Abstract
Background
The benefits of breastfeeding are well known, and the World Health Organization recommends exclusive breastfeeding for the first six months of life and continuing breastfeeding to age two. However, many women stop breastfeeding due to lactational breast abscesses. A breast abscess is a localised accumulation of infected fluid in breast tissue. Abscesses are commonly treated with antibiotics, incision and drainage (I&D) or ultrasound‐guided needle aspiration, but there is no consensus on the optimal treatment.
Objectives
To assess the effects of different treatments for the management of breast abscesses in breastfeeding women.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trial Register (27 February 2015). In addition we searched African Journals Online (27 February 2015), Google Scholar (27 February 2015), ProQuest Dissertations and Theses Databases (27 February 2015) and the WHO International Clinical Trials Registry Platform (ICTRP) search portal (27 February 2015). We also checked reference lists of retrieved studies and contacted experts in the field as well as relevant pharmaceutical companies.
Selection criteria
Randomised controlled trials (RCTs) investigating any intervention for treating lactational breast abscesses compared with any other intervention. Studies published in abstract form, quasi‐RCTs and cluster‐RCTs were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed risk of bias and extracted data. Data were checked for accuracy.
Main results
We included six studies. Overall, trials had an unclear risk of bias for most domains due to poor reporting. Two studies did not stratify data for lactational and non‐lactational breast abscesses, and these studies do not contribute to the results. This review is based on data from four studies involving 325 women.
Needle aspiration (with and without ultrasound guidance) versus incision and drainage (I&D)
Mean time (days) to complete resolution of breast abscess (three studies) ‐ there was substantial heterogeneity among these data (Tau2 = 47.63, I2 = 97%) and a clear difference between subgroups (with or without ultrasound guidance; Chi2 = 56.88, I2 = 98.2%, P = < 0.00001). We did not pool these data in a meta‐analysis. Two studies excluded women who had treatment failure when they calculated the mean time to complete resolution. One study found that the time to complete resolution of breast abscess favoured needle aspiration over I&D (mean difference (MD) ‐6.07; 95% confidence interval (CI) ‐7.81 to ‐4.33; n = 36), but excluded 9/22 (41%) women in the needle aspiration group due to treatment failure. Another study reported faster resolution in the needle aspiration group (MD ‐17.80; 95% CI ‐21.27 to ‐14.33; n = 64) but excluded 6/35 (17%) women in the needle aspiration group due to treatment failure. A third study also reported that needle aspiration was associated with a shorter time to complete resolution of breast abscess (MD ‐16.00; 95%CI ‐18.73 to ‐13.27; n = 60); however, the authors did not indicate the number of women who were lost to follow‐up for either group, and it is unclear how many women contributed to this result. Considering the limitations of the available data, we do not consider the results to be informative.
Continuation of breastfeeding, after treatment (success): results favoured the needle aspiration group, but we did not pool data from the two studies because of substantial unexplained heterogeneity (I2 = 97%). One study reported that women in the needle aspiration group were more likely to continue breastfeeding (risk ratio (RR) 2.89; 95% CI 1.64 to 5.08; n = 60), whereas the other study found no clear difference (RR 1.09; 95% CI 0.97 to 1.22 n = 70).
Treatment failure was more common among women treated with needle aspiration compared to those who underwent I&D (RR 16.12; 95% CI 2.21 to 117.73; two studies, n = 115, low quality evidence). In one study, treatment with needle aspiration failed in 9/22 women who subsequently underwent I&D to treat their breast abscess. In another study, treatment with needle aspiration failed in 6/35 women, who subsequently underwent I&D. All abscesses in the I&D group were successfully treated.
The included studies provided limited data for the review's secondary outcomes. No data were reported for adverse events. One study (60 women) reported that women in the needle aspiration group were more satisfied with their treatment than women who received I&D to treat their breast abscesses.
Incision and drainage (I&D) with or without antibiotics
One study (150 women) compared the value of adding a broad‐spectrum cephalosporin (single dose or a course of treatment) to women who underwent I&D for breast abscesses.
The mean time to resolution of breast abscess was reported as being similar in all groups (although women with infection were excluded). Mean time to resolution for women who received a course of antibiotics was reported as 7.3 days, 6.9 days for women who received a single dose of antibiotics and 7.4 days for women who did not receive antibiotics. Standard deviations, P values and CIs were not reported and prevented further analysis. No data were reported for any continuation of breastfeeding after treatment (success). For treatment failure, there was no clear difference between the groups of women who received antibiotics (either a single dose or a course of antibiotics) and those who did not (RR 1.00; 95% CI 0.36 to 2.76).
Included studies rarely reported this review's secondary outcomes (including adverse events). For post‐operative complications/morbidity, there was no difference in the risk of wound infections between the antibiotics and no antibiotics groups (RR 0.58; 95% CI 0.29 to 1.17), irrespective of whether women received a single dose or a course of antibiotics.
Authors' conclusions
There is insufficient evidence to determine whether needle aspiration is a more effective option to I&D for lactational breast abscesses, or whether an antibiotic should be routinely added to women undergoing I&D for lactational breast abscesses. We graded the evidence for the primary outcome of treatment failure as low quality, with downgrading based on including small studies with few events and unclear risk of bias.
Plain language summary
Treatments for breast abscesses in breastfeeding women
Some women develop a breast abscess while breastfeeding, called a lactational breast abscess. An abscess is a collection of infected fluid within the breast tissue. The aim of treatment is to cure the abscess quickly and effectively, ensuring maximum benefit to the mother with minimal interruption of breastfeeding.
Presently, lactational breast abscesses are treated by incision and drainage or needle aspiration, with or without diagnostic ultrasound. Antibiotics may or may not be prescribed. For incision and drainage the abscess is cut open with a scalpel (blade) to release the infected fluid. A drain may be inserted into the wound to help the infected fluid drain or may be left open so that the infected fluid drains naturally. A less invasive way to treat the breast abscess is by needle aspiration. A needle is inserted into the cavity of the breast abscess and a syringe is used to draw out the infected fluid, often using ultrasound guidance. As there are advantages in using this method e.g. no scars, reduced hospitalisation etc. the trend is to use this method more often.
We wanted to find evidence on the effectiveness of different treatments. We looked at the time taken for the abscess to heal using the different types of treatments, the number of women who continued to breastfeed after treatment and how many women had healed in the each group after treatment. The definition of healing varied across the studies.
We found six studies, of which four studies with a total of 325 woman contributed data. These studies compared needle aspiration versus incision and drainage. Needle aspiration appeared to decrease the healing time compared to incision and drainage, but large proportions of women were excluded from the analysis and it was therefore difficult to make conclusions. For the outcome continuation of breastfeeding, both of the studies showed that women treated with needle aspiration were more likely to continue breastfeeding compared to incision and drainage. In two studies, breast abscesses did not heal in some women who had needle aspiration and had to be treated with incision and drainage (low quality evidence). All breast abscesses that were treated with incision and drainage healed. We were not able to make any conclusions regarding unwanted effects or complications. Studies did not report sufficiently on the number of follow‐up visits, duration of continuation of breastfeeding, post‐operative complications, duration of hospital stay and adverse events. However, it appeared that women were more satisfied when treated with needle aspiration.
One study compared different regimens of antibiotics versus no antibiotics in breastfeeding women who were treated with incision and drainage for breast abscesses. We did not find any difference between groups for the outcome resolution of breast abscesses and infections after the procedure.
All of the studies were poorly conducted and/or reported and did not address all of the outcomes that we were interested in. Studies with better design and reporting are needed to properly assess these outcomes.
Summary of findings
Summary of findings for the main comparison. Needle aspiration compared with incision and drainage for breast abscesses in breastfeeding women.
| Needle aspiration compared with incision and drainage for breast abscesses in breastfeeding women | ||||||
| Patient or population: Breastfeeding women with breast abscesses Intervention: Needle aspiration Comparison: incision and drainage | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| incision and drainage | Needle aspiration | |||||
| Time to resolution of breast abscess (days) | This outcome was a dressed by three studies with severe heterogeneity (I2 = 97%), therefore the result was not pooled. |
|||||
| Continuation of breastfeeding | The result for this outcome was not pooled as it was provided by two studies of small sample size with severe heterogeneity (I2 = 97%) | |||||
| Treatment failure | Study population | RR 16.12 (2.21 to 117.73) | 115 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. CI: confidence interval; RR: risk ratio | ||||||
1Evidence provided by studies of unclear risk of bias
2Included studies were of small sample size with few events (<30)
Background
Description of the condition
The benefits of breastfeeding are well known and the World Health Organization (WHO) thus recommends exclusive breastfeeding for the first six months and continuing for up to two years and beyond. (WHO 2003; WHO 2012). However, there are many reasons why women stop breastfeeding; one of the most common being the complications of lactation (Dener 2003). Of note for this review, Amir 2004 found in a study of women who commenced breastfeeding that 0.4% (5/1183) developed a breast abscess.
Mastitis
Mastitis is an inflammatory condition of the breast that is usually associated with lactation and that can progress from the non‐infective stage, to infective mastitis and then to a breast abscess. The incidence of mastitis in lactating women is between 3% to 20% due to variations in the definition and follow‐up in the post partum period (Amir 2014). Mastitis is clinically characterised by a tender, hot, swollen, wedge‐shaped area of the breast associated with high temperatures (> 38.5°C ) and flu‐like symptoms. It may or may not be accompanied by an infection (Amir 2014). Some of the predisposing factors are limited feeding, poor positioning of the baby, illness of mother or baby, maternal malnutrition and cracked nipples. In infective mastitis, Staphylococcus aureus and Staphylococcus epidermidis are the commonest causative organisms (Amir 2014). Mastitis usually occurs during the first six weeks but can occur at any time during lactation (Amir 2014). The primary cause of mastitis is milk stasis (Hughes 1989). Conservative management includes efficient removal of milk, with the addition of antibiotics for possible bacterial infections (Baker 2010; Marchant 2002). Other measures include supportive care; rest and fluids, application of heat packs and analgesics. Antibiotics are recommended if symptoms have not improved (Lawrence 2005), although a Cochrane systematic review found insufficient evidence, due to a lack of studies, to confirm or refute when to use antibiotics in the treatment of mastitis (Jahanfar 2012).
Lactational breast abscess
A breast abscess is defined as a localised accumulation of infected fluid in breast tissue. Breast abscesses are usually puerperal (lactational) but can be non‐puerperal (Baker 2010). Three per cent of women with mastitis develop a lactational breast abscess ( Amir 2014).
The most common causative organism is Staphylococcus aureus (WHO 2003), although other organisms have been identified (Bertrand 1991; Dixon 1988; Karstrup 1993). A recent study has suggested that methicillin‐resistant Staphylococcus aureus (MRSA) is also beginning to play an important role (Branch‐Elliman 2012). Risk factors for developing lactational breast abscesses include: women over the age of 30, first pregnancies, gestational age ≥ 41 weeks and mastitis (Kvist 2005). A breast abscess usually presents as a hard, tender and sometimes fluctuant mass with overlying erythema (redness of the skin) (Barbosa‐Cesnick 2003). Diagnosis is usually made using ultrasound when a hypoechoic lesion with an irregular border is present (Dirbas 2011).
Three Cochrane reviews (Crepinsek 2012; Lumbiganon 2012; Mangesi 2010) have illustrated the need for education about breastfeeding during pregnancy and to determine effective treatments for the prevention of mastitis and engorgement, conditions which contribute towards the formation of lactational breast abscesses. For women at risk of developing a lactational breast abscesses, it is therefore necessary to examine existing studies on treatments for lactational breast abscesses, to understand its impact on maternal health, time to recovery and its effect on breastfeeding.
Description of the intervention
Approaches to treating breast abscesses include incision and drainage (I&D), usually carried out under general anaesthesia and needle aspiration, which may be a single aspiration with a drain left in situ or serial aspirations. Needle aspiration is usually done with a local anaesthetic. Antibiotics are recommended following either a needle aspiration or I&D (Abou‐Dakn 2010). Delayed, inappropriate or even inadequate treatment may result in more extensive lesions and permanent tissue damage, which could affect future lactation in about 10% of women. Breast abscesses that require extensive resection can cause disfigurement (World Health Organization 2000).
