Abstract
Background
Antimicrobial stewardship (AMS) programmes can improve the use of antimicrobial agents. However, there is limited experience in the implementation of such programmes in low- and middle-income countries (LMICs).
Objectives
To assess the effect of AMS measures in south-east Liberia on the quality of antimicrobial use in three regional hospitals.
Methods
A bundle of three measures (local treatment guideline, training and regular AMS ward rounds) was implemented and quality indicators of antimicrobial use (i.e. correct compounds, dosage and duration) were assessed in a case series before and after AMS ward rounds. Primary endpoints were (i) adherence to the local treatment guideline; (ii) completeness of the microbiological diagnostics (according to the treatment guideline); and (iii) clinical outcome. The secondary endpoint was reduction in ceftriaxone use.
Results
The majority of patients had skin and soft tissue infections (n = 108) followed by surgical site infections (n = 72), pneumonia (n = 64), urinary tract infection (n = 48) and meningitis (n = 18). After the AMS ward rounds, adherence to the local guideline improved for the selection of antimicrobial agents (from 34.5% to 61.0%, P < 0.0005), dosage (from 15.2% to 36.5%, P < 0.0005) and duration (from 13.2% to 31.0%, P < 0.0005). In total, 79.7% of patients (247/310) had samples sent for microbiological analysis. Overall, 92.3% of patients improved on Day 3 (286/310). The proportion of patients receiving ceftriaxone was significantly reduced after the AMS ward rounds from 51.3% to 14.2% (P < 0.0005).
Conclusions
AMS measures can improve the quality of antimicrobial use in LMICs. However, long-term engagement is necessary to make AMS programmes in LMICs sustainable.
Introduction
In the wake of the 2014–16 West African Ebola outbreak in Liberia, Guinea and Sierra Leone, the shortcomings of the national health systems became glaringly apparent.1 To improve the resilience to future health threats (e.g. epidemics, pandemics), West African countries have developed national policies to address health issues in line with the ‘Global action plan on antimicrobial resistance’.2 For instance, the Government of Liberia initiated their ‘Investment plan for building a resilient health system 2015–2021’, ‘National action plan for health security 2018–2022’ and ‘National infection prevention and control guideline 2018’.3 To avert the threat of antimicrobial resistance, the ‘National action plan on prevention and containment of antimicrobial resistance in Liberia 2018–2022’ was developed and numerous governmental and non-governmental health development partners were invited to align with the planned activities.3 Among others, strategic objectives of this national action plan on antimicrobial resistance are to improve awareness and understanding of antimicrobial resistance, to strengthen knowledge and evidence through surveillance and research and to optimize the use of antimicrobial agents.3
To address such objectives, antimicrobial stewardship (AMS) programmes can be effective to optimize the use of antimicrobial agents by applying a bundle of measures such as empirical therapy according to guidelines (e.g. if laboratory report is pending), bedside consultations/ward rounds with AMS teams and narrowing of antimicrobial therapy when appropriate.4 The Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH, funded by the Federal Ministry for Economic Cooperation and Development (BMZ), assigned Health Focus GmbH to set up an AMS programme in cooperation with Partners in Health (PIH) in south-east Liberia as part of its ‘Post-Ebola health systems strengthening and epidemic prevention’ and follow-on project ‘Health Systems Strengthening and Epidemic Prevention’.
While AMS programmes are well established in high income countries, there is limited experience in the implementation of such programmes in low- and middle-income countries (LMICs), particularly in Sub-Saharan Africa.5 However, studies from South Africa, Kenya, Sudan, Tanzania and Egypt showed that AMS programmes could be successfully implemented in Africa.6 Challenges of these programmes are inadequate financing and capacity, lack of access to appropriate technologies, overcrowded healthcare systems, limited knowledge and awareness, and a shortage of trained workforce.7–10
To support AMS activities and to define prescription targets, WHO developed a list of antimicrobials that were categorized into three groups: Access, Watch and Reserve (AWaRe).11 The Access group includes first- and second-line antimicrobials. The Watch and Reserve antimicrobials include critically important and last resort compounds, respectively. WHO has issued the target that at least 60% of antimicrobial consumption should be Access group antimicrobials.11 Surveys from Africa revealed that third-generation cephalosporins (Watch group) were the most commonly prescribed compounds (14.5%–28%).12,13 The reduction in use of these cephalosporins is therefore a common target in AMS programmes.
