Abstract
We report the engineering of Lactococcus lactis for the efficient conversion of sugar into diacetyl by combining NADH-oxidase overproduction and α-acetolactate decarboxylase inactivation. Eighty percent of the carbon flux was found to be rerouted via α-acetolactate to the production of diacetyl by preloading the cells with NADH-oxidase before their use as a cell factory.
Diacetyl has a strong, buttery flavor and is essential at low concentrations in many dairy products, such as butter, buttermilk, and fresh cheeses. It is also considered to be the most important off-flavor in the brewing process and in the wine industry.
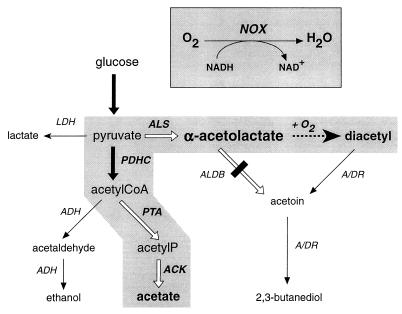
Diacetyl is a by-product of fermentation by many microorganisms. It is produced chemically by oxidative decarboxylation of the metabolic intermediate α-acetolactate (α-AL). One molecule of α-AL is produced from two molecules of pyruvate by the condensing enzyme, α-AL synthase (ALS) (14) (Fig. 1). In dairy fermentation, α-AL is mainly produced by lactic acid bacteria as a result of the metabolism of citric acid, a minor component of milk (7). The ability to utilize citric acid is found only in some Leuconostoc species and specific variants of Lactococcus lactis, namely, the biovar diacetylactis. Due to the balancing of redox equivalents, sugars are converted via pyruvate to lactic acid, while the more oxidized substrate citric acid is converted into α-AL and subsequently into acetoin via α-AL decarboxylase (ALDB) (Fig. 1). Specific L. lactis strains isolated from dairy cultures that produce large amounts of α-AL from citric acid were shown to lack the ALDB enzyme (8). In dairy fermentation, these mutants are responsible for production of relatively high levels of diacetyl, the direct product of chemical decarboxylation of α-AL. New selection methods (4, 6) and deletion of the aldB gene by genetic engineering (15) have made these mutants more readily available.
FIG. 1.
Glucose metabolism in an ALDB-deficient mutant of L. lactis overproducing the NOX enzyme. The rerouted pathways are highlighted in grey. Reactions or pathways producing NADH have large black arrows, those that are NADH independent have large white arrows, and those producing NAD+ have thin black arrows. The chemical oxidative decarboxylation of α-AL into diacetyl is displayed by a dotted arrow. ACK, acetate kinase; ADH, alcohol dehydrogenase; A/DR, acetoin/diacetyl reductase; PDHC, pyruvate dehydrogenase complex; PTA, phosphotransacetylase.
Based on the knowledge of the pathways involved in diacetyl production, several metabolic engineering strategies have been designed to improve diacetyl production by lactic acid bacteria. Since citric acid is only a minor component in milk, most efforts have been directed at converting lactose into diacetyl. Studies based on the overproduction of ALS (als [13] or ilvBN [2]), inactivation of lactate dehydrogenase (LDH) (3, 13), pyruvate formate-lyase (1), or ALDB (15), or a combination of these strategies (13, 15), have resulted in efficient conversion of lactose and glucose into acetoin, especially in the case of LDH inactivation (13).
However, diacetyl production from all these engineered strains was low. Attempts to combine both LDH and ALDB inactivation in order to maximize the rerouting towards α-AL and diacetyl have so far been unsuccessful. Studies by Lopez de Felipe et al. (11) demonstrated that overproduction of the Streptococcus mutans NADH oxidase (NOX) in L. lactis resulted in a phenotype similar to that of the LDH-deficient strain described by Platteeuw et al. (12). In aerated cultures of L. lactis, more than 80% of the fermented sugar (glucose) was converted into acetoin.
In this paper, we describe the combination of NOX overproduction with ALDB inactivation in L. lactis. Under aerobic conditions, the engineered strain can convert glucose into diacetyl far more efficiently than any recombinant strain described to date.
NOX overproduction in an ALDB-deficient strain.