Treatments
1. Antibiotics
Treatment of lactational breast abscesses with antibiotics, without removal of pus is considered to be ineffective (World Health Organization 2000). Following diagnostic or interventional ultrasound or I&D of breast abscesses, breast milk and fluid samples should be sent for culture to detect the presence of bacteria or resistant pathogens (Amir 2014). The most commonly found organism in a lactational breast abscess is Staphylococcus aureus with Steptococcus orEscherichia coli being less common. Antibiotics of choice such as dicloxacillin or flucloxacillin 500mg four times daily orally, or the recommended sensitive local antibiotic may be prescribed. First generation cephalosporins may also be an alternative. Women who may be allergic to penicillin may be prescribed cephalexin or clindamycin. In cases where Staphylococcus Aureus is resistant to penicillinase‐resistant penicillins (methicillin‐resistant Staphylococcus Aureus (MRSA)) is suspected, breast milk culture and assay of antibiotics sensitivities should be undertaken. Most strains of MRSA are sensitive to vancomycin or trimethoprim/sulphamethoxazole and less so to rifampin. One should presume that MRSA is resistant to treatment with macrolides and quinolones regardless of susceptibility test results (Amir 2014)
2. Surgical
Lactational breast abscesses have traditionally been treated with I&D, but more recently there is a growing tendency to use less invasive procedures. Where possible, all women with a suspected lactational breast abscess should have an ultrasound, which will be helpful in identifying all pockets of fluid. Management may depend on the state of the overlying skin. For skin that appears normal, drainage of the abscess is done by needle aspiration usually with ultrasound (see below). If the skin over the abscess is thin and shiny or the abscess appears as if it will burst, then I&D is recommended (Dirbas 2011).
Incision and drainage is done with local or general anaesthetic. An incision is made to allow for drainage of the infected fluid and if a drain is required a counter incision is then made. Daily washing out of the wound may be required until secretions decrease or are clear. By week four, the wound should be closed and without complications. I&D is recommended when the abscess is large or if there are multiple abscesses. A course of antibiotics is also advised (Abou‐Dakn 2010) (see below).
3. Needle aspiration
Breast abscesses are also treated with needle aspiration, using a local anaesthetic and under sterile conditions, with or without ultrasound guidance. The Society of Interventional Radiology (SIR) defines image‐guided percutaneous aspiration as "evacuation or diagnostic sampling of a fluid collection with the use of a catheter or a needle during a single imaging session, with removal of the catheter or needle immediately after the aspiration" while image‐guided percutaneous drainage is defined as "the placement of a catheter with the use of image guidance to provide continuous drainage of a fluid collection" (Wallace 2010, p432). It may be performed during a single session or as a staged procedure during multiple sessions (Wallace 2010).
The (WHO (World Health Organization 2000), supports the use of ultrasound guidance for diagnosis and treatment of lactational breast abscesses. Ulitzsch 2004 has shown that abscesses of less than three cm in diameter can be treated with single aspiration or serial aspirations until resolution. Failure was seen with abscesses greater than five cm in diameter. A probe or a drain is an alternative to using a needle to remove the infected fluid. If the aspirate is viscous, then a saline or antibiotic solution can be used to assist with the aspiration. Daily aspirations are recommended until the wound cannot be punctured anymore (< 4 mm). Serial aspirations are done between two to nine times. A course of antibiotics is usually recommended (Abou‐Dakn 2010).
Although needle aspiration is considered as being less invasive, not all lactational breast abscesses have been successfully treated by this method and have subsequently needed I&D (Ozseker 2008; Ulitzsch 2004). Some of the reasons cited for treatment failure include lack of clinical improvement, recurrence of abscess or formation of fistulas (Giess 2014).
Breastfeeding
Prior to drainage of the breast abscess, breastfeeding should continue from the unaffected breast. Breastfeeding from the affected breast should resume soon after drainage to prevent stasis of milk and relapse of the infection (World Health Organization 2000). Feeding from the affected breast is recommended, even if a drain is in place but care should be taken to ensure that the infant's mouth is not in contact with the infected fluid or breast tissue (Amir 2014). Failure to allow breastfeeding lends itself to the production of fluid that is viscous, which aggravates engorgement. Breastfeeding ensures drainage of the affected area and speedy resolution of the abscess (Walker 2011). Giess 2014 recommends that breastfeeding can and should continue from the lactating breast with the proviso that the prescribed antibiotics are safe for the infant. This encourages adequate drainage, which facilitates clearing of the infection and limits the bacterial culture medium.
How the intervention might work
The objective of any of the interventions employed in treating an abscess is to remove the infected fluid as speedily as possible, hastening resolution, thereby reducing the pain and discomfort and allowing the woman to continue breastfeeding her infant with little or no interruption. Maintaining the integrity of the breast is also important, i.e. the procedure should leave the woman complication‐free, with minimal or preferably no scarring, and the function of breastfeeding should be maintained.
Antibiotics and I&D have been viewed as standard therapy in managing lactational breast abscesses. More recently, however, there has been an emergence of studies favouring treatment of lactational abscesses with needle aspiration, which is considered a less invasive technique.
Christensen 2005 favoured the use of ultrasound‐guided drainage of breast abscesses as it caused less scarring, did not affect breastfeeding, did not require anaesthesia or hospitalisation, and was less expensive than surgery. Although I&D has the advantage of breaking down the loculi, if the procedure is carried out under a general anaesthetic, it will also involve hospitalisation and regular dressings. This is thought to cause considerable distress to both mother and baby during what is already a difficult time and the final cosmetic result may be unsatisfactory (Benson 1989; Dixon 1998). Scholefield 1987 expressed a similar view, suggesting that I&D is associated with a prolonged healing time, regular dressings, difficulties in breastfeeding, and the possibility of an unsatisfactory cosmetic outcome. Conversely Jones 1976 and Ajao 1994 found that I&D, curettage and primary closure of the abscess cavity had better scar formation and a reduction of cost of treatment.
Effective management of a lactational breast abscess is necessary to eliminate discomfort and reduce the risk of discontinuation of breastfeeding. Breastfeeding is regarded as fundamental to the growth and development of an infant, and it is therefore important that whatever the intervention is, it should not disrupt any momentum gained by the mother with regards to breastfeeding (Walker 2011).
Why it is important to do this review
There have been a number of Cochrane reviews addressing questions around prevention and treatment of breastfeeding complications (Crepinsek 2012; Lumbiganon 2012; Mangesi 2010).
Mangesi 2010 examined treatments for breast engorgement during lactation with one of its key outcomes being mastitis and the secondary outcome as breast abscess formation. One study showed that there was a difference in breast abscesses between the group that received acupuncture and those that did not, however, this study was underpowered and the results were not statistically significant.
Lumbiganon 2012 looked at antenatal breastfeeding education in increasing breastfeeding duration. As a secondary outcome, they also listed breastfeeding complications such as mastitis and breast abscesses. The authors reported that compared to formal breastfeeding education plus lactation consultation versus routine breastfeeding, education showed no significant difference in mastitis but a significant reduction in nipple pain. Crepinsek 2012 examined the effect of different interventions for the prevention of mastitis following childbirth. They showed that none of the interventions were effective in preventing mastitis. As appropriate studies were not available at the time the review by Jahanfar 2012 was done, the author was unable to support or deny the role antibiotics played in treating mastitis.
A recently published non‐Cochrane systematic review on the treatment of breast abscesses Lam 2014 included randomised controlled trials, non‐randomised trials as well as case series. Participants had lactational or non‐lactational breast abscesses and one study included men. Although the authors used SORT (Strength of Recommendation Taxonomy) to grade the quality of evidence and the recommendations made, it is not clear how judgements about risk of bias were made. The authors recommend the use of needle aspiration with or without the use of ultrasound as first line treatment of breast abscesses. No meta‐analysis was conducted to measure treatment effects.
Lam 2014 does not recommend breastfeeding from the affected breast due to Staphylococcal organisms, which places the infant at risk of pneumonia, lung abscesses and death. This recommendation contradicts other current literature (Amir 2014; Giess 2014).
Effective interventions for the prevention of engorgement and mastitis are still to be determined. In the absence of these interventions there is an increased likelihood of developing a breast abscess. Currently, there appears to be no consensus on which the best treatment for lactational breast abscess is and to this end there is a need to rigorously synthesise existing research to obtain clarity.
Objectives
To assess the effects of different treatments for the management of breast abscesses in breastfeeding women.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). Trials using cluster‐randomised or cross‐over designs were not eligible for inclusion. As per protocol, quasi‐randomised trials were also excluded as we identified RCTs. Studies only reported as abstracts were not included in the review. Future updates of this review may consider including quasi‐RCTs and cluster‐RCTs (due to paucity of data).
Types of participants
Breastfeeding women (exclusive breastfeeding or mixed‐feeding) presenting with breast abscesses in one or both breasts. Women with co‐morbidities were included (e.g. HIV, diabetes).
Types of interventions
Any intervention, surgical, non‐surgical, pharmacological, non‐pharmacological, invasive, non‐invasive, or a combination of treatments, to treat lactational breast abscesses, compared with any other intervention, surgical, non‐surgical, pharmacological, non‐pharmacological, invasive, non‐invasive, or a combination of treatments, aimed at treating lactational breast abscesses.
Types of outcome measures
Primary outcomes
Time to complete resolution of breast abscess (resolution of abscess was defined as no recurrence of abscess or need for any intervention). Time was defined by the authors as time of presentation for care or from time of hospitalisation.
Any continuation of breastfeeding after treatment (success).
Treatment failure.
Secondary outcomes
Number of follow‐up visits.
Duration of continuation of breastfeeding after treatment.
Maternal satisfaction with treatment.
Post‐operative complications/morbidity.
Duration of hospital stay.
Adverse events.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (27 February 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we carried out supplementary searches of African Journals Online (27 February 2015), dissertation databases, trial registries for ongoing studies and Google Scholar (27 February 2015). For dissertations we searchedProQuest Dissertations and Theses Databases (27 February 2015). For ongoing trials, we searched the WHO International Clinical Trials Registry Platform Search Portal (ICTRP) (27 February 2015). See Appendix 1 for search terms used in these databases.
Searching other resources
We checked the reference lists of included studies for relevant citations and contacted experts in the field in order to find any unpublished studies. We also contacted the following pharmaceutical companies: Aspen, Glaxo Smithkline, Novartis, Pfizer and Roche for relevant studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors (Hayley Irusen (HI) and Anke Rohwer (AR)) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We screened titles and abstracts of search results to exclude irrelevant studies. We then retrieved full text articles of seemingly relevant studies and examined them to see whether they met the inclusion criteria. We resolved any disagreement through discussion and by consultation with the third review author (Taryn Young (TY)).
Data extraction and management
We designed a form to extract data (Appendix 2). For eligible studies, two review authors (HI and AR) extracted the data using the agreed form. We resolved discrepancies through discussion and consultation of a third author (TY). We entered data into Review Manager software (RevMan 2014) and checked them for accuracy. When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details. Of the six included studies (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012), only one author responded in part (Naeem 2012). Where studies reported ranges, we used BMJ online 2014 as a resource to provide the statistical method to convert ranges to mean and standard deviations (Suthar 2012).
Assessment of risk of bias in included studies
Two review authors (HI and AR) independently made judgements about risk of bias. Discrepancies were resolved through discussion and by consultation with the third review author (TY) if they were not resolved.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could have put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings.
The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison of needle aspiration compared to I&D.
Time to complete resolution of breast abscess
Any continuation of breastfeeding after treatment (success)
Treatment failure
We used GRADE profiler (GRADEpro 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals. Outcomes with dichotomous data included any continuation of breastfeeding after treatment, resolution of abscess, and post‐operative complications/morbidity.
Continuous data
For continuous data, we used the mean difference, since outcomes were measured in the same way between trials. Outcomes with continuous data included time to complete resolution of abscess, number of follow‐up visits, duration of continuation of breastfeeding after treatment, maternal satisfaction with treatment and duration of hospital stay. For length of time to resolution of abscess, two studies (Chandika 2012; Saleem 2008) reported the range of values only. In these circumstances, we estimated mean and SD (BMJ online 2014).
Unit of analysis issues
Cluster‐randomised trials were not eligible for inclusion. However, due to paucity of data, cluster‐randomised trials will be eligible for inclusion in future updates.
Cluster‐randomised trials
In future updates, we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Trials using a cross‐over design were not eligible for inclusion in this review as this is not an appropriate study design for the interventions in this review.
