Abstract
Background
Immune checkpoint inhibitors (ICI) improve survival in patients with late-stage esophageal squamous cell carcinoma (ESCC) but have not been fully evaluated in locally advanced ESCC.
Method
We retrospectively assessed outcomes of consecutive, treatment-naïve locally advanced ESCC (stage III or IVA) adults treated with neoadjuvant ICI plus chemotherapy followed by surgery, who refused or lacked access to radiotherapy, with regards to surgery feasibility, pathological response, and relapse-free survival (RFS).
Results
We uneventfully treated 34 patients with the combined regimen in 2020. None reported grade III or higher toxic effects. All underwent surgery as planned: 32 received complete (R0) resections and 2 had microscopically positive margins (R1). Tumor downstaging occurred in 33 (97.1%) patients and 11 (32.4%) had pathologically complete response of the primary lesion. Median postoperative length of stay was 12 days (interquartile range: 11 to 17). All patients resumed a semi-liquid diet on discharge. The 90-day postoperative morbidity rate was 20.6% (7/34) with no mortalities. The 1-year RFS was 77.8% [95% CI, 64.2-94.2].
Conclusion
Neoadjuvant ICI plus chemotherapy was safe and resulted in significant downstaging, rendering inoperable tumors operable, relieving symptoms of dysphagia and prolonging survival for locally advanced ESCC patients who refused or lacked access to radiotherapy.
Keywords: esophageal squamous cell carcinoma, neoadjuvant therapy, immune checkpoint inhibitor, esophagectomy, perioperative outcomes, survival outcomes
Introduction
Esophageal cancer is the sixth most common cause of cancer-related death worldwide and is therefore a major global health challenge (1). Esophageal squamous cell carcinoma (ESCC) is the main histologic type in East Asian and Middle Eastern countries. At the time of their first diagnosis, 40-50% of ESCC present as locally advanced esophageal cancer that invades local structures or involves regional lymph nodes but without distant metastases (2, 3). Surgery is recognized as the definitive treatment for this cancer, but the prognosis is poor with esophagectomy alone, mostly due to relapse of residual disease (4, 5). Neoadjuvant chemoradiotherapy followed by surgery, has shown promising survival benefit and been recommended as the standard management for resectable ESCC patients (6–9). However, radiotherapy has been reported to have a high risk of side effects that could preclude the planned surgical procedure (10–13). Moreover, it is not always available due to the lack of access to radiotherapy worldwide, especially in many low- and middle-income countries (14).
Compared to the standard strategy of preoperative chemoradiotherapy, the current neoadjuvant chemotherapy regimen without radiotherapy has significantly low disease-control rate and inferior histopathologic outcomes for locally advanced ESCC (12). Therefore, it is imperative to develop novel alternative treatment options for those who refuse or lack of access to radiotherapy. Immune checkpoint inhibitors (ICI), including both pembrolizumab and camrelizumab, combined with chemotherapy have recently been reported to be safe and effective in patients with late-stage ESCC (15, 16). One ICI, nivolumab, improved relapse-free survival (RFS) when used as adjuvant therapy in stage II/III resected esophageal cancer (17). This study was aimed to explore the preliminary outcomes of neoadjuvant ICI plus chemotherapy followed by surgery for patients with treatment-naïve, locally advanced ESCC.
Patients and Methods
Study Design
We performed this retrospective analysis of prospective collected data at a single medical institute. From January 1st, 2020 to December 31st, 2020, data of consecutive ESCC patients were prospectively collected in Fudan University Shanghai Cancer Center (FUSCC). The Institutional Review Board of FUSCC approved this study. All the patients provided written informed consents.
Patient Eligibility
All patients underwent baseline tumor staging, including contrast-enhanced computed tomography (CT) of the chest and upper abdomen, ultrasound of the neck, and endoscopic ultrasound (EUS) of upper digestive tract with biopsy if necessary. Positron emission tomography (PET)/CT was suggested for those patients who could afford it as it was not covered by the common healthcare insurance yet (18–20).