The objective of this study was to assess the effect of the AMS programme in south-east Liberia on the quality of antimicrobial use.
Materials and methods
Study sites
The AMS programme was launched in the south-east of Liberia in three counties (River Gee, Grand Kru and Maryland) in 2019. One governmental hospital from each of these counties was part of this multicentre implementation (Table 1). These three counties were chosen as priorities by the Ministry of Health and GIZ to demonstrate how AMS implementation can bring about improvement in clinical care in rural health-care settings. The JJ Dossen Memorial Hospital (JJDH) in Harper City, Maryland County is the regional referral hospital that receives patients from Fish Town Hospital (FTH), Rally Time Hospital (RTH) and neighbouring Côte d’Ivoire.
Table 1.
Hospitals participating in the AMS programme in south-east Liberia
| Name | JJDH | FTH | RTH |
|---|---|---|---|
| Place, county | Harper, Maryland | Fishtown, River Gee | Grand Cess, Grand Kru |
| No. of beds | 109 | 100 | 75a |
| Referral hospital | Yes | Yes | Yes |
| Services | Paediatrics, internal medicine, surgery, obstetrics/gynaecology, mental health, eye care | Paediatrics, internal medicine, surgery, obstetrics/gynaecology, mental health, eye care | Paediatrics, internal medicine, surgery, obstetrics/gynaecology, mental health |
| Pharmacy | Yes | Yes | Yes |
| Microbiology laboratory | Yes | No | No |
The standard number of beds as defined by the Ministry of Health is 100 but was temporarily reduced due to refurbishments.
A newly established microbiology laboratory at JJDH provides culture and antimicrobial susceptibility testing from blood culture bottles, urine, CSF, stool and swabs. This service is available for all three hospitals in this AMS programme. The hospital receives technical, personnel and financial support from BMZ through GIZ (Germany) and PIH (USA).
AMS programme and bundles
No AMS structures were in place in any of the participating hospitals at the beginning of our programme. During a stakeholder planning workshop (June 2019) we first identified the five most important clinical conditions that should be the focus of the new AMS programme [i.e. skin and soft tissue infection (SSTI), meningitis, urinary tract infection (UTI), community-acquired pneumonia, surgical site infection]. These target diseases were agreed between stakeholders, government representatives and external advisors.
The AMS teams consisted of a pharmacist, nurse, physician, infection prevention and control practitioners, laboratory scientists, technicians and hospital administrators.
The AMS programme comprised a bundle of three measures: (i) implementation of a local treatment guideline for antimicrobial therapy; (ii) training of prescribers (Table S1, available as Supplementary data at JAC-AMR Online); and (iii) regular AMS ward rounds (e.g. thrice a week).
A local treatment guideline (Appendix S1), which aligned with the national therapeutic guidelines of Liberia, was developed and approved by all AMS teams of each hospital.14 This guideline included regional antimicrobial resistance data from Sub-Saharan Africa and EUCAST dosing recommendations (https://www.eucast.org/clinical_breakpoints/) complementary to the national therapeutic guideline.15–21 Paediatric dosages were used as suggested.14 The local guideline defines a minimum microbiological diagnostic workup for each target disease (Appendix S1). The specimens are defined for meningitis (blood culture, CSF, swabs from suspected sources), SSTI (swabs, aspiration from pus/abscess, blood culture, tissue), community-acquired pneumonia (sputum, blood culture), UTI (urine) and surgical site infection (swabs, aspiration from pus/abscess, blood culture). The guideline was handed over to all AMS team members and prescribers in the three hospitals and workshops were held to orientate the teams to the guidelines.
The first-of-its kind microbiology laboratory at the JJDH was implemented in October 2019 and has been serving the far south-east region of Liberia. The laboratory is able to perform culture, identification and antimicrobial susceptibility testing apart from other analyses. Antimicrobial susceptibility testing is done using disc diffusion and EUCAST clinical breakpoints. The laboratory is not yet enrolled in an external quality assurance programme. Specimens for microbiology analyses from FTH and RTH were transported to JJDH microbiology laboratory for further analysis through thrice-weekly shuttle (on Mondays, Wednesdays and Fridays, Table 1).
