Abstract
Objectives
Acute kidney injury (AKI) is a frequent complication among critical ill patients with COVID-19, but the actual incidence is unknown as AKI-incidence varies from 25% to 89% in intensive care unit (ICU) populations. We aimed to describe the prevalence and risk factors of AKI in patients with COVID-19 admitted to ICU in Norway.
Design
Nation-wide observational study with data sampled from the Norwegian Intensive Care and Pandemic Registry (NIPaR) for the period between 10 March until 31 December 2020.
Setting
ICU patients with COVID-19 in Norway. NIPaR collects data on intensive care stays covering more than 90% of Norwegian ICU and 98% of ICU stays.
Participants
Adult patients with COVID-19 admitted to Norwegian ICU were included in the study. Patients with chronic kidney disease (CKD) were excluded in order to avoid bias from CKD on the incidence of AKI.
Primary and secondary outcome measures
Primary outcome was AKI at ICU admission as defined by renal Simplified Acute Physiology Score in NIPaR. Secondary outcome measures included survival at 30 and 90 days after admission to hospital.
Results
A total number of 361 patients with COVID-19 were included in the analysis. AKI was present in 32.0% of the patients at ICU admission. The risk for AKI at ICU admission was related to acute circulatory failure at admission to hospital. Survival for the study population at 30 and 90 days was 82.5% and 77.6%, respectively. Cancer was a predictor of 30-day mortality. Age, acute circulatory failure at hospital admission and AKI at ICU admission were predictors of both 30-day and 90-day mortality.
Conclusions
A high number of patients with COVID-19 had AKI at ICU admission. The study indicates that AKI at ICU admission was related to acute circulatory failure at hospital admission. Age, acute circulatory failure at hospital admission and AKI at ICU admission were associated with mortality.
Keywords: COVID-19, acute renal failure, adult intensive & critical care
Strength and limitations of this study
The study is a national cohort of Norwegian patients with COVID-19 admitted to intensive care units (ICUs) in the study period.
The study has few missing data, and the inclusion rate is high.
The health system functioned within capacity during the study period, which renders that results are less likely to be biased due to capacity strains.
Acute kidney injury (AKI) in the ICU was defined according to renal Simplified Acute Physiology Score and does not fully comply with the Risk, Injury, Failure, Loss of kidney function and End-stage renal disease (RIFLE) or the Kidney Disease: Improving Global Outcomes (KDIGO) criteria due to the lack of creatinine-based measures of kidney function in the registry.
While the study provides complete data on AKI at ICU admission, it does not present the ICU trajectory of AKI in patients with COVID-19.
Introduction
COVID-19, an infectious disease caused by the novel coronavirus SARS-CoV-2, has quickly developed into a pandemic since the early outbreak in Wuhan, China, in December 20191.
Several studies report acute kidney injury (AKI) among hospitalised patients with COVID-19 while less data are obtained exclusively in intensive care unit (ICU) patients.2–10 To our knowledge, previous studies are not based on all ICU admissions within a large population. Furthermore, there is a call from the consensus report on COVID-19 associated AKI published by the 25th Acute Dialysis Quality Initiative (ADQI) Workgroup that studies should ‘incorporate the information about the proportion of different comorbidities in patients with and without AKI, including potential risk factors for the development of AKI’.11
National registries in Norway provide opportunities to perform nationwide registry studies in order to answer calls such as the one from the ADQI workgroup. The strength of such registry-based studies is that they are based on larger patient cohorts which make results more robust and generalisable. The drawback is that registry data set seldom are a direct fit for the research in question, making adaptations and extrapolations necessary.
This study is based on data available from the Norwegian Intensive Care and Pandemic Registry (NIPaR). NIPaR is a government funded national health registry constituted of two parts: the Norwegian Intensive Care Registry established in 1998 and the Norwegian Pandemic Registry established in March 2020.12 Registration is mandatory, and data are entered by hospital staff. For patients with COVID-19 in the ICU, we report the prevalence of AKI, factors associated with AKI and the association between AKI and mortality.
Methods
NIPaR contains two patient populations. NIPaR collects data on intensive care stays in predefined ICU, covering more than 90% of Norwegian ICU and 98% of ICU stays.13 Qualification criteria for ICU and intensive care patients are defined by NIPaR.14 The Norwegian Pandemic Registry includes patients admitted to hospital in Norway with a positive PCR test for SARS-CoV-2 during the previous 3 months, and includes 99% of pandemic patients admitted to hospital.13 Both registry parts employ automatic and manual validation to ensure data quality.
