-CR costs will be calculated based on the price list of medical expenses provided by hospital regarding professional wages (physiotherapist, cardiologist), exercise testing assessment costs and transportation costs to and from the patients’ homes to the hospital. In the TELE-CR group, the costs will include the purchase of the necessary equipment and consumables (internet connection subscription, telephone communication cost).
The cost/benefit analysis will result from the calculation of the incremental cost-effectiveness ratio (ICER):
ICER = (cost intervention group − cost control group) / (effectiveness intervention group−effectiveness control group).
Incremental cost refers to the difference/patient in the total average cost between the intervention group (TELE-CR) and the control group (CB-CR). Incremental effectiveness is defined as the difference in the mean change in QALYs between the study groups.
Anxiety and depression/smoking cessation
Anxiety/depression rates and nicotine dependence will be assessed at all three assessment points. Anxiety levels will be evaluated through the Greek version of the Hospital Anxiety and Depression Scale (HADS) that comprises seven items, each for anxiety and depression subscales.52 HADS is a validated measure to assess anxiety and depression symptoms, recommended for patients with CAD.53 54 Nicotine dependence will be assessed through the Fagerstrom Test for Nicotine Dependence.
Training adherence
Patients’ training adherence is defined as a percentage of the total number of completed training sessions (100%=36). Patients’ adherence in both study groups will be recorded by the supervising hospital outpatient clinic’s staff and will be evaluated at the end of intervention (A12). Based on the percentage of the sessions attended, participants will be categorised in adherence (>80%), partial adherence (20%–80%) and non-adherence (<20%).
Statistical analysis
Normality of data will be examined with Kolmogorov-Smirnov tests. Descriptive statistics will be used to report demographics and baseline characteristics. Between-group and within-group differences in the outcome measures will be evaluated using multivariate analysis of variance (MANOVA). The effectiveness of the control group (CB-CR) and intervention group (TELE-CR) will be examined with dependent t-test for each group (prescores and postscores). All participants will be included in an intention-to-treat analysis, regardless of adherence, for at least the assessment of the primary outcomes. Significance level will be set at p=0.05. Statistical Package for Social Sciences (SPSS) V.25 will be used for all data analyses.
Sample size calculation
The calculation of the sample size was performed with G*Power V.3.1.9.4 software. For test F, h detection of moderate effect size (f=0.3) after the interaction test (α level=0.05, 80%), a total of 111 participants were required to examine the recurrent MANOVA. After adjusting for potential dropouts (estimated attrition rate ≤10%), a minimum sample of 124 participants is required. Therefore, at least 62 participants will be recruited in each group.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the University of Thessaly (1108/01-12-2021) and by the Ethics Committee of the General University Hospital of Larissa. Written informed consent will be obtained from all study participants prior to their enrolment to the study intervention.
The findings of this study will be disseminated at local, national and international levels through publications in peer-reviewed journals, national and international conference presentations, and social, broadcast and print media. Additionally, all study participants will receive the study findings through electronic and postal mails.
Discussion
This trial is aiming to evaluate the efficacy, efficiency and safety of an exercise-based telerehabilitation programme using wearable sensors and web applications compared with a traditional supervised centre-based CR.
The objective assessment of functional capacity through CPET and the objective monitoring and recording of exercising and PA via the use of wearable sensors are the main features of this study that increase its reliability. The objective measurement of PA and training intensity using accelerometer and HR data is suggested to be more reliable than using questionnaires than self-reported PA55–57 or than the perceived rate of exertion on its own.55
Although a cost-effectiveness analysis is almost prerequisite for any novel intervention, only a few telerehabilitation studies have performed one. Frederix et al have shown the cost-effectiveness of an internet-based telerehabilitation programme28 while Kidholm et al outlined the non-cost-effectiveness of a telerehabilitation programme compared with centre-based CR.58 In our study, we intend to include a comprehensive cost-effectiveness analysis to evaluate any possible economic gains.
