Abstract
Due to their renowned regenerative capacity, adult zebrafish are a premier vertebrate model to interrogate mechanisms of innate spinal cord regeneration. Following complete transection of their spinal cord, zebrafish extend glial and axonal bridges across severed tissue, regenerate neurons proximal to the lesion, and regain their swim capacities within 8 weeks of injury. Recovery of swim function is thus a central readout for functional spinal cord repair. Here, we describe a set of behavioral assays to quantify zebrafish motor capacity inside an enclosed swim tunnel. The goal of these methods is to provide quantifiable measurements of swim endurance and swim behavior in adult zebrafish. For swim endurance, zebrafish are subjected to a constantly increasing water current velocity until exhaustion, and time at exhaustion is reported. For swim behavior assessment, zebrafish are subjected to low current velocities and swim videos are captured with a dorsal view of the fish. Percent activity, burst frequency, and time spent against the water current provide quantifiable readouts of swim behavior. We quantified swim endurance and swim behavior in wild-type zebrafish before injury and after spinal cord transection. We found that zebrafish lose swim function after spinal cord transection and gradually regain that capacity between 2 and 6 weeks post-injury. The methods described in this study could be applied to neurobehavioral, musculoskeletal, skeletal muscle regeneration, and neural regeneration studies in adult zebrafish.
Introduction
Adult zebrafish are eminently used to investigate mechanisms of neuromuscular and musculoskeletal development and disease modeling1, 2, 3. Zebrafish are capable of efficient, spontaneous repair of multiple tissues, including the brain, spinal cord, and skeletal muscle4, 5, 6, 7. The remarkable capacity to regenerate neuromuscular tissues and model diseases is attracting a growing scientific community into adult zebrafish research1, 2, 3. However, while assays of locomotion and swim behavior are available and standardized for larval zebrafish, there is a growing need to develop analogous protocols in adult fish8, 9, 10, 11. The goal of this study is to describe protocols to quantify swim endurance and swim behavior in adult zebrafish. We present these protocols in the context of spinal cord regeneration research. However, the behavioral protocols described here are equally applicable to studies of neural and muscle regeneration, neuromuscular and musculoskeletal development, as well as neuromuscular and musculoskeletal disease modeling.
Zebrafish reverse paralysis within 8 weeks of complete spinal cord transection. Unlike poorly regenerative mammals, zebrafish display pro-regenerative immune, neuronal, and glial injury responses that are required for functional spinal cord repair12, 13, 14. An ultimate readout of functional spinal cord repair is the ability of the lesioned tissue to regain its function after injury. A suite of standardized methods to assess functional regeneration in rodents include locomotor, motor, sensory, and sensorimotor tests15, 16, 17. Widely used tests in mouse spinal cord injury include the locomotor Basso Mouse Scale (BMS), forelimb motor tests, tactile sensory tests, and grid walking sensorimotor tests15, 17. In contrast with mammalian or larval zebrafish systems, behavioral tests in adult zebrafish are less developed, yet much needed to accommodate the growing needs of the tissue regeneration and disease modeling communities.
Complete spinal cord transections result in complete paralysis caudal to the injury site. Shortly after the injury, paralyzed animals are less active and avoid swimming as much as possible. To compensate for lost swim capacity, paralyzed animals display short, frequent bursts by overusing their pectoral fins, which lie rostral to the lesion. This compensatory swim strategy results in rapid exhaustion and lower swim capacity. As the zebrafish spinal cord regenerates, animals regain a smooth oscillatory swim function caudal to the lesion, allowing for increased swim endurance and improved swim behavior parameters. Here, we describe methods to quantify zebrafish swim endurance at increasing water current velocities and swim behavior at low current velocities.
Protocol
Adult zebrafish of the Ekkwill and AB strains were maintained at the Washington University Zebrafish Core Facility. All animal experiments were performed in compliance with IACUC institutional animal protocols.
NOTE: An example of the experimental setup is shown in Figure 1A. The calibration lid (customized), swim endurance lid (customized), and swim behavior lid (standard, enclosed tunnel lid) are shown in Figure 1B. The experimental workflow is presented in Figure 2.
Figure 1: Swim tunnel set up and customized lids.