Other unit of analysis issues
Studies with two or more than two treatment groups were included and were dealt with as recommended by the Cochrane Handbook for Systematic Interventions Reviews (Higgins 2011).When a multi‐arm study contributed more than one comparison to a particular meta‐analysis, we either combined treatment groups or divided the control group, so that the inclusion of data from the same woman more than once in the same analysis was avoided.
Dealing with missing data
No imputation of missing data was done. Where the required summary statistics were not reported, these were calculated from the available data according to the Cochrane Handbook Chapter 7.7 (Higgins 2011), specifically where means and confidence intervals and sample sizes per group were reported, standard deviations were calculated in the recommended manner.
For included studies, we noted levels of attrition. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number of participants randomised.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the T² was greater than zero and either the I² was greater than 30% or there was a low P (less than 0.10) in the Chi² test for heterogeneity. For significant heterogeneity, we used the random‐effects model or reported results narratively.
Assessment of reporting biases
We did not investigate reporting biases due to the limited number of included studies. In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where we detected substantial statistical heterogeneity (if the T² was greater than zero and either the I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity), we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
For random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, random‐effects analysis was used to produce it. We planned to carry out the following subgroup analyses. We assessed different definitions for the primary outcome.
Primiparas versus multigravidas.
Catheter aspiration (abscess ≥ 3 cm) versus needle aspiration (abscess < 3 cm).
Women under 30 years of age versus those over 30 years of age.
Urban settings versus rural settings.
Co‐morbidities versus no co‐morbidities.
Exclusive breastfeeding versus mixed breast‐bottle feeding.
High‐income settings versus low‐income settings.
Due to the limited amount of data in the included studies, we were not able to perform any of the pre‐specified subgroup analysis. In future updates of this review, we will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to perform sensitivity analysis on primary outcomes, to examine what effect excluding those studies at high risk of bias (for allocation concealment, and incomplete outcome data might have on the overall result of the meta‐analysis. However, since all of the included studies were of poor quality, we did not perform sensitivity analysis. We will carry out our planned sensitivity analysis in future updates of this review, if appropriate. In future updates, if we include cluster‐randomised trials in with the indivually randomised trials, we will also carry out sensitivity analysis to investigate the effect of the randomisation unit.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group’s Register retrieved four trial reports.The search for trial reports on the Proquest dissertation and theses databases yielded 2005 studies, Google Scholar retrieved 2501 studies, African Journals online database retrieved 22 studies and the WHO ICTRP search retrieved 38 studies. Screening of reference lists yielded 30 extra studies, while contact with experts yielded no studies. Of the pharmaceutical companies we contacted, Pfizer responded with seven reports, which were unsuitable for inclusion as they did not fulfil the inclusion criteria for this review of RCTS and Novartis was unable to assist. After screening abstracts for eligibility, 15 full text articles of seemingly relevant studies were obtained. Of these, we included six published studies (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012) and excluded nine studies (Blick 1980; Edino 2001; Florey 1946; Ozseker 2008; Peters 1991; Sheih 2009; Strauss 2003; Tewari 2006; Wang 2013). For included studies, we contacted all of the corresponding authors (seeCharacteristics of included studies).
For a summary of the search results, see (Figure 1).
1.
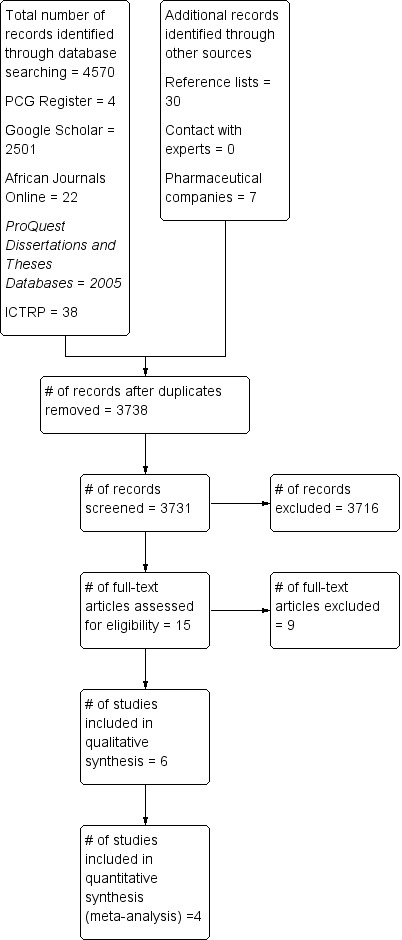
Study flow diagram.
Included studies
Six studies met our inclusion criteria. Four of the studies included 325 women (Table 2) and contributed data to the analyses (Eryilmaz 2005; Saleem 2008; Singla 2002; Suthar 2012). As the remaining two studies included lactational and non‐lactational breast abscesses and the results for the outcomes were not recorded separately for each abscess type, the studies were not included in the quantitative analysis (Chandika 2012; Naeem 2012) but are described qualitatively.
1. Summary of characteristics of studies.
| Study ID | Total number of participants | Intervention (n) | Comparison (N) | Country | *Primary outcomes reported | **Secondary outcomes reported | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||
| Eryilmaz 2005 | 45 | Needle aspiration without ultrasound (n = 22) | I&D (n = 23) | Turkey | Y | N | Y | N | N | Y | N | Y | N |
| Saleem 2008 | 60 | Percutaneous ultrasound‐guided drainage (n = 30) | I&D (n = 30) | Pakistan | Y | Y | N | Y | N | Y | Y | Y | N |
| Singla 2002 | 150 | All women underwent (I&D) Antibiotics (Group 1 n = 50; Group 2 n = 50) | Control (n = 50) | India | Y | N | Y | N | N | N | Y | N | N |
| Suthar 2012 | 70 | Percutaneous ultrasound‐guided needle aspiration (n = 35) | Open surgical drainage (n = 35) | India | Y | Y | Y | N | N | N | Y | N | Y |
*Primary and **Secondary outcomes
- Time to complete resolution.
- Any continuation of breastfeeding after treatment.
- Treatment failure .
- Number of follow‐up visits.
- Duration of continuation of breastfeeding after treatment.
- Maternal satisfaction with treatment.
- Post‐operative complications/morbidity.
- Duration of hospital stay.
- Adverse events.
I&D: incision and drainage
Study location
One study was based in Turkey (Eryilmaz 2005), a second in Pakistan (Saleem 2008) and two in India (Singla 2002; Suthar 2012). (Chandika 2012) was based in Uganda and (Naeem 2012) in Pakistan. All of the included studies were conducted within a hospital setting.
Types of intervention
The interventions included surgical (Chandika 2012; Eryilmaz 2005; Saleem 2008; Suthar 2012) as well as pharmacological interventions (Singla 2002). Only one study investigated two interventions against a control (Singla 2002) while each of the remaining five compared one intervention with another (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Suthar 2012).
Surgical interventions
Surgical interventions included incision and drainage (I&D) (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Suthar 2012), ultrasound‐guided needle aspiration/drainage (Saleem 2008; Suthar 2012;Chandika 2012; Naeem 2012), and needle aspiration without ultrasound (Eryilmaz 2005). Needle aspiration was compared with I&D in five studies (Eryilmaz 2005; Saleem 2008; Suthar 2012; Chandika 2012; Naeem 2012). All of the women in the studies randomised to I&D underwent general anaesthesia (Chandika 2012; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012). Eryilmaz 2005 reported that women in the I&D group received a local anaesthetic. Women in the ultrasound‐guided needle aspiration group (Chandika 2012Saleem 2008; Suthar 2012), received a local anaesthetic prior to the intervention. Participants in Naeem 2012 did not receive any local anaesthetic. It was unclear whether the participants in Eryilmaz 2005 study received any anaesthetic.
Intervention with antibiotics
Singla 2002 investigated different treatment regimens of a broad spectrum antibiotic (cefazolin) compared with no antibiotics. All three groups of women in Singla 2002 underwent I&D where the objective of the study was to evaluate the role of antibiotics in the management of lactational breast abscesses. One group received intravenous cefazolin during the procedure, followed by oral cefazolin for six days; the second intervention group received a single dose of intravenous cefazolin before the procedure and the control group did not receive any antibiotics.
Participants
The total number of women in the four studies that contributed data was 325. Two studies included women with non‐lactational breast abscesses (Chandika 2012; Naeem 2012). In Chandika 2012 66% (43/65) of women and in Naeem 2012 83% (53/64) presented with lactational breast abscesses. The outcomes in both studies were not stratified according to abscess types and therefore the data were not included in the meta‐analysis.
Age and parity of women
Most women were between the ages of 20 and 30 years. One study did not report on the age of women (Singla 2002). Saleem 2008; Chandika 2012 and Naeem 2012 included primiparous and multiparous women. Singla 2002 and Suthar 2012 did not report on the parity of women in their studies.
Abscess size
Abscess sizes differed between studies. Chandika 2012 and Naeem 2012 excluded all women with breast abscesses that were greater than 5 cm. The median abscess size in Saleem 2008 was 5.5 cm (range 2 cm to 12 cm). The mean abscess size in Suthar 2012 was 4.9 cm ± 2.5 cm (range 1 cm to 15 cm). The mean abscess size in Eryilmaz 2005 was 6.5 ± 2.7 cm (I&D) and 6.1 ± 2.8 cm (needle aspiration group). Singla 2002 did not report on the abscess size of participating women.
Duration of symptoms
Duration of symptoms varied across studies.
Methods used to diagnose breast abscess
Preliminary diagnosis of an abscess was made based on clinical features of pain, swelling, and redness of the breast associated with localised tenderness in Suthar 2012. These women had the diagnosis and size of breast abscess confirmed by ultrasound evidence. Eryilmaz 2005 and Naeem 2012 made the diagnosis via a clinical examination and ultrasound was not used. Saleem 2008 made the diagnosis based on presence of a palpable mass or focal tenderness in the clinical setting of mastitis. Singla 2002 did not report how the diagnosis of a lactational breast abscess was made.
Outcomes
None of the studies separated outcomes into primary and secondary outcomes.
Five studies reported on time to resolution of breast abscess (Eryilmaz 2005; Saleem 2008; Suthar 2012; Chandika 2012; Naeem 2012), three studies reported on continuation of breastfeeding (Saleem 2008; Naeem 2012; Suthar 2012), and three reported on resolution of breast abscess (Chandika 2012; Eryilmaz 2005; Suthar 2012). Secondary outcomes were not uniformly reported on in all studies. Only two of the six secondary outcomes were addressed. Two studies reported on maternal satisfaction with treatment (Eryilmaz 2005; Saleem 2008). Four studies reported on post‐operative complications (Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012).
Definitions employed by authors
Chandika 2012 defined breast resolution as absence of symptoms of inflammation and absence of fluid on sonar.
Naeem 2012 considered resolution of symptoms.
Saleem 2008 defined resolution as no recurrent abscess or need for surgery.
Suthar 2012 defined resolution in the needle aspiration group as absence of symptoms after four aspirations with no evidence of liquefaction using ultrasound. The definitions of resolution for the I&D group were unclear.
Eryilmaz 2005 defined time to healing as time from I&D to closure for the I&D group, and time until complete resolution of the mass up to a maximum of five aspirations for the needle aspiration group.
For this review, we considered healing time to be the same as time to resolution of abscess.
Excluded studies
Nine studies were excluded from the review (Blick 1980; Edino 2001; Florey 1946; Ozseker 2008; Peters 1991; Sheih 2009; Strauss 2003; Tewari 2006; Wang 2013). Reasons for study exclusions were non randomised controlled studies, non probability sampling, absence of comparator, case series and inclusion of non‐lactational breast abscesses. Excluded studies are summarised in the table of Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias for each included study is presented in the 'Risk of bias’ tables in the Characteristics of included studies. Figure 2 and Figure 3 illustrate the summary of risk of bias in all the studies. Across studies there was unclear risk of bias for most domains due to poor reporting.
2.
