Abstract
Introduction
Malignant peritoneal mesothelioma (MPM) is a rare, aggressive tumour arising primarily from the peritoneum. The only potentially curative treatment is cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC). However, the majority of patients are not eligible to undergo this treatment. The benefit of systemic treatment for these patients is limited at the cost of considerable morbidity. Hence, there is a need for appropriate palliative treatment options for patients with MPM. As MPM rarely disseminates outside the abdominal cavity, these patients might benefit from local treatment. A higher, more effective dose of chemotherapy can directly be delivered at the site of the disease. Systemic uptake will be limited, likely resulting in less toxicity. The aim of the INTERACT MESO trial is to determine the maximum tolerable dose of intraperitoneal paclitaxel monotherapy in patients with MPM. Secondary endpoints are to assess safety and toxicity, feasibility and the pharmacokinetic profile of this treatment.
Methods and analysis
The INTERACT MESO trial is a prospective, open-label, single-centre, phase I study with a classic three-plus-three dose escalation design. The study population consists of adult patients with primary MPM, without extra-abdominal disease, who are not eligible to undergo CRS-HIPEC. According to standard of care work-up for CRS-HIPEC, patients will undergo diagnostic laparoscopy to determine the feasibility of CRS-HIPEC. In case CRS-HIPEC is not considered feasible, a peritoneal port-a-cath (PAC) system will be placed. Through this PAC, 8–16 weekly cycles of intraperitoneal chemotherapy will be administered.
Ethics and dissemination
The Central Committee on Research Involving Human Subjects (CCMO, The Hague, The Netherlands) and the Medical Research Ethics Committee (METC, Rotterdam, The Netherlands) have granted permission to carry out this study protocol. The results of this trial will be submitted for publication in a peer-reviewed scientific journal.
Trial registration number
NL9718. EudraCT: 2021-003637-11.
Keywords: surgery, oncology, clinical trials, chemotherapy, toxicity
Strengths and limitations of this study.
The INTERACT MESO trial is the first trial that investigates paclitaxel monotherapy in patients with malignant peritoneal mesothelioma who are not eligible for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy.
In this phase I dose escalation trial the maximum tolerated dose (MTD), safety and feasibility of this treatment will be determined.
This trial will also determine the pharmacokinetic profile of intraperitoneal paclitaxel monotherapy.
Due to the nature of this trial, the efficacy of intraperitoneal paclitaxel cannot be determined; when the MTD is determined, larger phase II and III clinical trials will be conducted to determine the efficacy.
Introduction
Malignant peritoneal mesothelioma (MPM) is a rare but aggressive neoplasm with a poor prognosis, arising primarily from the serosal lining of the peritoneal cavity.1 Currently, the only possibly curative treatment is cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC).2 3 In the Netherlands, only a minority of patients undergo this treatment.1 For patients who are not eligible to undergo CRS-HIPEC, the treatment options are limited. Overall response rates to systemic chemotherapy are low (20%–25%), though morbidity rates are high, with a grade 3–4 haematological toxicity rate up to 38%.4–6 Moreover, the 2-year survival rate for these patients is only 20%.1 Combination checkpoint inhibition therapy with nivolumab and ipilimumab has been proposed as a new treatment option for patients with MPM. However, this treatment has comparable morbidity rates to that of systemic chemotherapy, and its benefit for patients with MPM is not proven.7 8 Because of the high morbidity rate, and the limited effectiveness of systemic treatment, it is debatable whether these therapies are suitable as palliative treatment for patients with MPM. Due to lack of appropriate palliative treatment options, the majority of patients with MPM in the Netherlands (63%) currently receive no antitumour treatment.1
For peritoneal metastases from several types of cancer, local treatment with intraperitoneal chemotherapy has been proposed as a palliative treatment option. This therapy can be delivered through an intraperitoneal port-a-cath (PAC), and potentially has major advantages over systemic treatment. A higher, more effective dose of chemotherapy can directly be delivered at the site of the disease, while systemic uptake is limited, likely resulting in fewer toxicity. Paclitaxel is a chemotherapeutic agent that is considered extremely favourable for intraperitoneal use.9 Due to its large molecular weight and lipophilic properties, it is slowly cleared from the peritoneal cavity when administered locally. This results in an area under the curve (AUC) after intraperitoneal administration that is up to a 1000-fold (3-log) higher than that in plasma, while peritoneal concentrations persist up to 48 hours after administration.10 This considerably increases drug activity.