Eligible patients were between 18 and 75 years of age, and had treatment-naïve ESCC located in the middle and lower thoracic esophagus and clinically staged as T3 to T4aN1 to N3 with no evidence of distant metastasis (M0) according to the American Joint Committee on Cancer (AJCC) 8th staging system (21). All the patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, adequate cardiopulmonary function, and no surgical contradictions. Key exclusion criteria were signs of esophageal perforation, immunodeficiency, ongoing systemic immunosuppressive therapy, active autoimmune or infectious disease, and clinically significant concurrent cancers.
Treatment Protocol
The patients received two doses of intravenous pembrolizumab or camrelizumab (both at a dose of 200 mg every 3 weeks) plus chemotherapy with paclitaxel (260 mg/m2 every 3 weeks) and cisplatin (75 mg/m2 every 3 weeks) for 2 cycles. Surgery was planned to be performed within 14 weeks after the last dose if the patients met the following surgical criteria: 1) the tumor was considered to completely resectable upon evaluation of the multidisciplinary team; 2) the patient had the physiological conditions for upper gastrointestinal reconstruction after esophagectomy; 3) there’s no contraindications to general anesthesia; 4) the patient refused radiation therapy. The primary end points were surgery feasibility rate, including the proportion of patients able to undergo surgery after neoadjuvant therapy, completeness of resection, and 90-day post-operative morbidity and mortality rates. The secondary end points were pathologic response and RFS rates. Drug toxicities were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). Changes in tumor size were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Surgical procedures of esophagectomy and lymph node dissection were conducted according to FUSCC institutional standards (22–25). The complications were specified and evaluated based on the International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy (26) and the Clavien-Dindo classification of surgical complications (27).
Pathological Assessment
Surgical specimens were assessed and staged according to the AJCC 8th criteria for evaluating tumor size, invasion depth, resection margin, and affected lymph nodes, for the percentage of residual viable tumor that was identified on routine hematoxylin and eosin staining (21). Pathologic response was evaluated and classified using the internationally recognized standards of tumor regression grade (TRG) system, based on two parameters of histomorphologic tumor regression and lymph node status (ypN) (28). Those with no evidence of vital residual tumor cells in both primary tumor and lymph nodes were considered to have pathological complete response (pCR).
Statistical Analysis
The patients were characterized by demographic and clinicopathologic variables. Differences in patient features were evaluated using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. All statistical analyses were two-sided, with p < 0.05 indicative of statistical significance, and performed using SPSS (version 22.0 IBM Corporation, Armonk, NY) and R 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Thirty-four patients underwent treatment with the combined protocol during the 1-year study period ( Table 1 ). They had either stage III (41.2%) or IVA (58.8%) ESCC and received two cycles of chemotherapy plus pembrolizumab (n=20) or camrelizumab (n=14). The median age of these patients was 61 years (range: 47-74). The majority of the cohort consisted of males (91.2%) and smokers (59%). Nearly half of the patients (47.1%) were documented to have a history of alcohol addiction. On initial evaluation by the thoracic surgeons, all patients were considered to have tumors that could not be completely resected.
Table 1.
Baseline demographics and clinical characteristics.