The study group included patients with COVID-19 above the age of 18 years admitted to ICU in the period between 10 March 2020 (the initial outbreak of SARS-CoV-2 in Norway), until 31 December 2020. Patients with chronic kidney disease (CKD), defined as previously diagnosed kidney disease on hospital admission, were excluded in order to avoid bias from CKD on the effects of AKI.
Data collection at hospital admission included age, gender, height, weight, comorbidities, pregnancy, regular medication of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB), smoking, S-creatinine (SCr) and organ complications at hospital admission including AKI, acute respiratory failure (ARF) and acute circulatory failure (ACF) recorded at the discretion of the attending physician.
Data collection from the ICU stay included primary reason for referral to ICU, clinical scoring systems in the ICU (Glasgow Coma Scale (GCS), Simplified Acute Physiology Score (SAPS II)), length of stay (LOS), mechanical ventilation, renal replacement therapy (RRT) (intermittent and continuous) and survival (ICU first 24 hours, in-hospital at ICU and at 30 and 90 days after admission to hospital).
Definitions
Due to lack of variables, it was not possible to employ creatinine values to define AKI in the ICU. The only available marker for renal function in the Norwegian Intensive Care Registry is contained within the SAPS II.15 As a result, renal SAPS II (rSAPSII) is the sole marker for AKI during ICU stay in this study. AKI in the ICU was defined as rSAPSII score of >4 (urine output (UO)/24 hours<1000 mL and/or blood urea nitrogen (BUN)>10 mmol/L). SAPS II is based on observations within the first 24 hours in the ICU.
While AKI at ICU admission was defined according to rSAPSII score, AKI at admission to hospital was defined according to RIFLE criteria. A serum creatinine increase of >1.5×baseline was available as a separate variable (RIFLE risk category). For missing data, AKI at hospital admission was based on serum creatinine at hospital admission and the Modification of Diet in Renal Disease (MDRD) equation for estimating baseline creatinine. An estimated glomerular filtration rate of 75 mL/min/1.73 m2 was used to calculate baseline creatinine.16 Data on ethnicity were not available for input in the equation.
ACF at admission to hospital was defined as acute deterioration in the patient circulation as compared with normal state, resulting in circulatory symptoms in high, moderate or light exertion or in rest. This includes cardiac arrythmia, symptoms of heart failure and/or cardiac ischaemia, regardless of vasopressor or inotrope treatment. Severe ACF was defined as circulatory symptoms in rest.
ARF at admission to hospital was defined as acute deterioration of respiratory function at admission to hospital as compared with normal state, resulting in respiratory symptoms in high, moderate or light exertion or in rest. This includes all conditions which can cause acute deterioration of respiratory function, including bacterial, viral, or cryptogenic pneumoniae, acute respiratory distress syndrome, pneumothorax, pleural fluid and bronchiolitis. Severe ARF was defined as respiratory symptoms in rest.
Comorbidities are defined as pre-existing diagnoses on admission to hospital. Comorbidities included chronic pulmonary disease (CPD), asthma, diabetes mellitus (DM) type 1 or 2, CKD, cardiovascular disease (CVD) including hypertension, liver disease, chronic neurological disease, cancer and immunocompromised condition (including HIV and immunosuppressive therapy).
The primary outcome was the development of AKI at admission to ICU, while secondary outcomes included survival at 30 and 90 days after admission to hospital.
Statistics
Statistical analysis was performed using IBM SPSS Statistics (V.26) and R V.4.0.4. If not stated otherwise, continuous variables are presented as median and/or mean if data are normally distributed, and categorical variables are presented as the number (n) of patients (valid % of the study population). Shapiro-Wilk test of normality was performed for continuous variables. Patient characteristics for patients with or without AKI were compared using Student’s t-test for continuous variables and Fisher exact test for categorical variables. A p<0.05 was considered statistically significant.
Univariable logistic regression analysis was performed to examine the predictors for AKI at ICU admission (as defined by rSAPSII-score >4). Independent variables included age, gender, comorbidities, smoking-status, medication with ACEi or ARB, ACF and ARF at admission to hospital. AKI at admission to hospital was not included as an independent variable in the analysis due to discrepancy in AKI definition. Variables with a p<0.1 in the univariable regression were included in the multivariable regression, where a p<0.05 was considered as statistically significant. Multicollinearity was evaluated using the variance inflation factor (VIF).
Univariable and multivariable logistic regression analysis as described was performed to assess risk factors associated with 30-day and 90-day mortality and the role of AKI at ICU admission for predicting survival. Independent variables in univariable logistic regression analysis included comorbidities, age, gender, smoking status, medication with ACEi or ARB, ACF and ARF at admission to hospital, and AKI at ICU admission. Multicollinearity was evaluated using the VIF.
Univariable and multivariable Cox regression analysis was performed in a similar fashion, as an additional approach to assess 30-day and 90-day mortality. The data were censored at 30 and 90 days.