Markman et al presented the first-phase one-dose escalation study of intraperitoneal paclitaxel in patients with ovarian cancer, pretreated with systemic chemotherapy.10 They established the maximum tolerable dose (MTD) to be 175 mg/m2 at a 2–3 weeks interval. Another dose escalation study was performed by Francis et al, delivering a lower dose at a weekly frequency.11 These patients were also pretreated with systemic chemotherapy. Severe abdominal pain was uncommon and only low-grade leucopenia, fatigue and stomatitis were observed. Grade 3–4 gastrointestinal complications were observed in 15% of the patients. Francis et al recommended a dose of 60–65 mg/m2 intraperitoneal paclitaxel in weekly cycles. Markman et al performed a phase II trial in 80 patients with ovarian cancer using 60 mg/m2 of intraperitoneal paclitaxel in 16 weekly cycles after pretreatment with systemic chemotherapy.12 The majority of patients (70%) received all planned 16 courses. Grade 3 complications were rare, with abdominal pain, neuropathy and neutropenia in one, two and one patient, respectively. Bowel perforation, a rare but potentially life-threatening complication, was observed once in the phase I trial (3%), but was not observed in the phase II trial. Five patients were removed from the study due to excessive toxicity, and three patients due to catheter malfunction. In total, 18 (24%) patients achieved a complete response.
As the effectiveness of systemic therapy is limited and MPM very rarely disseminates outside the abdominal cavity, the use of intraperitoneal paclitaxel monotherapy seems a logical and promising step. The group of Sugarbaker uses a long-term intraperitoneal administration of paclitaxel as an adjuvant treatment to CRS-HIPEC for patients with MPM. They use doses of 20 mg/m2 daily for five consecutive days monthly, starting 4–6 weeks postoperatively. Some of these patients showed remarkable survival, despite incompleteness of cytoreduction at CRS-HIPEC.13–15 Another major advantage of the suggested treatment is that ascites, a common MPM symptom that causes a major morbidity, can be drained through the same PAC system.
Currently, there are no studies that investigate intraperitoneal paclitaxel as non-adjuvant monotherapy in patients with MPM. The main objective of this clinical trial is to determine the MTD of intraperitoneal paclitaxel monotherapy in patients with MPM. Secondary endpoints are to assess safety and toxicity, feasibility and the pharmacokinetic (PK) profile of this treatment. When the MTD is determined, further research is needed to determine the effect on survival.
Methods and analysis
This protocol follows the Standard Protocol Items: Recommendations for Interventional Trials statement (online supplemental file 1).16
bmjopen-2022-062907supp001.pdf (79.3KB, pdf)
Study design
Trial setting
The INTERACT MESO trial is a prospective, open-label, single-centre, phase I study with a classic three-plus-three dose escalation design (figure 1). The defined dose levels are 100, 150 and 200 mg paclitaxel. This study is conducted at the Erasmus MC Cancer Institute, a tertiary referral hospital, located in Rotterdam, the Netherlands. Trial registration details are described in table 1. The study started recruitment in February 2022, and as of 17 May 2022 one patient has been enrolled. The end of the study is planned in February 2026.
Figure 1.
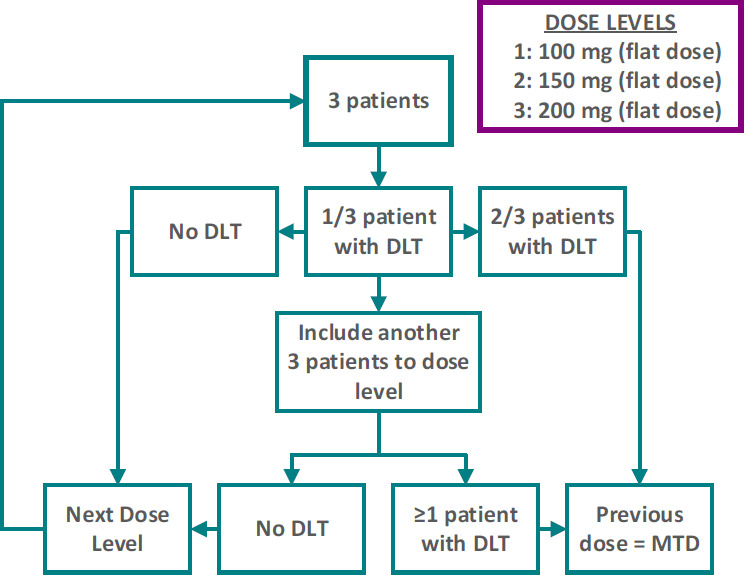
Three-plus-three dose escalation design. DLT, dose-limiting toxicity; MTD, maximum tolerable dose.
Table 1.