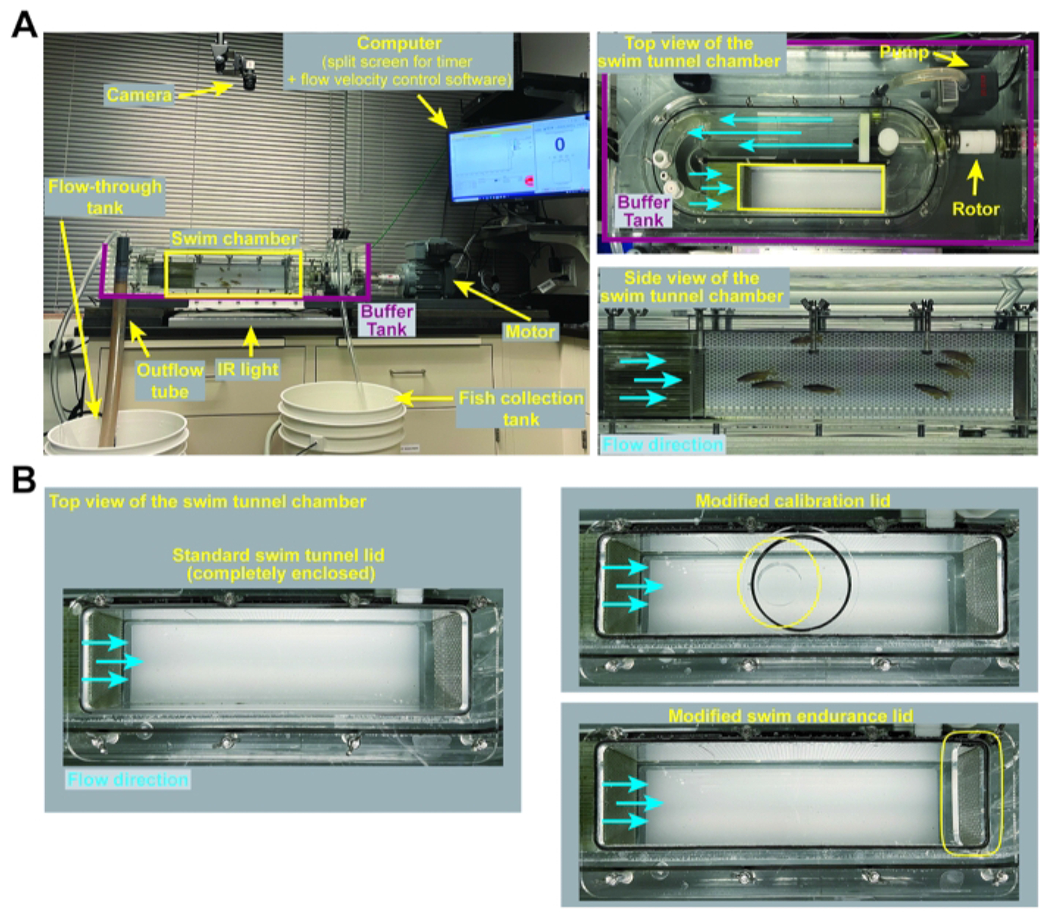
(A) Representative images of the swim tunnel set up including zoomed top and side views of the swim tunnel chamber. (B) Images of the swim tunnel lids used for the various applications described in this protocol. A standard, fully enclosed swim tunnel lid is used for swim behavior assays (section 3 of this protocol). A modified swim tunnel lid that accommodates a handheld digital flow meter is used for calibration (section 1 of this protocol). A modified swim endurance lid, containing a removable lid at the posterior end of the swim tunnel chamber, allows for the removal of exhausted fish during swim endurance testing (section 2 of this protocol). Please click here to view a larger version of this figure.
Figure 2: Experimental pipeline to assay for swim endurance and swim behavior in adult zebrafish.
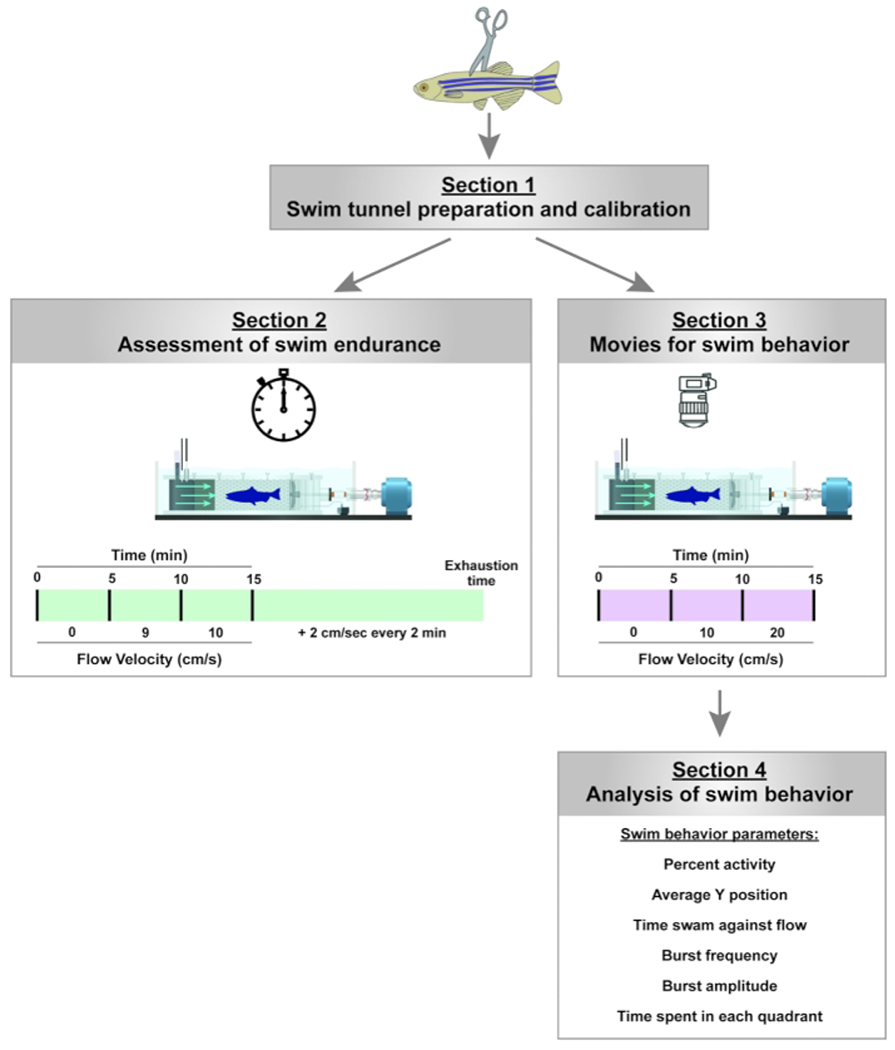
For swim endurance, fish swam against an increasing water current until exhaustion. For swim behavior, swim parameters are assessed in the absence of and at low current velocities. Please click here to view a larger version of this figure.
