Abstract
Electron microscope grids were submerged in Lake Washington, Seattle, Wash., in June 1996 as bait to which Caulobacter sp. swarmers would attach and on which they would then reproduce in situ. Enumeration of bands in the stalks of attached cells implied that the caulobacters were completing approximately three reproductive cycles per day. A succession of morphological types of caulobacters occurred, as well as an episode of bacteriovore grazing that slowed the accumulation of caulobacters and prevented the aging of the population.
To elucidate roles of bacteria in natural habitats, a wide variety of chemical and microscopical methods are now available that allow quantitative determinations of metabolic rates and estimations of viability. However, correlation of metabolic activity and reproductive potential with bacterial multiplication in situ remains elusive because the vast, diverse, and perpetual transformations effected by bacteria in nature are typically achieved by populations that do not fluctuate significantly in size. Some bacteriologists attribute this seeming paradox to the presence of high proportions of quiescent (i.e., not dividing), moribund (i.e., not active), or dead bacteria (9, 25), while others attribute the constancy of population size of lively, healthy, metabolically active bacteria to bacteriovory (13).
The present study was designed to determine the in situ reproductive rate of just one genus, Caulobacter, by exploiting the unique cell cycle marker, the stalk band, that is added to a caulobacter stalk once during each cell cycle (19, 24). Because of the stalk bands, a caulobacter cell that has divided several times since it was a flagellated swarmer cell incapable of cell division looks different from a stalked cell that has not yet divided. In artificial cultures, attachment by cells of dimorphic prosthecate bacteria is initiated predominantly in the swarmer stage, whether the substratum is glass (8, 12), cellulose fibers (26), the holdfasts of other cells (10, 15), or electron microscope (EM) grids (5) (reviewed in reference 16). By allowing caulobacters in Lake Washington, Seattle, Wash., to attach to submerged EM grids and examining the attached populations at intervals, we expected to find that the maximum number of bands per stalk would increase in proportion to the duration of submersion and that that number could be used to infer the number of reproductive cycles occurring daily.
The results revealed that caulobacters completed three reproductive cycles per day in Lake Washington in June 1996. In addition, direct microscopical evidence was obtained showing that attached caulobacters are susceptible to bacteriovore grazing.
Exposure, retrieval, and examination of substrata.
Formvar-coated nickel grids on sheets of Parafilm were attached to glass microscope slides by tape wound around the Parafilm. The slides were placed vertically in plastic slide boxes from which most of the bottoms had been cut out and whose corners had been fitted with plastic fishing line tethers. Five slides were placed in each of two such boxes; each box was enclosed within a ZipLoc plastic bag and lowered into the water; the bag was opened underwater, and the slide box was removed. The tethers were attached to the wooden supports of a marine light located approximately 100 m from shore so that the boxes hung suspended slightly more than 1 m below the lake surface, one on the south side of the light (site 1) and one on the north side (site 2). Grids were retrieved from site 1 after 1, 2, 3, 4, and 7 days of submersion and from site 2 after 1, 2, 3, and 4 days; one slide had fallen out of the box at site 2 between days 1 and 2. Grids were not retrieved on days 5 and 6.
To retrieve a slide of grids, a ZipLoc bag containing a plastic slide box with lid was lowered into the water. The bag was opened beneath the surface, a slide was transferred from the in situ slide box to the retrieving box, the retrieving box was closed with its lid, the bag was snapped closed, and the bag-box-slides assembly was lifted out of the water. The use of tightly closed plastic bags to introduce and retrieve the grids prevented contact of the grids with caulobacters present at and near the air-water interface of the lake, a problem not recognized in an earlier study (7). Within 15 to 20 min, the bag was opened and the water was allowed to drain out, the slide box lid was removed, and the slide was dipped in a Coplin jar containing 0.2% potassium phosphotungstate, pH 7. The surface of each grid was drained dry with a tissue, and the slide and grids were allowed to air dry.