WHO trial registration data set
| Primary registry and trial identifying number | EudraCT number: 2021-003637-11 Netherlands Trial Register: NL9718 |
| Date of registration in primary registry | September 2021 |
| Protocol version | Protocol version 4.0; 22 November 2021 |
| SPIRIT guidelines data set for clinical trials | See online supplemental file 1. |
| Secondary identifying numbers | Dutch competent authority (CCMO): NL78373.078.21 Local medical ethics committee (METC): MEC-2021-0697 |
| Source(s) of monetary or material support | Erasmus MC Foundation, Rotterdam, The Netherlands |
| Primary sponsor | Erasmus University Medical Center, Rotterdam, The Netherlands |
| Secondary sponsors | Not applicable |
| Contact for public queries | MVD, study coordinator Department of Surgical Oncology Erasmus MC, Rotterdam, The Netherlands m.dietz@erasmusmc.nl, (+31)010–7042125 |
| Contact for scientific queries | EVEM, principal investigator Department of Surgical Oncology Erasmus MC, Rotterdam, The Netherlands e.madsen@erasmusmc.nl, (+31)010–7041082 |
| Public title | Treatment of abdominal mesothelioma with intra-abdominal chemotherapy: INTERACT MESO |
| Scientific title | Intraperitoneal paclitaxel for patients with primary malignant peritoneal mesothelioma—a phase I/II dose escalation and safety study: INTERACT MESO |
| Countries of recruitment | The Netherlands |
| Health conditions or problems studied | Malignant peritoneal mesothelioma |
| Interventions | Patients undergo a diagnostic laparoscopy (DLS) according to standard work-up for CRS-HIPEC. If the disease is considered not resectable, a peritoneal port-a-cath (PAC) will be placed. Through this PAC, intraperitoneal paclitaxel will be administered in weekly cycles. |
| Key inclusion and exclusion criteria | Key inclusion criteria: Confirmed diagnosis of malignant peritoneal mesothelioma, WHO-ECOG performance status 0–1, aged ≥18 years old and adequate organ function and bone marrow reserves Key exclusion criteria: Extra-abdominal disease/metastatic disease, serious concomitant disease or active infections, any medical or psychological impediment to probable compliance with the protocol and pregnant or lactating women |
| Study type | Open-label single-centre phase I/II study |
| Date of first enrolment | Planned February 2022 |
| Target sample size | 11–21 according to dose escalation |
| Recruitment status | Pending |
| Primary outcome | Maximum tolerable dose (MTD) of intraperitoneal paclitaxel monotherapy in patients with MPM |
| Key secondary outcome(s) | Safety and toxicity, feasibility and the pharmacokinetic profile of intraperitoneal paclitaxel monotherapy |
CCMO, Central Committee on Research Involving Human Subjects; CRS, cytoreductive surgery; ECOG, Eastern Cooperative Oncology Group; HIPEC, hyperthermic intraperitoneal chemotherapy; METC, Medical Research Ethics Committee; MPM, malignant peritoneal mesothelioma; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Study population
The study population consist of adult patients with primary MPM, without extra-abdominal disease, who are not eligible to undergo CRS-HIPEC. Potentially eligible patients will be referred by their local clinician or through self-referral to a medical specialist. A member of the study team will inform patients about the trial at the outpatient clinic, and an eligibility assessment will be performed. In order to be eligible to participate in the study, potential subjects must meet all of the following inclusion criteria:
Histologically confirmed diagnosis of MPM.
Patients who are not eligible (or willing) to undergo CRS with HIPEC.
Aged ≥18 years.
Written informed consent by the patient according to the International Conference on Harmonisation Good Clinical Practie (ICH-GCP) and national/local regulations.
Patients must be ambulatory (WHO-Eastern Cooperative Oncology Group performance status 0 or 1).
Ability to return to the Erasmus MC for adequate follow-up as required by this protocol.
Patients must have normal organ function and adequate bone marrow reserve as assessed by the following laboratory requirements: absolute neutrophil count (ANC) >1.5×109/L, platelet count >100×109/L and haemoglobin >6.0 mmol/L. Patients must have a bilirubin <1.5× upper limit of normal (ULN), serum aspartate transaminase and alanine transaminase <2.5× ULN.
A potential subject who meets any of the following exclusion criteria will be excluded from participation in the study:
Incapacitated patients.
Extra-abdominal disease/metastatic disease established by preoperative CT scan of thorax-abdomen and/or positron emission tomography (PET) scan. Imaging not older than 2 months at time of surgery.
Medical or psychological impediment to probable compliance with the protocol.
