Abstract
To reduce treatment-related side effects in low-risk prostate cancer (PCa), both focal therapy and deferred treatments, including active surveillance (AS) and watchful waiting (WW), are worth considering over radical prostatectomy (RP). Therefore, this study aimed to compare long-term survival outcomes between focal therapy and AS/WW. Data were obtained and analyzed from the Surveillance, Epidemiology, and End Results (SEER) database. Patients with low-risk PCa who received focal therapy or AS/WW from 2010 to 2016 were included. Focal therapy included cryotherapy and laser ablation. Multivariate Cox proportional hazards models were used to compare overall mortality (OM) and cancer-specific mortality (CSM) between AS/WW and focal therapy, and propensity score matching (PSM) was performed to reduce the influence of bias and unmeasured confounders. A total of 19 292 patients with low-risk PCa were included in this study. In multivariate Cox proportional hazards model analysis, the risk of OM was higher in patients receiving focal therapy than those receiving AS/WW (hazard ratio [HR] = 1.35, 95% confidence interval [CI]: 1.02–1.79, P = 0.037), whereas no significant difference was found in CSM (HR = 0.98, 95% CI: 0.23–4.11, P = 0.977). After PSM, the OM and CSM of focal therapy and AS/WW showed no significant differences (HR = 1.26, 95% CI: 0.92–1.74, P = 0.149; and HR = 1.26, 95% CI: 0.24–6.51, P = 0.782, respectively). For patients with low-risk PCa, focal therapy was no match for AS/WW in decreasing OM, suggesting that AS/WW could bring more overall survival benefits.
Keywords: active surveillance, cancer-specific mortality, focal therapy, low-risk prostate cancer, overall mortality, watchful waiting
INTRODUCTION
Prostate cancer (PCa) is one of the most frequently diagnosed cancers worldwide, with approximately 191 930 new cases in the United States in 2020.1 Nowadays, with the widespread use of prostate-specific antigen (PSA) testing, the incidence of low-risk cancer is increasing quickly.2 Therefore, patients with low-risk PCa should be carefully considered. As low-risk PCa rarely has molecular alternation that results in progression to aggressive disease, radical prostatectomy (RP), as the gold standard of radical treatment, is considered unnecessary or an overtreatment due to its limited clinical benefit and considerable adverse effects.3
The 2021 European Association of Urology guidelines classify both active surveillance (AS) and watchful waiting (WW) as deferred treatment.4 AS is a treatment strategy that lets patients remain under close surveillance. Radical treatment such as RP will only be performed once tumor progression occurs.5 Therefore, AS aims to reduce side effects caused by overtreatment maximally while ensuring effective treatment for those in need.6 Many studies have shown that AS and RP could achieve similar survival outcomes for patients with low-risk PCa.7,8,9,10 WW is a conservative treatment for patients considered unsuitable for curative treatment; thus, palliative treatment will be carried out to maintain the quality of life when local or systemic disease progression occurs. A randomized controlled trial (RCT) including 731 patients compared AS and WW with RP and showed that surgery was not associated with significantly lower all-cause or PCa mortality than deferred treatment among men with localized PCa.9 At present, the use of AS and WW is increasing rapidly among patients with low-risk PCa. The number of low-risk patients who have received AS or WW has been higher than that of those who have received RP in the United States since 2013.11
Focal therapy, including high-intensity focused ultrasound (HIFU), cryotherapy, laser ablation, photodynamic therapy, electroporation, and focal radiotherapy, can be defined as part of the treatment for prostate diseases, aiming at reducing treatment-related side effects while maintaining the same oncological efficacy as compared to RP.12,13 The use of focal therapy is facilitated by the improved imaging technologies of PCa. A matched-pair analysis on 110 patients demonstrated that HIFU was comparable to robotic RP in controlling localized unilateral PCa, and no difference was found in requiring salvage therapies.14 Besides, some single-arm studies have also shown that focal therapy could achieve encouraging short-term oncological outcomes.15
Studies comparing clinical outcomes of both AS/WW and focal therapy as practical treatment for low-risk patients are still lacking as most focus on comparing these treatments with RP. In addition, recent studies mainly focused on the short-term oncological control efficacy, and thus, which treatment had better long-term survival outcomes remained unclear. Therefore, this study aimed to directly compare long-term survival outcomes between focal therapy and AS/WW in patients with low-risk PCa.
PATIENTS AND METHODS
Patients selection
Patients’ data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. The study was approved by the Ethics Committee of West China Hospital (Sichuan University, Chengdu, China). Given the retrospective nature of the study, requirement for informed consent was waived by the Institution review board of West China Hospital. Patients diagnosed with prostate adenocarcinoma from April 2010 to April 2016 were identified. The 7th edition of the American Joint Committee on Cancer Staging Manual was used to assess T stages.16 Figure 1 shows the details of inclusion and exclusion criteria. Patients were divided into two treatment groups: AS/WW group (n = 18 611) and focal therapy group (n = 681).