Evaluation
The AMS programme was evaluated from December 2019 to December 2021. Eligible patients (i.e. those receiving antimicrobial therapy) were identified by antimicrobial prescription chart reviews.
The inclusion criteria were (i) admission to any of the three hospitals and (ii) antimicrobial treatment for SSTI, meningitis, UTI, community-acquired pneumonia or surgical site infection. No exclusion criteria were applied. Primary endpoints were (i) adherence to the local treatment guideline; (ii) completeness of the microbiological diagnostics (according to the treatment guideline, Appendix S1); and (iii) clinical outcome.6 The secondary endpoint was reduction of ceftriaxone use.22
A standardized questionnaire was designed to record the prescriptions of the physicians in charge and the recommendation of the AMS team as well as medical data (infection, samples taken, outcome; Appendix S2).
The indication for antimicrobial treatment was based on the physician’s judgement. The selection of compounds was rated as ‘correct’ if compounds were prescribed (e.g. ceftriaxone and ampicillin in the empirical treatment of meningitis) according to the guideline (empirical treatment) or the microbiological laboratory report (targeted treatment). Empirical treatment was defined as a treatment without supporting microbiological laboratory results at the time of the ward round. Targeted treatment was defined as a treatment based on species identification and antimicrobial susceptibility testing for the individual patient. If one compound of a recommended combination for empirical treatment was not prescribed, it was rated as ‘incorrect’. Similarly, the dosage or duration were classified as ‘incorrect’ if one compound of a combination therapy was wrongly dosed or the duration of one compound was incorrect. An external advisor (F.S.) assessed both the prescribers and the AMS team for the adherence to the guideline.
At least one of the recommended specimens had to be sent for culture and susceptibility testing to consider the recommendation as fulfilled.
The outcome was assessed on Day 3 post-enrolment into the study (e.g. no change, improvement/discharge, deterioration, death, referral).
Two months after the start, the AMS programme was further evaluated using the seven core elements for the evaluation of AMS programmes that are applicable also in resource-limited settings.23 This checklist includes ‘leadership’, ‘accountability and responsibilities’, ‘expertise’, ‘education and practical training’, ‘responsible antimicrobial use’, ‘monitoring and surveillance’ and ‘reporting and feedback’.
Statistical analysis
The design of the study was a case series analysis. Proportions (e.g. correct use of antimicrobial agents before and after a ward round) were compared with the two-proportions z-test as implemented in R (version 3.6.1) and the package epiDisplay.
Ethical approval
Ethical approval was granted by the University of Liberia, Pacific Institute for Research and Evaluation Review Board (17-06-048).
Results
In total, 310 patients were included in the analysis (JJDH, Harper: n = 249; FTH, Fishtown: n = 39; RTH, Grand Cess: n = 22). The majority of patients were admitted to internal medicine (n = 101, 32.6%) followed by paediatrics (n = 100, 32.3%), surgery (n = 61, 19.7%) and obstetrics/gynaecology (n = 48, 15.5%). The majority of patients was female (n = 172, 55.5%) and the median age of all patients was 27.5 years (range: 0.01–95).
The majority had SSTI (n = 108, 34.8%) followed by surgical site infections (n = 72, 23.2%), pneumonia (n = 64, 20.7%), UTI (n = 48, 15.5%) and meningitis (n = 18, 5.8%).
Overall, 96.5% of patients (n = 299) received empirical therapy, while 11 patients (7 SSTI, 4 surgical site infection) had targeted therapy based on microbiological analyses and antimicrobial susceptibility testing (i.e. 3 MSSA, 2 Pseudomonas aeruginosa, 2 Klebsiella pneumoniae, 1 ESBL-producing Escherichia coli, 1 Acinetobacter sp., 1 Enterobacter cloacae, 1 Burkholderia cepacia). Recommended samples were taken and sent for microbiological analyses in 79.7% (n = 247) of patients.