Grids were examined in a JEOL 1200 tranmission EM or a Philips 208S transmission EM operated at 80 kV and 60 kV, respectively. Each grid was used only once; parallel rows of grid openings were scanned in a pattern that ensured that no opening on any grid was viewed more than once. Every identifiable caulobacter cell encountered was recorded photographically, even if the full length of its stalk was not observable (e.g., because it was embedded in debris or lay over a grid bar). All other organisms were enumerated, as well, but only occasionally photographed. Stalk bands were enumerated on the negative films and on contact prints examined with the aid of a dissecting microscope.
Attachment and reproduction of caulobacters.
Stalked caulobacters (swarmers cannot be identified as caulobacters) accounted for the majority (59%) of cells attached to the grids from two to seven days of submersion. Examples of attached caulobacters are illustrated in Fig. 1 through 4. The numbers of caulobacters encountered on the grids are shown in Table 1 and Fig. 5, which combine the observations on grids from both sites because there was no significant difference between sites for the first 4 days. As the numbers of caulobacters increased, it was clear that the accumulating cells comprised both new arrivals (cells with short stalks and zero or one band per stalk) and stably attached cells that were dividing in place. Cells with relatively short stalks without bands were found on every grid, but cells with long stalks with more than three bands were not found until the 4th day. These observations strongly imply that attachment to natural substrata is initiated by swarmers, as it is in monocultures.
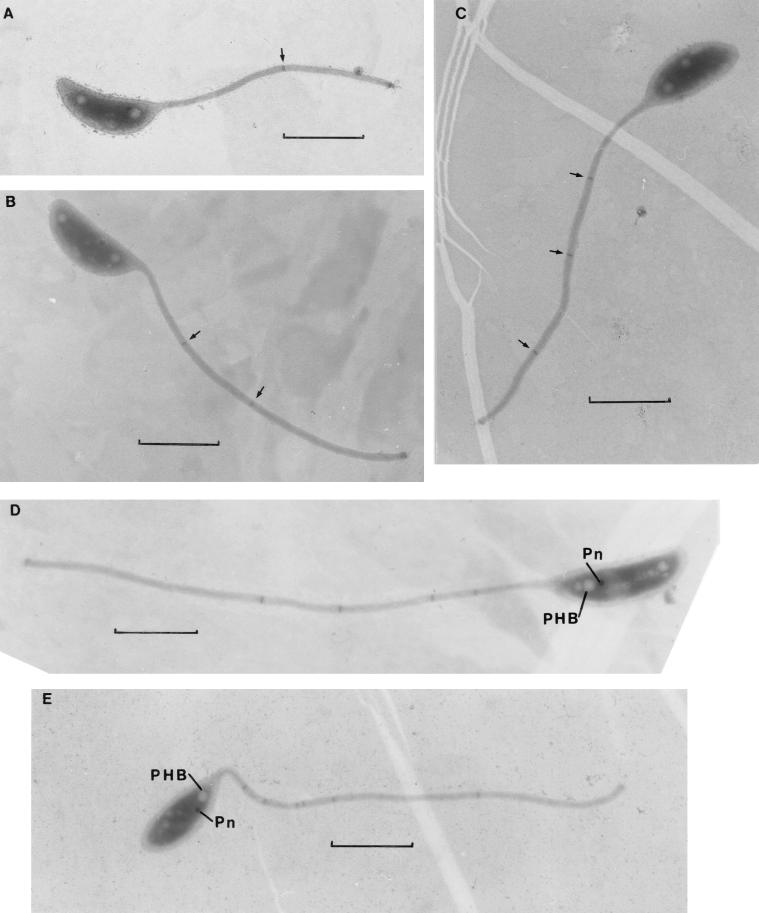
FIG. 1.