1. Swim tunnel preparation and calibration
Fill the swim tunnel and surrounding buffer tank with zebrafish system water (10 L filtered water; alkalinity: 50-150 mg/L CaCO3; pH: 6.8-7.5; temperature: 26-28.5°C; nitrate < 50 mg/L; nitrite < 0.1 mg/L; and salinity < 0.5-1 g/L).
Fill an additional flow-through tank with zebrafish system water (≈7.5 L). Position the swim tunnel and flow-through tank to allow for excess zebrafish system water to flow from the buffer tank into the flow-through tank through an outflow tube secured on the side of the buffer tank.
- Once the tunnel and buffer tank are filled, perform the following.
- Place the flush pump inside the buffer tank and connect it to the adjacent swim tunnel with PVC tubes. Place the flow-through pump inside the flow-through tank and connect it to the wall of the buffer tank.
-
Turn on the flush pump located inside the buffer tank and the flow-through pump located in the flow-through tank to begin water circulation.NOTE: The dual pump system will ensure continuous water flow into the swim tunnel (from the flush pump) and into the buffer tank (from the flow-through pump).
-
Clear any air bubbles trapped inside the swim tunnel by gradually increasing the water current velocity from 10 cm/s to 100 cm/s in intervals of 10 cm/s. Decrease the velocity back to 0 cm/s in intervals of 10 cm/s. To control flow velocity, click the up and down arrows in the Velocity section of the flow velocity control software (see Table of Materials).NOTE: The motor and rotor are connected to the accompanying computer. The flow velocity control software communicates with the motor to create the desired flow velocity. The use of the flow velocity control software is optional. The alternative is to manually control the water current motor.
-
Calibration
NOTE: Calibration is required before each experiment.-
Use the calibration lid to close the swim tunnel.NOTE: The calibration lid is customized with a reinforced central opening that fits the flow meter probe used for calibration (Figure 1B). Eight wingnuts are used to secure all the lids to the tunnel.
- Turn on the digital flow meter and connect it to the flow meter probe. Place the flow meter probe inside the swim tunnel via the calibration lid. Position the blades of the flow meter probe perpendicular to the direction of the flow.
- Calibrate the output of the swim tunnel motor (controlled with the flow velocity control software) using the digital flow meter. To do this, perform the following steps.
- Open the flow velocity control software and click Calibration.
-
Change the options on the top left to RPM vs Voltage. Increase the flow velocity from 0 cm/s to 100 cm/s in 5 cm/s increments, by typing the values in the Velocity section of the flow velocity control software. At each step, click the “+” button and record the current velocity indicated by the digital flow meter.NOTE: The resulting linear relationship should have an R2 value close to 1.
- To confirm calibration, increase the water current velocities from 0 to 10, 25, 50, 75, and 100 cm/s, and then decrease velocities to 75, 50, 25, 10 cm/s using the up and down arrows in the Uwater [cm/s] section of the flow velocity control software. At each velocity (from the software), measure and record the corresponding velocity indicated by the digital flow meter.
- Consider the tunnel calibrated and accurate if the measured water current velocities are within a deviation of ±2 cm/s. If the deviation is beyond ±2 cm/s, repeat steps 1.4.4 to 1.4.6 to ensure proper calibration.
-
Materials
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| AutoSwim software | Loligo Systems | MI10000 | Optional - for Automatic control of current velocity |
| Customized lid | Loligo Systems | MI10001 | This customized lid is used for swim endurance |
| DAQ-BT | Loligo Systems | SW10600 | Optional - for Automatic control of current velocity |
| Eheim pump | Loligo Systems | PU10160 | 20 L/min. This pump is placed in the flow-through tank. |
| Fiji | Fiji | Freely available through Image J (Fiji) | Specific script available at https://github.com/MokalledLab/ SwimBehavior |
| Flowtherm | Loligo Systems | AC10000 | Handheld digital flow meter - for calibration |
| High Speed Camera | Loligo Systems | VE10380 | USB 3.0 color video camera (4MP) |
| IR light panel | Loligo Systems | VE10775 | 450 x 210 mm, placed under the swim tunnel chamber |
| Monofocal lens | Loligo Systems | VE10388 | 25mm manual lens |
| PVC Tubing | VWR | 60985-534 | 5/16 x 7/16” Wall thickness: 1/16” |
| R Studio | R Studio | Freely available. Version 3.6 with extra packages. | Specific script available at https://github.com/MokalledLab/ SwimBehavior |
| Swim tunnel respirometer | Loligo Systems | SW10060 | 5L (120V/60Hz). The system includes the swim chamber, motor, manual control of water current velocity, 1 pump placed inside the chamber, standard swim tunnel lid for swim behavior, and modified swim tunnel lid for calibration |
| uEye Cockpit | IDS | Freely available software to control camera parameters | Alternative cameras and accompanying softwares could be used |
| Vane wheel flow probe | Loligo Systems | AC10002 | Digital flow probe - for calibration |
2. Assessment of swim endurance
NOTE: Experimental groups are divided into groups of 10 or fewer animals for swim endurance.