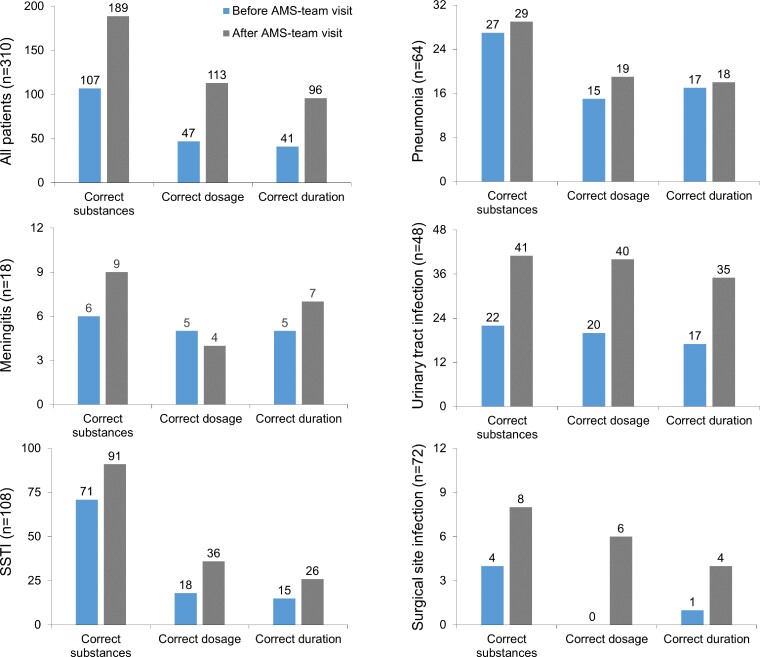
We assessed the quality of antimicrobial use (correct substances, dosage and duration) of prescribers and the recommendation of the AMS team in a structured case report from (Appendix S2). Overall, the selection of appropriate antimicrobials according to the guideline or laboratory reports improved after the AMS ward round from 34.5% (107/310) to 61.0% (189/310, P < 0.0005). The selection of antimicrobial compounds improved after the AMS round for each type of infection excluding pneumonia where there was still a low prescription rate of azithromycin (36%, 23/64) even after the AMS ward round (Figure 1).
Figure 1.
Quality of antimicrobial use before and after the ward round by the AMS team. The correct substance based on the local guideline (empirical treatment) or microbiological laboratory reports (targeted treatment), correct dosage and correct duration was rated for all patients (n = 310) or stratified to cases with meningitis (n = 18), SSTI (n = 108), community-acquired pneumonia (n = 64), urinary tract infection (n = 48) and surgical site infection (n = 72). The numbers on the bars represent the total number of patients with correct substances, dosage or duration.
The dosage was correct as prescribed by the treating physician in 15.2% of patients (47/310) prior to AMS ward rounds and improved to 36.5% (113/310, P < 0.0005) after the recommendation of the AMS team. Correct dosing was particularly challenging in cases with meningitis where the proportion of correct dosage dropped after the AMS visit. The major error was underdosing.
The documented duration of antimicrobial treatment was appropriate in only 13.2% of cases (41/310) before AMS rounds and improved to 31.0% (96/310, P < 0.0005) after the AMS ward rounds. In cases with pneumonia, the recommended duration was incorrect in 72% (46/64). In the majority of cases (before and after the AMS ward round), antimicrobial agents were prescribed for longer periods compared with the guideline recommendation. For instance, although the guideline recommends treatment for 5–7 days, ceftriaxone was prescribed for >7 days in 32.4% (56/173) or cloxacillin >7 days in 52.0% (118/227) of cases.
Overall, prescribers most frequently used ceftriaxone (51.3%, 159/310), followed by metronidazole (50.3%, 156/310) and cloxacillin (24.2%, 85/310, Table 2).
Table 2.