| Variables (%) | All Patients (N=34) | Pembrolizumab (N=20) | Camrelizumab (N=14) |
|---|---|---|---|
| Sex | |||
| Female | 3 (8.8%) | 0 | 3 (21.4%) |
| Male | 31 (91.2%) | 20 (100%) | 11 (78.6%) |
| Age | |||
| Median (range) | 61 (47-74) | 60.5 (47-74) | 63 (55-68) |
| ECOG | |||
| 0 | 25 (73.5%) | 15 (75%) | 10 (71.4%) |
| 1 | 9 (26.5%) | 5 (25%) | 4 (28.6%) |
| BMI, kg/m2 | |||
| Median (range) | 22.2 (14.7-29.8) | 21.6 (14.7-26.0) | 22.4 (17.5-29.8) |
| Smoking | 20 (59%) | 14 (70%) | 6 (43%) |
| Alcohol addiction | 16 (47.1) | 12 (60%) | 4 (28.5%) |
| Tumor location | |||
| Middle | 25 (73.5%) | 13 (65%) | 12 (85.7%) |
| Lower | 9 (26.5%) | 7 (35%) | 2 (14.3%) |
| Clinical T stage | |||
| cT3 | 26 (76.5%) | 15 (75%) | 11 (78.6%) |
| cT4a | 8 (23.5%) | 5 (25%) | 3 (25%) |
| Clinical N stage | |||
| cN2 | 16 (47%) | 9 (45%) | 7 (50%) |
| cN3 | 18 (53%) | 11 (55%) | 7 (50%) |
| Clinical stage | |||
| III | 14 (41.2) | 8 (40%) | 6 (43%) |
| IVA | 20 (58.8) | 12 (60%) | 8 (57%) |
| Grade | |||
| G1 | 1 (3%) | 1 (5%) | 0 |
| G2 | 13 (38.2%) | 9 (45%) | 4 (28.6%) |
| G3 | 9 (26.5%) | 4 (20%) | 5 (35.7%) |
| GX | 11 (32.3%) | 6 (30%) | 5 (35.7%) |
Safety and Feasibility
Therapy-related adverse events of any grade during the neoadjuvant regimen occurred in 58.8% (20/34) patients, but none were grade III or higher. The most common adverse incidents were grade 1 digestive tract-associated side effects (8/34), such as nausea, vomiting and diarrhea. Another adverse event of high incidence was reactive capillary endothelial proliferation (7/34; grade 1 in 6 patients and grade 2 in one patient), which was commonly associated with camrelizumab (16). The median interval between the administration of the second dose and surgery was 5 weeks (range 4-8 weeks), and there were no therapy-related surgical delays. FUSCC multidisciplinary team for thoracic cancer evaluated the medical data of each patient including the symptoms, endoscopic and radiological findings. All patients showed partial response (PR) or stable disease (SD) and underwent surgery with intent to curative treatment; 16 (47%) received esophagectomy with 2-field lymphadenectomy whereas 18 (53%) had 3-field lymphadenectomy. Regarding the anastomotic site, half of the patients underwent intra-thoracic anastomosis and half cervical procedure. The average operating time was 211 ± 47 min, and the intraoperative blood loss was 144 ± 126 ml. There were 7 (20.6%) patients who experienced postoperative complications, which were all below grade IIIa according to Clavien-Dindo classification. No patients died within 90 days after surgery. All patients resumed a semi-liquid diet at the time of discharge which relieved their chief complaint of dysphagia noted at the initial clinic visit. ( Tables 2 , 3 ).
Table 2.
Adverse events during neoadjuvant immune checkpoint inhibitors plus chemotherapy and postoperative complications.
| Events (%) | All patients (N=34, %) | Grade | Pembrolizumab (N=20) | Camrelizumab (N=14) |
|---|---|---|---|---|
| All events during neoadjuvant therapy | 20 (58.8%) | / | 6 (30%) | 14 (100%) |
| Nausea/vomiting/diarrhea | 8 | I | 4 | 4 |
| Reactive capillary endothelial proliferation | 7 | I/II | 0 | 7 |
| Fatigue | 3 | I | 1 | 2 |
| Leukopenia | 2 | I | 1 | 1 |
| All postoperative complications | 7 (20.6%) | / | 5 (25%) | 2 (14.3%) |
| Anastomotic leak | 3 | II | 2 | 1 |
| Pneumonia | 2 | II | 2 | 0 |
| hoarseness | 1 | I | 1 | 0 |
| Subcutaneous emphysema | 1 | I | 0 | 1 |
| 90-day Postoperative mortality | 0 | / | 0 | 0 |
Drugs toxicity was assessed and graded according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). Postoperative complications were evaluated by the Clavien-Dindo classification.
Table 3.