-
Set up the flow velocity control software.
NOTE: The use of the flow velocity control software in this section is optional. The alternative is to manually control the water current motor. For manual water current control, proceed to step 2.3 and manually increase the water current velocity in the specified increments in steps 2.5 and 2.6.- Open the flow velocity control software. Click the box labeled Experiment. Uncheck Uswim and Uwater.
- Change the flow speed in the Uwater [cm/s] box on the bottom left for adjusting water current velocities.
- To begin an automated protocol, click on the Start Logging box. In the dialog window that opens, choose Automated from the dropdown list.
- To choose a previously saved protocol file, click on the file icon next to Protocol File to open the desired protocol.
- Set up the output file by clicking on the file icon next to Log File. In the file explorer window that opens, name the output file and save it in the desired location.
Set up a split lap timer window. Make sure to have simultaneous access to the flow velocity control software and timer windows on the computer screen.
- Set up a fish collection tank to house exhausted fish after their removal from the swim tunnel. Fill the collection tank with zebrafish system water (0.75 L). Fill a long PVC tube with zebrafish system water.
- Place one end (end 1) of the prefilled PVC tube in the collection tank and the other end (end 2) in the buffer tank. Make sure water can freely flow from the buffer tank into the collection tank.
- Clamp the upper end of the PVC tube (end 2) with a binder clip to prevent water flow. Use the binder clip to control the outflow of water as needed.
-
Close the swim tunnel using the swim endurance lid.
NOTE: The swim endurance lid is customized with a swim tunnel window to remove exhausted fish from the swim tunnel, without interrupting the rest of the assay (Figure 1B).
-
Place one group of fish inside the swim tunnel. Start the split lap timer while adjusting current velocity to 0 cm/s for 5 min, 9 cm/s for 5 min, and 10 cm/s for 5 min by typing these values in the Uwater [cm/s] section of the flow velocity control software.
NOTE: This step will acclimate animals to the swim tunnel and flow direction.
-
Following acclimation of fish, start the automated flow velocity control program that will increase water current velocity by 2 cm/s every min.
NOTE: Fish will swim until exhaustion. Exhausted fish are pushed toward the back end of the swim tunnel.
-
When a fish is exhausted, unclamp the fish collection tube, open the swim tunnel window and collect the fish in the collection tank. Record the time at exhaustion using the split lap timer.
NOTE: Fish can occasionally drift to the back end of the swim tunnel without being exhausted. To ensure a fish is exhausted, gently tap the back end of the tunnel or create a shadow over that area to stimulate the fish to swim. Exhausted fish do not respond to the startle stimulus and lay flat at the back end of the tunnel.
-
Repeat step 2.7 until all the fish are exhausted and collected in the collection tank. Click the Emergency Stop button on the flow velocity control software and stop the timer.
NOTE: Double-check throughout the swim whether the number of fish removed from the swim tunnel chamber matches the times recorded.
-
Repeat steps 2.5 to 2.8 for each group of fish.
NOTE: The protocol can be paused here, but to be accurate to the time point after injury, it is recommended that movies and endurance swims be performed on the same day. The tunnel can continue circulating while experiments are paused.
3. Capturing videos for swim behavior assay
NOTE: Only up to five animals can be tracked at a time. If experimental groups are larger than five animals, multiple videos can be taken for each group, where the first video tracks five or fewer animals and the second video tracks the other five or fewer animals. For longitudinal studies that aim to track individual animals over time, fish can be individually housed and tracked across multiple time points. All scripts for tracking and analyzing are available via GitHub (see Table of Materials).
-
Turn on the Infrared light panel located under the swim tunnel. Secure the camera on a ceiling mount on top of the swim tunnel. Adjust focus and aperture rings.
NOTE: Focus and aperture settings depend on the distance between the camera and the swim tunnel, as well as the light environment.
- Open the camera recording software (see Table of Materials). Set the software settings as follows.
- Click the 1:4 aspect display. Make sure that the field of view covers the entire swim tunnel. Turn off auto-contrast and auto-white balance to normalize the background and contrast across groups.
- Open the Camera Properties window by clicking on the wrench icon. Set the parameters as follows: Pixel Clock: 344 MHz, Frame Rate: 70 fps, click on the box next to Hold to check it, Exposure Time: 0.290 ms. Do not close this window.
- Crop the Recording Window to cover only the swim chamber of the tunnel by tilting/turning the camera as needed.