Stalked vibrioid caulobacter cells attached to grids at 2 (A to C) and 4 (D and E) days of submersion in Lake Washington, displaying one (A), two (B), three (C), four (D), and six (E) stalk bands (marked by arrows in panels A to C). PHB, granule with the appearance of poly-β-hydroxybutyrate; Pn, granule with the appearance of inorganic polyphosphate. Each marker is 1 μm.
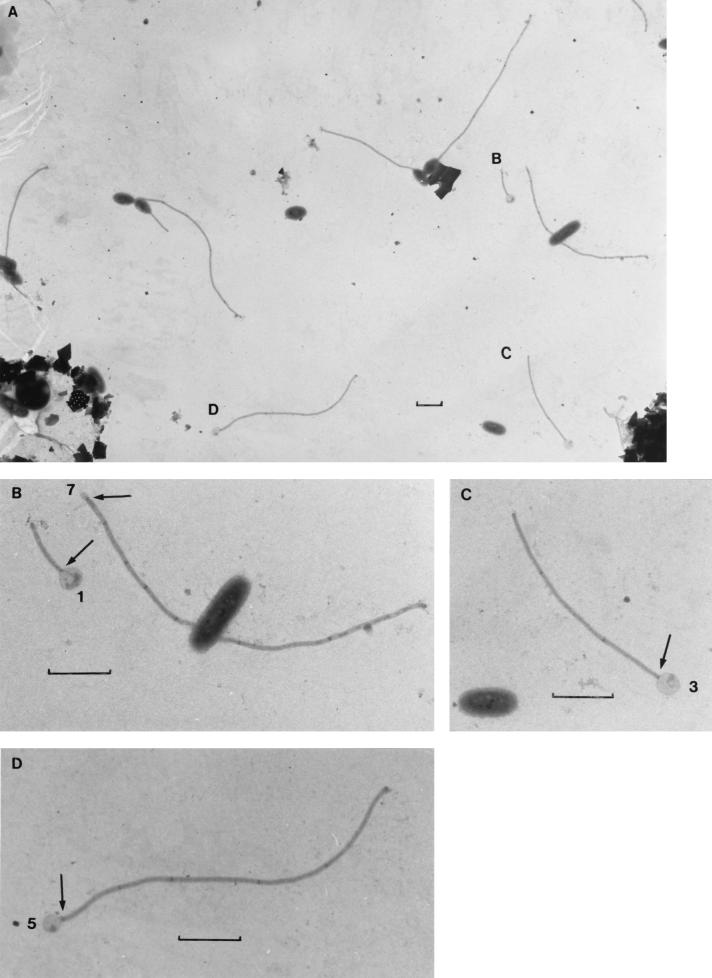
FIG. 4.
(A) Stalked caulobacter cells and stalk remnants attached to grids at 7 days of submersion in Lake Washington. (B to D) The stalk remnants are shown at higher magnification. The stalk band typically separates the intact region of the stalk remnant from the collapsed, gnawed end that was previously connected to the cell (arrows). The number of stalk bands remaining is indicated at the gnawed end of each remnant; the numbers (1, 3, 5, and 7) suggest that grazing was not selective for cells of a particular age. Each marker is 1 μm.
TABLE 1.
Tally of bands in stalks visible from cell pole to holdfast
| Days of submersion | No. of stalked cells scored
|
No. of bands/stalkb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vibrioid
|
Fusiform
|
Bacteroid
|
All types
|
Median | Maximum | |||||
| Newa | Total | New | Total | New | Total | New | Total | |||
| 1 | 4 | 5 | 0 | 1 | 1 | 1 | 5 | 7 | 1 | 2 |
| 2 | 8 | 11 | 2 | 4 | 0 | 0 | 10 | 15 | 1 | 2 |
| 3 | 15 | 22 | 4 | 7 | 2 | 2 | 21 | 31 | 1 | 3 |
| 4 | 26 | 75 | 2 | 9 | 0 | 7 | 28 | 91 | 2 | 9 V |
| 7 | 17 | 51 | 13 | 62 | 0 | 0 | 30 | 113 | 2 V, 3 F | 8 V, 9 F |
New arrivals are defined as stalked cells with zero or one band.