Figure 1.
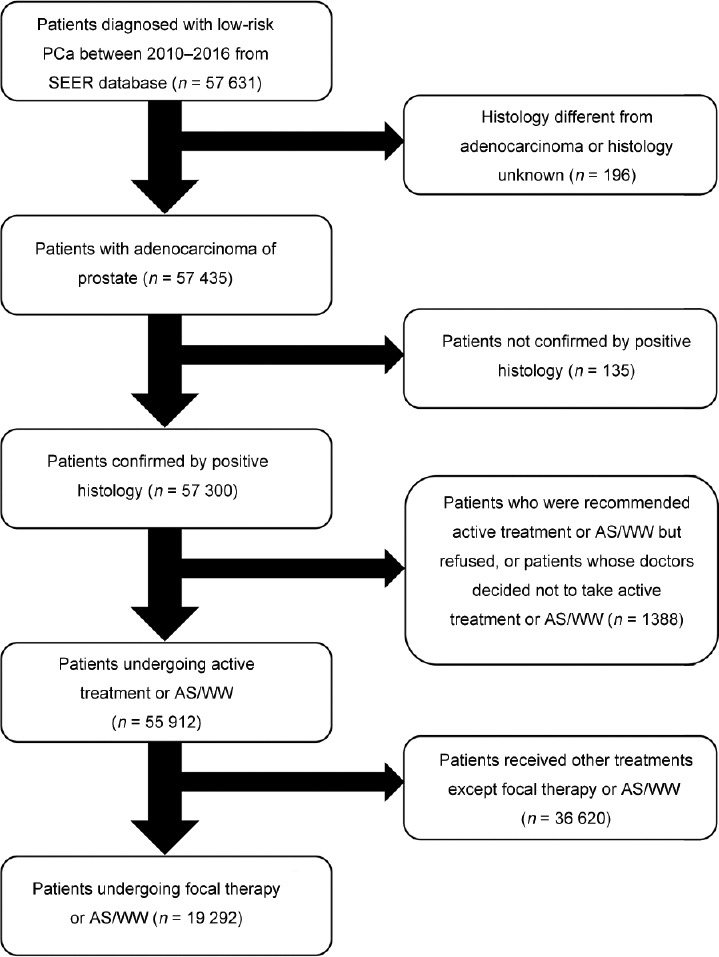
Flowchart describing the selection of patients. PCa: prostate cancer; SEER: Surveillance, Epidemiology, and End Results; AS: active surveillance; WW: watchful waiting.
Focal therapy included cryotherapy (n = 451) and laser ablation (n = 230), as patients receiving other types of focal therapy are relatively few. Considering that the sample size of the cryotherapy and laser ablation groups was far smaller than the AS/WW group, our study regarded these two modes as one general therapy.
Propensity score matching (PSM)
We conducted PSM (1:1 matching algorithm, nearest-neighbor matching with caliper width of 0.05) to help patients receiving AS/WW (n = 681) and focal therapy (n = 681) obtain similar characteristics, simulate randomized trial design, and reduce selection bias. Adjustment variables included age, PSA level, and T stage (Supplementary Table 1). Gleason score was not included as total Gleason score between AS/WW group and focal therapy group was not significantly different.
Supplementary Table 1.
Propensity score parameter list
| The variables used in calculating the propensity matching | Age, PSA, T stage |
|---|---|
| Propensity scoring algorithm | Logistic regression model |
| C-statistical | 0.64 |
| Matching method | Greedy matching within specified caliper distances |
| Distance metric | 0.05 |
| Matching ratio | (AS/WW) 1:1 (focal therapy) |
| Use of replacement | With replacement |
| Matching sample size | AS/WW (681 cases) vs. focal therapy (681 cases) |
AS: active surveillance; WW: watchful waiting; PSA: prostate-specific antigen
Statistical analyses
Baseline characteristics between AS/WW and focal therapy cohorts were compared. Two-tailed t-test was performed and presented as mean ± standard deviation (s.d.) to evaluate differences of continuous variables, whereas two-tailed χ2 test or Fisher's exact test was performed to estimate differences of categorical variables and presented as the frequency and its proportion. Survival time was measured subsequent to the diagnosis. Multivariate Cox proportional hazards models were performed to test the overall mortality (OM) and cancer-specific mortality (CSM) between two treatment groups using crude and adjusted-covariate models (adjusted for age, PSA, race, and total Gleason score), both in unmatched and propensity score matched cohorts. Another group of multivariate Cox proportional hazards models was additionally conducted, in which focal therapy group was divided into cryotherapy and laser ablation groups, to assess the OM and CSM between AS/WW and cryotherapy or laser ablation separately. In original cohorts, Kaplan–Meier methods were used to obtain the cumulative incidence survival curves. Inverse probability of treatment weighting (IPTW) and standardized mortality ratio weighting (SMRW), which were calculated using the propensity score, were performed in the entire cohort to confirm the robustness of our results. Considering the potential effect of age on survival outcomes, the smooth curve was performed to explore the possible nonlinear relationship between OM and age. Then, subgroup analysis was performed to evaluate the treatment efficacy among different age groups.