- Open the Recording Window by clicking on the film reel icon. Set the recording settings as follows:
- Check the box for the maximum number of frames.
- Manually input 63,000 for the number of frames.
- Check the box for Calc. Frame Rate. This allows the program to pull the frame rate defined in step 3.2.2 (70 fps).
- Change the JPEG Quality to 30.
- Record a test run.
- Click on Create and name the new file Test and save it on the desktop.
- Go back to the recording window and click Record. Let the test movie run for the entire duration of the protocol (15 min).
- Once the test is finished, ensure that there are no dropped frames and that 63,000 frames are recorded.
-
Place a group of fish in the swim tunnel and close the tunnel using a standard fully enclosed lid (Figure 1B).
NOTE: Ensure all the fish are in the tunnel before fully tightening the lid. Make sure there are no air bubbles under the lid. This will otherwise affect the results.
-
Open a new recording window and name the file. Example: 2_A_1_00001_WildtypeGroupA.avi
NOTE: Ensure that the settings are according to the parameters in steps 3.2 and 3.3. The JPEG Quality will always go back to default and needs to be reset for each new movie.
CAUTION: Do not click record yet.
-
Begin a new experiment using the flow velocity control software.
NOTE: To begin an automated protocol, click the Start Logging box. In the dialog window that opens, choose Automated from the dropdown list. To choose a previously saved protocol file, click on the file icon next to Protocol File to open the desired protocol.- To begin a manual protocol, set the flow speed to 0 cm/s for 5 min, 10 cm/s for 5 min, followed by 20 cm/s for 5 min using the Uwater [cm/s] box in the flow velocity control software.
-
Save the new data output file (will be saved as a .csv file) under the same name as the movie file and in the same folder.CAUTION Do not click start yet.
Place a paper towel or piece of fabric on the side of the swim tunnel to ensure all behaviors are due to fish swimming and not due to a startle response caused by movement in the environment.
In quick succession, make sure water is calm and no ripples are moving across the frame. Click Record in the camera software window to start recording the movie file. Click Start in the flow velocity control software to begin the protocol, which will continue uninterrupted.
Watch the movie to make sure that no frames are dropped, that there are no bubbles in the field of view, and that all fish are recorded.
Once the movie recording is completed, click on Emergency Stop to end the flow velocity control protocol. Check for the data output file that is saved automatically. Click Close on the Recording Window to save the movie file.
Remove the lid. Carefully retrieve the fish and return them to their tank.
Repeat steps 3.5 to 3.12 for all groups of fish.
-
Once the movie recording is finished for all the groups, convert movies from 70 fps videos to 20 fps videos with the MovieProcessing_v5.bat script. To do this, move the script file to the folder containing the raw videos. Right-click on the file and choose Run.
NOTE: The script runs automatically. A command prompt window will come up showing the progress of the script. The above step is optional. It reduces the number of frames in a 15 min video from 63,000 to 18,000 frames and makes the SwimBehavior_v7.R script run more quickly.
Empty the tunnel and put away all the equipment.
4. Analyzing movies for swim behavior assessment
NOTE: Movie recording and analysis can be completed on separate days.
Open the Tracking_v2.ijm script in Fiji and click Run to begin the program. In the window that pops up, choose the folder containing the swim behavior movies to track and click Open. Look for frame 1 of the first movie, a dialog box, and the region of interest (ROI) manager that will come up.
-
Follow the directions given in the dialog box. Create an ROI of the bottom of the swim tunnel chamber and click OK. Ensure that no black corners are seen.
NOTE: The threshold window will open along with an edited, thresholded Frame 1.
-
Change the color scheme from B&W to Red. Adjust the Max Value until frame 1 shows the fish in red and nothing else. Record the threshold. Click OK in the dialog box.
NOTE: The program will automatically run. The ROI that was made for Frame 1 will be continuously selected and unselected for subsequent frames. A progress bar will monitor the process in the bottom-right corner of the Fiji window. Tracking takes about 40 min per movie. When all the movies are tracked, the Fiji program will stop. The ROI will stop being selected. The folder containing the movies will now have a _raw.csv file for each movie. Fiji can be closed at this point.
- Aligning, assembling, and acquiring descriptive statistics
- Open the Swim Behavior_v7.R script in R Studio.
-
Click on Source in the top-right corner of the script section. In a new window that opens, choose the folder containing the _raw.csv files generated by Fiji. Click Open.NOTE: The program will run automatically.
-
In a dialog box that pops up asking to confirm the number of fish in each movie, click Yes if the numbers given are correct, or click No if the numbers are incorrect.NOTE: If No was clicked, a message will pop up asking that the movies be re-tracked with a new ROI and threshold. If Yes was clicked, the program will proceed.
-
In the new window that opens asking whether the non-swimming fish should be removed, click Yes or No.NOTE: Non-swimming fish is defined as fish with less than 50% activity at 10 cm/s. It is not recommended that non-swimming fish be removed.
- In a new pop-up window that asks whether the groups are unblinded and whether the user would like to combine any groups, unblind or combine groups if there is more than one control group.
- After a response is given for the previous question, ensure that the program gives a message Aligning file X of Y, where X is the current file being aligned and Y is the total number of files to align. It takes about 30 s for each file to be aligned.