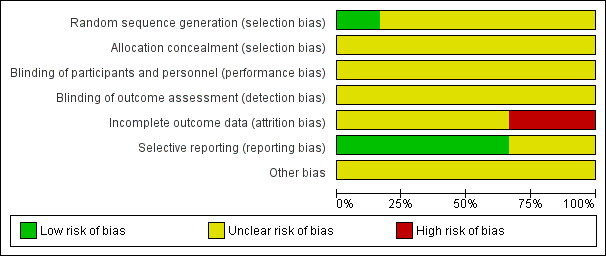
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
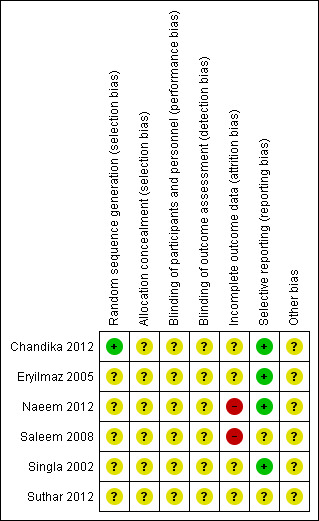
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was assessed as adequate in one study Chandika 2012, where a table of random numbers was generated via a computer (Microsoft excel version 5.0).
Random sequence generation was unclear in the remaining five studies as the authors did not adequately report on methods used to generate a random sequence (Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012).
Allocation concealment was unclear in all six studies as methods for performing allocation concealment were not described (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012).
Blinding
The nature of the intervention would have rendered it difficult to blind personnel, participants and outcome assessors involved in these studies. If data analysts were used and were independent of the research team, this was not made clear in the study and we therefore judged all studies as having an unclear risk for performance and detection bias. (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012).
Incomplete outcome data
Four studies were judged as having an unclear risk of attrition bias (Chandika 2012; Eryilmaz 2005; Singla 2002; Suthar 2012). In Chandika 2012 four participants were lost to follow‐up in the needle aspiration group, while in the I&D group one was lost to follow‐up. It is unclear what the outcomes for these abscesses were as this was not reported. In Eryilmaz 2005, nine participants in the needle aspiration group were excluded from the analysis. Their healing times were not included in the results as it was not known how long these abscesses took to heal. The study reported the healing rate as 41% (13/22), which is incorrect. In Singla 2002, all results were given as percentages and therefore made it difficult to comment on the attrition rate. In Suthar 2012, the authors indicated that the lactational breast abscesses for 29 women had resolved and six women were excluded from the study. It is unclear what happened to the six women.
Naeem 2012 was judged as having a high risk of attrition due to the fact that there were missing participants in both groups. In addition, correspondence by the author revealed that an additional three participants were missing. This information was not reported in the study. In Saleem 2008, risk of incomplete outcome data was judged high due to exclusions of participants. The authors stated that four abscesses perforated before treatment and three women underwent surgery because the abscesses were not suited for ultrasound. It is unclear to which groups these women belonged and whether they were included in the analysis. Nine women who had mastitis did not have breast abscesses and it is not known whether they were exposed to any intervention and included in the analysis.
Selective reporting
Four of the studies reported adequately on specified outcomes (Chandika 2012; Eryilmaz 2005; Naeem 2012; Singla 2002). Saleem 2008 prespecified resolution and complication rates but reported on other outcomes as well and we were therefore uncertain if these fell under complication and resolution rate. We therefore judged the study as having an unclear risk of bias. Suthar 2012 was judged as having unclear risk of bias as none of the outcomes were prespecified in the methods section.
Other potential sources of bias
We judged all six studies as having an unclear risk of other potential sources of bias (Chandika 2012; Eryilmaz 2005; Naeem 2012; Saleem 2008; Singla 2002; Suthar 2012). In Chandika 2012, women who were resistant to cloxacillin were removed from the study after randomisation and the authors have not described how many were resistant to cloxacillin and what happened to these women with regards to the abscess. In Naeem 2012, the authors reported that skin indurations around abscesses were present in 93.75% of abscesses in the I&D group, whereas 71.8% of women in the needle aspiration group had indurations. The authors report that this baseline difference was significant, yet the reported P value was 0.20, which is not statistically significant. We contacted the authors regarding this inconsistency but no response was received. In Saleem 2008, a table comparing baseline characteristics between groups was not available, which made it difficult to judge whether the groups were similar at the start of the study. In addition, groups were not treated similarly whereby in the ultrasound‐drainage group women had an ultrasound to confirm diagnosis and resolution but it is unclear what was done for the I&D group to confirm diagnosis and resolution. Also, the authors reported that women who had abscesses greater than 5 cm had a catheter inserted, but they did not report how many women had a catheter inserted. In Singla 2002, the authors reported most of the results as percentages and absolute numbers were not provided. The report also did not contain a table of participant characteristics to judge whether these groups were similar or not.
In Suthar 2012, a table of baseline characteristics was absent and it was therefore difficult to judge whether women in both groups were similar at the start of the study.
Effects of interventions
See: Table 1
1. Needle aspiration versus incision and drainage I&D)
Three studies (n = 160) were included under this comparison (Eryilmaz 2005; Saleem 2008; Suthar 2012). In Eryilmaz 2005, needle aspiration was done without ultrasound, while Saleem 2008 and Suthar 2012 both used ultrasound guidance for needle aspiration. In light of heterogeneity for all three outcomes, we did not pool data and the overall effected was not reported. Results have been summarised in the Table 1.
Primary outcomes
1.1 Time to complete resolution of breast abscess
Three studies reported on the mean time to complete resolution of breast abscess (Eryilmaz 2005; Saleem 2008; Suthar 2012) ‐ see Analysis 1.1. Eryilmaz 2005 and Suthar 2012 excluded women who had treatment failure when they calculated the mean time to complete resolution.
1.1. Analysis.
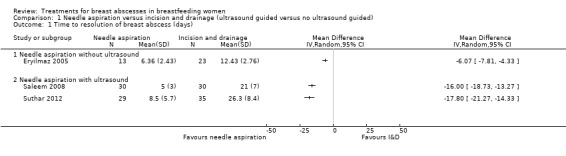
Comparison 1 Needle aspiration versus incision and drainage (ultrasound guided versus no ultrasound guided), Outcome 1 Time to resolution of breast abscess (days).
Eryilmaz 2005 found that the time to complete resolution of breast abscess was significantly less in the needle aspiration group compared to the I&D group (mean difference (MD) ‐6.07; 95% confidence interval (CI) ‐7.81 to ‐4.33; n = 36), but excluded 9/22 (41%) women in the needle aspiration group due to treatment failure.
Suthar 2012 found a significant reduction in time to complete resolution in the needle aspiration group (MD ‐17.80; 95% CI ‐21.27 to ‐14.33; n = 64), but excluded 6/35 (17%) women in the needle aspiration group due to treatment failure.
Saleem 2008 also found a significant reduction in time to complete resolution of breast abscess in the needle aspiration group (MD ‐16.00; 95% CI ‐18.73 to ‐13.27; n = 60), but did not indicate the number of women who were lost to follow‐up for either group and it is therefore not known on how many women the calculation of average time to resolution of breast abscess was based on.
Taking into consideration the limitations of the available data of all three studies, we do not consider the results to be informative.
1.2 Any continuation of breastfeeding after treatment (success)
Two studies (n = 130) reported on this outcome (Saleem 2008; Suthar 2012). We did not pool the data, since there were high levels of unexplained heterogeneity in the random‐effects meta‐analysis (Tau² = 1.38; Chi² = 32.88: P < 0.00001; I² = 97%) (Analysis 1.2).
1.2. Analysis.
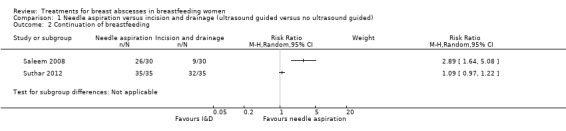
Comparison 1 Needle aspiration versus incision and drainage (ultrasound guided versus no ultrasound guided), Outcome 2 Continuation of breastfeeding.
In Saleem 2008, women in the needle aspiration group were more likely to continue breastfeeding (risk ratio (RR) 2.89; 95% CI 1.64 to 5.08; n = 60). In Suthar 2012, continuation of breastfeeding showed a trend towards needle aspiration, however this was not statistically significant (RR 1.09; 95% CI 0.97 to 1.22 n = 70).
1.3 Treatment failure
Two studies (n = 115) reported on treatment failure (Eryilmaz 2005; Suthar 2012). In Eryilmaz 2005, treatment with needle aspiration failed in 9/22 women who proceeded to have I&D. All abscesses in the I&D group were successfully treated. In Suthar 2012, treatment with needle aspiration failed in 6/35 women, who then underwent I&D. Treatment failure rate was high among women who were treated with needle aspiration (RR 16.12; 95% CI 2.21 to 117.73; participants = 115; studies = two) Analysis 1.3. We graded this evidence as low quality (see Table 1).
1.3. Analysis.
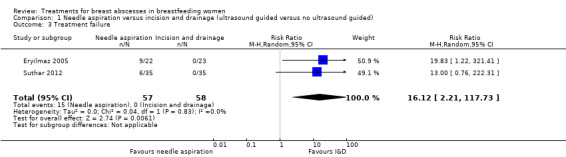
Comparison 1 Needle aspiration versus incision and drainage (ultrasound guided versus no ultrasound guided), Outcome 3 Treatment failure.
Secondary outcomes
Secondary outcomes were poorly reported in all studies and only limited data were available to include in the analysis.
1.4 Number of follow‐up visits
An assessment of the number of follow‐up visits would have provided information on the recovery following the intervention. However, no data were available. Post intervention follow‐up visits were not specified in Eryilmaz 2005, Singla 2002, and Suthar 2012. Saleem 2008 followed up women for up to two months in the needle aspiration group, at weeks four and eight after the procedure.
1.5 Duration of continuation of breastfeeding after treatment
None of the studies reported on the duration of continuation of breastfeeding.
1.6 Maternal satisfaction with treatment
Singla 2002 and Suthar 2012 did not discuss maternal satisfaction with the procedure. Eryilmaz 2005 reported that 16/23 (70%) of women in the I&D group were satisfied with the outcome but did not report on the needle aspiration group. Saleem 2008 indicated that there was 100% (30/30) satisfaction with treatment in the percutaneous ultrasound group whereas in the I&D group only 17/30 (55%) women were satisfied with the procedure (RR 1.74; 95% CI 1.28 to 2.38) (Analysis 1.4).
1.4. Analysis.
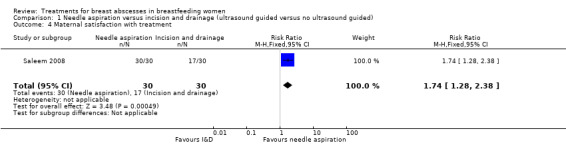
Comparison 1 Needle aspiration versus incision and drainage (ultrasound guided versus no ultrasound guided), Outcome 4 Maternal satisfaction with treatment.
1.7 Post‐operative complications/morbidity
In Saleem 2008, one woman developed a milk fistula, 1/30 (3%) woman had a residual abscess in the needle aspiration group, and 5/30 (16%) women developed milk fistulas in the I&D group. In Suthar 2012, 20% (14/70) of women complained of intolerable pain in the needle aspiration group and one woman developed a milk fistula in the I&D group.
1.8 Duration of hospital stay
Duration of hospital stay was not reported in Suthar 2012. Eryilmaz 2005 treated both groups of women on a outpatient basis. Saleem 2008, described having admitted (4/30) 13% of women in the ultrasound group, but did not state for how long. The rest of this group were treated as outpatients. The women in the I&D group were admitted for a mean of four days (two to eight days).
1.9 Adverse events
An adverse event for this review was considered in the context of events arising from drugs that may have been prescribed for women during the interventions and complications associated with the prescription thereof. None of the studies reported on adverse events. Complications arising from the procedure itself were described under post‐operative complications and morbidity.
2. Incision and drainage (I&D): antibiotic use versus no antibiotic use
One study (Singla 2002) involving 150 women, compared two different antibiotic regimens to no antibiotic administration. All three groups of women underwent I&D. Two of the groups were given antibiotics and were compared with a similar group of women who were not given any antibiotics. Group A (n = 50) received cefazolin 1 g intravenously (IV) at the time of induction of anaesthesia and 500 mg eight hourly IV for 24 hours. This was followed by oral cefalexin 500 mg six hourly for six days. Group B (n = 50) received a single dose of cefazolin 1 g IV 30 minutes before surgery. Group C (n = 50) did not receive any antibiotics.
Primary outcomes
2.1 Time to resolution of breast abscess
The mean time to resolution of breast abscess was similar in all groups, although women with an infection were excluded. Mean time to resolution for women who received a course of antibiotics was 7.3 days, 6.9 days for women who received a single dose of antibiotics, and 7.4 days for women who did not receive antibiotics. Standard deviations (SDs), P values and confidence intervals (CIs) were not reported and prevented further analysis.