Surgery, pathologic response and survival outcomes.
| Variables (%) | All Patients (N=34) | Pembrolizumab (N=20) | Camrelizumab (N=14) |
|---|---|---|---|
| Operative duration, mean (SD), min | 211 (47) | 204 (40) | 222 (55) |
| Estimated blood loss, mean (SD), mL | 144 (126) | 144 (126) | 144 (127) |
| Postoperative hospital stay, median (IQR), day | 12 (11-17) | 13 (11-23) | 12 (10-13) |
| Completeness of Resection | |||
| R0 | 32 (94.1%) | 19 (95%) | 13 (92.9%) |
| R1 | 2 (5.9%) | 1 (5%) | 1 (7.1%) |
| Lymph nodes resected, median (IQR), No | 30 (25-38) | 31 (25-37) | 27 (23-37) |
| Complete response of primary tumor | 11 (32.4%) | 6 (30%) | 5 (35.7%) |
| TRG1 | 8 (23.4) | 4 (20) | 4 (28.6) |
| TRG | |||
| 1 | 8 (23.5%) | 4 (20%) | 4 (28.6%) |
| 2 | 3 (8.8%) | 2 (10%) | 1 (7.1%) |
| 3 | 7 (20.6) | 5 (25%) | 2 (14.3%) |
| 4 | 16 (47.1%) | 9 (45%) | 7 (50%) |
| ypT | |||
| 0 | 11 (32.4%) | 6 (30%) | 5 (35.7%) |
| 1 | 6 (17.6%) | 5 (25%) | 1 (7.2%) |
| 2 | 5 (14.7%) | 3 (15%) | 2 (14.2%) |
| 3 | 12 (35.3%) | 6 (30) | 6 (42.9%) |
| ypN+ | 19 (55.9%) | 11 (55%) | 8 (57%) |
| ypStage | |||
| I | 13 (38.2%) | 7 (35%) | 6 (42.9%) |
| II | 2 (5.9%) | 5 2(10%) | 0 |
| III | 12 (35.3%) | 6 (30%) | 6 (42.9) |
| IV | 7 (20.6%) | 5 (25%) | 2 (14.2%) |
| 1-year relapse events | 7 (20.6%) | 5 (25%) | 2 (14.2%) |
| 1-year RFS,% (95%CI) | 77.8 (64.2-94.2) | 72.7 (54.5-97) | 85.7 (69.2-100) |
IQR, interquartile range; pCR, pathological complete response; defined as no evidence of residual viable tumor cells in the resected primary tumor and lymph nodes. TRG, tumor regression grade, based on two parameters of histomorphologic tumor regression and lymph node status (ypN) (28). RFS, relapse-free survival.
Radiologic and Pathologic Response
Representative radiologic responses after two preoperative doses of ICI plus chemotherapy are shown in Figure 1 . The evaluation and comparison of the radiographic results before and after the neoadjuvant therapy were described previously (29). Surgical pathology revealed 32 (94.1%) patients had complete resection (R0) of the primary tumor and the local lymph nodes, while 2 (5.9%) had macroscopic negative resection but positive circumferential margins microscopically (R1) on the resected esophagus. The median number of resected lymph nodes for each patient was 30 (interquartile range (IQR): 25 to 38). Based on the 8th AJCC system for clinical and pathologic staging, all patients were down-staged after neoadjuvant therapy; 28 (82.3%) patients had significant tumor (T) shrinkage, and 27 (79.4%) had nodal (N) downstaging. Tumor regression grade (TRG) I, II, III and IV, defined by two parameters of histomorphologic tumor regression and lymph node status (ypN) (28), were observed in 23.5%, 8.8%, 20.6% and 47.1% of patients, respectively. Complete pathologic response of the primary tumor site (ypT0) was seen in 11 (32.4%) patients, but 3 of them had residual cancer cells in the resected lymph nodes ( Table 3 and Supplementary Table ).