Kaplan-Meier survival analyses for the time to death was performed to compare the group with AKI at ICU admission versus the group with no AKI. The comparison was done using log-rank test. Level of significance was considered p <0.05. Days from ICU admission to death (event) or 15 May 2021 (censoring), considered the time of analysis.
Ethics
The study was approved by Regional Committees for Medical and Health Research Ethics West (approval number 169604). Informed consent was waived based on information to participants in NIPaR about the registry and their right to withdraw from NIPaR.
Results
A total of 394 adult patients were admitted to ICU with COVID-19 in the study period. Thirty-three of the patients were excluded due to CKD, resulting in a study population of 361 ICU patients, 100 women and 261 men. From these, 105 (29.1%) had AKI at hospital admission. Median age was 63.6 (IQR; 53.5–72.5) years and median body mass index was 27.7 (24.8–32.0) kg/m2. Current smokers constituted 2.5% of the patients. None of the female patients were pregnant. Median LOS at the ICU was 11.6 (5.7–19.5) days. Mechanical ventilation was initiated in 81.2% of the patients.
Comorbidity was reported in 68.1% of the study population, and 29.1% had two or more comorbidities. Regular medication of ACEi and/or ARB was used by 23.4% of the study population (table 1).
Table 1.
Patient characteristics by acute kidney injury (AKI) status at intensive care unit (ICU) admission
| Patient demographics | All patients (N=361) |
Missing data (no. of patients) | AKI (n=114) |
No AKI (n=242) |
P value (AKI vs no AKI) |
| Age in years, median (IQR) | 63.6 (53.5–72.5) | – | 65.6 (58.4–73.6) | 61.6 (52.0–72.3) | 0.003 |
| Male | 261 (72.3%) | – | 86 (75.4%) | 172 (71.1%) | 0.233 |
| Female | 100 (27.7%) | – | 28 (24.6%) | 70 (28.9%) | 0.233 |
| BMI, median (IQR) | 27.7 (24.8–32.0) | 141 | 27.3 (23.0–30.6) | 28.3 (25.1–32.4) | 0.132 |
| BMI>30 | 83 (37.7%) | 141 | 21 (31.8%) | 61 (40.4%) | 0.147 |
| Current smoker | 9 (2.5%) | – | 3 (2.6%) | 6 (2.5%) | 0.592 |
| Comorbidity/ies | 246 (68.1%) | – | 81 (71.1%) | 161 (66.5%) | 0.233 |
| 1 | 141 (39.1%) | – | 44 (38.6%) | 94 (38.8%) | 0.530 |
| >2 | 105 (29.1%) | – | 37 (32.5%) | 67 (27.7%) | 0.212 |
| CVD | 158 (43.8%) | – | 58 (50.9%) | 98 (40.5%) | 0.042 |
| DM | 74 (20.5%) | – | 23 (20.2%) | 50 (20.7%) | 0.518 |
| Asthma | 55 (15.2%) | – | 15 (13.2%) | 39 (16.1%) | 0.288 |
| CPD | 37 (10.2%) | – | 14 (12.3%) | 23 (9.5%) | 0.266 |
| Immunocompromised | 20 (5.5%) | – | 5 (4.4%) | 14 (5.8%) | 0.394 |
| Cancer | 17 (4.7%) | – | 6 (5.3%) | 11 (4.5%) | 0.476 |
| CND | 12 (3.3%) | – | 5 (4.4%) | 7 (2.9%) | 0.329 |
| Liver disease | 3 (0.8%) | – | 1 (0.9%) | 2 (0.8%) | 0.687 |
| ACEi/ARB | 83 (23.4%) | 7 | 34 (30.6%) | 47 (19.7%) | 0.019 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CND, chronic neurological disease; CPD, chronic pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus.
Patients with AKI at admission to the ICU were older than patients with no AKI. They also had more CVD and more often used ACEi or ARB (table 1). Patients with AKI at admission to ICU were more likely to have reduced GCS (table 2).
Table 2.