Once the files are aligned, check for a new .csv file generated with the same name (_aligned.csv). Ensure that the program combines the data, runs statistics, and plots output graphs. Check for the analysis files generated in a new folder labeled Results within the parent folder that contains the _raw.csv and _aligned.csv files.
-
Within the Results folder, check for two folders named Diagnostics and Graphs and four .csv files named BulkData_Avg, BulkData_Full, SummaryData_Avg, and SummaryData_Full.
NOTE: The SummaryData_Full.csv contains the individual data for each fish in each group at each time point. This data is automatically plotted but can be extracted and plotted elsewhere.- Ensure that the Graphs folder contains the graphs generated by the program and .csv files containing the data points for each graph.
-
Ensure that the Diagnostics folder contains a single .csv file with diagnostic data for aligned files.NOTE: Columns in the Diagnostics.csv file include the following: a) The number of frames containing extra objects, which should be less than 100. Too many frames with extra objects suggest a problem with the tracking. b)The number of frames with missing or merged objects. It is normal for this metric to be high. Fish that have not regenerated well will frequently be swept to the back of the tunnel and will be counted as missing. c) Frames with more than 240-pixel jumps. This number increases with the number of objects (fish) in a single movie. A detailed explanation of how the behavior metrics were calculated is provided in Supplementary File 1.
Representative Results
We set up the swim tunnel as described in section 1 of this protocol (Figure 1). We assessed the swim endurance (section 2 of this protocol) as well as swim behavior (sections 3 and 4 of this protocol) of adult zebrafish at baseline and after spinal cord injury (Figure 2).
For establishing baseline motor function, we examined the swim endurance of wild-type zebrafish under increasing water current velocities (Figure 3A). In this assay, wild-type zebrafish swam for 41 min before getting exhausted. Fish were then subjected to complete spinal cord transections as previously described and swim endurance assays were performed6. After anesthetizing zebrafish using MS-222, a small incision is made with fine scissors to transect the spinal cord 4 mm caudal to the brainstem region. A complete transection was confirmed visually. To confirm the loss of swim capacity after spinal cord surgery, injured animals were assessed at 2 or 3 days post-injury (dpi). At this time point, zebrafish are completely paralyzed caudal to the lesion site. Swim endurance was assessed at 2, 4, and 6 weeks post-injury (wpi). At 2 wpi, lesioned fish lost 60% of their swim endurance capacity (Figure 3A). Regenerating fish gradually regained swim endurance at 4 and 6 wpi. These results indicated that wild-type zebrafish are capable of regaining swim endurance capacity after spinal cord injury.
Figure 3: Functional recovery in wild-type zebrafish after spinal cord injury.
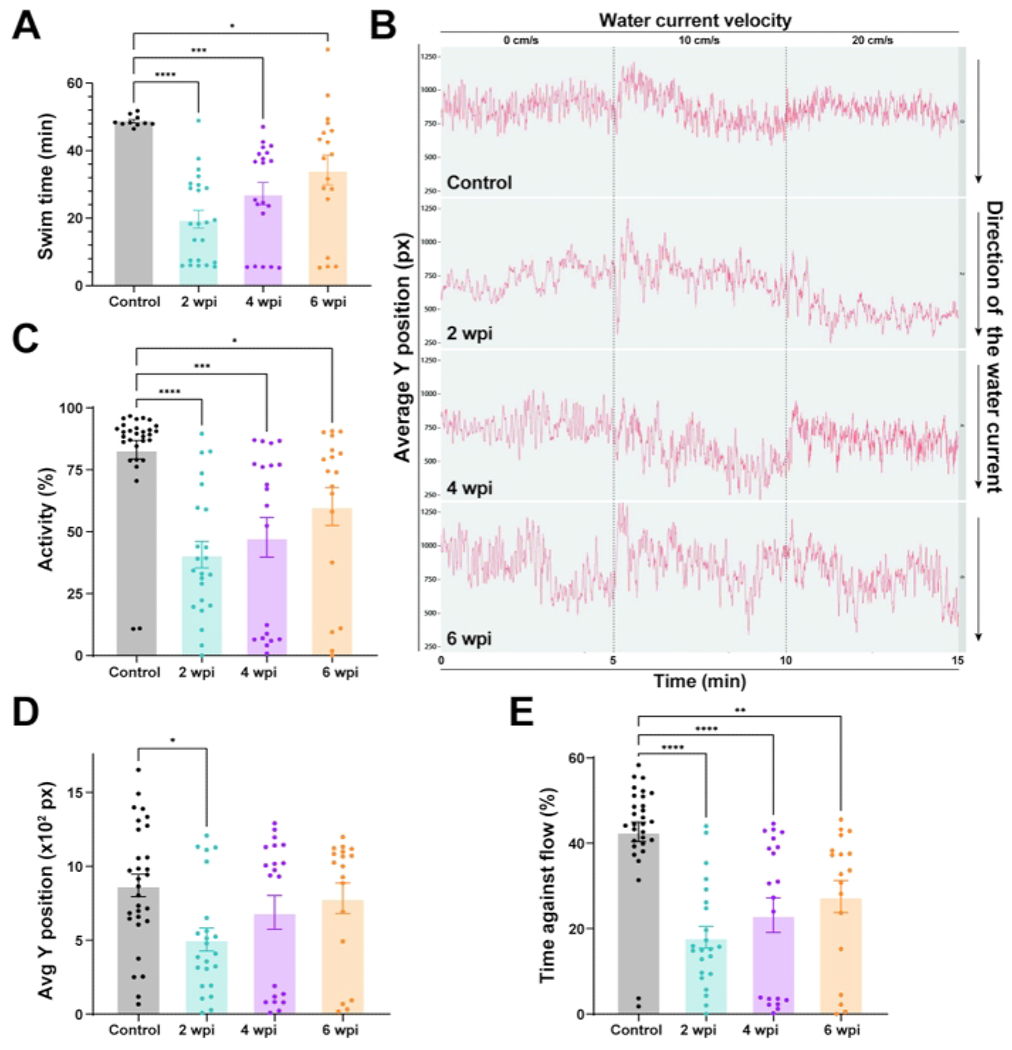
(A) Motor function determined by swim endurance assays for wild-type zebrafish at baseline and 2, 4, and 6 wpi. Dots denote individual animals from two independent experiments. (B) Swim behavior assays tracked wild-type zebrafish under low water current velocities. The average Y position is shown at each time point throughout tracking (0 cm/s for 5 min, 10 cm/s for 5 min, and 20 cm/s for 5 min). (C–E) Percent activity (C), average Y position in the tunnel (D), and time swam against the flow (E) were quantified at 20 cm/s. For all quantifications, two independent experiments are shown. n = 30 in the pre-injury condition; n = 23 at 2 wpi, n = 20 at 4 wpi, n = 18 at 6 wpi. One-way ANOVA was used for statistical analyses. Error bars represent the Standard Error of the Mean (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Please click here to view a larger version of this figure.