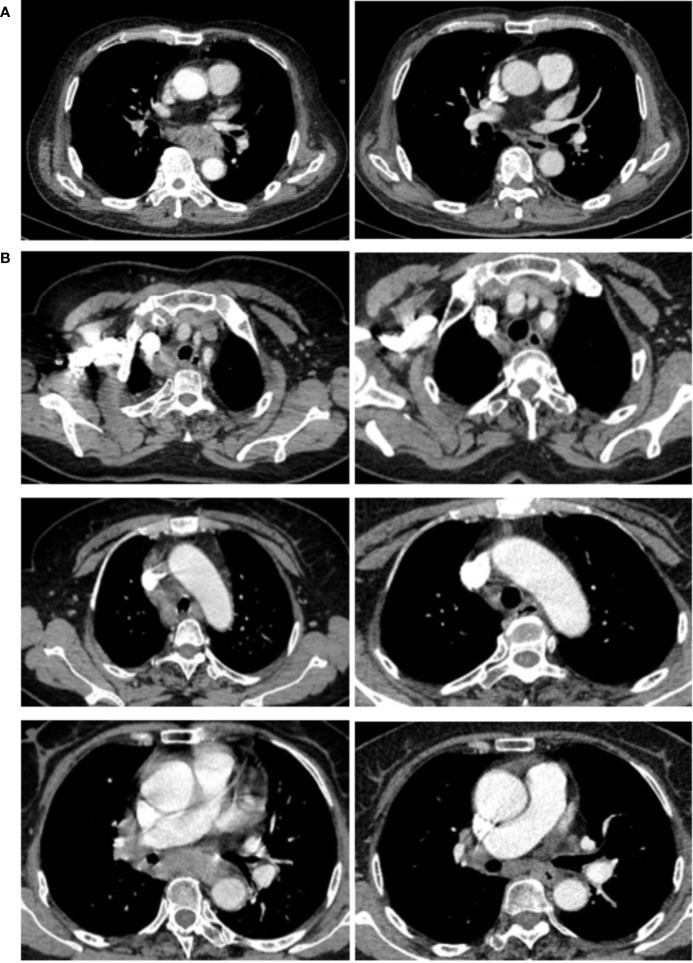
Figure 1.
Cases of radiological responses after neoadjuvant immune checkpoint inhibitors and chemotherapy. (A) This shows the radiological images of a 67-year-old male (patient 1) with a stage IVA ESCC before and after neoadjuvant treatment. This patient achieved pathological regression of 100% for esophageal lesion with no residual lymph node metastasis according to postoperative specimen (B) This shows the images of a 68-year-old female (patient 2), who had a stage IVA ESCC before neoadjuvant treatment. This patient had 100% pathological regression of the primary tumor but had residual metastatic lymph nodes.
Survival Outcomes
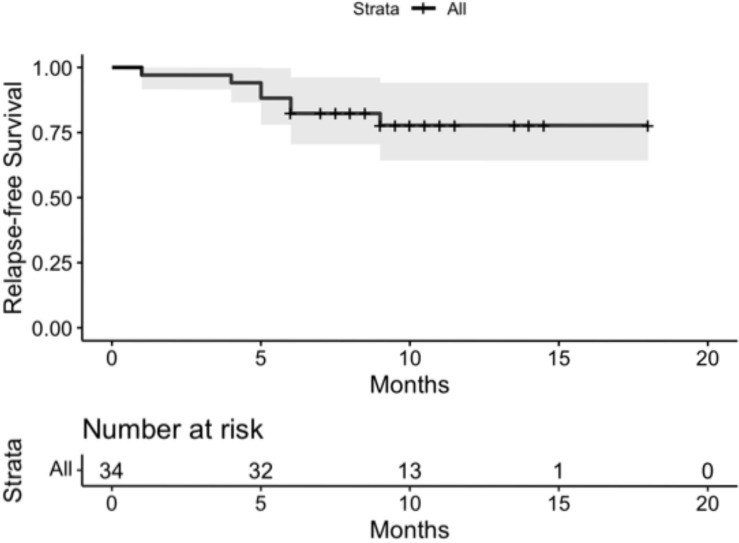
The median follow-up time was 9.5 months (IQR: 8.5 to 11 months. 7 patients had documented disease recurrence, of whom 3 developed supraclavicular lymph node metastases and 4 distant spread, including bone, brain and liver involvement. At the time of their most recent follow up, no deaths occurred as a result of esophageal cancer; one patient died while undergoing treatment for primary kidney cancer. For the entire cohort, the 1-year RFS was 77.8% (95% confidential interval (CI) 64.2% to 94.2%) ( Figure 2 ). No significant difference in survival was observed between the 2 ICI drugs of pembrolizumab and camrelizumab. Overall survival data were not mature ( Table 3 ).
Figure 2.
For the entire cohort, the 1-year Relapse-free survival (RFS) was 77.8% (95% confidential interval (CI) 64.2% to 94.2%).