All tests were two-sided, with P < 0.05 considered statistically significant. To perform the analyses, EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and statistical software packages R (http://www.R-project.org, The R Foundation) were used.
RESULTS
Our results included 19 292 individuals, of whom 681 patients received focal therapy, whereas 18 611 patients received AS/WW. Among 681 patients receiving focal therapy, 451 received cryotherapy, whereas 230 received laser ablation. The mean follow-up duration in the AS/WW group was 35.9 (s.d.: 23.5) months, whereas that in the focal therapy group was 45.6 (s.d.: 23.4) months. Patients receiving focal therapy had a higher mean age, T stage, and Gleason score (all P < 0.001), whereas those who received AS/WW had a higher average PSA level (P < 0.001). In the focal therapy group, patients receiving cryotherapy had a higher T stage than those receiving laser ablation (P < 0.001), whereas Gleason score and total Gleason score between two types of treatments were not significantly different (P = 0.762 and P = 0.475, respectively). Details on baseline characteristics of patients are shown in Table 1.
Table 1.
Descriptive characteristics of patients undergone active surveillance/watchful waiting or focal therapy before and after propensity score matching
| Propensity score matching | Characteristic | AS/WW | Focal therapy | Cryotherapy | Laser ablation | aP | bP |
|---|---|---|---|---|---|---|---|
| Before matching | Patients (n) | 18 611 | 681 | 451 | 230 | ||
| Age (year), mean±s.d. | 64.0±7.6 | 67.5±7.8 | 67.3±7.7 | 67.8±8.0 | <0.001 | 0.471 | |
| PSA level (ng ml−1), mean±s.d. | 5.62±1.88 | 5.23±2.07 | 5.25±1.99 | 5.18±2.21 | <0.001 | 0.660 | |
| Year of diagnosis, n (%) | <0.001 | 0.002 | |||||
| 2010 | 1840 (9.9) | 129 (18.9) | 88 (19.5) | 41 (17.8) | |||
| 2011 | 2352 (12.6) | 135 (19.8) | 98 (21.7) | 37 (16.1) | |||
| 2012 | 2456 (13.2) | 123 (18.1) | 90 (20.0) | 33 (14.4) | |||
| 2013 | 3018 (16.2) | 97 (14.2) | 67 (14.9) | 30 (13.0) | |||
| 2014 | 2743 (14.7) | 80 (11.8) | 50 (11.1) | 30 (13.0) | |||
| 2015 | 2914 (15.7) | 61 (9.0) | 29 (6.4) | 32 (13.9) | |||
| 2016 | 3288 (17.7) | 56 (8.2) | 29 (6.4) | 27 (11.7) | |||
| Race, n (%) | 0.008 | 0.214 | |||||
| White | 14 606 (78.5) | 530 (77.8) | 348 (77.2) | 182 (79.1) | |||
| Black | 2512 (13.5) | 115 (16.9) | 82 (18.2) | 33 (14.4) | |||
| Other | 1025 (5.5) | 26 (3.8) | 17 (3.8) | 9 (3.9) | |||
| Unknown | 468 (2.5) | 10 (1.5) | 4 (0.9) | 6 (2.6) | |||
| Region, n (%) | <0.001 | 0.533 | |||||
| Alaska | 6 (<0.01) | 0 (0) | 0 (0) | 0 (0) | |||
| East | 6719 (36.1) | 339 (49.8) | 219 (48.6) | 120 (52.2) | |||
| Northern plains | 1351 (7.3) | 101 (14.8) | 66 (14.6) | 35 (15.2) | |||
| Pacific coast | 9876 (53.1) | 200 (29.4) | 135 (29.9) | 65 (28.3) | |||
| Southwest | 659 (3.5) | 41 (6.0) | 31 (6.9) | 10 (4.4) | |||
| T stage, n (%) | <0.001 | <0.001 | |||||
| T1a | 0 (0) | 60 (8.8) | 9 (2.0) | 51 (22.2) | |||
| T1b | 0 (0) | 12 (1.8) | 4 (0.9) | 8 (3.5) | |||
| T1c | 17 235 (92.6) | 537 (78.8) | 386 (85.6) | 151 (65.6) | |||
| T1NOS | 68 (0.4) | 11 (1.6) | 3 (0.7) | 8 (3.5) | |||
| T2a | 1308 (7.0) | 61 (9.0) | 49 (10.9) | 12 (5.2) | |||
| Gleason score, n (%) | <0.001 | 0.762 | |||||
| Unknown | 107 (0.6) | 40 (5.9) | 27 (6.0) | 13 (5.6) | |||
| 1+2 | 2 (<0.01) | 0 (0) | 0 (0) | 0 (0) | |||
| 2+2 | 17 (0.1) | 0 (0) | 0 (0) | 0 (0) | |||