To examine zebrafish swim behavior during spinal cord regeneration, we tracked the swim behavior of wild-type animals at 0 cm/s water current velocity or under constant, low current velocities of 10 and 20 cm/s (Figure 3B). Average tracks of fish position in the swim tunnel chamber were used for visual assessment of swim behavior at low current velocities (Figure 3B). In this assay, uninjured controls swam steadily in the front part of the swim tunnel chamber (closer to the source of water current), which corresponds to an elevated Y position (Figure 3B). In contrast, at 2 wpi, injured fish were not able to maintain steady swim capacity against the current. Consequently, their swim tracks are more irregular with an overall decrease in Y position (Figure 3B). Y position increased at 4 and 6 wpi, indicating that regenerating animals gradually regained their ability to swim in the front of the swim tunnel chamber. To quantify swim behavior parameters, we calculated the percent activity, position in the swim tunnel (Y position), and time swam against the current (Figure 3C–E). Relative to uninjured controls, lesioned animals at 2 wpi were markedly less active (Figure 3C), stalled in the rear quadrant of the swim tunnel (Figure 3D), and lost their ability to swim against low current velocities (Figure 3E). Consistent with their innate ability to achieve functional recovery, lesioned animals gradually normalized swim behavior parameters at 4 and 6 wpi (Figure 3C–E). The swim endurance and swim behavior parameters together offered quantifiable readouts of swim function and functional spinal cord repair in zebrafish.
Discussion
Adult zebrafish are a popular vertebrate system for modeling human diseases and studying mechanisms of tissue regeneration. CRISPR/Cas9 genome editing has revolutionized reverse genetic studies for modeling disease in zebrafish; however, large-scale genetics in adult zebrafish has been hindered by biological and technical challenges, including the unavailability of adult zebrafish tissues to high-throughput phenotyping. Given the complex anatomy of adult zebrafish, prolonged histological processing is required to obtain and analyze tissue architecture. The swim endurance and swim behavior assays described in this study can be used to pre-screen for neural, muscular, or skeletal phenotypes at a medium throughput before histology. Moreover, as studies of tissue regeneration aim to improve functional tissue repair, the protocols described in this study will be widely applicable, if not essential, for studies of neural, muscular, and skeletal regeneration research.
Functional locomotion assays have been integral to our understanding of neural development and regeneration. Standard locomotor assays are widely available in vertebrate species, including rats, mice, and larval zebrafish. Mouse and rat model systems have behavioral and functional locomotion assays such as the BMS16 and BBB17, 18, respectively. Similarly, a host of protocols have been described to measure locomotion, startle response, and behavior in larval zebrafish. These protocols are efficient to reveal behavioral differences among experimental groups in light and dark environments, predator evasion, and activity19, 20, 21. Here, we describe quantifiable, reproducible methods to measure functional recovery after spinal cord injury in adult zebrafish.
Note that this study presents several limitations. First, behavioral studies are highly dependent on genetic and environmental factors. To control for genetic variability, we used siblings to control for age, sex, and the genetic background across experimental groups22, 23. To control for environmental factors, we made sure experiments are performed at the same time of the day, under controlled temperature and lighting conditions20. Second, while the swim behavior assay is less sensitive to biased analysis, the researcher running the swim endurance assay determines when a fish reaches exhaustion and proceeds to remove exhausted fish from the swim tunnel chamber without interrupting the rest of the fish cohort. Thus, our swim endurance experiments were performed by a single researcher who is blinded to experimental conditions. It is particularly important to avoid researcher-to-researcher variability for longitudinal studies of experimental groups over time. Finally, collisions between fish can complicate the tracking analysis in swim behavior assays. We thus recommend performing swim behavior assays for groups of five or fewer fish to minimize the chances of collisions between fish.