Laboratory findings and organ complications by acute kidney injury (AKI) status at intensive care unit (ICU) admission
| Variables at admission to hospital | All patients (N=361) |
Missing data (no. of patients) | AKI (n=114) |
No AKI (n=242) |
P value (AKI vs no AKI) |
| SCr in µmol/L, median (IQR) | 85.0 (70.3–104.0) | 1 | 98.0 (73.5–128.0) | 80.5 (69.5–96.0) | <0.000 |
| Estimated baseline SCr, median (IQR) | 92.5 (76.0–95.6) | – | 92.5 (85.0–94.5) | 92.7 (75.3–96.1) | 0.826 |
| AKI at hospital admission | 105 (29.1%) | 4 | 62 (54.4%) | 42 (17.4%) | <0.000 |
| Severe ARF | 319 (88.6%) | 1 | 103 (91.2%) | 212 (87.2%) | 0.213 |
| Severe ACF | 124 (35.4%) | 11 | 49 (45.0%) | 74 (31.4%) | 0.010 |
| Variables at admission to ICU | |||||
| GCS | |||||
| 14–15 | 323 (89.5%) | – | 89 (78.1%) | 229 (94.6%) | <0.000 |
| <13 | 38 (10.5%) | – | 25 (21.9%) | 13 (5.4%) | <0.000 |
| SAPS II score, median (IQR) | 34.0 (26.0–42.0) | – | 43.0 (37.0–50.0) | 31.0 (24.0–36.0) | <0.000 |
| BUN in mmol/L | 5 | ||||
| <10 | 275 (77.2%) | – | 33 (28.9%) | 242 (100.0%) | <0.000 |
| 10–29.9 | 79 (21.9%) | – | 79 (69.3%) | ||
| >30 | 2 (0.6%) | – | 2 (1.8%) | ||
| UO in mL per 24 hours | |||||
| >1000 | 307 (85.0%) | – | 60 (52.6%) | 242 (100.0%) | <0.000 |
| 500–999 | 35 (9.7%) | – | 35 (30.7%) | ||
| <500 | 19 (5.3%) | – | 19 (16.7%) | ||
| AKI at ICU admission | 114 (32.0%) | 5 |
ACF, acute circulatory failure; ARF, acute respiratory failure; BUN, blood urea nitrogen; GCS, glasgow coma scale; SAPS II, Simplified Acute Physiology Score II; SCr, serum-creatinine; UO, urine output.
The distribution of organ failure at admission to hospital was 88.6%, 35.4% and 29.1% for ARF, ACF and AKI (as defined by RIFLE criteria), respectively. ACF at hospital admission was significantly more prevalent in patients who suffered AKI at ICU admission (p<0.05).
A total of 114 (32.0%) patients had AKI in the ICU. From these, 79 (69.3%) and 2 (1.8%) had BUN=10–29.9 mmol/L and >30 mmol/L, respectively. UO of 500–999 mL/24 hours and <500 mL/24 hours was presented by 30.7% and 16.7%. More than half of the patients who had AKI at ICU admission also had AKI at admission to hospital.
RRT was required in 8.0% (n=29) of the total patient group during the ICU stay (table 3). Continuous RRT (CRRT) was initiated in 28 patients, and intermittent RRT (IRRT) was initiated in 7 patients. Median time with CRRT was 9.0 (5.0–14.0) days and 6.5 (5.0–7.5) days with IRRT.
Table 3.
Treatment and patient outcome by acute kidney injury (AKI) status at intensive care unit (ICU) admission
| Treatment | All patients (N=361) |
Missing data (no. of patients) | AKI (n=114) |
No AKI (n=242) |
P value (AKI vs no AKI) |
| LOS in ICU, median (IQR) | 11.6 (5.7–19.5) | – | 13.5 (5.9–25.6) | 10.9 (5.7–19.0) | 0.125 |
| Mechanical ventilation | 293 (81.2%) | – | 99 (86.8%) | 192 (79.3%) | 0.057 |
| RRT | 29 (8.0%) | – | 16 (14.0%) | 13 (5.4%) | 0.006 |
| CRRT | 28 (7.8%) | – | 15 (13.2%) | 13 (5.4%) | 0.012 |
| Median days (IQR) | 9.0 (5.0–14.0) | – | 8.0 (5.0–12.0) | 11.5 (5.5–16.3) | 0.863 |
| IRRT | 7 (1.9%) | – | 6 (5.3%) | 0 (0.0%) | 0.001 |
| Median days (IQR) | 6.5 (5.0–7.5) | – | 6.5 (5.0–7.5) | – | – |
| Outcome | |||||
| Survival first 24 hours in ICU | 358 (99.2%) | – | 111 (97.4%) | 242 (100.0%) | 0.032 |
| Survival at hospital discharge | 295 (81.7%) | – | 80 (70.2%) | 210 (86.8%) | <0.000 |
| Survival at 30 days | 298 (82.5%) | – | 77 (67.5%) | 217 (89.7%) | <0.000 |
| Survival at 90 days | 280 (77.6%) | – | 70 (61.4%) | 206 (85.1%) | <0.000 |
CRRT, continuous renal replacement therapy; IRRT, intermittent renal replacement therapy; LOS, length of stay; RRT, renal replacement therapy.