| 2+3 | 43 (0.2) | 1 (0.2) | 1 (0.2) | 0 (0) | |||
| 2+4 | 2 (<0.01) | 0 (0) | 0 (0) | 0 (0) | |||
| 3+2 | 26 (0.1) | 0 (0) | 0 (0) | 0 (0) | |||
| 3+3 | 18 414 (98.9) | 640 (94.0) | 423 (93.8) | 217 (94.4) | |||
| Total Gleason score, n (%) | 0.573 | 0.475 | |||||
| 3 | 9 (<0.01) | 0 (0) | 0 (0) | 0 (0) | |||
| 4 | 18 (0.1) | 0 (0) | 0 (0) | 0 (0) | |||
| 5 | 72 (0.4) | 1 (0.2) | 1 (0.2) | 0 (0) | |||
| 6 | 18 512 (99.5) | 680 (99.8) | 450 (99.8) | 230 (100.0) | |||
| Survival (month), median (Q1–Q3) | 34.0 (15.0–55.0) | 48.0 (28.0–66.0) | 50.0 (33.0–66.0) | 43.5 (21.0–64.0) | <0.001 | 0.003 | |
| After matching | Patients (n) | 681 | 681 | 451 | 230 | ||
| Age (year), mean±s.d. | 67.2±7.9 | 67.5±7.8 | 67.3±7.7 | 67.8±8.0 | 0.912 | 0.471 | |
| PSA level (ng ml−1), mean±s.d. | 5.25±2.07 | 5.23±2.07 | 5.25±1.99 | 5.18±2.21 | 0.832 | 0.660 | |
| Race, n (%) | 0.085 | 0.214 | |||||
| White | 541 (79.4) | 530 (77.8) | 348 (77.2) | 182 (79.1) | |||
| Black | 88 (12.9) | 115 (16.9) | 82 (18.2) | 33 (14.4) | |||
| Other | 38 (5.6) | 26 (3.8) | 17 (3.8) | 9 (3.9) | |||
| Unknown | 14 (2.1) | 10 (1.5) | 4 (0.9) | 6 (2.6) | |||
| T stage, n (%) | <0.001 | <0.001 | |||||
| T1a | 0 (0) | 60 (8.8) | 9 (2.0) | 51 (22.2) | |||
| T1b | 0 (0) | 12 (1.8) | 4 (0.9) | 8 (3.5) | |||
| T1c | 602 (88.4) | 537 (78.9) | 386 (85.6) | 151 (65.6) | |||
| T1NOS | 3 (0.4) | 11 (1.6) | 3 (0.7) | 8 (3.5) | |||
| T2a | 76 (11.2) | 61 (9.0) | 49 (10.9) | 12 (5.2) | |||
| Gleason score, n (%) | <0.001 | 0.762 | |||||
| Unknown | 3 (0.4) | 40 (5.9) | 27 (6.0) | 13 (5.6) | |||
| 2+3 | 2 (0.3) | 1 (0.1) | 1 (0.2) | 0 (0) | |||
| 3+2 | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | |||
| 3+3 | 674 (99.0) | 640 (94.0) | 423 (93.8) | 217 (94.4) | |||
| Total Gleason score, n (%) | 0.089 | 0.475 | |||||
| 5 | 5 (0.7) | 1 (0.1) | 1 (0.2) | 0 (0) | |||
| 6 | 676 (99.3) | 680 (99.9) | 450 (99.8) | 230 (100.0) |
aComparison between AS/WW and focal therapy; bcomparison between cryotherapy and laser ablation. PCa: prostate cancer; AS: active surveillance; WW: watchful waiting; s.d.: standard deviation; PSA: prostate-specific antigen; Q1–Q3: quartile 1–quartile 3; T1NOS: T1 not otherwise specified
In the multivariate Cox proportional hazards model analysis, patients who received focal therapy had a higher risk of OM (hazard ratio [HR] = 1.76, 95% confidence interval [CI]: 1.33–2.33, P < 0.001). After adjusting confounders, including age, PSA level, race, and Gleason score, focal therapy still led to a higher OM than AS/WW (HR = 1.35, 95% CI: 1.02–1.79, P = 0.037). With regard to the CSM analysis, focal therapy was not inferior to AS/WW according to results of both crude model (HR = 1.19, 95% CI: 0.29–4.98, P = 0.810) and adjusted model (HR = 0.98, 95% CI: 0.23–4.11, P = 0.977), as shown in Table 2. In subgroup analysis according to the type of focal therapy, OMs of both cryotherapy and laser ablation showed no significant difference compared with AS/WW (HR = 1.27, 95% CI: 0.90–1.80; and HR = 1.51, 95% CI: 0.97–2.37, respectively). The CSM between AS/WW and both cryotherapy and laser ablation also showed no significant difference (HR = 0.73, 95% CI: 0.10–5.35; and HR = 1.52, 95% CI: 0.20–11.21, respectively; Figure 2). In the Kaplan–Meier curves, focal therapy was no match for AS/WW in decreasing OM (P < 0.001), whereas the CSM between two treatments had no obvious difference (P = 0.809), as shown in (Figure 3).