Serious concomitant disease or active infections.
History of autoimmune disease or organ allografts, or with active or chronic infection, including HIV and viral hepatitis.
Serious intercurrent chronic or acute illness such as pulmonary (chronic obstructive pulmonary disease or asthma) or cardiac (New York Heart Association class III or IV) or hepatic disease or other illness considered by the study coordinator to constitute an unwarranted high risk for participation in this study.
Pregnant or lactating women; for all women of childbearing potential a negative urine pregnancy test will be required as well as the willingness to use adequate contraception during the study until 4 weeks after finishing treatment.
Absence of assurance of compliance with the protocol.
An organic brain syndrome or other significant psychiatric abnormality which would comprise the ability to give informed consent, and preclude participation in the full protocol and follow-up.
Patient timeline and additional procedures
A flow chart of the study is shown in figure 2. A more detailed description of (additional) study procedures is presented in table 2.
Figure 2.
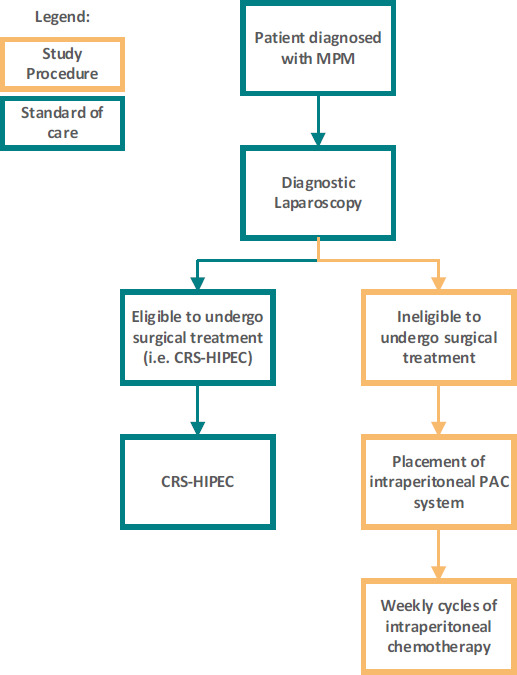
Study workflow. After patients are diagnosed with malignant peritoneal mesothelioma (MPM), they will undergo a diagnostic laparoscopy (DLS) as a part of standard care. If the disease is deemed resectable, patients will undergo cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) as part of standard care. If the disease is considered not resectable during DLS, patients are eligible for inclusion in the currents study. A port-a-cath (PAC) system will be placed subcutaneously while the catheter tip is placed inside the peritoneal cavity. After surgery, patients will receive weekly cycles of intraperitoneal chemotherapy. CRS-HIPEC, cytoreductive surgery with hyperthermic intraperitoneal chemotherapy.
Table 2.
Study procedures
| Before first visit | First visit | Second visit | DLS | First postoperative visit | Intraperitoneal chemotherapy (CTx) | Response evaluation | Intraperitoneal CTx 9th–16th cycles* |
Response evaluation | Last study visit | ||||||||
| First cycle | Second cycle | Third cycle | Fourth cycle | Fifth cycle | Sixth cycle | Seventh cycle | Eighth cycle | ||||||||||
| MTB† | X | X | |||||||||||||||
| Medical history | X | X | |||||||||||||||
| Inclusion/exclusion criteria | X | ||||||||||||||||
| Provide information about the study | X | X | |||||||||||||||
| Written informed consent | X | ||||||||||||||||
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Physical examination (including weight)‡ | X | X | X‡ | X‡ | X‡ | X‡ | X‡ | X‡ | X‡ | X‡ | X‡ | ||||||
| Operability check (anaesthetist) | X | ||||||||||||||||
| Haematology and blood chemistry | X | X‡ | X | X | X | X | X | X | X | X | X | ||||||
| Viral serology | X | ||||||||||||||||
| Pregnancy test‡ | X | ||||||||||||||||
| Placement of peritoneal PAC§ | X | ||||||||||||||||
| Visit medical oncologist | X | X | X | X | |||||||||||||
| CT scan chest/abdomen | X¶ | X** | X | X‡‡ | |||||||||||||
| Intraperitoneal chemotherapy | X | X | X | X | X | X | X | X | X | ||||||||
| Performance status | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Chemotherapy toxicity evaluation (CTCAE 5.0) | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Collection of blood and peritoneal fluid for PK analysis | X | X | |||||||||||||||
| Removal of peritoneal PAC | X†† | ||||||||||||||||
*In case of no progression of disease (ie, CR, PR or SD) and if the patient is willing.