Evaluation of prescribers and the recommendation of the AMS teams at three hospitals in south-east Liberia
| Overall (n = 310) | Meningitis (n = 18) | SSTI (n = 108) | Community-acquired pneumonia (n = 64) | UTI (n = 48) | Surgical site infection (n = 72) | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years, median (range) | 27.5 (0.01–95) | 13 (0.1–52) | 32 (0.03–95) | 4 (0.01–82) | 36 (0.1–78) | 28.5 (0.7–76) |
| Female, % (n) | 55.5 (172) | 50 (9) | 52.8 (57) | 50 (32) | 69 (33) | 65 (47) |
| Prescription | ||||||
| Empirical therapy, % (n) | 96.5 (299) | 100 (18) | 93.5 (101) | 100 (64) | 100 (48) | 94 (68) |
| No. of antimicrobials non-indicateda, median (range) | 1 (0–3) | 1 (0–2) | 2 (0–3) | 1 (0–2) | 1 (0–2) | 2 (0–3) |
| Ranking of prescribed antimicrobial agents (Physician), drug, % (n) | ||||||
| First | Ceftriaxone, 51.3 (159) | Ceftriaxone, 89 (16) | Metronidazole, 61.1 (66) | Gentamicin, 78 (64) | Metronidazole, 48 (23) | Metronidazole, 75 (54) |
| Second | Metronidazole, 50.3 (156) | Metronidazole, 44 (8) | Cloxacillin, 52.8 (57) | Ampicillin, 56 (36) | Ciprofloxacin, 44 (21) | Ceftriaxone, 65 (47) |
| Third | Cloxacillin, 27.4 (85) | Ampicillin, 28 (5) | Ceftriaxone, 23.4 (56) | Ceftriaxone, 44 (28) | Doxycycline, 40 (19) | Cloxacillin, 38 (27) |
| Ranking of prescribed antimicrobial agents (AMS team), drug, % (n) | ||||||
| First | Cloxacillin, 45.8 (142) | Ceftriaxone, 78 (14) | Cloxacillin, 75 (81) | Azithromycin, 61 (39) | Ciprofloxacin, 88 (42) | Cloxacillin, 85 (61) |
| Second | Metronidazole, 36.5 (113) | Ampicillin, 56 (10) | Metronidazole, 54.6 (59) | Ampicillin, 56 (36) | Metronidazole, 4 (2) | Metronidazole, 69 (50) |
| Third | Ciprofloxacin, 20.6 (64) | Metronidazole, 11 (2) | Ciprofloxacin, 11 (12) | Ceftriaxone, 44 (28) | Ampicillin, 4 (2) | Ciprofloxacin, 14 (10) |
| Adherence to the guideline, % (n) | ||||||
| Correct sampling | 79.7 (247) | 67 (12) | 82.4 (89) | 61 (39) | 85 (41) | 92 (66) |
| Recommendation followed | 84.2 (261) | 98 (16) | 78.7 (85) | 83 (53) | 85 (41) | 92 (66) |
| Non-availability of recommended drug | 2.5 (8) | 0 (0) | 0.9 (1) | 2 (1) | 10 (5) | 1 (1) |
| Outcome, % (n) | ||||||
| No change | 2.3 (7) | 6 (1) | 1.9 (2) | 2 (1) | 0 (0) | 4 (3) |
| Improvement/discharge | 92.3 (286) | 89 (16) | 90.7 (98) | 94 (60) | 69 (46) | 92 (66) |
| Deterioration | 1.6 (5) | 0 (0) | 0 (0) | 2 (1) | 4 (2) | 3 (2) |
| Death | 3.2 (10) | 6 (1) | 5.6 (6) | 3 (2) | 0 (0) | 1 (1) |
| Referral | 0.7 (2) | 0 (0) | 1.9 (2) | 0 (0) | 0 (0) | 0 (0) |
According to the guideline.
The proportion of patients receiving ceftriaxone was significantly reduced after the AMS ward rounds from 51.3% (159/310) to 14.2% (44/310, P < 0.0005). Similarly, a reduction was also observed for gentamicin (22.9% versus 5.2%, P < 0.0005) and metronidazole (50.3% versus 36.5%, P < 0.0005). While ampicillin was equally used before and after the AMS visit (19.0% versus 15.5%, P = 0.3), usage increased for oxacillin (24.2% versus 45.8%, P < 0.0005) and ciprofloxacin (13.9% versus 20.3%, P = 0.04).
In general, the majority of the recommendations of the AMS team were followed (84.2%, 261/310, Table 2); only a small proportion of recommendations could not be followed due to non-availability of drugs. The majority of patients improved on Day 3 (92.3%, 286/310, Table 2). Death (n = 10) was not associated with incorrect recommendation nor with not following the recommendations (P > 0.5).
Seven months after preparing the infrastructure for the project and 2 months after starting the AMS ward rounds, the AMS programme at JJDH was systematically assessed in the context of established core elements of hospital AMS programmes.23 While accountability and responsibility were clearly defined including a written AMS programme strategy and formal organizational multidisciplinary structure, we identified major challenges. These refer to senior hospital management leadership towards AMS (e.g. insufficient sustainable financial support), available expertise on infection management (e.g. limited access to experienced healthcare professionals), education and practical training (e.g. limited educational resources and regular training), monitoring and surveillance (e.g. compliance with one or more of the specific interventions, susceptibility rates and antimicrobial use are not regularly monitored) and reporting and feedback (e.g. reports on antibiotic susceptibility rates and antimicrobial use were not shared with prescribers).