Survival for the total study population at 30 and 90 days was 82.5% and 77.6%, respectively. Survival at 30 and 90 days in patients with AKI at ICU admission was 67.5% and 61.4%, respectively, which was significantly lower compared with 89.7% and 85.1% in patients with no AKI.
A total of 337 patients with no missing data were included in three logistic regression analyses to assess risk factors for AKI at ICU admission and risk factors associated with mortality.
In the first multivariable model, only ACF was significantly associated with the development of AKI at ICU admission (OR 1.19; 95% CI 1.05 to 1.35) (table 4). Multicollinearity was evaluated using the VIF. VIF ranged from 1.02 (ACF) to 1.51 (CVD). The area under the curve (AUC) was 0.64 (95% CI 0.57 to 0.70).
Table 4.
Odds for acute kidney injury (AKI) at intensive care unit (ICU) admission
| Variable | Univariable logistic regression | Multivariable logistic regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Immunocompromised | 0.5 | 0.11 to 1.58 | 0.281 | |||
| Liver disease | 1.11 | 0.05 to 11.67 | 0.935 | |||
| Cancer | 0.8 | 0.22 to 2.39 | 0.701 | |||
| CND | 1.27 | 0.33 to 4.31 | 0.705 | |||
| Current smoker | 1.11 | 0.23 to 4.29 | 0.886 | |||
| Gender | 0.88 | 0.52 to 1.46 | 0.619 | |||
| Age | 1.02 | 1.01 to 1.04 | 0.013 | 1.02 | 1.00 to 1.04 | 0.121 |
| CVD | 1.5 | 0.94 to 2.38 | 0.089 | 1 | 0.56 to 1.78 | 0.996 |
| DM | 0.95 | 0.52 to 1.69 | 0.867 | |||
| Asthma | 0.79 | 0.39 to 1.49 | 0.474 | |||
| CPD | 1.17 | 0.54 to 2.42 | 0.673 | |||
| ACEi/ARB | 1.77 | 1.04 to 3.00 | 0.033 | 1.52 | 0.82 to 2.83 | 0.187 |
| ACF | 1.21 | 1.07 to 1.37 | 0.002 | 1.19 | 1.05 to 1.35 | 0.006 |
| ARF | 1.08 | 0.85 to 1.42 | 0.561 | |||
| (Intercept) | 0.09 | 0.03 to 0.31 | <0.000 | |||
ACEi, angiotensin-converting enzyme inhibitor; ACF, acute circulatory failure; ARB, angiotensin II receptor blocker; ARF, acute respiratory failure; CND, chronic neurological disease; CPD, chronic pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus.
In the second multivariable model, risk factors associated with 30-day mortality were cancer, age, AKI at ICU admission and ACF (table 5). VIF ranged from 1.03 (ACF) to 1.47 (CVD). The AUC was 0.87 (95% CI 0.83 to 0.92).
Table 5.
Odds for survival at 30 days
| Variable | Univariable logistic regression | Multivariable logistic regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Immunocompromised | 0.29 | 0.02 to 1.47 | 0.235 | |||
| Liver disease | 2.28 | 0.11 to 24.21 | 0.503 | |||
| Cancer | 3.24 | 1.05 to 9.35 | 0.032 | 4.39 | 1.17 to 15.90 | 0.024 |
| CND | 0.44 | 0.02 to 2.38 | 0.442 | |||
| Current smoker | 1.30 | 0.19 to 5.55 | 0.746 | |||
| Gender | 1.54 | 0.85 to 2.75 | 0.149 | |||
| Age | 1.08 | 1.05 to 1.11 | <0.000 | 1.07 | 1.04 to 1.11 | <0.000 |
| CVD | 2.33 | 1.33 to 4.15 | 0.004 | 0.93 | 0.40 to 2.11 | 0.857 |
| DM | 1.14 | 0.56 to 2.20 | 0.707 | |||
| Asthma | 1.09 | 0.49 to 2.25 | 0.818 | |||
| CPD | 4.17 | 1.97 to 8.73 | <0.000 | 2.50 | 0.98 to 6.43 | 0.055 |
| ACEi/ARB | 2.02 | 1.09 to 3.66 | 0.023 | 1.24 | 0.53 to 2.89 | 0.625 |
| ACF | 1.78 | 1.50 to 2.14 | <0.000 | 1.70 | 1.41 to 2.09 | <0.000 |
| ARF | 1.18 | 0.87 to 1.79 | 0.349 | |||
| AKI at ICU admission | 4.32 | 2.44 to 7.78 | <0.000 | 3.78 | 1.90 to 7.67 | <0.000 |
| (Intercept) | 0.00 | 0.00 to 0.002 | <0.000 | |||
ACEi, angiotensin-converting enzyme inhibitor; ACF, acute circulatory failure; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; ARF, acute respiratory failure; CND, chronic neurological disease; CPD, chronic pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ICU, intensive care unit.