Some data for vibrioid (V) and fusiform (F) cells are specified.
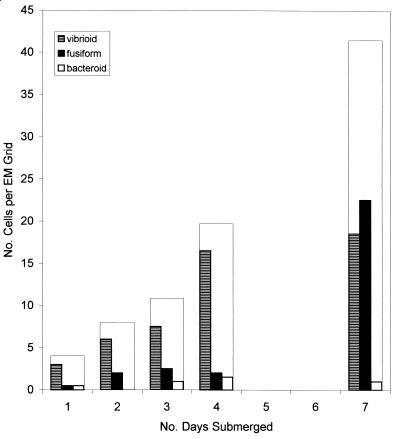
FIG. 5.
Numbers of stalked caulobacter cells found attached to grids on each day of submersion in Lake Washington. Cell numbers were totaled for the two sites and normalized per grid for each day's collection. Each wide bar represents the total number of caulobacter cells, of all three types, and includes cells whose band numbers could not be determined (see text).
Three cell types of caulobacters appeared: vibrios first and then fusiform cells and a bacteroid type, which was infrequently encountered. During the first 4 days, only vibrioid caulobacters appeared on the grids in significant numbers. Attached vibrios with one or two bands per stalk were detected on day 2. Two- and three-banded stalks were present on day 3. By day 4, accumulation of caulobacters and other bacteria was considerably heavier on the grids, with one to five cells in many of the openings. The most common band number on day 4 was still only two, but five- and six-banded stalks were as numerous as were two- and three-banded stalks on day 3. On day 4, the maximum number of bands was nine. On the conservative assumptions that the earliest vibrioid caulobacter swarmers attached during the first 1 to 2 days and had two bands per stalk by day 2 and nine by day 4, and that one or two bands were installed during the first cell cycle and one band was installed during each subsequent cycle (19), the vibrioid caulobacters completed at least eight cycles between days 1 and 4 (2.7 cycles/day) or seven cycles between days 2 and 4 (3.5 cycles/day).
Few fusiform caulobacters with banded stalks were detectable on day 4, but on day 7 the fusiform cells were more abundant than vibrios (Table 1; Fig. 5); by that time they displayed up to nine bands per stalk. Using the same assumptions as for the vibrios, if most of the fusiform cells attached after day 4 and survived until day 7, then they appeared to have completed nine cycles within those 3 days (three cycles/day).
The vibrioid and fusiform caulobacters thereby displayed similar in situ reproductive rates. Three cycles per day (an average of 8 h per cycle) is well within the physiologic capabilities of bacteria that exhibit generation times averaging 4 to 5 h in pure cultures. Whether reproduction on the submerged grids occurred throughout each day or only during a part of the day cannot be inferred from this study.
Episode of predation.