Moreover, the geographical features of Greece, with many islands and remote areas, contribute to the care inequality being observed, combined with the high variability of access to primary care professionals.59 CR is almost absent from the Greek public health system, partly owing to the lack of clinics and training in its delivery. Furthermore, while some studies have already investigated the implications of telerehabilitation in other diseases, such as chronic obstructive pulmonary disease, with favourable outcomes,60 no similar study, to our knowledge, has not yet been carried out for patients with CAD, leaving a great gap open. In accordance to these statements, recent guidelines support the implementation of home-based CR, telehealth and mHealth interventions, with the use of wearable activity trackers to increase cardiac patients’ participation rates and long-term adherence to healthy behaviours.11 Furthermore, although digital literacy of patients with CVD is presented as a barrier to CR participation,61 data from a recent study reveal encouraging results concerning the successive use of smartphones and wearable technology by an elderly cardiac population.62 Additionally, adherence in telerehabilitation interventions appears to present higher rates.63–65
Therefore, there is an urgent need for innovative, safe, more cost-effective CR strategies. If an exercise-based telerehabilitation programme, using wearable sensors, meets these prerequisites, it can act as a supplement and/or substitution (according to the needs) to traditional centre-based CR in patients with CAD of low to moderate cardiac risk, thus allowing more patients to have access to CR with the least possible economic burden.
Supplementary Material
Footnotes
Twitter: @AndrewXanthopo1
Contributors: VA and GP conceived the study design. AX, GG, KIG, JS, CD, EK and VS contributed to the conception of the design. VA, GP and LB drafted the manuscript and all authors reviewed several drafts of the manuscript. All authors approved the final manuscript to be published.
Funding: This work was supported by the Ministry of Health of the Czech Republic project for conceptual development in research organisations (reference number: 65269705) (University Hospital Brno, Czech Republic).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015;201 Suppl 1:S1–7. 10.1016/S0167-5273(15)31026-3 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368–76. 10.1016/j.ijcard.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 4.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abreu A, Pesah E, Supervia M, et al. Cardiac rehabilitation availability and delivery in Europe: how does it differ by region and compare with other high-income countries?: endorsed by the European association of preventive cardiology. Eur J Prev Cardiol 2019;26:1131–46. 10.1177/2047487319827453 [DOI] [PubMed] [Google Scholar]
- 6.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019;26:824–35. 10.1177/2047487318825350 [DOI] [PubMed] [Google Scholar]
- 8.Touloumi G, Karakosta A, Kalpourtzi N, et al. High prevalence of cardiovascular risk factors in adults living in Greece: the EMENO National health examination survey. BMC Public Health 2020;20:1665. 10.1186/s12889-020-09757-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepoli MF, Hoes AW, Brotons C, et al. Main messages for primary care from the 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur J Gen Pract 2018;24:51–6. 10.1080/13814788.2017.1398320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol 2021;28:460–95. 10.1177/2047487320913379 [DOI] [PubMed] [Google Scholar]
- 11.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 12.Sandercock GRH, Cardoso F, Almodhy M, et al. Cardiorespiratory fitness changes in patients receiving comprehensive outpatient cardiac rehabilitation in the UK: a multicentre study. Heart 2013;99:785–90. 10.1136/heartjnl-2012-303055 [DOI] [PubMed] [Google Scholar]
- 13.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 14.Salzwedel A, Jensen K, Rauch B, et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the cardiac rehabilitation outcome study (CROS-II). Eur J Prev Cardiol 2020;27:1756–74. 10.1177/2047487320905719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries H, Kemps HMC, van Engen-Verheul MM, et al. Cardiac rehabilitation and survival in a large representative community cohort of Dutch patients. Eur Heart J 2015;36:1519–28. 10.1093/eurheartj/ehv111 [DOI] [PubMed] [Google Scholar]
- 16.Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev 2019;1:Cd003331. 10.1002/14651858.CD003331.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neubeck L, Freedman SB, Clark AM, et al. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol 2012;19:494–503. 10.1177/1741826711409326 [DOI] [PubMed] [Google Scholar]