NOX overproduction in the presence of oxygen results in a large reduction of the intracellular pool of NADH (O2 + NADH → H2O + NAD+). Consequently, the pyruvate pool is rerouted through NADH-independent pathways (ALS, ALDB, phosphotransacetylase, and acetate kinase) and through the NAD+-dependent pyruvate dehydrogenase complex (Fig. 1), resulting in a mixture of acetoin and acetate. ALDB deficiency in this context, combined with a large reduction of the NADH-dependent diacetyl reductase activity (Fig. 1), should lead to prevention of acetoin formation, resulting in the production of large amounts of α-AL and diacetyl.
To facilitate nox overexpression using the nisin-controlled expression system (10) in an ALDB-deficient lactococcal background, the nisRK genes, necessary for nisin regulation, were integrated at the pepN locus of strain FI8076 (an aldB deletion derivative of MG1363 [15]), as described by de Ruyter et al. (5). The resulting strain, NZ9050, was transformed with plasmid pNZ2600, which contains the nox gene from S. mutans cloned under the control of the regulated nisA promoter (12).
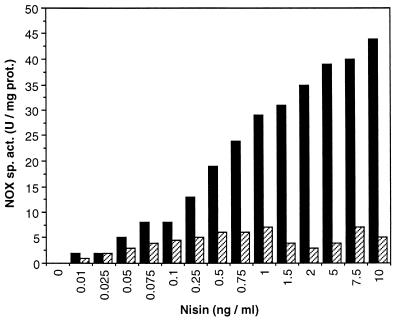
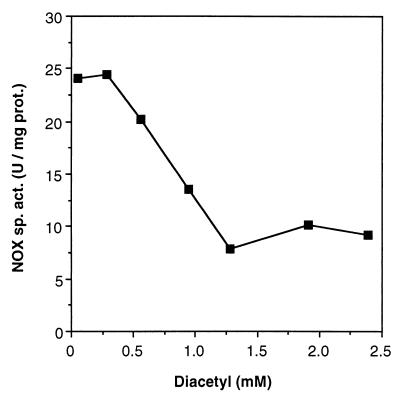
Cultures of L. lactis NZ9050(pNZ2600) were grown in GM17 (M17 medium [Merck] supplemented with 0.5% [wt/vol] glucose) with different concentrations of nisin (0 to 10 ng/ml). Cultivation conditions were either aerobic (with 300 rpm shaking in a G76 water bath; New Brunswick Scientific, Edison, N.J.) or unaerated (static cultivation). A high level of nisin-induced production of NOX was observed in unaerated cultures (Fig. 2). At a concentration of 10 ng of nisin per ml, NOX activity was more than 40 U of total proteins per mg of cell extracts from full-grown (16 h of cultivation) cultures. This activity is 400-fold higher than the endogenous NOX activity observed under uninduced conditions (0.1 U/mg) and 1,000-fold higher than in the wild-type strain NZ9050 (0.04 U/mg) under identical conditions. Under aerobic conditions, however, NOX activity did not exceed 5 U/mg (Fig. 2), although the total growth and the growth rate were very similar to growth under unaerated conditions. This relatively low NOX activity resulted in only a partial rerouting of the metabolic flux towards α-AL and diacetyl production (data not shown). The lower NOX activity under aerobic conditions than under unaerated conditions seemed to be a direct result of the changes in product formation. Exposing cells that were grown unaerated for 16 h in the presence of 2 ng of nisin per ml for high NOX induction (25 U/mg in cell extracts) to oxygen for 24 h did not lead to a decrease of enzyme activity. However, exposure of the same cells to diacetyl for 24 h resulted in a partial, but irreversible, decrease of the NOX activity, even under unaerated conditions (Fig. 3). Since diacetyl does not influence the catalytic reaction directly, decrease of NOX activity under aerobic conditions can only be explained by direct inactivation of the NOX enzyme by diacetyl. Noteworthy in this respect is the fact that diacetyl is generally considered to be an antimicrobial agent because of the presence of two reactive carbonyl moieties in the molecule (11). This inhibitory effect of diacetyl was not observed for any of the other metabolic enzymes tested (data not shown) and has not been specifically described to date.
FIG. 2.
Nisin-dependent induction of NOX activity in L. lactis NZ9050(pNZ2600) grown under aerobic (hatched bars) and unaerated (black bars) conditions. Cells were grown in the presence of the indicated nisin concentrations for 16 h at 30°C in GM17.