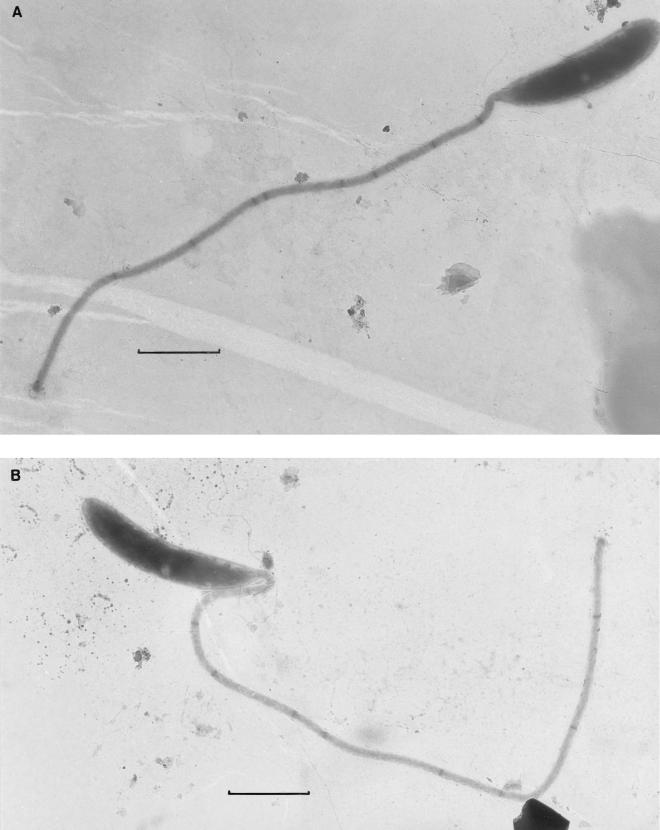
On day 7, there was clear evidence of intensive grazing at some time between 4 and 7 days, as well as a shift within the caulobacter population from predominantly vibrioid to predominantly fusiform cells. The most reasonable interpretation of the significant change in caulobacter populations on the grids is that the once-predominant vibrioid caulobacters were grazed after grids were retrieved on day 4; continued arrival of vibrios was accompanied by fusiform cells more frequently than before day 4. The grazing was evident as remnants of stalks, which remained attached to the grids like rooted stems of plants from which the fruits had been plucked. Typically, one end of the stalk remnant had collapsed, and the remaining bleb usually terminated at a stalk band (arrows, Fig. 4). Other damaged regions of stalks were also typically bounded by bands (arrows, Fig. 3), implying that the band can serve to seal off such regions, as suggested by Schmidt (21).
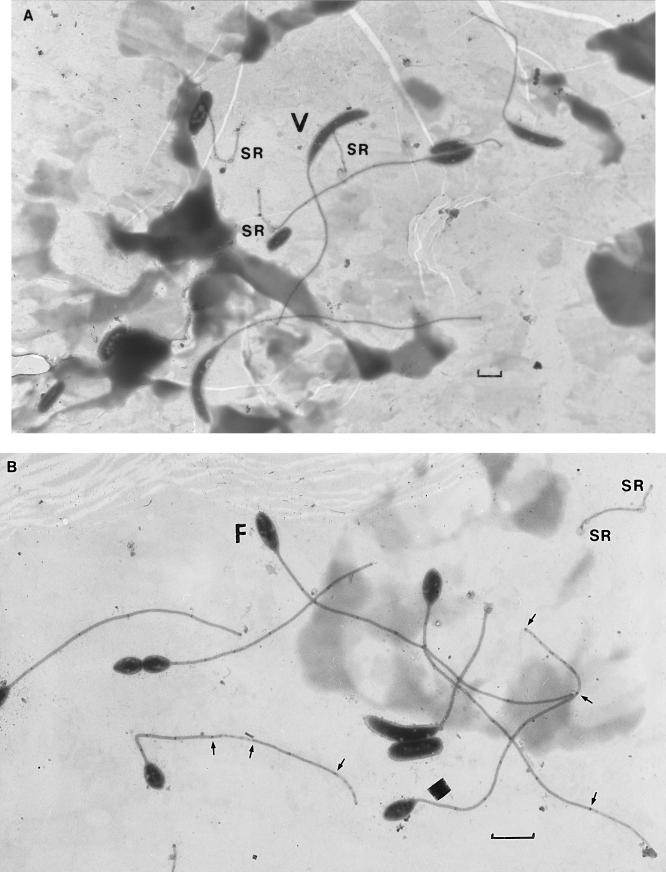
FIG. 3.
Stalked caulobacter cells attached to grids at 7 days of submersion in Lake Washington, displaying various numbers of stalk bands. (A) cluster of vibrioid (V) cells; (B) cluster of predominantly fusiform (F) cells. SR, stalk remnant. Arrows indicate bands at the ends of damaged regions of stalks. Each marker is 1 μm.