2.2 Any continuation of breastfeeding
The study did not report on this outcome.
2.3 Treatment failure
This study reported on recurrence of abscess, which was considered as treatment failure. There was no difference between groups (RR 1.00; 95% CI 0.36 to 2.76; one study, n = 150, fixed‐effect meta‐analysis). There was no difference between the group that received a course of antibiotics and the group that received a single dose of antibiotics (Test for subgroup differences Chi² = 0.00, P = 1.00, I² = 0%) (Analysis 2.1).
2.1. Analysis.
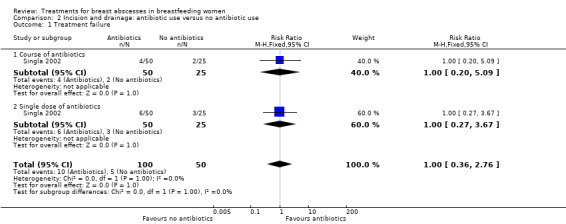
Comparison 2 Incision and drainage: antibiotic use versus no antibiotic use, Outcome 1 Treatment failure.
Secondary outcomes
2.4 Number of follow‐up visits
The study did not report on this outcome.
2.5 Duration of continuation of breastfeeding after treatment
The study did not report on this outcome.
2.6 Maternal satisfaction with treatment
The study did not report on this outcome.
2.7 Post‐operative complications/morbidity
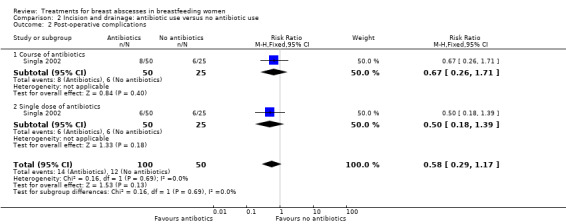
The only post‐operative complication Singla 2002 reported on was wound infection. There was no clear difference in the risk of wound infections between women who received antibiotics compared to women who did not receive antibiotics (RR 0.58; 95% CI 0.29 to 1.17; one study; n = 150). There was no difference between the group that received a course of antibiotics and the group that received a single dose of antibiotics (Test for subgroup differences: Chi² = 0.16, P = 0.69, I² = 0%) (Analysis 2.2).
2.2. Analysis.
Comparison 2 Incision and drainage: antibiotic use versus no antibiotic use, Outcome 2 Post‐operative complications.
2.8 Duration of hospital stay
The study did not report on this outcome.
2.9 Adverse events
The study did not report on this outcome.
Discussion
Summary of main results
We aimed to compare interventions used for treating lactational breast abscesses. Six studies met our inclusion criteria. Four of these studies included 325 women (Table 2) and contributed data to the analyses (Eryilmaz 2005; Saleem 2008; Singla 2002; Suthar 2012). A meta‐analysis was not possible. the other two studies did not stratify data for lactational and non‐lactational breast abscesses (Chandika 2012; Naeem 2012) and these studies did not contribute any data to the results of this review.
We did not report the overall effect for any of the outcomes, since data obtained were of poor quality and there was significant overall heterogeneity. Although results for the outcome time to resolution of abscess favoured needle aspiration in all three studies, Eryilmaz 2005 and Suthar 2012 excluded 9/22 (41%) and 6/35 (17%) of women respectively. These women were all in the needle aspiration group, had treatment failure and thus underwent I&D. Saleem 2008 did not report on any loss to follow‐up. Taking these limitations into consideration, we do not believe that these results are meaningful. For the outcome continuation of breastfeeding, results favoured needle aspiration. One study (Saleem 2008), showed a significant result, while the other did not (Suthar 2012). Treatment failure only occurred in the needle aspiration groups. All women with treatment failure proceeded to have I&D. In Eryilmaz 2005, treatment with needle aspiration failed in 9/22 women and in Suthar 2012, treatment with needle aspiration failed in 6/35 women.
Studies did not provide sufficient data on the number of follow‐up visits, duration of continuation of breastfeeding, duration of hospital stay, and adverse events to contribute data for this review. There appeared to be greater maternal satisfaction with needle aspiration compared to I&D; it is unclear as to which procedure is associated with complications post intervention.
Singla 2002, (n = 150 women), compared different antibiotic regimens versus no antibiotics in the context of I&D. Very low quality evidence suggests that there was no difference between groups for the outcome treatment failure, which was defined as recurrence of abscess in this context, and rates of infection.
Overall completeness and applicability of evidence
One study was based in Turkey (Eryilmaz 2005), one in Pakistan (Saleem 2008) and two in India (Singla 2002; Suthar 2012), all of which are low‐ and middle‐income countries. The overall sample sizes were small, n = 160 for the comparison needle aspiration versus I&D: and n = 150 for the comparison antibiotic use versus no antibiotic use with I&D. None of the studies included sample size calculations. It is thus difficult to generalise the findings of this review across countries and settings.
It is unclear how contextual, ethnic and cultural factors may have had an impact on the primary outcomes in the included studies. Most of the studies included women of Indian origin where social norms and mores are a way of life, particularly during the postpartum period and therefore may differ from cultural practices in other countries. For example, a study based in India by (Bandyopadhyay 2009) reported that more than half of the women commenced breastfeeding 24 hours or later following childbirth because colostrum was considered ‘harmful’ to the baby. It is not known how many of the women in the study were exposed to such practices and the contribution this may have had in the development of lactational breast abscesses. Women were also isolated for defined periods of time because of the “impure and polluting effects of childbirth”, an act which could potentially delay women seeking medical assistance (Bandyopadhyay 2009 p4).
The HIV status of women in the included studies was not described in any of the studies and it was therefore difficult to comment on its impact on the primary outcomes. However, studies show that women with HIV and low CD4 counts are at increased risk of developing breast abscesses (Kapatamoyo 2010). This would be an important factor to consider especially in sub‐Saharan Africa, where HIV/AIDS prevalence is high.
Women living in high‐income countries may seek treatment a lot earlier than women living in lower‐income countries, due to better access to health facilities, available resources and insight into breast abscess formation. These contextual factors, as well as other ethnic and cultural factors may also affect the response to treatment of lactational abscesses in different settings.
In the included studies, outcomes were not stratified according, to e.g. income, setting, parity, whether infants were exclusively breast fed, existing co‐morbidities and age, which may have explained the high levels of heterogeneity. Thus, it was not possible to assess their impact on the primary outcomes. A range of abscess sizes were included in the studies. The study by Suthar 2012 categorised abscesses as > 5 cm or < 5 cm but was not stratified according to the outcomes of interest using these ranges and would have provided insight into resolution and time to resolution for the different interventions.
There were missing data in all the studies. We requested information on the missing data from all of the authors and received no response. A more complete data set may have influenced our results.
Singla 2002 reported that the results for the outcomes time to resolution and resolution of abscess as similar across all three groups of women. It was difficult to analyse the results any further as means and standard deviations were not provided
We were not able to pool any data in a meta‐analysis. For the outcome time to resolution of breast abscess, there were high proportions of missing data and for the outcome continuation of breastfeeding, there were high levels of unexplained heterogeneity. Data were poorly reported across studies and outcomes were also measured differently, e.g. some used subjective measures for outcomes like signs and symptoms, while others used objective means e.g. ultrasound to determine resolution. In one instance, Saleem 2008 defined what resolution was in the ultrasound group, but did not explain how resolution was measured in the I&D group. Another possible explanation of heterogeneity could be the lack of methodological rigour. It was difficult to make judgements about risk of bias, since reporting across studies was very poor. Risk of bias was judged as being unclear for most of the domains across studies. Saleem 2008 was assessed as having high risk of attrition bias with no intention‐to‐treat analysis done. Suthar 2012 was assessed as having a high level of selective reporting bias.
The unexplained heterogeneity influencing the outcomes may also be due to factors beyond these interventions e.g. inconsistent multidisciplinary team support/approaches during and after the interventions for lactational breast abscesses.
Quality of the evidence
We used GRADE Profiler software to assess the quality of the evidence by rating the quality of evidence for one of the primary outcomes (treatment failure) under the main comparison "needle aspiration versus incision and drainage". Factors taken into consideration include study limitations, imprecision, inconsistency of results, indirectness of evidence and publication bias (Guyatt 2011). The evidence was graded to be of low quality for the outcome of treatment failure. Downgrading of evidence was based on including studies of unclear risk of bias small sample sizes with few events. We were unable to assess the quality of findings for the continuation of breastfeeding or time to resolution of breast abscess ‐ this is because the results were not pooled due to presence of severe heterogeneity.
Potential biases in the review process
We followed the Cochrane Handbook (Higgins 2011) and used the standard methods text of the Cochrane Pregnancy and Childbirth Group. We did not exclude studies in foreign languages and we aimed to find all published and unpublished studies with our extensive search strategy. We obtained all relevant studies identified from search results. We independently reviewed all potentially relevant studies and resolved disagreement by discussion. Potential bias in the review process should be minimal. We were not able to use a funnel plot to assess reporting bias, since we only included four studies in the review.
We considered randomised controlled trials for inclusion and made judgements about risk of selection bias according to the standard Cochrane 'Risk of bias' tool. None of the studies adequately described how the random sequence was generated and we therefore judged them as having unclear risk of selection bias.
Agreements and disagreements with other studies or reviews
Ulitzsch 2004, carried out a retrospective study, involving 56 lactating women, to evaluate the use of ultrasound‐guided needle aspiration and concluded that ultrasound‐guided needle aspiration in women with lactational breast abscesses smaller than 3 cm, and catheter drainage for abscesses larger than 3 cm, were useful methods in treating lactational breast abscesses
The review by Lam 2014 recommended the use of needle aspiration for the treatment of breast abscesses. The authors included a combination of 35 randomised controlled trials, non randomised trials and case series comparing needle aspiration versus I&D of breast abscesses. Participants had lactational and non‐lactational abscesses, and included women and men. SORT (Strength of Recommendation Taxonomy) was used to grade the evidence and make recommendations. It is however unclear as to how risk of bias was assessed. The review recommended needle aspiration, with or without ultrasound as the first line of treatment of breast abscess. Lam 2014 did not recommend breastfeeding from the affected breast due to Staphlococcal organisms, which placed the infant at risk of pneumonia, lung abscesses and death, and which is in contradiction with current literature (Amir 2014; Giess 2014).
Authors' conclusions
Implications for practice.
Current research is insufficient to determine whether needle aspiration is a more effective option to incision and drainage (I&D) for treating lactational breast abscesses or whether an antibiotic should be routinely added to women undergoing I&D for lactational breast abscesses. It was difficult to determine what the influence of the interventions were on the secondary outcomes due to the absence of data, e.g. duration of continuation of breastfeeding.
Implications for research.
As needle aspiration is a less invasive method compared to I&D, there is a need for a high‐quality, large randomised controlled trial to inform best practice. Future research design would include studies with adequate power (sample size) and rigorous methods. Follow‐up of participants would be all‐encompassing and therefore include duration of feeding and whether women had to supplement breastfeeding as a result of the intervention used, the impact of HIV, maternal morbidity and preferences for the intervention, cost‐analysis (the latter could ideally be considered in future updates of this review if such information becomes available), the number of women who recovered from the intervention and complications in the management of lactational breast abscesses. Consideration would be given to abscess size as there is still uncertainty around what the optimal size would be for ultrasound‐guided needle aspiration to be effective. High‐risk groups would be included in the sample size, e.g. smokers, rural versus urban, younger versus older women and outcomes would be stratified based on these risks.
Acknowledgements
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Review authors AR and TY are supported in part by the Effective Health Care Research Consortium, which is funded by UKaid from the UK Government Department for International Development.