Table 2.
Multivariate Cox regression analyses for cancer-specific mortality and overall mortality in the total cohort and matched population
| Outcome | Treatment | Nonadjusted model, HR (95% CI) | Adjusted model, HR (95% CI) | PSM model, HR (95% CI) |
|---|---|---|---|---|
| CSM | AS/WW | Reference | Reference | Reference |
| Focal therapy | 1.19 (0.29–4.89) | 0.98 (0.23–4.11) | 1.26 (0.24–6.51) | |
| P | 0.810 | 0.977 | 0.782 | |
| OM | AS/WW | Reference | Reference | Reference |
| Focal therapy | 1.76 (1.33–2.33) | 1.35 (1.02–1.79) | 1.26 (0.92–1.74) | |
| P | <0.001 | 0.037 | 0.149 |
Adjusted model: adjusted for age, PSA level, race, T stage, and total Gleason score. PSM model: matched according to age, PSA level and T stage. CSM: cancer-specific mortality; OM: overall mortality; AS: active surveillance; WW: watchful waiting; PSM: propensity score matching; PSA: prostate-specific antigen; HR: hazard ratio; CI: confidence interval
Figure 2.
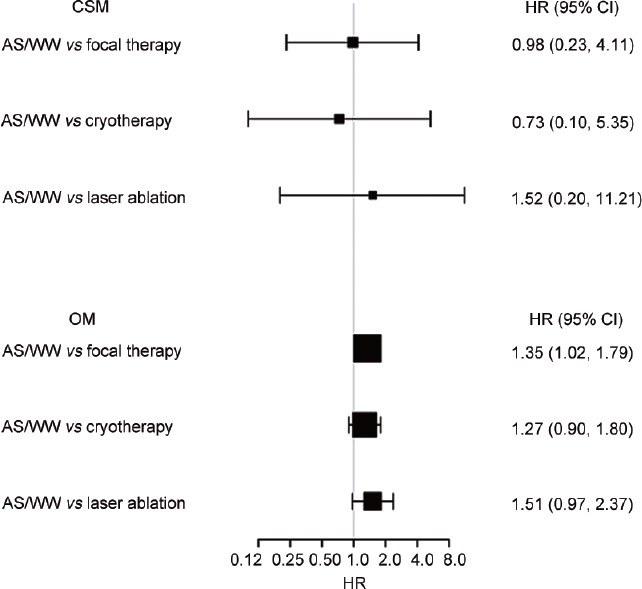
Subgroup analyses of OM and CSM in the comparison between AS/WW and each type of focal therapy. Adjusted for age, PSA level, race, T stage, and total Gleason score. OM: overall mortality; CSM: cancer-specific mortality; AS: active surveillance; WW: watchful waiting; PSA: prostate-specific antigen; HR: hazard ratio; CI: confidence interval.
Figure 3.