Discussion
We used a case series to analyse the quality of a newly implemented AMS programme in an LMIC. Our focus was not to measure the effectiveness (e.g. reduced length of stay, reduction of colonization or infection with drug-resistant bacteria) but to assess the quality of antimicrobial use and to identify challenges.
The total number of 310 documented cases during a 2 year observation period may appear small but should be interpreted against the background of the ongoing SARS-CoV2 pandemic (e.g. AMS activities needed to be reduced, on-site training by external experts was cancelled).
The good use of the microbiology laboratory on the one hand (79.7% of patients had a sample sent for culture/susceptibility) and the high proportion of empirical therapy on the other hand (96.5%, Table 2) could point towards two issues. Either results do not reach prescribers (due to excessive turnaround time) or are not used to modify the antimicrobial treatment despite being available. A Gabonese laboratory with a quality management system also observed that laboratory reports were rarely used to change antimicrobial treatment of SSTI pointing towards post-analytical errors.24 These findings underline the perception that clinicians may not be appreciative of the value of microbiological testing; hence, education on the use of microbiological data and communication between clinicians and the microbiology laboratory need to be improved.
Although the AMS ward rounds improved the quality of antimicrobial prescriptions in terms of agent, dose and duration, there was still a high proportion of wrong selection of compounds (39%, 121/310), incorrect dosage (63.5%, 197/310) and incorrect duration (69%, 214/310) after the AMS review (Figure 1). This is in line with a high proportion of wrong choice of antimicrobials for the treatment of pneumonia in Ghana (67.5%).25 There is now agreement that only long-term and multifaceted interventions can lead to behaviour changes an improvement in prescribing.26 These long-term engagements should be flanked by regular educational activities and point-prevalence studies to identify areas for improvement.27
Short-term achievements in appropriate antimicrobial use can be turned back or even return to baseline if the intervention is too short or does not effectively involve local prescribers, as shown in Sierra Leone.28
AMS is only one tool to stem the tide of antimicrobial resistance. Another powerful tool in resource-limited settings is improved diagnostics. For instance, the implementation of point-of-care testing for common viruses (influenza, SARS-CoV) in combination with biomarker assays (PCT, CRP) could reduce the use of antimicrobial agents by 83%–35%.29
When reducing antibiotics, one must not lose sight of the quality of medical care. This becomes even more complicated in settings such as LMICs where access to antimicrobials is a bigger challenge as the non-availability of antibiotics most likely causes more deaths than antimicrobial resistance.30 Thus, AMS ‘is not only about reducing inappropriate use but also about assuring access to effective treatment’ as Cox et al. bring it to the point.5
Our study has limitations. First, the evaluation of the adherence to the guideline depends on the quality of the guideline. Such a local guideline is in many aspects a compromise of (differing) national and international recommendations (e.g. dosage, duration or selection of compound). Therefore, non-adherence to the guideline could be ultimately positive for patients if the guideline is not tailored to the local needs. Second, our case series might not be representative for all LMICs, as all prescribers had access to a microbiology service; an advantage that is not available for many working in LMICs. Third, the study could be biased. We relied on provider diagnosis/classification of the five conditions (SSTI, UTI, pneumonia, meningitis and surgical site infection) without any secondary verification (reporting bias); we also relied on ward teams to identify all of the cases for AMS rounds so it is possible that some cases were missed (selection bias). Fourth, we focused on the evaluation of antimicrobial treatment and omitted prescriptions for prophylaxis. The latter was the main reason for antimicrobial use (e.g. in obstetric or gynaecological surgery) and could be a target of future interventions.31
In conclusion, AMS programmes can improve the quality of antimicrobial use in LMICs. In addition to changing prescribing behaviour, AMS programmes face the challenge of underfunding and limited access to experts, expertise and training. Long-term engagement is therefore necessary to make AMS programmes in LMICs sustainable.
Supplementary Material
Acknowledgements
We would like to thank the Ministry of Health & Social Welfare, Republic of Liberia; International Development Partners (Gesellschaft für Internationale Zusammenarbeit, Partners in Health, Health Focus GmbH); members of the Maryland, Grand Kru and River Gee County Health Teams; and the participating communities in south-east Liberia for their support.