In the third model, age, AKI at ICU admission and ACF were associated with 90-day mortality (online supplemental table s1). VIF in this model ranged from 1.02 (ACF) to 1.46 (CVD). The AUC was 0.87 (95% CI 0.82 to 0.91).
bmjopen-2021-059046supp001.pdf (734.6KB, pdf)
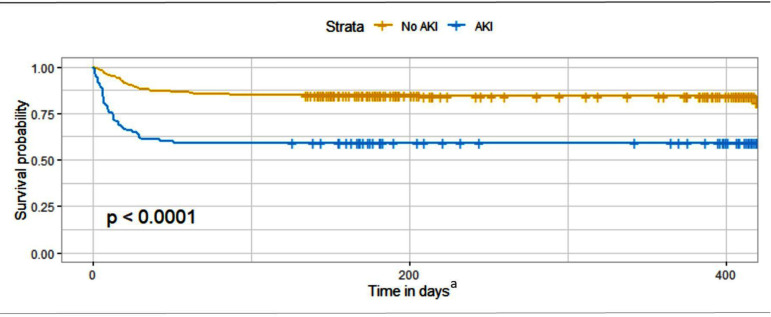
The results of Kaplan-Meier survival analysis stratified by AKI status at admission to ICU showed that patients with AKI had significantly lower survival than patients without AKI (log-rank p<0.001) (figure 1). The difference in survival was constrained to the first 50 days.
Figure 1.
Kaplan-Meier survival analysis stratified by AKI status at ICU admission.
The results from Cox regression analysis for survival at 30 days were in agreement with the results from logistic regression analysis. For survival at 90 days, the results were also in agreement, while CPD and regular medication of ACEi and/or ARB were additional significant predictors of mortality (online supplemental table s2 & s3).
Discussion
We performed a nationwide study of 361 adult patients with COVID-19 admitted to ICU. Prevalence of AKI at ICU admission was 32.0%. ACF at hospital admission predicted AKI at ICU admission. Age, cancer, ACF and AKI at ICU admission were risk factors for mortality at 30 days.
The COVID-19 pandemic in Norway, with its population of 5.4 million people,17 has been relatively well contained. During the study period, a total 50 145 cases of SARS-CoV-2 were reported, of which 2185 were admitted to hospital and 394 to the ICU.18 As in other countries, patients with COVID-19 in Norwegian ICU tend to be younger and more likely men compared with the general ICU population.14 Most patients in the study population were overweight, and a large proportion were obese, which is markedly different than in the general Norwegian population.19 Comorbidities such as CVD, DM and asthma were also more prevalent in the study population than in the general Norwegian population.20
The definition of AKI at ICU admission in our study does not fully comply with the AKI staging criteria due to the lack of creatinine-based measures of kidney function in the Norwegian Intensive Care Registry.21 The SAPS II criteria for reduced UO are similar to the staging criteria from the AKI network consensus, corresponding to AKI stage 2 and 3 (online supplemental table s4). BUN may increase by factors unrelated to kidney function, for instance due to steroid use which is regularly prescribed to patients with COVID-19 in the ICU.22 This would lead to an overestimate of AKI at ICU admission in our study, given standard enteral nutrition practices in Norwegian ICU. Creatinine is also influenced by several factors unrelated to kidney function but was chosen over BUN as the preferred biochemical parameter in the consensus process leading up to AKI definitions due to its widespread use.21 23
While not fully in line with current AKI definitions, the combination of UO and BUN should provide an estimate of AKI sufficiently similar to that of creatinine and UO to be relevant in a registry study. The significant difference in SCr at admission to hospital between patients with and without AKI at ICU admission in our material supports this assumption. Prevalence of AKI at ICU admission in our study is also similar to previous findings in the general ICU population. Bagshaw et al report that on the day of ICU admission, 36% of the general ICU population suffer AKI as defined by RIFLE criteria, 16.3% in the risk group and 19.9% in the injury and failure groups combined.24 A narrative review in COVID-19 found 23% prevalence of AKI in the ICU.25 In our study, 30.2% (n=114) of patients with COVID-19 admitted to ICU had AKI (table 2).
Due to the lack of granularity in our data, there are findings in our material that warrant further investigation. In our study group, 40.4% (n=42) of the patients with AKI at admission to hospital did not present AKI at ICU admission. It is likely that different AKI criteria applied at hospital admission and ICU admission affects this difference. However, we cannot rule out, for instance, that patients with mild prerenal AKI at admission were clinically stabilised to normal kidney function in a hospital ward prior to ICU admission due to respiratory failure. This would not contradict the impression that many patients with COVID-19 in the ICU have single organ respiratory dysfunction.26 On the other hand, 5.4% (n=13) of patients with no AKI at ICU admission received RRT during their ICU stay (table 3). We would expect some patients with long ICU stays do develop AKI during their ICU stays, but we cannot rule out losing cases of AKI at ICU admission due to lack of creatinine values in the ICU. In order to establish the timeline of AKI in patients with COVID-19 in ICU, studies with higher granularity data including serial UO and creatinine measurements are needed.