In consideration of critical steps, we note that injured fish can be fragile especially in the early days after injury. We thus recommend handling fish with extreme care. For swim endurance assays, collecting fish with the PVC tube as quickly as possible, either head or tail first, reduces the chance of secondary injuries during the collection process. For swim behavior assays, the flush pump can occasionally create waves, distorting the movies and causing analysis errors. In this case, the flush pump can be briefly turned off. However, we do not recommend turning off the flush pump for extended times to ensure water is constantly circulating between the swim tunnel chamber and the buffer tank. Monitoring movie recording allows for immediate termination and restarting of the movie if a frame has been dropped or a fish strays from the recording area. In additional considerations, while performing the tracking analysis, if the R code gives an error during file processing, the most likely problem is in the naming of the files. The program is made to function under a very specific naming strategy: Timepoint_Group_Subgroup_Stock number_Anything else (for example, 0_A_1_00001_WildtypeGroupA.avi). This naming allows for multiple time points, groups, and subgroups to be plotted and aligned together. Finally, while checkpoints have been built into the analysis script to ensure proper tracking, it is important to carefully check the analysis output. The program will automatically ask whether the fish number is correct, and prompt movies for re-analysis if the fish number is incorrect. Straight-line artifacts may appear in the Average Y position plot, indicating that an extra object has been recognized as a fish. In this instance, the best course of action is to carefully watch the movie to exclude extra artifacts that tend to appear at a higher flow speed.
Supplementary Material
Supplementary File 1: Please click here to download this File.
Acknowledgments
We thank the Washington University Zebrafish Shared Resource for animal care. This research was supported by the NIH (R01 NS113915 to M.H.M.).
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/63240.
Disclosures
The authors have no conflicts of interest.
References
- 1.Becker CG, Becker T Neuronal regeneration from ependymo-radial glial cells: cook, little pot, cook! Developmental Cell. 32 (4), 516–527 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Mokalled MH, Poss KD A regeneration toolkit. Developmental Cell. 47 (3), 267–280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orger MB, de Polavieja GG Zebrafish behavior: opportunities and challenges. Annual Review of Neuroscience. 40, 125–147 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Becker CG, Becker T Adult zebrafish as a model for successful central nervous system regeneration. Restorative Neurology and Neuroscience. 26 (2-3), 71–80 (2008). [PubMed] [Google Scholar]
- 5.Gurevich DB et al. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science. 353 (6295), aad9969 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Mokalled MH et al. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science. 354 (6312), 630–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kizil C, Kaslin J, Kroehne V, Brand M Adult neurogenesis and brain regeneration in zebrafish. Developmental Neurobiology. 72 (3), 429–461 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Wolman MA et al. A genome-wide screen identifies PAPP-AA-mediated IGFR signaling as a novel regulator of habituation learning. Neuron. 85 (6), 1200–1211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granato M et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 123, 399–413 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Brockerhoff SE et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proceedings of the National Academy of Sciences of the United States of America. 92 (23), 10545–10549 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moens CB, Yan YL, Appel B, Force AG, Kimmel CB Valentino: a zebrafish gene required for normal hindbrain segmentation. Development. 122 (12), 3981–3990 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Cavone L et al. A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Developmental Cell. 56 (11), 1617–1630.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Reimer MM et al. Motor neuron regeneration in adult zebrafish. Journal of Neuroscience. 28 (34), 8510–8516 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatt Shaw D et al. Localized EMT reprograms glial progenitors to promote spinal cord repair. Developmental Cell. 56 (5), 613–626.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed RU, Alam M, Zheng YP Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 5 (3), e01324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajoohesh-Ganji A, Byrnes KR, Fatemi G, Faden AI A combined scoring method to assess behavioral recovery after mouse spinal cord injury. Neuroscience Research. 67 (2), 117–125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma. 12 (1), 1–21 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Scheff SW, Saucier DA, Cain ME A statistical method for analyzing rating scale data: the BBB locomotor score. Journal of Neurotrauma. 19 (10), 1251–1260 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Li Q et al. Differential behavioral responses of zebrafish larvae to yohimbine treatment. Psychopharmacology (Berl). 232 (1), 197–208 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Wakamatsu Y, Ogino K, Hirata H Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Scientific Reports. 9 (1), 16307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed O, Seguin D, Gerlai R An automated predator avoidance task in zebrafish. Behavioral Brain Research. 216 (1), 166–171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conradsen C, McGuigan K Sexually dimorphic morphology and swimming performance relationships in wild-type zebrafish Danio rerio. Journal of Fish Biology. 87 (5), 1219–1233 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Leris I, Sfakianakis DG, Kentouri M Are zebrafish Danio rerio males better swimmers than females? Journal of Fish Biology. 83 (5), 1381–1386 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1: Please click here to download this File.