Contributor Information
Abraham S Alabi, Health Focus GmbH, Friedrich-Ebert-Straße 33, 14469 Potsdam, Germany.
Stephen W Picka, Health Focus GmbH, Friedrich-Ebert-Straße 33, 14469 Potsdam, Germany.
Reubvera Sirleaf, Health Focus GmbH, Friedrich-Ebert-Straße 33, 14469 Potsdam, Germany.
Pacifique R Ntirenganya, Partners In Health, Sophie Road Oldest, Congo Town, Monrovia, Liberia.
Arnold Ayebare, Partners In Health, Sophie Road Oldest, Congo Town, Monrovia, Liberia.
Nidia Correa, Partners In Health, Boston, MA, USA.
Sarah Anyango, Partners In Health, Sophie Road Oldest, Congo Town, Monrovia, Liberia.
Gerald Ekwen, Partners In Health, Sophie Road Oldest, Congo Town, Monrovia, Liberia.
Emmanuel Agu, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Rebecca Cook, Partners In Health, Sophie Road Oldest, Congo Town, Monrovia, Liberia; Partners In Health, Boston, MA, USA.
John Yarngrorble, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Ibrahim Sanoe, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Henry Dugulu, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Emmanuel Wiefue, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Diana Gahn-Smith, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Francis N Kateh, Ministry of Health, Capitol Bye-Pass, Monrovia, Liberia.
Ezekiel F Hallie, School of Pharmacy, University of Liberia, Monrovia, Liberia.
Christiane G Sidonie, Centre de Recherches Medicales, CERMEL, Lambarene, Gabon.
Aaron O Aboderin, Obafemi Awolowo University, Ile-Ife, Nigeria.
David Vassellee, German Corporation for International Cooperation, GIZ, Tubman Boulevard, Congo Town, Monrovia, Liberia.
Damien Bishop, German Corporation for International Cooperation, GIZ, Tubman Boulevard, Congo Town, Monrovia, Liberia.
Daniel Lohmann, German Corporation for International Cooperation, GIZ, Tubman Boulevard, Congo Town, Monrovia, Liberia.
Manja Naumann-Hustedt, Health Focus GmbH, Friedrich-Ebert-Straße 33, 14469 Potsdam, Germany.
Alois Dörlemann, Health Focus GmbH, Friedrich-Ebert-Straße 33, 14469 Potsdam, Germany.
Frieder Schaumburg, Institute of Medical Microbiology, University of Münster, Münster, Germany.
Funding
This work was supported by the Federal Ministry for Economic Cooperation and Development (BMZ) through Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ), Liberia (Health Project No: 15.2174.9-001.00).
Transparency declarations
Abraham S. Alabi, Stephen W. Picka, Reubvera Sirleaf, Manja Naumann-Hustedt and Alois Dörlemann are employed through Health Focus GmbH; Frieder Schaumburg received reimbursement for assisting with preparing the manuscript from Health Focus GmbH. All other authors: none to declare.
Supplementary data
Table S1 and Appendices S1 and S2 are available as Supplementary data at JAC-AMR Online.
References
- 1. CDC . 2014-2016 Ebola Outbreak in West Africa. https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html. [DOI] [PMC free article] [PubMed]
- 2. WHO . Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/publications/i/item/9789241509763.