†Scans and reports of (referred) patients are first discussed in a multidisciplinary tumour board. When patients are considered candidates for HIPEC procedure, they are seen in the outpatient clinic.
‡If applicable.
§In case complete cytoreduction is deemed impossible.
¶If not performed by referring centre.
**Maximum of 4 weeks before start of study treatment.
††Optional, according to patient preference and life expectancy.
‡‡At cycle 16 if applicable.
CR, complete response; CTCAE, Common Terminology Criteria for Adverse Events; DLS, diagnostic laparoscopy; HIPEC, hyperthermic intraperitoneal chemotherapy; MTB, multidisciplinary tumour board; PAC, port-a-cath; PK, pharmacokinetic; PR, partial response; SD, stable disease.
Screening
The multidisciplinary tumour board will review all referred patients who are possibly eligible to participate in the study. Potential candidates for CRS-HIPEC will visit the surgical oncology outpatient clinic, where they will be informed about the treatment options, including the study, and undergo standard screening procedures. The standard of care CRS-HIPEC screening procedure includes a CT scan of the thorax and abdomen (not older than 2 months before surgery), laboratory testing (including kidney and liver panels, and blood cell count), anaesthetic assessment and a diagnostic laparoscopy (DLS). If the disease is considered not resectable during DLS, and if the patient meets the inclusion/exclusion criteria, the patient is eligible for inclusion. Patients who are considered ineligible for CRS-HIPEC, based on parameters that were obtained before DLS, but have no contraindication for intraperitoneal chemotherapy can also participate in the study.
Surgical procedures
Patients will be operated under general anaesthesia, according to local hospital procedures. During the diagnostic laparoscopy, the extent of disease is assessed according to the ‘peritoneal carcinomatosis index’. Ascites fluid will be collected for storage in the local MPM biobank. The surgeon will determine the feasibility of complete cytoreduction. If it is deemed impossible to achieve complete cytoreduction, a PAC system will be placed subcutaneously, while the catheter tip is placed inside the peritoneal cavity. After surgery, patients may leave the hospital that same day, with careful (including written) instructions for, example, hygiene. Patients are seen in the outpatient clinic approximately a week after surgery by a medical oncologist. The start date of the first treatment cycle of chemotherapy will be determined.
Chemotherapy
Patients will receive intraperitoneal paclitaxel (dose according to current dose level) dissolved in 1 L of saline (0.9% NaCl), prewarmed to 37°C through the PAC that was placed during laparoscopy. Patients will receive all necessary premedications prior to infusion, according to the local standard protocol for intravenous administration of paclitaxel. If present, prior to infusion, ascites will be drained through the PAC, and stored in the MPM biobank. Administration of intraperitoneal chemotherapy will take about 1.5–2 hours. After infusion, patients are instructed to switch position frequently to maximise distribution of chemotherapy in the peritoneal cavity. Patients will be observed for 2 hours after chemotherapy administration. If no adverse events (AE) occur during this period, patients will be discharged with careful instructions to contact the hospital if any alarming symptoms do develop. During the first and the fourth cycles of intraperitoneal chemotherapy, additional blood samples and intraperitoneal fluid samples will be collected for PK analysis. The 24-hour AUC will be calculated for systemic and intraperitoneal paclitaxel. Other PK parameters such as the maximum concentration (Cmax) and the elimination half life (t1/2) will also be determined.
Patients will initially receive eight weekly cycles of intraperitoneal chemotherapy. After the start of the first cycles, following cycles can be delayed at the discretion of the medical oncologist in case of a medical indication (eg, neutropenia). If a cycle is delayed for more than 2 weeks, this is considered a dose-limiting toxicity (DLT). After the first eight cycles, response evaluation will take place. Depending on this outcome, another eight cycles can be initiated. In case of ongoing therapy response, there is no limit to the number of cycles.
Follow-up
As the current proposal is a phase I trial, a long-term follow-up is not applicable. However, (PET)-CT scans are performed at baseline, during response evaluation (if possible, according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria) and every 4 months after the last treatment. By doing so, valuable preliminary data on the effectiveness of this treatment can be acquired. Also, in case of treatment response after 16 cycles, a second diagnostic laparoscopy can be performed to definitively assess response and possibly assess eligibility for surgical treatment.