FIG. 3.
Inhibition of NOX activity by the presence of diacetyl in the growth medium. NOX activity was measured in cell extracts of L. lactis NZ9050(pNZ2600) grown in GM17 under unaerated conditions and induced with 2 ng of nisin per ml.
Use of resting cells as biocatalysts for α-AL and diacetyl production.
Since NOX activity was severely reduced in an irreversible manner during growth under aerobic conditions, we created a high-diacetyl-producing L. lactis system based on a two-step fermentation process. For this purpose, cultures of L. lactis strain NZ9050 harboring pNZ2600 were grown in GM17 under unaerated conditions and nisin was added at different concentrations (0 to 10 ng/ml) in the mid-exponential growth phase (optical density at 600 nm [OD600] = 0.5). Unaerated cultivation was continued until the cultures reached the end of the exponential growth phase (OD600 = 2.0). Cells were then harvested and resuspended in a small volume of 100 mM potassium phosphate buffer (pH 6.0) supplemented with glucose (15 mM), and the concentrated cell suspension (OD600 = 20) was incubated for 24 h at 30°C. Although NOX activity gradually decreased in nongrowing cells under aerobic incubation (reduction from 76 to 25 U/mg in 24 h), the net NOX activity remained much higher in the nongrowing cells than in growing cultures.
Glucose utilization and product formation was monitored in the nongrowing cell suspensions under aerobic and unaerated conditions. Glucose, organic acids, acetoin, ethanol, α-AL, and diacetyl were analyzed as previously described (8). The various end products formed from glucose fermentation after 24 h at maximal NOX induction are shown in Table 1. The carbon balances (corrected for CO2 production) calculated from the concentrations of the various end products were close to unity. Under unaerated conditions at maximal NOX induction, glucose was mainly converted into lactic acid (86% [percentage of total amount of carbon converted]), but some α-AL (9%), diacetyl (2%), and acetoin (2%) were also detected since the conditions were not completely anaerobic (static fermentations). Under aerobic conditions, lactic acid production decreased and α-AL and diacetyl production increased in correlation with NOX overproduction. In cells with a maximal induction of NOX, no more lactic acid production was observed and glucose was converted to α-AL (57%), diacetyl (16%), acetoin (5%), and acetic acid plus CO2 (21%) (Table 1). For wild-type cultures (L. lactis NZ9050), fermentation was completely (99%) homolactic under unaerated conditions, and under aerobic conditions lactic acid (75%), acetic acid (18%), α-AL (6%), diacetyl (0.7%), and acetoin (0.3%) were formed from glucose.
TABLE 1.
End product formation from cell suspensions of L. lactis NZ9050(pNZ2600)
| Growth conditions | Concn of end product (conversion efficiency [%])a
|
||||
|---|---|---|---|---|---|
| α-AL | Diacetyl | Acetoin | Lactic acid | Acetic acid | |
| Unaerated | 0.9 (9) | 0.3 (3) | 0.1 (1) | 17.2 (86) | 0.0 (0) |
| Aerobic | 5.7 (57) | 1.6 (16) | 0.5 (5) | 0.0 (0) | 4.2 (21) |
Cells were incubated in 100 mM potassium phosphate buffer containing 0.5% glucose for 24 h under unaerated and aerobic conditions. Before harvesting, cells were induced for NOX overexpression by the addition of 2 ng of nisin/ml during mid-exponential phase (OD600 = 1.0). Values are expressed in millimolar units produced per 10 mM glucose converted; values in parentheses are percentages of glucose converted. Standard deviations of the concentration data are routinely 0.1 mM.
Concluding remarks.
The efficient diacetyl production from glucose by L. lactis reported here results from the combination of two successful strategies aimed at increasing diacetyl production: (i) inactivation of the aldB gene to avoid enzymatic conversion of the diacetyl precursor α-AL to acetoin as described previously by several authors (4, 6, 8, 15) and (ii) high level NOX overproduction to reroute metabolic flux away from lactic acid production towards more oxidized products as described recently in our laboratory (12). Eighty percent of the pyruvate resulting from glucose breakdown was redirected towards production of α-AL in our engineered L. lactis strain. This efficiency of diacetyl production from glucose is by far the highest reported for engineered lactic acid bacteria (1, 2, 3, 4, 6, 8, 12, 13, 15). The only by-products from this metabolic engineering approach are acetate and CO2 formed via the pyruvate dehydrogenase complex pathway. By disrupting one of the pdh genes, an even more efficient production of α-AL and diacetyl could possibly be achieved.