We acknowledge Tonya Esterhuizen who assisted with statistical aspects of the review. We also acknowledge Prof Paul Garner from the Liverpool School Tropical Medicine and Co‐ordinating Editor of the Cochrane Infectious Diseases Group for his contribution to this review.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for Google Scholar, African Journals Online, ICTRP and dissertation databases
African Journals Online, ICTRP and dissertation databases
(breastfeed* OR lactation OR lactational OR lactating OR puerper* OR postpartum OR post‐partum OR postnatal) AND abscess* AND (manage* OR therap* OR treat* OR intervent* ) AND random*
Google Scholar
(breastfeed* OR lactation OR lactational OR lactating OR puerper* OR postpartum OR post‐partum OR postnatal) AND abscess* AND (manage* OR therap* OR treat* OR intervent* ) AND random*
Appendix 2. DATA extraction form
SYSTEMATIC REVIEW ‐ LACTATIONAL BREAST ABSCESS
STUDY ELIGIBILITY FORM
STUDY ID:
DATE:
EXTRACTOR (INITIALS):
STUDY REFERENCE:
STUDY ELIGIBILITY
| Eligible | Not eligible | ||
| Participants | |||
| Intervention | |||
| Comparison | |||
| Outcomes | |||
| Study design |
TRIAL METHODOLOGY
| Study Design/type | |
| Total study duration |
| Domain | Judgment | Quotes/comments |
| Selection bias | ||
| Adequate sequence generation Was the allocation sequence adequately generated? |
High Low Unclear |
|
| Allocation concealment | ||
| Was allocation adequately concealed |
High Low Unclear |
|
| Performance Bias | ||
|
Blinding of participants/providers Was knowledge of the allocated interventions adequately prevented during the study |
High Low Unclear |
|
| Detection Bias | ||
|
Blinding of outcome assessors Was knowledge of the allocated interventions adequately prevented during measurement? |
High Low Unclear |
|
| Attrition bias | ||
|
Incomplete outcome data addressed Were incomplete outcome data adequately addressed? |
High Low Unclear |
|
| Reporting bias | ||
|
Free of selective reporting Are reports of the study free of suggestion of selective outcomes reporting? |
High Low Unclear |
|
| Other bias | ||
|
Free of other bias Was the study apparently free of other problems that could put it at high risk of bias |
High Low Unclear |
Source: Chapter 8 Assessing risk of bias in included studies. Higgins JPT. Cochrane Handbook for Systematic Review of Interventions (Higgins 2011).
TRIAL CHARACTERISTICS
| Date of study | |
| Total number of participants randomised | |
| Total number of participants analysed | |
| Level of attrition (%)Total number | |
| Setting | |
| Country | |
| Ethics approval obtained |
PARTICIPANT CHARACTERISITICS
| Age (years) | |
| Gestational age at delivery | |
| Parity | |
| Duration of symptoms at enrolment (days) | |
| Abscess size at enrolment (cm) | |
| Characteristics of breast abscess: e.g. unilateral/bilateral… | |
| Diagnosis of breast abscess | |
| Duration of lactation (months) | |
| Abscess/mastitis‐previous pregnancy | |
| Co‐morbities (list) |
INTERVENTIONS
| Total number of intervention groups | |||
| Details | Intervention 1 | Intervention 2 | Control group |
| State specific intervention | |||
| Number of participants randomised | |||
| Number of participants analysed | |||
| Catheter size | |||
| Needle size | |||
| Intervention details (sufficient for replication if feasible | |
|
|
OUTCOMES
RESULTS PER OUTCOME
| Primary outcome 1: | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Primary outcome 2 | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Primary outcome 3: | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Secondary outcome 1 | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
|
Secondary outcome 2 |
|
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Secondary outcome 3 | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Secondary outcome 4 | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Secondary outcome 5 | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| OTHER: | |
| Collected YES/NO | |
| Reported YES/NO | |
| How was outcome defined | |
| Unit of measurement | |
| Primary outcome/secondary outcome |
Primary outcome 1:
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD) /risk |
Primary outcome 2:
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
Primary outcome 3:
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
Secondary outcome: 1
| Intervention1 |
Intervention2 |
|
| Number of participants with more than one visit | ||
| Missing participants | ||
| Mean (SD)/Risk |
Secondary outcome 2
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
Secondary outcome 3
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
Secondary outcome 4
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
Secondary outcome 5
| Intervention 1 |
Intervention 2 |
|
| Number of participants | ||
| Missing participants | ||
| Mean (SD)/Risk |
MISCELLANEOUS
| Funding source | |
| Key conclusions of the study | |
| Miscellaneous comments from study authors | |
| References to other relevant studies | |
| Author’s contact details | |
| Correspondence required | |
| Miscellaneous comments by the review authors |
Data and analyses
Comparison 1. Needle aspiration versus incision and drainage (ultrasound guided versus no ultrasound guided).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of breast abscess (days) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Needle aspiration without ultrasound | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Needle aspiration with ultrasound | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Continuation of breastfeeding | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Treatment failure | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 16.12 [2.21, 117.73] |
| 4 Maternal satisfaction with treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.28, 2.38] |
Comparison 2. Incision and drainage: antibiotic use versus no antibiotic use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.76] |
| 1.1 Course of antibiotics | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.20, 5.09] |
| 1.2 Single dose of antibiotics | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.27, 3.67] |
| 2 Post‐operative complications | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.17] |
| 2.1 Course of antibiotics | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.26, 1.71] |
| 2.2 Single dose of antibiotics | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chandika 2012.
| Methods |
Study design: randomised controlled trial. Duration of study: October 2006 and March 2007. |
|
| Participants |
Sample size: 65. Only 66% of the women presented with lactational breast abscesses diagnosed using ultrasound. Inclusion criteria: women 14 years and older with breast abscesses up to 5 cm in diameter as determined via ultrasound, who presented to the Accident and Emergency department and breast clinic with breast abscess. Exclusion criteria: women with recurrent or chronic breast abscesses and those with necrotic skin overlying the abscess or abscesses that were already draining. Women with clinical features of immune suppression (WHO clinical stage 111 and 1V) and those known to be allergic to penicillin and antibiotics were also excluded. Setting: Accident and emergency and breast clinic of Mulago Hospital complex, Kampala City. Country: Uganda. |
|
| Interventions |
1. Ultrasound‐guided needle aspiration (n = 33) This was done in the department of radiology ultrasound room in the OPD. Aspiration was done using ultrasound guidance using local anaesthetic. Infected fluid samples were sent for culture and sensitivity. Aspiration was done until there was no infected fluid. Cloxacillin 500 mg orally 8 hourly for 10 days and Diclofenac 75 mg IM stat and 50 mg 8 hourly for 3 days respectively. Follow‐up was done via the OPD by the principal investigator on days 7, 14 and 30. If abscess persisted, aspiration was done on day 7, if it persisted on day 14 ‐ this was considered treatment failure. The women was then sent for I&D. Women were asked to resume breastfeeding on both breasts as soon pain during breastfeeding was tolerable. 2. Incision and drainage (n = 32) Procedure was done in the operating theatre under general anaesthesia. Women were hospitalised overnight and discharged the next day. The participant was placed in a supine position. The affected breast was swabbed using Chlorhexidine Centrimide. A skin incision was made at the area of maximum fluctuation along skin lines and a sinus forceps used to reach the abscess cavity. Infected fluid samples were sent for culture and sensitivity. The infected fluid was then evacuated and loculi broken down digitally, the wound was then packed with sterile gauze. Dicolfenac 75 mg injection (IM) stat and 50 mg 8 hourly for 3 days Cloxacillin 500 mg 8 hourly for 10 days. On discharge, wound dressings were done at nearby clinic until the wound healed. Women who were resistant to Cloxacillin were excluded from the study and antibiotics changed based on sensitivity studies. Women were asked to resume breastfeeding on both breasts as soon pain during breastfeeding was tolerable. |
|
| Outcomes | Authors did not differentiate between primary and secondary outcomes. Time to breast abscess resolution. Breast abscess recurrence. Acceptance of ultrasound‐guided needle aspiration procedure. Cost of the procedure. Definition of healing: healing was defined as achieving breast abscess resolution. Breast abscess resolution was defined as clinically no breast tenderness, swelling or wound at previous site of abscess and sonographically complete absence of fluid collection, normal breast glandular and fibro fat tissue with no edema. |
|
| Notes |
Funding: not reported. Conflict of interest: the authors declared no competing interest. Ethics approval: approval issued by Faculty of medicine research committee, National science and research council, Mulago hospital complex and the Department of surgery, Mulago hospital. Author contacted: yes, no response received. Below are the questions sent to the authors via email:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel version 5 was used to generate a random number list. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there was no blinding this would not affect the resolution of the abscess. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors reported that no blinding was done. However blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 33 women were randomised to the ultrasound needle aspiration group with 32 allocated to the I&D group. The authors indicated that 29 were effectively treated by ultrasound‐guided needle aspiration group and 31 in the incision group with no reason being given. These numbers have not been included in the analysis and no ITT was done. Authors contacted and awaiting response. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the methods section have been addresses. |
| Other bias | Unclear risk | Women in both arms were excluded from the study if they were shown to be resistant to cloxacillin after randomisation and it is not known how many women were resistant to cloxacillin in both arms. Authors declared no conflict of interest. |
Eryilmaz 2005.
| Methods |
Study design: prospective RCT. Duration of study: January 2000 to July 2003. |
|
| Participants |
Sample size: 45. Inclusion criteria: lactating women with breast abscesses presenting at the surgical clinic. Women were treated for mastitis prior to development of the abscess. Exclusion criteria: not reported. Setting: Department of surgery Vakif Gureba training hospital, Instanbul. Country: Turkey. |
|
| Interventions |
1. Incision and drainage (n = 23) The procedure was done via the surgical OPD using local anaesthesia . The abscess was incised and the infected fluid was evacuated. The wound was left open to drain and dressed daily until the wound healed. A sample of the infected fluid was sent for bacteriological examination. Antibiotics were prescribed post‐operatively. Women were encouraged to feed from the unaffected breast and the breast with the abscess was emptied using breast pump. 2. Needle aspiration. Ultrasound guidance was not used (n = 22) It is unclear as to whether any anaesthesia was used during this intervention. Aspirations were repeated on alternate days until the abscess had completely resolved or until 5 needle aspirations had been performed. A sample of the infected fluid was sent for bacteriological examination. Antibiotics were prescribed post‐operatively Breastfeeding as per I&D group. |
|
| Outcomes | Primary outcomes and secondary outcomes. Authors did not differentiate between primary and secondary outcomes.
Definition of healing for incision and drainage group: healing was defined as time from incision and drainage to wound closure. Definition of failure for needle aspiration group: if after 5 aspirations which were done every other day the abscess was not resolved, this was considered a failure of treatment. |
|
| Notes |
Funding: not reported. Ethics approval: not stated. Informed consent obtained from the participants. Author contacted: yes, no response received. Below are the questions sent to the authors via email.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised 1:1. Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there was no blinding, it is unclear whether the resolution of the abscess would be affected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding was done. Blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 women were excluded from the analysis in the needle aspiration group (mean healing time). The authors have not described how long the abscesses took to heal even after I&D. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported on. |
| Other bias | Unclear risk | Funding: authors did not declare any competing interests. Ethics approval: not stated. Informed consent obtained from the participants. Author contacted: yes. |
Naeem 2012.