- 18.Dunlay SM, Witt BJ, Allison TG, et al. Barriers to participation in cardiac rehabilitation. Am Heart J 2009;158:852–9. 10.1016/j.ahj.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruano-Ravina A, Pena-Gil C, Abu-Assi E, et al. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol 2016;223:436–43. 10.1016/j.ijcard.2016.08.120 [DOI] [PubMed] [Google Scholar]
- 20.Sari DM, Wijaya LCG. Cardiac rehabilitation via telerehabilitation in COVID-19 pandemic situation. Egypt Heart J 2021;73:31. 10.1186/s43044-021-00156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnier F, Gayda M, Nigam A, et al. Cardiac rehabilitation during quarantine in COVID-19 pandemic: challenges for Center-Based programs. Arch Phys Med Rehabil 2020;101:1835–8. 10.1016/j.apmr.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosetti M, Abreu A, Cornelissen V, et al. Delphi consensus recommendations on how to provide cardiovascular rehabilitation in the COVID-19 era. Eur J Prev Cardiol 2021;28:541–57. 10.1093/eurjpc/zwaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batalik L, Dosbaba F, Hartman M, et al. Long-Term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med 2021;57:807–14. 10.23736/S1973-9087.21.06653-3 [DOI] [PubMed] [Google Scholar]
- 24.Batalik L, Pepera G, Papathanasiou J, et al. Is the training intensity in phase two cardiovascular rehabilitation different in telehealth versus outpatient rehabilitation? J Clin Med 2021;10:4069. 10.3390/jcm10184069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batalik L, Konecny V, Dosbaba F, et al. Cardiac rehabilitation based on the walking test and telerehabilitation improved cardiorespiratory fitness in people diagnosed with coronary heart disease during the covid-19 pandemic. Int J Environ Res Public Health 2021;18:1–11. 10.3390/ijerph18052241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraal JJ, Peek N, Van den Akker-Van Marle ME, et al. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home study. Eur J Prev Cardiol 2014;21:26–31. 10.1177/2047487314552606 [DOI] [PubMed] [Google Scholar]
- 27.Piotrowicz E, Piotrowicz R, Opolski G, et al. Hybrid comprehensive telerehabilitation in heart failure patients (TELEREH-HF): a randomized, multicenter, prospective, open-label, parallel group controlled trial-Study design and description of the intervention. Am Heart J 2019;217:148–58. 10.1016/j.ahj.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 28.Frederix I, Solmi F, Piepoli MF, et al. Cardiac telerehabilitation: a novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017;24:1708–17. 10.1177/2047487317732274 [DOI] [PubMed] [Google Scholar]
- 29.Maddison R, Rawstorn JC, Stewart RAH, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart 2019;105:122. 10.1136/heartjnl-2018-313189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouwers RWM, van Exel HJ, van Hal JMC, et al. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth Heart J 2020;28:443–51. 10.1007/s12471-020-01432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalheiro AH, Silva Cardoso J, Rocha A, et al. Effectiveness of Tele-rehabilitation programs in heart failure: a systematic review and meta-analysis. Health Serv Insights 2021;14:11786329211021668–68. 10.1177/11786329211021668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin K, Khonsari S, Gallagher R, et al. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review and meta-analysis. Eur J Cardiovasc Nurs 2019;18:260–71. 10.1177/1474515119826510 [DOI] [PubMed] [Google Scholar]
- 33.Subedi N, Rawstorn JC, Gao L, et al. Implementation of Telerehabilitation interventions for the self-management of cardiovascular disease: systematic review. JMIR Mhealth Uhealth 2020;8:e17957. 10.2196/17957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batalik L, Filakova K, Batalikova K, et al. Remotely monitored telerehabilitation for cardiac patients: a review of the current situation. World J Clin Cases 2020;8:1818–31. 10.12998/wjcc.v8.i10.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eysenbach G, CONSORT-EHEALTH Group . CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res 2011;13:e126. 10.2196/jmir.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrosetti M, Abreu A, Corrà U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol 2020:2047487320913379. 10.1177/2047487320913379 [DOI] [PubMed] [Google Scholar]
- 38.Hansen D, Abreu A, Ambrosetti M, et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: why and how: a position statement from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol 2022;29:230–45. 10.1093/eurjpc/zwab007 [DOI] [PubMed] [Google Scholar]
- 39.Hansen D, Mathijs W, Michiels Y, et al. Phase III multidisciplinary exercise-based rehabilitation is associated with fewer hospitalizations due to adverse cardiovascular events in coronary artery disease patients. Eur J Prev Cardiol 2022;28:e17–20. 10.1093/eurjpc/zwaa038 [DOI] [PubMed] [Google Scholar]