Discussion
Our study shows that two cycles of preoperative ICI plus chemotherapy were well tolerated in locally-advanced ESCC patients, without therapy-related surgical delays. Furthermore, the preoperative regimen provided significant disease downstaging, turning unresectable ESCC into completely resectable tumors. More importantly, our data showed the introduction of preoperative ICI drugs did not increase the surgical difficulty or the postoperative complication, including treatment-related mortality. On the short-term follow-up, our study cohort demonstrated favorable 1-year RFS without any deaths from the late-stage disease. Therefore, this preoperative strategy allowed locally advanced ESCC that were unlikely to be surgical candidates at the first diagnosis to be completely removed eventually, without the need for radiotherapy. In this way, the novel treatment method could relieve dysphagia symptom of these patients, but also potentially extend their long-term survival.
Several clinical trials are currently evaluating the neoadjuvant role of ICI combined with chemoradiotherapy for esophageal cancer (NCT03604991, NCT03087864, NCT03044613, NCT02844075 and NCT03792347), some of which have reported preliminary outcomes confirming the high degree of safety and feasibility of the treatment strategy (30). Our previous work demonstrated that the use of neoadjuvant ICI plus chemotherapy could achieve a rate of over 40% of major pathologic response (MPR) in ESCC, without increasing the complication rates during the therapy and surgery (29).
Our study also brings special attention to a particular dilemma regarding treatment response. We noted that in 8.8% (3/34) of patients, although the primary site had pCR following neoadjuvant therapy, persistent disease was still present within involved lymph nodes. A similar observation has also been made by other investigators. PALACE-1 which is a phase II multicenter study aiming to evaluate preoperative pembrolizumab combined with chemoradiotherapy for resectable ESCC recently published its preliminary results, showing 11% (2/18) achieved pCR in primary tumor, however, had residual cancer cells in resected lymph nodes (30). The management of these patients with persistent nodal disease has opened up a new challenge for oncologists. It is unclear whether there is any benefit for adjuvant therapy using the original regimen after complete resection of ESCC. On the one hand, these patients who harbored residual cancer cells in the lymph nodes showed definite pathologic proof that their tumor was well responsive to ICI plus chemotherapy. On the other, current guidelines suggest that there is no proven benefit of adjuvant therapy for ESCC (3, 31). CheckMate 577 (17), a global, randomized, double-blind, placebo-controlled phase 3 trial to evaluate adjuvant therapy with ICI in esophageal or gastroesophageal junction cancer, reported that among patients who underwent resection after neoadjuvant chemoradiotherapy, RFS was significantly longer with nivolumab adjuvant therapy compared to placebo therapy. It should be noted that the majority (71%) of CheckMate 577 participants had adenocarcinoma and only 29% ESCC. We anticipate that studies focusing on the ICI adjuvant therapy for ESCC patients will provide more data, and could possibly result in new guidelines.
Our study has certain limitations that should be addressed. First, although this study may have the largest sample size of ESCC patients receiving neoadjuvant ICI plus chemotherapy followed by surgery to date, it is likely that some sort of selection bias was present due to the nature of monocentric series in a single institution. The inclusion of a validation cohort would have strengthened the findings of the study. Second, the study population was limited to East Asians, thereby raising concerns about the generalizability of our results, since disease spectra as well as biological and pathologic characteristics may differ among ethnic groups. Third, this is a retrospective single-armed study, and the results should be further validated by multi-institutional prospective randomized-controlled trials. Encouragingly, there are several ongoing prospective clinical trials which will provide more evidence in this area.
In summary, neoadjuvant ICI plus chemotherapy was safe for those patients with locally advanced ESCC who refused or lacked access to radiotherapy. This treatment regimen provided significant tumor downstaging rendering inoperable tumors operable, relieving symptoms of dysphagia and prolonging survival.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author Contributions
JX and YiZ conceptualized and designed the study; XM and WZ collected data, follow-up, wrote the manuscript; BL, YY and YM performed data cleansing and statistical analysis; BL, YaZ, JX and YiZ participated in the surgery and perioperative management. MT and YiZ established the clinical management protocols and edited the manuscript. All authors have reviewed, discussed, and approved the manuscript.
Funding
This work is supported by Shanghai Pujiang Program (2020PJD014).