Cell-free stalks are not encountered in monocultures of caulobacters, whether the cells are grown suspended (19) or attached to glass beads (24) and whether they are grown in batch or perpetual cultures (19). In monocultures, stalks with as many as 13 to 19 bands have been reported. To remove stalks from cells requires either shearing in a blender (6, 18, 21, 22) or a stalk abscission mutation (17, 18). A vigorous mechanical force must have been exerted on individual stalked cells on the grids, a force that was not exerted on the entire population; stalk remnants were found among cells with various numbers of stalk bands (Fig. 3 and 4). Bands still visible in the stalk remnants were enumerated and ranged from zero to eight bands per fragment, suggesting that the feeders fed at random among caulobacters of different ages.
Other observations.
The vertical position of the slides was effective in avoiding the accumulation of debris, dead microorganisms, and nonadhesive microorganisms that settle by gravity. The result was slide (and grid) surfaces free of macroscopic deposits of material that accumulated on horizontal surfaces such as the rim of the slide box. Microscopically, noncellular debris accumulated only slowly and did not significantly interfere with observations, even after 7 days of submersion.
Besides caulobacters, two other morphologically distinctive microorganisms were encountered: a sheathed rod, presumably Sphaerotilus sp., and a diatom, presumably (from its shape and manner of attachment) Diatoma sp. All other attached organisms were bacteria, predominantly rods, but occasionally a coccus; bacteriophage virions were detected only once, as a cluster of podovirus-like particles surrounding a flagellated rod. All of the microbial cells appeared to have attached actively, not to have settled passively on the grids. The diatom and the Sphaerotilus were attached by puddles of adhesive material from one point only on each organism's surface. Like caulobacters, Sphaerotilus initiates attachment in the swarmer stage (2); it appeared to reproduce in situ on our grids, accumulating up to six cells within each sheath. Single rod-shaped bacteria were typically flagellated, cocci were surrounded by fimbriae and/or fibrous extracellular slime, and the stalked caulobacters exhibited a progressively longer mean stalk length as well as increasing band numbers. Dividing cells were encountered at frequencies well above those usually reported for aquatic assemblages (3, 11), which may well include cells not actively reproducing in situ (25). The metabolic activity of the attached bacteria was further evidenced by the presence of reserve granules in many cells. Electron-translucent granules were interpreted as carbon reserves, either polysaccharide or poly-β-hydroxybutyrate, and sharp-edged, electron-dense granules were interpreted as polyphosphate. Both types of granules were present in many cells and in nearly all of the stalked cells (Fig. 1D and E.).
We infer from all of these appearances that the bacteria found on the grids were alive and were metabolically active, reproducing members of the microbial community of Lake Washington.
Interpretations.
The reproductive rate of three cycles per day inferred for caulobacters in this study is within the range of 2 to 10 h per generation calculated for aquatic bacteria that developed as colonies on submerged glass microscope slides (1). It is possible that these observations reflect peak activity of the caulobacters and that a similar study conducted periodically during a full year's turn of seasons would reveal whether the reproductive rate varies with water temperature or other environmental variables.
The succession from vibrioid to fusiform caulobacters was probably fortuitous. In a companion study of caulobacters in Lake Washington from February through August 1996 (to be reported separately), there was an observable succession of Caulobacter species, with vibrioid types predominating from March through early June, fusiforms predominating in early summer, and then a second bloom of different vibrioid species in late July and August. That this succession was reflected on the grids in mid-June is nevertheless regarded as pure coincidence and a result of uneven and varying distribution of caulobacter types within the lake as well as with time and season. None of the physical (temperature or turbidity), chemical (salinity, nitrate, phosphate, or silicon) or biotic (chlorophyll, algae, protozoa, invertebrate animals, or total chemoheterotrophic aerobic bacteria) parameters monitored during the long-term study varied significantly within the short period of submersion of the grids.