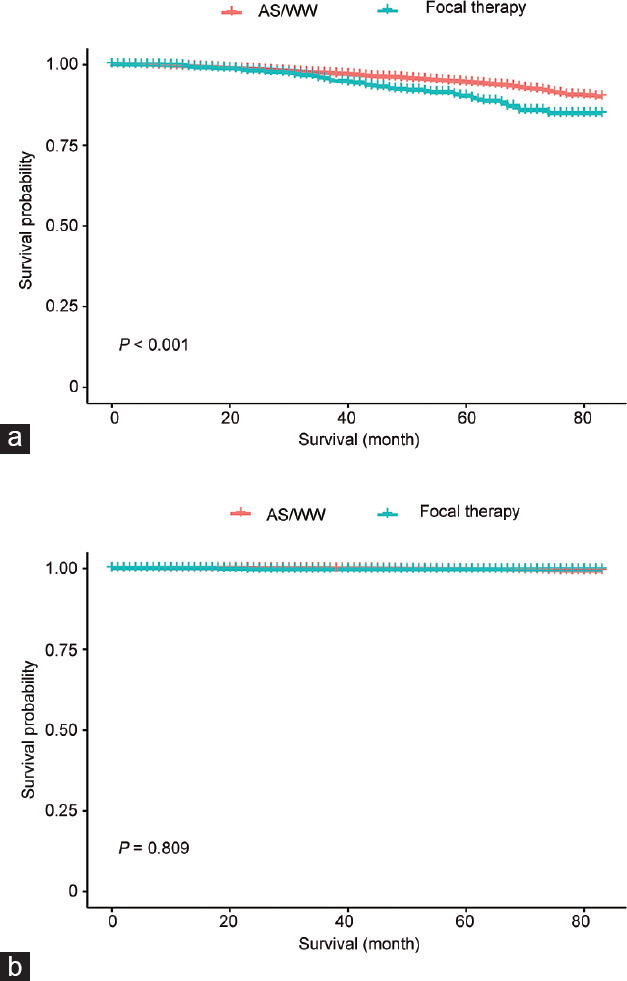
Kaplan–Meier survival curve of OM and CSM. (a) Kaplan–Meier survival curve of OM in the comparison of AS/WW and focal therapy. (b) Kaplan–Meier survival curve of CSM in the comparison of AS/WW and focal therapy. OM: overall mortality; CSM: cancer-specific mortality; AS: active surveillance; WW: watchful waiting.
After PSM, we screened a total of 1362 patients into matched cohorts, with 681 patients in each type of treatment. Another multivariate Cox proportional hazards model analysis was performed in the matched cohort, and no significant difference was found between AS/WW and focal therapy for OM (HR = 1.26, 95% CI: 0.92–1.74, P = 0.149) and CSM (HR = 1.26, 95% CI: 0.24–6.51, P = 0.782).
With regard to sensitivity analyses, results of IPTW-adjusted model demonstrated that focal therapy may be associated with higher risk of OM (HR = 1.34, 95% CI: 1.21–1.47, P < 0.001), whereas the CSM between two treatments showed no significant difference (HR = 0.76, 95% CI: 0.47–1.23, P = 0.268). The SMRW-adjusted model showed no obvious superiority in terms of decreasing OM and CSM between the AS/WW and focal therapy (HR = 1.32, 95% CI: 0.84–2.07, P = 0.222; and HR = 1.16, 95% CI: 0.13–10.09, P = 0.895, respectively).
The smooth curve showed intersection between the focal therapy and AS/WW groups in terms of OM (Figure 3). Based on this result, all patients were divided into three groups according to their ages, with threshold of 60 years and 80 years. The AS/WW group showed obvious superiority of survival outcome in the 60–80 years group (HR = 1.62, 95% CI: 1.17–2.23, P = 0.003; Table 3). However, the effect of interaction was not significant (P = 0.290), reflecting the tendency that focal therapy led to higher risks of OM existed in all patients with different ages (Supplementary Figure 1 (129.9KB, tif) ).
Table 3.
Age-related subgroup analysis for overall mortality between active surveillance/watchful waiting and focal therapy
| Age at diagnosis (year) | Treatment | Patients (n) | HR (95% CI) | P |
|---|---|---|---|---|
| <60 | AS/WW | 5070 | Reference | |
| Focal therapy | 110 | 1.65 (0.52–5.27) | 0.394 | |
| ≥60 and <80 | AS/WW | 13 215 | Reference | |
| Focal therapy | 539 | 1.62 (1.17–2.23) | 0.003 | |
| ≥80 | AS/WW | 326 | Reference | |
| Focal therapy | 32 | 1.08 (0.43–2.72) | 0.868 |
OM: overall mortality; AS: active surveillance; WW: watchful waiting; HR: hazard ratio; CI: confidence interval
DISCUSSION
Radical treatments, either RP or radiotherapy, are associated with considerable side effects. Preservation of healthy prostate tissues can potentially promote better continence and sexual potency outcomes.17 Both AS/WW and focal therapy are based on the principle of preserving the maximum tissue whenever possible. AS/WW, which keeps patients under close surveillance, can help patients preserve the maximal prostate tissue, whereas focal therapy can damage the cancer tissue while preserving the surrounding healthy tissues. Some studies have shown focal therapy could confirm better functional outcomes than RP;18 however, financial burdens and psychological pressure caused by focal therapy cannot be ignored when compared with AS/WW. With the development of diagnostic approaches, greater diagnostic precision conferring the exact location of prostate cancer can help in risk stratification of patients. By offering focal therapy and having prostate preserved to a well-characterized subgroup of men, more patients will achieve better continence and sexual potency outcomes.