Conflict of Interest
Author MT was employed by Mayo Clinic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.810898/full#supplementary-material
Abbreviations
AJCC, American Joint Committee on Cancer; CI, Confidential interval; CT, Computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; ECOG, Eastern Cooperative Oncology Group; ESCC, Esophageal squamous cell carcinoma; EUS, Endoscopic ultrasound; FUSCC, Fudan University Shanghai Cancer Center; HR, Hazard ratio; ICI, Immune checkpoint inhibitors; pCR, pathologic complete response; PET, Positron emission tomography; RECIST, Response Evaluation Criteria in Solid Tumors; RFS, Relapse-free survival; TNM, Tumor Nodes Metastases; TRG, Tumor regression grade.
References
- 1. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal Cancer. Nat Rev Dis Primers (2017) 3:17048. doi: 10.1038/nrdp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer Incidence and Mortality in China, 2015. J Natl Cancer Center (2020) 1(1):2–11. doi: 10.1016/j.jncc.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol (2020) 38:2677–94. doi: 10.1200/JCO.20.00866 [DOI] [PubMed] [Google Scholar]
- 4. Leng X, He W, Yang H, Chen Y, Zhu C, Fang W, et al. Prognostic Impact of Postoperative Lymph Node Metastases After Neoadjuvant Chemoradiotherapy for Locally Advanced Squamous Cell Carcinoma of Esophagus: From the Results of NEOCRTEC5010, a Randomized Multicenter Study. Ann Surg (2019) 274(6):e1022–29. doi: 10.1097/SLA.0000000000003727 [DOI] [PubMed] [Google Scholar]
- 5. Wan T, Zhang XF, Liang C, Liao CW, Li JY, Zhou YM, et al. The Prognostic Value of a Pathologic Complete Response After Neoadjuvant Therapy for Digestive Cancer: Systematic Review and Meta-Analysis of 21 Studies. Ann Surg Oncol (2019) 26:1412–20. doi: 10.1245/s10434-018-07147-0 [DOI] [PubMed] [Google Scholar]
- 6. Eyck BM, van Lanschot JJB, Hulshof M, Wilk BJ, Shapiro J, Hagen P, et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614 [DOI] [PubMed] [Google Scholar]
- 7. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BPL, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 9. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival After Neoadjuvant Chemotherapy or Chemoradiotherapy for Resectable Oesophageal Carcinoma: An Updated Meta-Analysis. Lancet Oncol (2011) 12:681–92. doi: 10.1016/S1470-2045(11)70142-5 [DOI] [PubMed] [Google Scholar]
- 10. Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, et al. Meta-Analysis of Postoperative Morbidity and Perioperative Mortality in Patients Receiving Neoadjuvant Chemotherapy or Chemoradiotherapy for Resectable Oesophageal and Gastro-Oesophageal Junctional Cancers. Br J Surg (2014) 101:321–38. doi: 10.1002/bjs.9418 [DOI] [PubMed] [Google Scholar]
- 11. Bosch DJ, Muijs CT, Mul VE, beukema JC, Hospers GAP, Burgerhof JGM, et al. Impact of Neoadjuvant Chemoradiotherapy on Postoperative Course After Curative-Intent Transthoracic Esophagectomy in Esophageal Cancer Patients. Ann Surg Oncol (2014) 21:605–11. doi: 10.1245/s10434-013-3316-8 [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and Mortality of Patients Who Underwent Minimally Invasive Esophagectomy After Neoadjuvant Chemoradiotherapy vs Neoadjuvant Chemotherapy for Locally Advanced Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Surg (2021) 156(5):444–51. doi: 10.1001/jamasurg.2021.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klevebro F, Elliott JA, Slaman A, Vermeulen BD, Kamiya S, Rosman C, et al. Cardiorespiratory Comorbidity and Postoperative Complications Following Esophagectomy: A European Multicenter Cohort Study. Ann Surg Oncol (2019) 26:2864–73. doi: 10.1245/s10434-019-07478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gospodarowicz M. Global Access to Radiotherapy-Work in Progress. JCO Glob Oncol (2021) 7:144–5. doi: 10.1200/GO.20.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol (2020) 38:4138–48. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 16. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab Versus Investigator's Choice of Chemotherapy as Second-Line Therapy for Advanced or Metastatic Oesophageal Squamous Cell Carcinoma (ESCORT): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 17. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Cutsem EV, Piessen G, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125 [DOI] [PubMed] [Google Scholar]