Withdrawal of individual subjects
Subjects can leave the study at any time for any reason if they wish to do so without any consequences. The investigator can decide to withdraw a subject from the study for urgent medical reasons. Should a patient or the study coordinator decide to withdraw, all efforts will be made to complete and report the observations as thoroughly as possible. Patients will receive treatment according to standard of care. Three patients within a dose level must be observed for 2 weeks (two cycles of chemotherapy) before proceeding to the next higher dose level. If a patient is withdrawn from the study prior to completing two cycles of therapy and 1 week of follow-up without experiencing a DLT prior to withdrawal, an additional patient may be added to that dose level. The investigators also have the right to withdraw patients from the study if one of more of the following events occur:
Significant protocol violation or non-compliance on the part of the patient or investigator.
Refusal of the patient to continue treatment or observations.
Any change in the condition of the patient that justifies discontinuation of treatment.
Decision by the study coordinator that termination is in the patient’s best medical interest.
Unrelated medical illness or complication.
Objectives and analysis
Primary objective
The primary objective is to determine the MTD of intraperitoneal paclitaxel monotherapy for patients with MPM who are ineligible to undergo CRS-HIPEC. The MTD will be determined during the first eight cycles of intraperitoneal chemotherapy by a classic three-plus-three dose escalation design with three dose levels (ie, 100, 150 and 200 mg flat dose paclitaxel; see figure 1). To determine the MTD, DLTs are predefined. DLTs are graded according to the Common Terminology Criteria for Adverse Events version 5.0. If less than 33% of the subjects in a dose cohort experience DLT (ie, one subject out of a maximum of six subjects in a dose cohort), the next higher dose cohort will be assessed. Dose levels higher than 200 mg will not be assessed. If ≥33% of the subjects experience DLT in the first dose cohort (ie, 100 mg), a dose de-escalation to 80 mg will be assessed. There will be no dose escalation within the patients. The following events will be considered DLTs:
Haematological:
ANC <0.5×109/L (grade 4), lasting longer than 7 days.
Febrile neutropenia (ANC <1.0×109/L with fever ≥38.5°C) (grades 3–4).
Platelet count <25×109/L (grade 4).
Non-haematological:
-
Grade ≥3 non-haematological AEs, except nausea/vomitus, diarrhoea or fatigue, for which the following DLT definition will apply:
Nausea grade ≥3, despite optimal antiemetic use.
Diarrhoea grade ≥3, despite optimal loperamide use.
Fatigue grade ≥3 lasting longer than 7 days.
Delay of next cycle by >2 weeks due to any medical reason.
Secondary objective(s)
Secondary objectives are to assess the safety, toxicity and feasibility of this treatment, and to establish the PK profile of intraperitoneal paclitaxel. During the study, ascites and tumour material will be systematically collected, processed and stored for translational research purposes.
Sample size calculation and statistical analysis
Because of the dose escalation design, the needed number of participants depends on data obtained during different dose levels (see figure 1). The minimum number of patients is 4, if the first two patients in the first dose cohort immediately experience DLT, as well as the first two patients in the dose de-escalation cohort. The minimum number of patients required to reach the primary endpoint (ie, to find the MTD) is 11. If the first three patients experience no DLT, but the first two patients in the second dose cohort both experience DLT, then five patients were already included, after which an additional six patients have to be included at the first dose level, to come to nine patients treated at the MTD. The maximum number of patients that can possibly be required to reach the primary endpoint is 21. There are six patients required in each dose cohort to reach the MTD, after which an additional three patients have to be included in the final dose cohort, to come to nine patients treated at the MTD.
The statistical analyses/data summaries will be performed using R and RStudio. Other tools may be used for exploratory summaries and graphical presentations. Descriptive statistics will be used to describe paclitaxel PK, dose linearity and its relation to paclitaxel-related side effects. Systemic bioavailability of peritoneal administration will be analysed by comparing the AUC with the results of our many other pharmacological studies with paclitaxel. Relationship between toxicity and paclitaxel exposure will be explored graphically and with logistic regression (two sided and p<0.05).
Harms and auditing
All AEs, serious adverse events (SAE) or suspected unexpected serious adverse reactions (SUSARs) will be recorded. All (S)AEs and SUSARs as related to the administration of intraperitoneal paclitaxel will be reported through the web portal ToetsingOnline to the accredited Medical Research Ethics Committee (METC) that approved the protocol, within 7 days of first knowledge for SAEs that result in death or are life threatening, followed by a period of maximum of 8 days to complete the initial preliminary report. All other SAEs will be reported within a period of maximum of 15 days after the sponsor has first knowledge of the SAEs. In addition to the expedited reporting of SUSARs, the sponsor will submit, once a year throughout the clinical trial, a safety report to the accredited METC, competent authority and competent authorities of the concerned member states. The sponsor (Erasmus MC Cancer Institute, the Netherlands) is insured to provide cover for any patients who suffer harm from study participation.