Several studies have supported selected patients with low- or intermediate-risk PCa but low-burden PCa to choose the focal therapy. Among the available therapies, HIFU, cryotherapy, photodynamic therapy, and laser ablation have shown encouraging short-term outcomes.19 However, the long-term survival data of focal therapy are notably absent, and few studies focused on the effectiveness and safety of AS/WW and focal therapy for low-risk PCa. Long-term survival outcomes were examined in this study. Based on our results, focal therapy showed no significant inferiority in decreasing CSM compared with AS/WW for low-risk PCa. However, focal therapy showed higher risk of OM, indicating that AS/WW could bring more overall survival benefits. Approximately 40% of patients receiving AS/WW for the first time have been reported to convert to radical treatments,20 which may explain why the AS/WW group in this study achieved better survival results. Although no higher OM was detected when comparing cryotherapy and laser ablation with AS/WW in the subgroup analysis according to focal therapy type, there was a trend that cryotherapy and laser ablation would lead to more deaths than AS/WW. A multivariate Cox proportional hazards model was also performed to compare OM and CSM between cryotherapy and laser ablation, and no significant result was detected (Supplementary Table 2), indicating both cryotherapy and laser ablation were responsible for increasing OM.
Supplementary Table 2.
Analysis for overall mortality and cancer-specific mortality between cryotherapy and laser ablation
| Outcome | Treatment | Nonadjusted model | Adjusted model |
|---|---|---|---|
| CSM | Cryotherapy | Reference | Reference |
| Laser ablation | 2.13 (0.13–34.06) P=0.593 | 1.72 (0.10–29.66) P=0.708 | |
| OM | Cryotherapy | Reference | Reference |
| Laser ablation | 1.36 (0.78–2.37) P=0.274 | 1.23 (0.70–2.15) P=0.466 |
Adjusted model: adjusted for age, PSA level, race, and total Gleason score. CSM: cancer-specific mortality; OM: overall mortality; PSA: prostate-specific antigen
A phase III RCT compared short-term oncological control outcomes of AS and vascular-targeted photodynamic therapy. Patients in the photodynamic therapy group who had disease progression were fewer than those in the AS group at 24 months (P < 0.001).21 However, focal therapy showed no significant difference in decreasing CSM compared to AS/WW in our study, and the risk of OM was greater in the focal therapy group. This could be explained by the fact that our study focused on survival outcomes whereas the RCT focused on oncological control efficacy. Once disease progression occurred, radical treatments would be performed to promote survival in AS/WW group. Moreover, the follow-up duration of this RCT was 24 months; thus, further disease progression would be recorded in the photodynamic therapy group if it had a longer follow-up duration.
In several single-arm designed studies, the efficacy and safety of focal therapy were analyzed. A longitudinal outcome study, including 25 patients who received laser ablation, reported that 96% of patients in postoperative target biopsy showed no evidence of PCa, and the mean decrease of PSA between baseline and 3 months was 2.3 ng ml−1.22 These studies showed an encouraging short-term oncological control efficacy of laser ablation. However, these studies did not analyze long-term survival outcomes of focal therapy and failed to compare focal therapy with AS/WW. Another large sample-sized study included 1160 patients who received cryotherapy. About 75.7% of them were biochemical recurrence-free at 36 months, and only 3.7% became positive in postoperative biopsy.23 Although this study had a longer follow-up duration and larger sample size, it also failed to compare the survival outcomes between focal therapy and AS/WW.
Although the subgroup analysis according to age showed a significant superiority of survival outcome in the intermediate age group (60–80 years), no obvious interaction effect was found, reflecting the tendency that focal therapy led to higher risks of OM in all patients at different ages.
The primary advantage of this study is that our data were obtained from the large sample database, allowing us to compare the retrospective data of AS/WW and focal therapy directly by using a large sample size. Moreover, contrary to recent studies that mainly focused on short-term oncological control, our study focused on long-term survival outcomes. Furthermore, a series of statistical analyses was used to reduce bias and confounding factors, confirming that focal therapy was similar to AS/WW in decreasing CSM but still did not match AS/WW in decreasing OM.
This study still has some limitations. First, although PSM was performed to assume randomization in this study, the bias could not be overcome entirely. For example, data of detailed health status are not available in the SEER database, which may result in a selection bias. Few RCTs comparing focal therapy and AS/WW have been reported so far; therefore, more high-quality RCTs are still required to better evaluate the safety and effectiveness of focal therapy. Second, as majority of patients with low-risk PCa can survive for >10 years,24 the follow-up duration in this study remains too short when analyzing survival outcomes. Third, as relevant data are lacking, the therapy conversion rate in the AS/WW group cannot be measured, which is an important factor affecting survival outcomes of patients. In addition, although focal therapy shows higher OM than AS/WW, the sample size of those who died was still insufficient to determine that focal treatment led to death directly. However, data we used in the SEER database had already covered most regions in the USA accompanied with a relatively large sample size. Thus, further studies are needed to evaluate the effectiveness of focal therapy and to verify our outcomes.