- 3. Government of Liberia-Ministry of Health . National Action Plan on Prevention and Containment of Antimicrobial Resistance in Liberia 2018-2022. Government of Liberia-Ministry of Health, 2018. [Google Scholar]
- 4. Schuts EC, Hulscher M, Mouton JW et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. [DOI] [PubMed] [Google Scholar]
- 5. Cox JA, Vlieghe E, Mendelson M et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. [DOI] [PubMed] [Google Scholar]
- 6. Akpan MR, Isemin NU, Udoh AE et al. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist 2020; 22: 317–24. [DOI] [PubMed] [Google Scholar]
- 7. Kakkar AK, Shafiq N, Singh G et al. Antimicrobial stewardship programs in resource constrained environments: understanding and addressing the need of the systems. Front Public Health 2020; 8: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kpokiri EE, Taylor DG, Smith FJ. Development of antimicrobial stewardship programmes in low and middle-income countries: a mixed-methods study in Nigerian hospitals. Antibiotics (Basel) 2020; 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huong VTL, Ngan TTD, Thao HP et al. Assessing feasibility of establishing antimicrobial stewardship programmes in two provincial-level hospitals in Vietnam: an implementation research study. BMJ Open 2021; 11: e053343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalungia AC, Mwambula H, Munkombwe D et al. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: implications for future policy and practice. J Chemother 2019; 31: 378–87. [DOI] [PubMed] [Google Scholar]
- 11. WHO . 2021 AWaRe Classification. https://www.who.int/publications/i/item/2021-aware-classification. [Google Scholar]
- 12. Hsia Y, Lee BR, Versporten A et al. Use of the WHO access, watch, and reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health 2019; 7: e861–e71. [DOI] [PubMed] [Google Scholar]
- 13. Pauwels I, Versporten A, Drapier N et al. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother 2021; 76: 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Government of Liberia-Ministry of Health . National Standard Therapeutic Guidelines and Essential Medicines List. Government of Liberia-Ministry of Health, 2017. [Google Scholar]
- 15. Emele F. Etiologic spectrum and pattern of antimicrobial drug susceptibility in bacterial meningitis in Sokoto, Nigeria. Acta Paediatr 2000; 89: 942–6. [DOI] [PubMed] [Google Scholar]
- 16. Alabi AS, Frielinghaus L, Kaba H et al. Retrospective analysis of antimicrobial resistance and bacterial spectrum of infection in Gabon, Central Africa. BMC Infect Dis 2013; 13: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aston SJ, Ho A, Jary H et al. Etiology and risk factors for mortality in an adult community-acquired pneumonia cohort in Malawi. Am J Respir Crit Care Med 2019; 200: 359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aston SJ, Rylance J. Community-acquired pneumonia in Sub-Saharan Africa. Semin Respir Crit Care Med 2016; 37: 855–67. [DOI] [PubMed] [Google Scholar]
- 19. Bernabé KJ, Langendorf C, Ford N et al. Antimicrobial resistance in West Africa: a systematic review and meta-analysis. Int J Antimicrob Agents 2017; 50: 629–39. [DOI] [PubMed] [Google Scholar]
- 20. Lai PS, Bebell LM, Meney C et al. Epidemiology of antibiotic-resistant wound infections from six countries in Africa. BMJ Glob Health 2017; 2: e000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allegranzi B, Bagheri Nejad S, Combescure C et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011; 377: 228–41. [DOI] [PubMed] [Google Scholar]
- 22. Bishaw B M, Tegegne GT, Berha AB. Appropriate use of ceftriaxone in Sub-Saharan Africa: a systematic review. Infect Drug Resist 2021; 14: 3477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pulcini C, Binda F, Lamkang AS et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin Microbiol Infect 2019; 25: 20–5. [DOI] [PubMed] [Google Scholar]
- 24. Alabi A, Kazimoto T, Lebughe M et al. Management of superficial and deep-seated Staphylococcus aureus skin and soft tissue infections in Sub-Saharan Africa: a post hoc analysis of the StaphNet cohort. Infection 2018; 46: 395–404. [DOI] [PubMed] [Google Scholar]
- 25. Sefah IA, Essah DO, Kurdi A et al. Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: findings and implications for the future. JAC Antimicrob Resist 2021; 3: dlab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pouly E, Coppry M, Rogues AM et al. Systematic review of factors promoting behaviour change toward antibiotic use in hospitals. Clin Microbiol Infect 2022; 10.1016/j.cmi.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 27. Godman B, Egwuenu A, Haque M et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life (Basel) 2021; 11: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamilton D, Bugg I. Improving antimicrobial stewardship in the outpatient department of a district general hospital in Sierra Leone. BMJ Open Qual 2018; 7: e000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ciccone EJ, Kabugho L, Baguma E et al. Rapid diagnostic tests to guide case management of and improve antibiotic stewardship for pediatric acute respiratory illnesses in resource-constrained settings: a prospective cohort study in Southwestern Uganda. Microbiol Spectr 2021; 9: e0169421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laxminarayan R, Matsoso P, Pant S et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–75. [DOI] [PubMed] [Google Scholar]
- 31. D'Arcy N, Ashiru-Oredope D, Olaoye O et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics (Basel) 2021; 10: 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.