| Methods |
Study design: RCT Duration of study: August 2008 and August 2010 |
|
| Participants |
Sample size: 64 (only 52 (86.67%) were lactational breast abscesses. From the text it appears that 11 had non‐puerperal breast abscesses, but that the percentages do not correspond to the actual numbers. We have contacted authors for clarification. Inclusion criteria: Any female with a single abscess smaller than 5 cm in a reproductive‐aged group who was not pregnant at the time and not being treated for any other breast pathology. Exclusion criteria: Women with sinus/fistula of breast abscess, prolonged history and necrosis of the skin. Setting: KVVS hospital, Karachi. Country: Pakistan. |
|
| Interventions |
1. Incision and drainage (n = 32) The intervention was carried out in a hospital setting. Women were admitted between 1 to 3 days. A general anaesthetic was given. Before surgery the infected fluid was sent for culture and sensitivity and cytology. 625 mg capsule of co‐amoxiclav 3 times a day were and 400 mg metronidazole (non‐lactational breast abscesses) until culture reports were received. IV analgesia was initially given. IM analgesia was initially given and then followed by oral analgesia. Daily dressings were for 1 to 3 weeks Women were followed up for 8 weeks after the procedure. Women were unable to feed with the affected breast so milk was discarded after expressing with breast pump according to the authors. 2. Ultrasound‐guided needle aspiration. (n = 32) The procedure was done in the OPD therefore requiring no hospital admission. No anaesthetic was given. The aspiration was done using ultrasound guidance. Prior to the procedure, samples were sent for culture and sensitivity and cytology. 625 mg capsule of co‐amoxiclav 3 times a day and (400 mg metronidazole) was given to non‐puerperal abscesses. The cavity was washed with normal saline and a follow‐up ultrasound was done on the third day. If the abscess was seen, the canula was left in place. IM analgesia was initially given. Follow‐up was done for 8 weeks after the procedure. Women continued to feed. |
|
| Outcomes | Authors did not differentiate between primary and secondary outcomes. 1.Time taken to resolve symptoms (point tenderness; erythema; hyperthermia) 2.Recurrence of breast abscess 3.Healing time |
|
| Notes |
Funding: not reported. Conflict of interest: authors did not declare any competing interests. Ethics approval: not stated. Author contacted: Partial response received from corresponding author below: "Our review is assessing puerperal breast abscesses only and the management thereof". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not stated how they were randomly divided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there was no blinding the resolution of the abscess would not be affected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Table 1: Group A, n = 32 however outcomes for 30 only addressed. For group B there is also one missing, the values only add up to 31. The response from the author was that in the analysis there were: Group A ‐ 30 women and group B 31. Upon requesting information from the corresponding author he mentioned that 3 participants were missing at week 8 follow‐up ‐ no reasons were given as to what happened to them. This information was not in the study. |
| Selective reporting (reporting bias) | Low risk | Healing time was addressed and abscess recurrence. However with regards to time to resolving of symptoms, only breast pain was addressed, erythema and hyperthermia not addressed. |
| Other bias | Unclear risk | The authors report that skin indurations around abscesses were present in Group A in 93.75% whereas in Group B only 71.8%. They say the P value is significant, but it is 0.20. There is also no table of baseline characteristics of both groups, so we cannot really judge whether there were any baseline differences between groups. |
Saleem 2008.
| Methods |
Study design: RCT. Duration of study: Jan 2005 to June 2007. |
|
| Participants |
Sample size: 60. Inclusion criteria: women with clinically suspected breast abscesses. All women had mastitis. Exclusion criteria: not given. Setting: Allied Hospital, Faisalabad. Country: Pakistan. |
|
| Interventions |
1. Percutaneous drainage under ultrasound guidance (n = 30) 26/30 women had needle aspiration done via OPD and 4/30 were admitted to hospital. Only women with abscesses larger than 3 cm were given an anaesthetic. The abscess was diagnosed using ultrasound. An ultrasound‐guided needle puncture was made to confirm the diagnosis. Samples of the infected fluid were sent for culture and sensitivity. Abscesses that were smaller than 3 cm were aspirated; for those that were 3 cm and larger a catheter was inserted to allow the abscess to drain. The wound was irrigated 3 to 5 times with sterile saline until the aspirate ran clear. Antibiotics were prescribed. Women were followed up to 2 months. Women who were discharged with catheters were taught how to clean the catheter. On return to the facility, the catheter was removed when abscess was no longer visible on ultrasound. Women were encouraged to breastfeed. 2. Incision and drainage (n = 30) 30 women were admitted to hospital for a mean duration of 4 days. General anaesthetic was given. Samples of the infected fluid were sent off for culture and sensitivity. Antibiotics were prescribed. Women were followed up to 2 months. Daily dressings were done. Women were encouraged to breastfeed. |
|
| Outcomes | Primary outcomes and secondary outcomes. Authors did not differentiate between primary and secondary outcomes.
|
|
| Notes |
Funding: not reported. Conflict of interest: authors did not declare any competing interests. Ethics approval: not indicated. Informed consent obtained. Author contacted: yes and no response received. Below are the questions sent to the authors via email.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Authors only state that participants were “divided into two groups randomly”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although blinding was not done this may not have affected resolution. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. Blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors state that 4 women had abscesses that perforated before treatment, 3 women underwent surgery because the abscesses were not suited for ultrasound drainage. Authors do not state to which group these women belonged and whether they were included in the analysis or not. In group A, 9 participants did not have breast abscesses, and it is uncertain if these women were included in the analysis since there is no mention of the sample size for table III. In the I&D group it is unclear if all women were operated on regardless of whether they had an abscess or if there was an obvious diagnosis of a breast abscess and were all allocated to Group B. It is for these reasons that it was assessed as high risk. |
| Selective reporting (reporting bias) | Unclear risk | Authors only pre‐specified resolution rate and complications. They described recurrence, breastfeeding cessation, fistulas. It was unclear if the latter was considered under the banner of complications and therefore was assessed as unclear. |
| Other bias | Unclear risk | We did not have a table comparing baseline characteristics between groups. This made it very difficult to judge whether groups were actually similar at the start of the study. Also, groups were not treated equally. Group A had ultrasound to confirm diagnosis and resolution of abscess and were unclear how was this done in group B as it was not reported. We were also uncertain as to whether participants in Group A had a catheter inserted or only aspiration. |
Singla 2002.
| Methods |
Study design: prospective RCT. Duration of study:1989 to 1998. |
|
| Participants |
Sample size: n = 150; 50 women per group. Inclusion criteria: women with lactational breast abscesses. Exclusion criteria: women with diabetes (3) and those on previous antibiotics (29) were excluded from the study. Setting: Department of surgery, Pt PD Sharma PGIMS, Rohtak Haryana, PIN‐124001, India. Country: India. |
|
| Interventions | Women in all 3 groups women were treated with I&D under general anaesthetic for their abscesses. Women in group A and B were then given a broad spectrum antibiotic and compared to group C, a control group. Group A: n = 50. Women received cefazolin (1 g) IV at the time of induction of anaesthesia and 500 mg 8 hourly IV for 24 hours. This was followed by cefalexin (500 mg) capsules for 6 hourly for 6 days. Group B: n = 50. Women received IV cefazolin (1 g), given IV 30 minutes before surgery only. Group C: n = 50. Women were not given any antibiotics. Under general anaesthesia the abscess was drained and the infected fluid sent for culture and sensitivity. The cavity was curetted and packed for 5 minutes. It was then washed with normal saline with visible active bleeders ligated and the wound closed over a suction drain. The cavity was not obliterated. Three blood culture specimens were taken, one at 30 minutes before the procedure, one at 30 minutes after the procedure and the last, an hour after the procedure. It is unclear as to what procedures were followed after the intervention nor the advice on lactation if any. |
|
| Outcomes | Primary outcomes and secondary outcomes. Authors did not differentiate between primary and secondary outcomes.
|
|
| Notes |
Funding: not reported. Conflict of interest: authors did not declare any competing interests. Ethics approval: not described. Only informed consent taken from women. Author contacted: the hospital director was contacted via email for contact details of author. No response received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors say women were "randomly allocated" but did not describe how this was done. |
| Allocation concealment (selection bias) | Unclear risk | This was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there was no blinding the resolution of the abscess would not be affected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. Blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Difficult to say as all results are reported in percentages. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the methods section were addressed. |
| Other bias | Unclear risk | The study does not contain a lot of detail. Baseline characteristics for participants were not provided and cannot be certain if women in the groups were similar. The latter makes it difficult to determine whether participants in the various groups were the same. |
Suthar 2012.
| Methods |
Study design: RCT. Duration of study: not stated. |
|
| Participants |
Sample size: 70. Inclusion criteria: women with puerperal breast abscess. Exclusion criteria: women with diabetes mellitus, renal failure, steroid therapy, suspected malignancy, history of malignancy, recurrent breast abscess, active pulmonary TB, tuberculous lymphadenitis, sub‐areolar breast abscess imminent necrosis of skin on breast were all excluded. Setting: not described. It is unclear from where the participants were sourced. Country: authors are based in Gujarat India. |
|
| Interventions |
1. Percutaneous ultrasound‐guided needle aspiration (n = 35) Women were treated on an OPD basis. Local anaesthesia was given. Post‐procedural ultrasound images were obtained to evaluate residual fluid collections. Aspirations were done at 4‐ to 5‐day intervals until resolution, using ultrasound evidence, which was considered as an end point of management. Treatment was considered a failure after 4 aspirations and with evidence of liquefaction. Oral antibiotics were prescribed. It is unclear whether women had to adhere to post discharge visits though all women were encouraged to breastfeed under hygienic conditions. 2. Open surgical drainage under general anaesthetic indoors (n = 35) Women were hospitalised and the procedure was done using general anaesthetic. Daily dressings with packing gauze were carried out until signs and symptoms resolved and there was ultrasound evidence of complete healing. Oral antibiotics were prescribed. Women were encouraged to feed from the opposite side and to express milk from the operated side. It is not known what the post discharge procedures were as these were not described. |
|
| Outcomes | Primary outcomes and secondary outcomes. Authors did not differentiate between primary and secondary outcomes.
Failure for the ultrasound group was defined as persistence of signs and symptoms after 4 aspirations with ultrasound evidence of liquefaction. |
|
| Notes |
Funding: not reported. Conflict of interest: authors did not declare any competing interests. Ethics approval: the authors do not state whether this was received. Consent was obtained from women. Author contacted: yes and no response received. Below are the questions sent to the authors via email.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described as authors only state that they made use of "random sampling". |
| Allocation concealment (selection bias) | Unclear risk | Not reported on. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. Although blinding was not described the resolution of the abscess would not be affected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. Blinding of outcome assessors is not possible due to the nature of the interventions and therefore difficult to judge whether this has an impact. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were accounted for. The number of participants were deduced from the tables provided. No flow chart was provided and nowhere do they mention how many participants were allocated to the different groups. |
| Selective reporting (reporting bias) | Unclear risk | Although all stated outcomes were discussed. These were not prespecified in the methods section. |
| Other bias | Unclear risk | There is no table of baseline characteristics of participants, therefore very difficult to judge whether groups were similar at the start of the study. |
I&D: incision and drainageI IM: intramuscular ITT: intention‐to‐treat IV: intravascular OPD: outpatients department RCT: randomised controlled trial SD: standard deviation stat: immediately TB: tuberculosis UGA: ultrasound‐guided aspiration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blick 1980 | A variety of abscesses were presented with only 3/80 puerperal abscesses. |
| Edino 2001 | Prospective study. |
| Florey 1946 | This is not a comparative study, it looks at cases that presented and were treated. |
| Ozseker 2008 | Case series. |
| Peters 1991 | No comparator group. |
| Sheih 2009 | Non probability convenience sampling and different outcomes for intervention and comparator groups. |
| Strauss 2003 | Retrospecitve case series, not a clinical trial. |
| Tewari 2006 | No comparator group, descriptive study. |
| Wang 2013 | No comparator group, descriptive study. |
Differences between protocol and review
There are some differences between our published protocol (Irusen 2013) and the full review ‐ these are detailed below.
Index to thesis in Great Britian and Ireland and Dissonline have not been searched for studies for the review as Stellenbosch University library does not have access to these databases.
We have changed the third primary outcome 'Resolution of breast abscess' to 'Treatment failure'. After data extraction, we realised that 'Treatment failure' is a more pertinent outcome. We also realised that there was an error in the protocol that neither the review team, nor the peer reviewers picked up before publication. We intended including the primary outcome 'Resolution of abscess' and had added it to the list of primary outcomes. With various iterations and corrections on the protocol within the review team, it was accidentally deleted. However, in the published protocol, under the heading Measures of treatment effect, we referred to this outcome under the subheading Dichotomous data'. We thus definitely had the intention of including it in the protocol.
We changed the name of the primary outcome 'Time to resolution of abscess' to 'Time to resolution of breast abscess' for clarity.
Future updates of this review may consider including quasi‐RCTs and cluster‐RCTs due to paucity of data.
Methods for sensitivity analysis has been updated to reflect possible inclusion of cluster‐RCTs in future updates of this review.
Contributions of authors
Hayley Irusen is guarantor for the review and she developed the protocol. Anke Rohwer and Taryn Young gave input on the protocol development and draft. Professor Daniël Wilhelm Steyn commented on the clinical aspects of the protocol and review.
Hayley Irusen was involved in screening the results and eligibility assessment of the studies. She was involved in data extraction, data management, 'Risk of bias' assessment, data analysis and data interpretation. She was responsible for writing the initial draft of the review.
Anke Rohwer as the second author was independently involved in the screening of search results, analysis and interpretation of the data, and commented on and revised the manuscript.
Taryn Young provided methodological support for the review. She oversaw the project. She assisted in resolving disagreements, contributed to data analysis and interpretation of results and commenting on and revising the manuscript. All authors approved the final version of the review.
Sources of support
Internal sources
Centre for Evidence‐based Health Care, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa.