CONCLUSIONS
Patients with low-risk PCa who received focal therapy and AS/WW achieved similar survival outcomes in regard to decreasing CSM. However, focal therapy was no match for AS/WW in decreasing OM, suggesting that AS/WW could result in more overall survival benefits.
AUTHOR CONTRIBUTIONS
All authors contributed to the planning and design of the study. QMY wrote the main manuscript. THL modified the manuscript text. KJ and SQ were involved in review of the raw data and directly involved in the analysis. LY and QW provided analytical feedback based on aggregated results. XHZ and DJ prepared figures and tables. JKL provided substantive review and commentary on multiple drafts. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Association of age at diagnosis and risk of OM. OM: overall mortality; AS: active surveillance; WW: watchful waiting.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (SQ2017YFSF090096), the National Natural Science Foundation of China (81770756), and the Sichuan Science and Technology Program (2017HH0063).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Moschini M, Carroll PR, Eggener SE, Epstein JI, Graefen M, et al. Low-risk prostate cancer: identification, management, and outcomes. Eur Urol. 2017;72:238–49. doi: 10.1016/j.eururo.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Costello AJ. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat Rev Urol. 2020;17:177–88. doi: 10.1038/s41585-020-0287-y. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, Bellmunt J, Briers E, Bolla M, Bourke L, et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Arnhem: EAU Guidelines Office. 2021:51. [Google Scholar]
- 5.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, et al. EAU guidelines on prostate cancer.Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Briganti A, Fossati N, Catto JW, Cornford P, Montorsi F, et al. Active surveillance for low-risk prostate cancer: The European Association of Urology Position in 2018. Eur Urol. 2018;74:357–68. doi: 10.1016/j.eururo.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Das M. Surgery no better than observation for localised prostate cancer. Lancet Oncol. 2017;18:e512. doi: 10.1016/S1470-2045(17)30565-X. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of conservative management for low-risk prostate cancer in the Veterans Affairs Integrated Health Care System from 2005-2015. JAMA. 2018;319:2231–3. doi: 10.1001/jama.2018.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132–42. doi: 10.1056/NEJMoa1615869. [DOI] [PubMed] [Google Scholar]
- 10.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 11.Mahal BA, Butler S, Franco I, Spratt DE, Rebbeck TR, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010-2015. JAMA. 2019;321:704–6. doi: 10.1001/jama.2018.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Poel HG, van den Bergh RC, Briers E, Cornford P, Govorov A, et al. Focal therapy in primary localised prostate cancer: The European Association of Urology Position in 2018. Eur Urol. 2018;74:84–91. doi: 10.1016/j.eururo.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13:622–32. doi: 10.1016/S1470-2045(12)70121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albisinni S, Aoun F, Bellucci S, Biaou I, Limani K, et al. Comparing high-intensity focal ultrasound hemiablation to robotic radical prostatectomy in the management of unilateral prostate cancer: a matched-pair analysis. J Endourol. 2017;31:14–9. doi: 10.1089/end.2016.0702. [DOI] [PubMed] [Google Scholar]
- 15.Rischmann P, Gelet A, Riche B, Villers A, Pasticier G, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71:267–73. doi: 10.1016/j.eururo.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. p. 461. [Google Scholar]
- 17.Klotz L, Emberton M. Management of low risk prostate cancer-active surveillance and focal therapy. Nat Rev Clin Oncol. 2014;11:324–34. doi: 10.1038/nrclinonc.2014.73. [DOI] [PubMed] [Google Scholar]
- 18.Scheltema MJ, Chang JI, Böhm M, van den Bos W, Blazevski A, et al. Pair-matched patient-reported quality of life and early oncological control following focal irreversible electroporation versus robot-assisted radical prostatectomy. World J Urol. 2018;36:1383–9. doi: 10.1007/s00345-018-2281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13:641–53. doi: 10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]
- 20.Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, et al. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70:760–6. doi: 10.1016/j.eururo.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Azzouzi AR, Vincendeau S, Barret E, Cicco A, Kleinclauss F, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017;18:181–91. doi: 10.1016/S1470-2045(16)30661-1. [DOI] [PubMed] [Google Scholar]
- 22.Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol. 2015;68:924–6. doi: 10.1016/j.eururo.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 2012;109:1648–54. doi: 10.1111/j.1464-410X.2011.10578.x. [DOI] [PubMed] [Google Scholar]
- 24.Tosoian JJ, Mamawala M, Epstein JI, Landis P, Macura KJ, et al. Active surveillance of grade group 1 prostate cancer: long-term outcomes from a large prospective cohort. Eur Urol. 2020;77:675–82. doi: 10.1016/j.eururo.2019.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of age at diagnosis and risk of OM. OM: overall mortality; AS: active surveillance; WW: watchful waiting.