In the regression model for AKI at ICU admission, only ACF at admission to hospital was found to be significantly associated. ACF is an uncommonly reported parameter at admission to hospital, and thus often not included in analysis for prediction of AKI. Although we biologically would expect collinearity between ACF and AKI at ICU admission, this was contradicted by low VIF in the statistical analysis. The results suggests that AKI at ICU admission is more closely associated to circulatory status than any other factors in critically ill patients with COVID-19.
In the regression model on survival, the factors age, cancer, ACF and AKI at ICU admission were significantly associated with increased risk of death during first 30 days. Age, ACF and AKI at ICU admission were significantly associated with increased risk of death during first 90 days. AKI at ICU admission contributed considerably to the regression model. The finding puts AKI at ICU admission up as a strong and clinically important marker for survival in critically ill patients with COVID-19. While cancer also had a high contribution to the model, only 17 patients with cancer are included in the study, which reduces the clinical impact of this finding. CPD also contributes to the model but is only borderline significant. However, in the supplementary Cox regression model CPD was found significantly associated with risk of death during both first 30 and 90 days. Additionally, CPD is a risk factor in a larger group of the study population, 37 in total, and as such may be a more clinically relevant risk factor than cancer. Furthermore, respiratory disease, in addition to age, CVD and diabetes, is a previously well-recognised risk factor for severe disease progression and mortality in COVID-19.27
More than one out of three patients with AKI diagnosed first 24 hours of ICU stay were deceased after 30–90 days. The Kaplan-Meier analysis illustrates that the mortality is predominantly in the short term within 50 days, in essence predominantly during the acute phase of illness. The finding supports that AKI at ICU admission is a clinically important marker for poor outcome in COVID-19 (figure 1). Low VIF in both regression models means that the effects of collinearity in the models are low. This puts further emphasis on AKI at ICU admission as an important prognostic factor for mortality in COVID-19.
We recognise several strengths and limitations in this study. The study is a national cohort containing complete data of all Norwegian patients with COVID-19 (N=394) admitted to ICU in the study period. Furthermore, because of the mandatory obligation by the Norwegian authorities to deliver data, the number of missing data was negligible. ICU admission criteria and treatment traditions are also similar in Norwegian ICUs which renders that data are comparable across centres. Furthermore, during the COVID-19 pandemic in Norway patients were not denied ICU care due to capacity concerns, thereby reducing selection bias due to triage decisions.
While national data increase generalisability, a major limitation in this study is that NIPaR does not contain creatinine-based measures for AKI. Although the combination of UO and BUN in rSAPSII should provide an estimate of AKI sufficiently similar to that of UO and creatinine to be relevant, these indicators mandates that the results be interpreted with caution and limit generalisability. We also lack data regarding the timeline of AKI in COVID-19, and the use of vasopressor in the ICU. While the statistical analyses are rigorous, we nevertheless recommend that the results are treated as a basis for further investigation.
Conclusion
In this national cohort of patients with COVID-19 admitted to ICUs in Norway 32.0% (n=114) developed AKI during first 24 hours of ICU admission. The majority presented clinical and/or biochemical signs of AKI at admission to hospital. The study indicates that ACF at hospital admission was the most important risk factor for AKI at admission to ICU, and that age, cancer, ACF and AKI at ICU admission were associated with mortality at 30 days after hospital admission.
Supplementary Material
Footnotes
Contributors: EAA (submitting author) conceptualised and designed the study, performed data cleaning and assisted with the data analyses, drafted the initial manuscript, and reviewed and revised the manuscript. PK and SMA conceptualised and designed the study, contributed to the interpretation of the results, and critically reviewed the manuscript. FZG conducted the data analyses and critically reviewed the manuscript. EAB conceptualised and designed the study, collected the data, contributed to the interpretation of the results, and critically reviewed the paper. EAB act as guarantor. All authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data cannot be shared publicly because of GDPR restrictions. Data are available from Norwegian Intensive Care and Pandemic Registry (NIPaR) upon application containing necessary approvals.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by Regional Committees for Medical and Health Research Ethics West (approval number 169604). Informed consent was waived based on information to participants in Norwegian Intensive care and Pandemic Registry (NIPaR) about the registry and their right to withdraw from NIPaR. Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . Coronavirus Disease (COVID-19) Situation Report 51 [Internet]. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 [Accessed March 11, 2020].