- 40.La Scala Teixeira CV, Evangelista AL, Pereira PEdeA, et al. Complexity: a novel load progression strategy in strength training. Front Physiol 2019;10:839. 10.3389/fphys.2019.00839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WWM, Choi KC, Yum RWY, et al. Effectiveness of motivational interviewing on lifestyle modification and health outcomes of clients at risk or diagnosed with cardiovascular diseases: a systematic review. Int J Nurs Stud 2016;53:331–41. 10.1016/j.ijnurstu.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 42.Pepera GB, Sandercock PD, Gavin RH. A pilot study to investigate the safety of exercise training and testing in cardiac rehabilitation patients. Br J Cardiol 2013;20:78. 10.5837/bjc.2013.012 [DOI] [Google Scholar]
- 43.Bethell HJN. Exercise-based cardiac rehabilitation. Medicine 2006;34:195–6. 10.1383/medc.2006.34.5.195 [DOI] [Google Scholar]
- 44.Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2018;39:1144–61. 10.1093/eurheartj/ehw180 [DOI] [PubMed] [Google Scholar]
- 45.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation 2013;128:873–934. 10.1161/CIR.0b013e31829b5b44 [DOI] [PubMed] [Google Scholar]
- 46.Santos-Lozano A, Santín-Medeiros F, Cardon G, et al. ActiGraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med 2013;34:975–82. 10.1055/s-0033-1337945 [DOI] [PubMed] [Google Scholar]
- 47.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 2005;37:S544–54. 10.1249/01.mss.0000185674.09066.8a [DOI] [PubMed] [Google Scholar]
- 48.Van Remoortel H, Raste Y, Louvaris Z, et al. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One 2012;7:e39198. 10.1371/journal.pone.0039198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Healy GN, Matthews CE, Dunstan DW, et al. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 2011;32:590–7. 10.1093/eurheartj/ehq451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papathanasiou G, Georgoudis G, Papandreou M, et al. Reliability measures of the short international physical activity questionnaire (IPAQ) in Greek young adults. Hellenic J Cardiol 2009;50:283–94. [PubMed] [Google Scholar]
- 51.Kontodimopoulos N, Pappa E, Niakas D, et al. Validity of the EuroQoL (EQ-5D) instrument in a Greek general population. Value Health 2008;11:1162–9. 10.1111/j.1524-4733.2008.00356.x [DOI] [PubMed] [Google Scholar]
- 52.Rishi P, Rishi E, Maitray A, et al. Hospital anxiety and depression scale assessment of 100 patients before and after using low vision care: a prospective study in a tertiary eye-care setting. Indian J Ophthalmol 2017;65:1203–8. 10.4103/ijo.IJO_436_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 54.Michopoulos I, Kalkavoura C, Michalopoulou P, et al. [Hospital anxiety and depression scale (HADS): Validation in a Greek general hospital sample]. Psychiatriki 2007;18:217–24. [PubMed] [Google Scholar]
- 55.Butte NF, Ekelund U, Westerterp KR. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc 2012;44:S5–12. 10.1249/MSS.0b013e3182399c0e [DOI] [PubMed] [Google Scholar]
- 56.Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity - a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17:127–39. 10.1097/HJR.0b013e32832ed875 [DOI] [PubMed] [Google Scholar]
- 57.Westerterp KR. Reliable assessment of physical activity in disease: an update on activity monitors. Curr Opin Clin Nutr Metab Care 2014;17:401–6. 10.1097/MCO.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 58.Kidholm K, Rasmussen MK, Andreasen JJ, et al. Cost-Utility analysis of a cardiac Telerehabilitation program: the Teledialog project. Telemed J E Health 2016;22:553–63. 10.1089/tmj.2015.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Network HFP . Heart failure policy and practice in Europe: Greece, 2020. [Google Scholar]
- 60.Vasilopoulou M, Papaioannou AI, Kaltsakas G, et al. Home-Based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J 2017;49:1602129. 10.1183/13993003.02129-2016 [DOI] [PubMed] [Google Scholar]
- 61.Falter M, Scherrenberg M, Dendale P. Digital health in cardiac rehabilitation and secondary prevention: a search for the ideal tool. Sensors 2021;21:12. 10.3390/s21010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snoek JA, Prescott EI, van der Velde AE, et al. Effectiveness of home-based mobile guided cardiac rehabilitation as alternative strategy for Nonparticipation in clinic-based cardiac rehabilitation among elderly patients in Europe: a randomized clinical trial. JAMA Cardiol 2021;6:463–8. 10.1001/jamacardio.2020.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batalik L, Dosbaba F, Hartman M, et al. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: a randomized controlled trial. Medicine 2020;99:e19556–e56. 10.1097/MD.0000000000019556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur J Prev Cardiol 2017;24:1260–73. 10.1177/2047487317710803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang R, Bruning J, Morris NR, et al. Home-Based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother 2017;63:101–7. 10.1016/j.jphys.2017.02.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.