Unexpectedly, the observations reported here provided direct evidence that periodic waves of bacteriovory can account for lack of massive accumulation (blooming) of bacterial populations, even while they are reproducing at a significant rate. Although caulobacter swarmers are probably susceptible to ingestion by flagellates and by ciliates, amebae and ciliates have been observed to ignore prosthecate bacteria as they meander over mixed bacterial populations attached to glass slides (Poindexter, unpublished data). Amebae, in particular, appear to react to the prostheca as an extension of the cell, which is then judged to be a particle too large to engulf, as though it were an inedible filamentous bacterium (see references 4, 14, and 23). The water temperature at the submersion sites ranged from 16.4 to 18.7°C (average, 17.5°C) at mid-day. The temperature had risen from 7°C in early February, and the rise in temperature during the spring had been accompanied by a bloom of small animals, especially in May, most of which fed on suspended microbes and had nearly decimated the phytoplankton community by mid-June. Two types of invertebrate animals were present that could have gnawed caulobacter cells from their stalks: tardigrades and rotifers. Because the tardigrades occurred much closer to shore than the submersion sites, W. T. Edmondson (personal communication) recommended that the grazing episode be attributed to rotifer feeding. Some rotifers are sessile and feed on suspended particles swept into the gullet on feeding currents generated by cilia, but others are mobile and feed by means of protruding mouth parts capable of grinding and gnawing prey. Stalked caulobacter cells tenaciously attached to a large (relative to rotifers) substratum apparently were susceptible to such predators.
This view of natural populations is consistent with experimental studies with natural bacterial populations (see, e.g., reference 20) as well as with the inferred role of grazing in the maintenance of stable climax communities in municipal water and sewage treatment facilities. It is not consistent with the view that populations of bacteria that are stable in density in their natural habitats are necessarily quiescent. On the contrary, such populations may provide a significant, or at least a supplemental, proportion of the diet of animals as well as of protozoa while simultaneously contributing to the biogeochemical transformations for which they are known to be responsible.
FIG. 2.
Stalked vibrioid caulobacter cells attached to grids at 4 days of submersion in Lake Washington, displaying eight (A) and nine (B) stalk bands. Each marker is 1 μm.
Acknowledgments
The outdoor work in this study was conducted during 1996 while J.S.P. was on academic leave, generously hosted by J.T.S. and his laboratory at the University of Washington in Seattle. The boat used was made available through the generosity of the laboratory of W. T. Edmondson, Department of Zoology, University of Washington, Seattle.
The work was supported in part by a Barnard College Faculty Grant to J.S.P.
Footnotes
This report is dedicated to the memory of W. T. Edmondson, who so faithfully defended the quality of life of the inhabitants of Lake Washington for most of the twentieth century.