Since this is a phase I dose escalation study, all (S)AEs and SUSARs will be evaluated by the study team before the decision will be made to continue with the next dose level. Therefore, no data safety monitoring board will be installed.
Patient and public involvement
There was no patient or public involvement in the design, conduct, reporting or dissemination plans of the INTERACT MESO trial. However, the design of this trial has been shared with the Asbestos Victims Association of the Netherlands (in Dutch, ‘Asbestslachtoffers Vereniging Nederland’), and they support this research.
Ethics and dissemination
This study will be conducted in agreement with both the Declaration of Helsinki (latest amendment: 64th WMA General Assembly, Fortaleza, Brazil, October 2013), the Dutch laws and regulations with the WMO (‘Wet Medisch-wetenschappelijk Onderzoek met mensen’) in particular. In case of protocol modifications, the research medical ethics committee (METC) and the Dutch competent authority (Central Committee on Research Involving Human Subjects, CCMO) will be informed. The new protocol has to be approved by the METC and CCMO before it can be implemented. Data collection, data assessment and data analysis will be performed according to the local guidelines for data management of the Erasmus MC.
The sponsor will submit a summary of the progress of the trial to the accredited METC once a year. Information will be provided on the date of inclusion of the first subject, numbers of subjects included and numbers of subjects that have completed the trial, SAEs/serious adverse reactions, other problems and amendments.
The results of this clinical trial will be submitted for publication in a peer-reviewed scientific journal.
Discussion
The main objective of the INTERACT MESO trial is to determine the MTD of intraperitoneal paclitaxel monotherapy in patients with MPM. Secondary endpoints are to assess safety and toxicity, feasibility and the PK profile of this treatment. To our knowledge, the INTERACT MESO trial is the first clinical trial that investigates intraperitoneal paclitaxel as non-adjuvant monotherapy in patients with MPM who are not eligible for CRS-HIPEC.
Currently, the majority of patients with MPM in the Netherlands receive no antitumour treatment.1 The morbidity of systemic treatment is high, and the effectiveness is limited.4–8 Hence, there is a lack of appropriate palliative treatment for patients with MPM. As MPM rarely disseminates outside the abdominal cavity, the use of intraperitoneal chemotherapy seems a logical and promising step. This has major advantages over systemic treatment, as a higher, more effective dose of chemotherapy can directly be delivered at the site of the disease, while systemic uptake is limited. This will likely result in fewer systemic toxicity, and thus an increase in quality of life. In rare cases where metastases do develop, a switch can be made to systemic treatment. By first applying local treatment, most patients will be spared a toxic and often ineffective systemic therapy. The placement of the intraperitoneal PAC is performed during standard of care diagnostic laparoscopy, thus not associated with extra visits or procedures. The Erasmus MC Cancer Institute is experienced with the placements of intraperitoneal PACs and the administration of intraperitoneal chemotherapy. The INTERACT trial, a phase I dose escalation study with concomitant intraperitoneal irinotecan combined with FOLFOX in patients with peritoneal metastases from colorectal carcinoma, was conducted in the Erasmus MC Cancer Institute.17 This trial recently finished and shows promising results. Another advantage of the peritoneal PAC is that ascites, a common symptom of MPM, causing major morbidity, can repeatedly be drained through the PAC system.
Paclitaxel is a well-known chemotherapeutic agent and is considered extremely favourable for intraperitoneal use.9 Due to its large molecular weight and lipophilic properties, it is slowly cleared from the peritoneal cavity when administered locally. This results in an AUC after intraperitoneal administration that is up to a 1000-fold (3-log) higher than that in plasma, while peritoneal concentrations persist up to 48 hours after administration.10 Based on earlier studies, intraperitoneal paclitaxel is expected to be a more effective treatment for patients with extensive peritoneal mesothelioma, compared with the current available systemic chemotherapy. Though systemic administration has not shown to result in survival benefit for patients with MPM, the fact that up to a 1000-fold AUC can be achieved by peritoneal administration provides the rationale for the hypothesis that intraperitoneal treatment can be effective.
The starting dose in this dose escalation study will be a 100 mg flat dose. In earlier phase I and II studies that investigated the use of intraperitoneal paclitaxel in patients with ovarian cancer in weekly cycles, the MTD was 60–65 mg/m2.11 12 This translates to a 120–130 mg flat dose. The patients with ovarian cancer in these studies were heavily pretreated with systemic chemotherapy. As intraperitoneal paclitaxel will be used as first-line monotherapy in the current study, a higher MTD is anticipated. Currently, the systemic effective dosage is 175–200 mg (flat dose). As intraperitoneal administration can reach up to a 1000-fold higher AUC, there is no clinical rationale to pursue a dose escalation beyond a 200 mg flat dose.