- 2.Sardu C, Gambardella J, Morelli MB, et al. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med 2020;9. 10.3390/jcm9051417. [Epub ahead of print: 11 05 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farouk SS, Fiaccadori E, Cravedi P, et al. COVID-19 and the kidney: what we think we know so far and what we don't. J Nephrol 2020;33:1213–8. 10.1007/s40620-020-00789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y-T, Shao S-C, Hsu C-K, et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care 2020;24:346. 10.1186/s13054-020-03009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch JS, JH N, Ross DW. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J 2020;13:354–61. 10.1093/ckj/sfaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Yang X, Yang L, et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care 2020;24:394. 10.1186/s13054-020-03098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Xu D, Fu S, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care 2020;24:219. 10.1186/s13054-020-02939-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luther T, Bülow-Anderberg S, Larsson A, et al. COVID-19 patients in intensive care develop predominantly oliguric acute kidney injury. Acta Anaesthesiol Scand 2021;65:364-372. 10.1111/aas.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinot M, Eyriey M, Gravier S, et al. Predictors of mortality, ICU hospitalization, and extrapulmonary complications in COVID-19 patients. Infect Dis Now 2021;51:518–25. 10.1016/j.idnow.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) Workgroup. Nat Rev Nephrol 2020;16:747–64. 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasjonalt Servicemiljø for medisinske kvalitetsregistre . Norsk Intensiv- og pandemiregister [Internet]. Available: https://www.kvalitetsregistre.no/registers/norsk-intensiv-og-pandemiregister [Accessed 23 Sep 2020].
- 13.Buanes EA, Kvåle R, Barratt-Due A. Årsrapport for 2020 med plan for forbetringstiltak. Norsk intensiv- og pandemiregister, 2021. https://helse-bergen.no/norsk-intensivregister-nir/arsrapportar [Google Scholar]
- 14.Buanes EA, Kvåle R, Barratt-Due A. Årsrapport for 2019 med plan for forbetringstiltak. Norsk Intensivregister, 2020. https://helse-bergen.no/norsk-intensivregister-nir/arsrapportar [Google Scholar]
- 15.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPs II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Uchino S, Cruz D, et al. A comparison of observed versus estimated baseline creatinine for determination of rifle class in patients with acute kidney injury. Nephrol Dial Transplant 2009;24:2739–44. 10.1093/ndt/gfp159 [DOI] [PubMed] [Google Scholar]
- 17.Statistisk Sentralbyrå . Befolkning [Internet] 2021. Available: https://www.ssb.no/befolkning/folketall/statistikk/befolkning [Accessed 19 Aug 2021].
- 18.Folkehelseinstituttet . Statistikk om koronavirus og covid-19 [Internet]. Available: https://www.fhi.no/sv/smittsomme-sykdommer/corona/dags-og-ukerapporter/dags-og-ukerapporter-om-koronavirus/#table-pagination-32719729 [Accessed 9 Jun 2021].
- 19.Statistisk Sentralbyrå . Helseforhold, levekårsundersøkelsen. In: 06181: Levevaner (prosent) etter statistikkvariabel, år og kjønn, 2019, 2021. https://www.ssb.no/statbank/table/06181/tableViewLayout1/ [Google Scholar]
- 20.Statistisk Sentralbyrå . Helseforhold, levekårsundersøkelsen. In: 11190: Sykelighet. Sykdom, skade eller funksjonshemming, etter type sykelighet, alder, statistikkvariabel, år og kjønn, 2019, 2021. https://www.ssb.no/statbank/table/11190/tableViewLayout1/ [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Perrone RD, Sterns RH, ed. Assesment of kidney function. Waltham, MA: UpToDate, 2021. [Google Scholar]
- 23.Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 2008;15:222–34. 10.1053/j.ackd.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM, George C, Dinu I, et al. A multi-centre evaluation of the rifle criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008;23:1203–10. 10.1093/ndt/gfm744 [DOI] [PubMed] [Google Scholar]
- 25.Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 2020;46:1339–48. 10.1007/s00134-020-06153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laake JH, Buanes EA, Småstuen MC, et al. Characteristics, management and survival of ICU patients with coronavirus disease-19 in Norway, March-June 2020. A prospective observational study. Acta Anaesthesiol Scand 2021;65:618–28. 10.1111/aas.13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Peng F, Xu B. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059046supp001.pdf (734.6KB, pdf)
Data Availability Statement
No data are available. Data cannot be shared publicly because of GDPR restrictions. Data are available from Norwegian Intensive Care and Pandemic Registry (NIPaR) upon application containing necessary approvals.