REFERENCES
- 1.Bott T L, Brock T D. Growth and metabolism of periphytic bacteria: methodology. Limnol Oceanogr. 1970;15:333–342. [Google Scholar]
- 2.Bott T L, Brock T D. Growth rate of Sphaerotilus in a thermally polluted environment. Appl Microbiol. 1970;19:100–102. doi: 10.1128/am.19.1.100-102.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagström Å, Larsson U, Hörstedt P, Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979;37:805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn M W, Höfle M G. Grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio strain CB5 in chemostat cocultures. Appl Environ Microbiol. 1998;64:1910–1918. doi: 10.1128/aem.64.5.1910-1918.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch P. Budding bacteria. Annu Rev Microbiol. 1974;28:391–444. doi: 10.1146/annurev.mi.28.100174.002135. [DOI] [PubMed] [Google Scholar]
- 6.Jones H C, Schmidt J M. Ultrastructural study of crossbands occurring in the stalks of Caulobacter crescentus. J Bacteriol. 1973;116:466–470. doi: 10.1128/jb.116.1.466-470.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan T L, Staley J T. Electron microscopic study of succession in the periphyton community of Lake Washington. Microb Ecol. 1976;2:241–251. doi: 10.1007/BF02011645. [DOI] [PubMed] [Google Scholar]
- 8.Leifson E. The bacterial flora of distilled and stored water. II. Caulobacter vibrioides Henrici & Johnson 1935 in distilled water. Int Bull Bacteriol Nomencl Taxon. 1962;12:155–159. [Google Scholar]
- 9.Lewis D L, Gattie D K. The ecology of quiescent microbes. ASM News. 1991;57:27–32. [Google Scholar]
- 10.Moore R L, Marshall K C. Attachment and rosette formation by hyphomicrobia. Appl Environ Microbiol. 1981;42:751–757. doi: 10.1128/aem.42.5.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell S Y, Christian R R. Frequency of dividing cells as an estimator of bacterial productivity. Appl Environ Microbiol. 1981;42:23–31. doi: 10.1128/aem.42.1.23-31.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton A. Role of transcription in the temporal control of development in Caulobacter crescentus. Proc Natl Acad Sci USA. 1972;69:447–451. doi: 10.1073/pnas.69.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overmann J, Beatty J T, Hall K H. Purple sulfur bacteria control the growth of aerobic heterotrophic bacterioplankton in a meromictic salt lake. Appl Environ Microbiol. 1996;62:3251–3258. doi: 10.1128/aem.62.9.3251-3258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pernthaler J, Posch T, Šimek K, Vrba J, Amann R, Psenner R. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl Environ Microbiol. 1997;63:596–601. doi: 10.1128/aem.63.2.596-601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poindexter J S. Biological properties and classification of the Caulobacter group. Bacteriol Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poindexter J S. Dimorphic prosthecate bacteria: the genera Caulobacter, Asticcacaulis, Hyphomicrobium, Pedomicrobium, Hyphomonas, and Thiodendron. In: Balows A, Trüper H G, Dworkin M, Harder W, Schliefer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 2176–2196. [Google Scholar]
- 17.Poindexter J S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978;135:1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poindexter J S, Hagenzieker J G. Novel peptidoglycans in Caulobacter and Asticcacaulis spp. J Bacteriol. 1982;150:332–347. doi: 10.1128/jb.150.1.332-347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poindexter J S, Staley J T. Caulobacter and Asticcacaulis stalk bands as indicators of stalk age. J Bacteriol. 1996;178:3939–3948. doi: 10.1128/jb.178.13.3939-3948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter K G. Natural bacteria as food resources for zooplankton. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C.: American Society for Microbiology; 1984. pp. 340–345. [Google Scholar]
- 21.Schmidt J M. Effect of lysozyme on crossbands in stalks of Caulobacter crescentus. Arch Mikrobiol. 1973;89:33–40. [Google Scholar]
- 22.Schmidt J M, Swafford J R. Ultrastructure of crossbands in prosthecae of Asticcacaulis species. J Bacteriol. 1975;124:1601–1603. doi: 10.1128/jb.124.3.1601-1603.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Šimek K, Vrba J, Pernthaler J, Posch T, Hartmen P, Nedoma J, Psenner R. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol. 1997;63:587–595. doi: 10.1128/aem.63.2.587-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staley J T, Jordan T L. Crossbands of Caulobacter crescentus stalks serve as indicators of cell age. Nature. 1973;246:155–156. doi: 10.1038/246155a0. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson L H. A case for bacterial dormancy in aquatic systems. Microb Ecol. 1978;4:127–133. doi: 10.1007/BF02014283. [DOI] [PubMed] [Google Scholar]
- 26.Umbreit T H, Pate J L. Characterization of the holdfast region of wild-type cells and holdfast mutants of Asticcacaulis biprosthecum. Arch Microbiol. 1978;118:157–168. [Google Scholar]