Earlier studies have shown that intraperitoneal administration of paclitaxel causes mild toxicity. Common toxicities that occur from systemic administration, such as neuropathy, were not observed after intraperitoneal administration.10–12 Bowel perforation is a rare but potentially serious complication from intraperitoneal treatment. This was extremely rare in previous studies that investigated a similar treatment strategy.
During this study, ascites and tumour material will also be collected, processed and stored for translational research purposes. As MPM is a rare disease, this could result in valuable information for all patients with MPM.
If the MTD for intraperitoneal paclitaxel in the current study population is determined, and the treatment is found to be safe, a larger phase III clinical trial should be conducted to determine the effect on survival outcomes. Because the incidence of MPM in the Netherlands alone is low, a phase III clinical trial would have to be conducted internationally.
Supplementary Material
Footnotes
JPvK and MVD contributed equally.
Contributors: JPvK and MVD drafted this manuscript. All authors drafted the original study protocol and revised the manuscript. EVEM, JGJVA, CV and RHJM initiated the trial and supervised the drafting of the study protocol and manuscript. NADG, ARMB-K, SLWK and JWAB contributed to the study conceptualisation and development of the study protocol. EVEM acquired funding for implementation of the trial protocol and is the primary clinical investigator. All authors approved the final manuscript.
Funding: This work was supported by the Erasmus MC Foundation.
Disclaimer: The funding body had no role in the study design, the collection, analysis, or interpretation of data, or in writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Dutch competent authority (CCMO): NL78373.078.21. Local medical ethics committee (METC): MEC-2021-0697. Participants gave informed consent to participate in the study before taking part.
References
- 1.de Boer NL, van Kooten JP, Damhuis RAM, et al. Malignant peritoneal mesothelioma: patterns of care and survival in the Netherlands: a population-based study. Ann Surg Oncol 2019;26:4222–8. 10.1245/s10434-019-07803-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686–93. 10.1245/s10434-014-3978-x [DOI] [PubMed] [Google Scholar]
- 3.Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237–42. 10.1200/JCO.2009.23.9640 [DOI] [PubMed] [Google Scholar]
- 4.Jänne PA, Wozniak AJ, Belani CP, et al. Open-Label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer 2005;7:40–6. 10.3816/CLC.2005.n.020 [DOI] [PubMed] [Google Scholar]
- 5.Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567–72. 10.1200/JCO.2007.15.2868 [DOI] [PubMed] [Google Scholar]
- 6.Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International expanded access program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211–8. 10.1016/j.lungcan.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 7.Baas P, Scherpereel A, Nowak AK, et al. First-Line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375–86. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 8.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 9.Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol 2010;2:109–16. 10.4251/wjgo.v2.i2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markman M, Rowinsky E, Hakes T, et al. Phase i trial of intraperitoneal taxol: a gynecoloic oncology group study. J Clin Oncol 1992;10:1485–91. 10.1200/JCO.1992.10.9.1485 [DOI] [PubMed] [Google Scholar]
- 11.Francis P, Rowinsky E, Schneider J, et al. Phase I feasibility and pharmacologic study of Weekly intraperitoneal paclitaxel: a gynecologic oncology group pilot study. J Clin Oncol 1995;13:2961–7. 10.1200/JCO.1995.13.12.2961 [DOI] [PubMed] [Google Scholar]
- 12.Markman M, Brady MF, Spirtos NM, et al. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a gynecologic oncology group study. J Clin Oncol 1998;16:2620–4. 10.1200/JCO.1998.16.8.2620 [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH, Stuart OA. Unusually favorable outcome of 6 consecutive patients with diffuse malignant peritoneal mesothelioma treated with repeated doses of intraperitoneal paclitaxel. A case series. Surg Oncol 2020;33:96–9. 10.1016/j.suronc.2020.01.009 [DOI] [PubMed] [Google Scholar]
- 14.Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res 2018;7:599–608. 10.21037/tlcr.2018.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. Eur J Surg Oncol 2017;43:1228–35. 10.1016/j.ejso.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer NL, Brandt-Kerkhof ARM, Madsen EVE, et al. Concomitant intraperitoneal and systemic chemotherapy for extensive peritoneal metastases of colorectal origin: protocol of the multicentre, open-label, phase I, dose-escalation interact trial. BMJ Open 2019;9:e034508. 10.1136/bmjopen-2019-034508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062907supp001.pdf (79.3KB, pdf)