- 18. Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of Endoscopic Ultrasonography (EUS), Positron Emission Tomography (PET), and Computed Tomography (CT) in the Preoperative Locoregional Staging of Resectable Esophageal Cancer. Surg Endosc (2010) 24:1380–6. doi: 10.1007/s00464-009-0783-x [DOI] [PubMed] [Google Scholar]
- 19. Nishimaki T, Tanaka O, Ando N, Ide H, Watanabe H, Shinoda M, et al. Evaluation of the Accuracy of Preoperative Staging in Thoracic Esophageal Cancer. Ann Thorac Surg (1999) 68:2059–64. doi: 10.1016/S0003-4975(99)01171-6 [DOI] [PubMed] [Google Scholar]
- 20. Li B, Li N, Liu S, Li Y, Qian B, Zhang Y, et al. Does [18F] Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Have a Role in Cervical Nodal Staging for Esophageal Squamous Cell Carcinoma? J Thorac Cardiovasc Surg (2020) 160:544–50. doi: 10.1016/j.jtcvs.2019.11.046 [DOI] [PubMed] [Google Scholar]
- 21. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol (2017) 12:36–42. doi: 10.1016/j.jtho.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang Y, et al. Esophagectomy With Three-Field Versus Two-Field Lymphadenectomy for Middle and Lower Thoracic Esophageal Cancer: Long-Term Outcomes of a Randomized Clinical Trial. J Thorac Oncol (2021) 16:310–7. doi: 10.1016/j.jtho.2020.10.157 [DOI] [PubMed] [Google Scholar]
- 23. Li B, Hu H, Zhang Y, Zhang J, Miao L, Ma L, et al. Three-Field Versus Two-Field Lymphadenectomy in Transthoracic Oesophagectomy for Oesophageal Squamous Cell Carcinoma: Short-Term Outcomes of a Randomized Clinical Trial. Br J Surg (2020) 107:647–54. doi: 10.1002/bjs.11497 [DOI] [PubMed] [Google Scholar]
- 24. Li B, Hu H, Zhang Y, Zhang J, Miao L, Ma L, et al. Extended Right Thoracic Approach Compared With Limited Left Thoracic Approach for Patients With Middle and Lower Esophageal Squamous Cell Carcinoma: Three-Year Survival of a Prospective, Randomized, Open-Label Trial. Ann Surg (2018) 267:826–32. doi: 10.1097/SLA.0000000000002280 [DOI] [PubMed] [Google Scholar]
- 25. Li B, Xiang J, Zhang Y, Li H, Zhang J, Sun Y, et al. Comparison of Ivor-Lewis vs Sweet Esophagectomy for Esophageal Squamous Cell Carcinoma: A Randomized Clinical Trial. JAMA Surg (2015) 150:292–8. doi: 10.1001/jamasurg.2014.2877 [DOI] [PubMed] [Google Scholar]
- 26. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg (2015) 262:286–94. doi: 10.1097/SLA.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 27. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann Surg (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 28. Schneider PM, Baldus SE, Metzger R, Kocher M, Bongartz R, Bollschweiler E, et al. Histomorphologic Tumor Regression and Lymph Node Metastases Determine Prognosis Following Neoadjuvant Radiochemotherapy for Esophageal Cancer: Implications for Response Classification. Ann Surg (2005) 242:684–92. doi: 10.1097/01.sla.0000186170.38348.7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and Safety of Neoadjuvant Chemotherapy and Immunotherapy in Locally Resectable Advanced Esophageal Squamous Cell Carcinoma. J Thorac Dis (2021) 13:3518–28. doi: 10.21037/jtd-21-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative Pembrolizumab Combined With Chemoradiotherapy for Oesophageal Squamous Cell Carcinoma (PALACE-1). Eur J Cancer (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 31. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17:855–83. doi: 10.6004/jnccn.2019.0033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.