The engineered strain described in this report was shown to be nearly optimal for sugar fermentation into a mixture of α-AL and diacetyl. However, for optimal production of diacetyl alone, a more efficient chemical conversion of α-AL into diacetyl should be developed. This may be achieved by extended aeration of the fermentation fluid, preferably at an even lower pH than used here for the fermentations.
Acknowledgments
This research was carried out in the framework of the European Community Research Programme BIOTECH (contract no. BIO4-CT96-0498). P.H. holds an EC BIOTECH postdoctoral training grant (contract no. BIO4-CT96-5093).
We thank F. Lopez de Felipe for supplying L. lactis strain NZ9800 containing plasmid pNZ2600 and for stimulating discussions. We are grateful to R. Holleman for high-pressure liquid chromatography analyses of culture supernatants.
REFERENCES
- 1.Arnau J, Jørgensen F, Madsen S M, Vrang A, Israelsen H. Cloning, expression, and characterization of the Lactococcus lactis pfl gene, encoding pyruvate formate-lyase. J Bacteriol. 1997;179:5884–5891. doi: 10.1128/jb.179.18.5884-5891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson K H, Godon J-J, Renault P, Griffin H G, Gasson M J. Effect of ilvBN-encoded α-acetolactate synthase expression on diacetyl production in Lactococcus lactis. Appl Microbiol Biotechnol. 1996;45:107–111. [Google Scholar]
- 3.Boumerdassi H, Monnet C, Desmazeaud M, Corrieu G. Isolation and properties of Lactococcus lactis subsp. lactis biovar diacetylactis CNRZ 483 mutants producing diacetyl and acetoin from glucose. Appl Environ Microbiol. 1997;63:2293–2299. doi: 10.1128/aem.63.6.2293-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curic M, Stuer-Lauridsen B, Renault P, Nilsson D. A general method for selection of α-acetolactate decarboxylase-deficient Lactococcus lactis mutants to improve diacetyl formation. Appl Environ Microbiol. 1999;65:1202–1206. doi: 10.1128/aem.65.3.1202-1206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Ruyter P G G A, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goupil N, Corthier G, Ehrlich S D, Renault P. Imbalance of leucine flux in Lactococcus lactis and its use for the isolation of diacetyl-overproducing strains. Appl Environ Microbiol. 1996;62:2636–2640. doi: 10.1128/aem.62.7.2636-2640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugenholtz J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:165–178. [Google Scholar]
- 8.Hugenholtz J, Starrenburg M J C. Diacetyl production by different strains of Lactococcus lactis subsp. lactis var. diacetylactis and Leuconostoc ssp. Appl Microbiol Biotechnol. 1992;38:17–22. [Google Scholar]
- 9.Jay J M. Antimicrobial properties of diacetyl. Appl Environ Microbiol. 1982;44:525–532. doi: 10.1128/aem.44.3.525-532.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuipers O P, de Ruyter P G G A, Kleerebezem M, de Vos W M. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21. [Google Scholar]
- 11.Lopez de Felipe F, Kleerebezem M, de Vos W M, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platteeuw C, Hugenholtz J, Starrenburg M, van Alen-Boerrigter I, de Vos W M. Metabolic engineering of Lactococcus lactis: influence of the overproduction of α-acetolactate synthase in strains deficient in lactate dehydrogenase as a function of culture conditions. Appl Environ Microbiol. 1995;61:3967–3971. doi: 10.1128/aem.61.11.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snoep J L, Teixeira de Mattos M J, Starrenburg M J C, Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and α-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992;174:4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swindell S R, Benson K H, Griffin H G, Renault P, Ehrlich S D, Gasson M J. Genetic manipulation of the pathway for diacetyl metabolism in Lactococcus lactis. Appl Environ Microbiol. 1996;62:2641–2643. doi: 10.1128/aem.62.7.2641-2643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]