External sources
-
Department for International Development, UK.
AR and TY are supported in part by the Effective Health Care Research Consortium, which is funded by UKaid from the UK Government Department for International Development.
Declarations of interest
Hayley Irusen: None known.
Anke C Rohwer is supported in part by the Effective Health Care Research Consortium, which is funded by UKaid from the UK Government Department for International Development. This DFID grant is aimed at ensuring the best possible systematic reviews, particularly Cochrane Reviews, are completed on topics relevant to the poor, particularly women, in low‐ and middle‐income countries. DFID does not participate in the selection of topics, in the conduct of the review, or in the interpretation of findings.
D Wilhelm Steyn: None known.
Taryn Young is supported in part by the Effective Health Care Research Consortium, which is funded by UKaid from the UK Government Department for International Development. This DFID grant is aimed at ensuring the best possible systematic reviews, particularly Cochrane Reviews, are completed on topics relevant to the poor, particularly women, in low‐ and middle‐income countries. DFID does not participate in the selection of topics, in the conduct of the review, or in the interpretation of findings.
New
References
References to studies included in this review
Chandika 2012 {published data only}
- Chandika A, Gakwaya AM, Kiguli‐Malwadde E, Chalya PL. Ultrasound guided needle aspiration versus surgical drainage in the management of breast abscesses: a Ugandan experience. BMC Research Notes 2012;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eryilmaz 2005 {published data only}
- Eryilmaz R, Sahin M, Tekelioglu MH, Daldal E. Management of lactational breast abscesses. Breast 2005;14(5):375‐9. [DOI] [PubMed] [Google Scholar]
Naeem 2012 {published data only}
- Naeem M, Rahimnajjad MK, Rahimnajjad NA, Ahmed QJ, Fazel PA, Owais M. Comparison of incision and drainage against needle aspiration for the treatment of breast abscess. American Surgeon 2012;78(11):1224‐7. [PubMed] [Google Scholar]
Saleem 2008 {published data only}
- Saleem S, Farooq T, Khan N, Shafiq M, Azeem M, Dab RH. Puerperal breast abscesses; Percutaneous ultrasound guided drainage compared with conventional incision and drainage. Professional Medical Journal 2008;15(4):431‐6. [Google Scholar]
Singla 2002 {published data only}
Suthar 2012 {published data only}
- Suthar KD, Mewada BN, Surati KN, Shah JK. Comparison of percutaneous ultrasound guided needle aspiration and open surgical drainage in management of puerperal breast abscess. International Journal of Medical Science and Public Health 2013;2(1):69‐72. [Google Scholar]
References to studies excluded from this review
Blick 1980 {published data only}
- Blick PWH, Flowers MW, Marsden AK, Wilson DH, Ghoneim ATM. Antibiotics in surgical treatment of acute abscesses. British Medical Journal 1980;281(6233):111‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edino 2001 {published data only}
- Edino ST, Ihezue CH. Management of acute abscesses in Jos, Nigeria. Nigerian Journal of Surgical Research 2001;3(1):24‐8. [Google Scholar]
Florey 1946 {published data only}
- Florey ME. Treatment of breast abscesses with penicillin. British Medical Journal 1946;2(4483):845‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozseker 2008 {published data only}
- Ozseker B, Ozcan UA, Rasa K, Cizmeli OM. Treatment of breast abscesses with ultrasound‐guided aspiration and irrigation in the emergency setting. Emergency Radiology 2008;15:105‐8. [DOI] [PubMed] [Google Scholar]
Peters 1991 {published data only}
- Peters F, Flick‐Filliés D. Drainage of large breast abscesses with reference to esthetic and functional aspects. Geburtshilfe und Frauenheilkunde 1991;51(11):901‐4. [DOI] [PubMed] [Google Scholar]
Sheih 2009 {published data only}
- Sheih WI, Suleri M, Kulsoom M. Comparison of multiple needle aspirations and open surgical drainage in management of breast abscess. Journal of Rawalpindi Medical College (JRMC) 2009;13(1):30‐3. [Google Scholar]
Strauss 2003 {published data only}
- Strauss A, Middendorf K, Müller‐Egloff S, Heer IM, Untch M, Bauerfeind. Sonographically guided percutaneous needle aspiration of breast abscesses ‐ a minimal‐invasive alternative to surgical incision. Ultraschall in der Medizin 2003;24(6):393‐8. [DOI] [PubMed] [Google Scholar]
Tewari 2006 {published data only}
- Tewari M, Shukla HS. An effective method of drainage of puerperal breast abscess by percutaneous placement of suction drain. Indian Journal of Surgery 2006;68(6):330‐3. [Google Scholar]
Wang 2013 {published data only}
- Wang K, Ye Y, Sun G, Xu Z. The Mammotome biopsy system is an effective treatment strategy for breast abscess. American Journal of Surgery 2013;205:35‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Abou‐Dakn 2010
- Abou‐Dakn M, Richardt A, Schaefer‐Graf U, Wöckel A. Inflammatory breast diseases during lactation: milk stasis, puerperal mastitis, abscesses of the breast, and malignant tumors ‐ current and evidence‐based strategies for diagnosis and therapy. Breast Care 2010;5(1):33‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajao 1994
- Ajao OG, Ladipo JK, al‐Saigh AA, Malatani T. Primary closure of breast abscess compared with the conventional gauze packing and daily dressings. West African Journal of Medicine 1994;13(1):28‐30. [PubMed] [Google Scholar]
Amir 2004
- Amir LH, Forster D, McLachlan H, Lumley J. Incidence of breast abscess in lactating women: report from an Australian cohort. BJOG: an international journal of obstetrics and gynaecology 2004;111:1378–81. [DOI] [PubMed] [Google Scholar]
Amir 2014
- Amir LH, The Academy of Breastfeeding Medicine Protocol Committee. ABM Clinical Protocol #4: Mastitis, Revised March 2014. Breastfeeding Medicine 2014;9(5):239‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2010
- Baker CR. In: Teo James T H, Reese George Dr editor(s). Rapid Surgery. 2nd Edition. Oxford: Wiley‐Blackwell, 2010:1. [Google Scholar]
Bandyopadhyay 2009
- Bandyopadhyay M. Impact of ritual pollution on lactation and breastfeeding practices in rural West Bengal, India. International Breastfeeding Journal 2009;4(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbosa‐Cesnick 2003
- Barbosa‐Cesnick C, Schwartz K, Foxman B. Lactation mastitis. JAMA 2003;289(13):1609‐12. [DOI] [PubMed] [Google Scholar]
Benson 1989
- Benson EA. Management of breast abscesses. World Journal of Surgery 1989;13(6):753‐6. [DOI] [PubMed] [Google Scholar]
Bertrand 1991
- Bertrand H, Rosenblood LK. Stripping out pus in lactational mastitis: a means of preventing breast abscess. Canadian Medical Association Journal 1991;145(4):299‐306. [PMC free article] [PubMed] [Google Scholar]
BMJ online 2014
- Mean and Standard deviation. http://www.bmj.com/about‐bmj/resources‐readers/publications/statistics‐square‐one/2‐mean‐and‐standard‐deviation (accessed 05 December 2014).
Branch‐Elliman 2012
- Branch‐Elliman W, Golen TH, Gold HS, Yassa DS, Baldini LM, Wright SB. Risk factors for Staphylococcus aureus post partum breast abscess. Clinical Infectious Disease 2012;54:71‐7. [DOI] [PubMed] [Google Scholar]
Christensen 2005
- Christensen AF, Al‐Suliman N, Nielsen KR, Vejborg I, Severinsen N, Christensen H, et al. Ultrasound‐guided drainage of breast abscesses: results in 151 patients. British Journal of Radiology 2005;78(927):186‐8. [DOI] [PubMed] [Google Scholar]
Crepinsek 2012
- Crepinsek MA, Crowe L, Michener K, Smart NA. Interventions for preventing mastitis after childbirth. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007239] [DOI] [PubMed] [Google Scholar]
Dener 2003
- Dener C, Inan A. Breast abscesses in lactating women. World Journal of Surgery 2003;27(2):130‐3. [DOI] [PubMed] [Google Scholar]
Dirbas 2011
- Dirbas FM, Scott‐Conner CEH. Breast Surgical Techniques and Interdisciplinary Management. Springer, 2011. [Google Scholar]
Dixon 1988
- Dixon JM. Repeated aspiration of breast abscesses in lactating women. BMJ 1988;297(6662):1517‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 1998
- Dixon JM, Venizelos B, Matheson L. Breast lumps in young women. New technique has excellent cosmetic results. BMJ 1998;317(7152):209‐10. [PubMed] [Google Scholar]
Giess 2014
- Giess CS, Golshan M, Flahery K, Birdwell RL. Clinical experience with aspiration of breast abscesses based on size and etiology at an Academic Medical Center. Journal of Clinical Ultrasound 2014;42:513‐21. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, BrozekJ, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1989
- Hughes LE, Mansel RE, Webster DJT. Infection of the breast. Benign Disorders and Diseases of the Breast. London: Bailliere Tindall, 1989:143–9. [Google Scholar]
Jahanfar 2012
- Jahanfar S, Ng CJ, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD005458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1976
- Jones NAG, Wilson DH. The treatment of acute abscesses by incision, curettage and primary suture under antibiotic cover. British Journal of Surgery 1976;63(6):499‐501. [DOI] [PubMed] [Google Scholar]
Kapatamoyo 2010
- Kapatamoyo B, Andrews B, Bowa K. Association of HIV with breast abscess and altered microbial susceptibility patterns. Medical Journal of Zambia 2010;37(2):58‐63. [PMC free article] [PubMed] [Google Scholar]
Karstrup 1993
- Karstrup S, Solvig J, Nolsoe CP, Nilsson P, Khattar S, Loren I, et al. Acute puerperal breast abscesses: US‐guided drainage. Radiology 1993;188(3):807‐9. [DOI] [PubMed] [Google Scholar]
Kvist 2005
- Kvist LJ, Rydhstroem H. Factors related to breast abscess after delivery: a population‐based study. BJOG: an international journal of obstetrics and gynaecology 2005;112(8):1070‐4. [DOI] [PubMed] [Google Scholar]
Lam 2014
- Lam E, Chan T, Wiseman SM. Breast abscess: evidence based management recommendations. Expert Review of Anti‐infective Therapy 2014;12(7):753‐62. [DOI] [PubMed] [Google Scholar]
Lawrence 2005
- Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. 7th Edition. Elsevier Mosby, 2005:11‐25. [Google Scholar]
Lumbiganon 2012
- Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006425.pub3] [DOI] [PubMed] [Google Scholar]
Mangesi 2010
- Mangesi L, Dowswell T. Treatments for breast engorgement during lactation. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD006946.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchant 2002
- Marchant DJ. Inflammation of the breast. Obstetrics and Gynecology Clinics of North America 2002;29(1):89‐102. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scholefield 1987
- Scholefield JH, Duncan JL, Rogers K. Review of a hospital experience of breast abscesses. British Journal of Surgery 1987;74(6):469‐70. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Ulitzsch 2004
Walker 2011
- Walker M. Breastfeeding Management for the Clinician: Using the Evidence. 2nd Edition. Sudbury, Mass: Jones and Bartlett Publishers, 2011. [Google Scholar]
Wallace 2010
- Wallace MJ, Chin KW, Fletcher TB, Bakal CW, Cardella JF, Grassi CJ, et al. Quality improvement guidelines for percutaneous drainage/aspiration of abscess and fluid collections. Journal of Vascular and Interventional Radiology : JVIR 2010;21(4):431–5. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization, Dept of Child & Adolescent Health & Development. Implementing the Global Strategy for Infant and Young Child Feeding. 3‐5 Feb 2003 Meeting Report. Geneva: WHO, 2003. [Google Scholar]
WHO 2012
- World Health Organization. Nutrition. www.who.int/nutrition/topics/exclusive_breastfeeding/en (accessed 2013) 2012 July 29.
World Health Organization 2000
- WHO 2000. World Health Organization: Mastitis: Causes and Management. Publication Number WHO/FCH/CAH/00.13, World Health Organization, Geneva, 2000..
References to other published versions of this review
Irusen 2013
- Irusen H, Rohwer AC, Steyn DW, Young T. Treatments for breast abscesses in breastfeeding women. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010490] [DOI] [PMC free article] [PubMed] [Google Scholar]


