Abstract
Storage of Salmonella enterica serovar Typhimurium strains in soil and water microcosms resulted in loss of culturability on standard plating media. Prior incubation in buffered peptone water supplemented with ferrioxamine E markedly extended the time that bacteria were recoverable by plating, except in the case of mutants deficient in ferrioxamine E uptake.
Salmonella species are important food-borne pathogens that represent a significant and increasing public health problem in industrialized countries. Responding to the need for rapid and sensitive methods for detecting Salmonella spp., we recently reported the use of the hydroxamate siderophore ferrioxamine E as a semiselective growth supplement in standard enrichment procedures to increase the speed and sensitivity of detection of Salmonella organisms in environmental samples, in particular from food (9, 12). Ferrioxamines are highly effective iron sources for Salmonella serovars of subspecies I, II, and IIIb, which together account for >99% of human clinical isolates (6), but not for a number of quite closely related species. The current investigation arose from attempts to exploit this effectiveness and selectivity to study the behavior of Salmonella enterica serovar Typhimurium strains in mixed populations in soil samples. The persistence of bacteria in the environment (e.g., in soil, water, sewage, etc.) depends on the long-term survival of heavily stressed cells, particularly the so-called viable-but-nonculturable (VNC) organisms, that cannot grow on conventional laboratory plating media but may revive in vivo and cause disease (2, 3, 8). We demonstrate here that ferrioxamine E was able to resuscitate stressed serovar Typhimurium cells, thus highlighting the need to reassess methods for the analysis of bacteriological safety.
Soil microcosms were made by inoculating 7-kg batches of nonsterile or heat-sterilized (2 h at 134°C) humic soil with fresh tryptic soy agar (TSA; BD Heidelberg, Heidelberg, Germany) cultures of bacterial strains at 107 to 108 CFU g−1 and stored at 12 to 15°C in normal daylight. At intervals, 25-g samples of soil were suspended in 250 ml of 0.85% (wt/vol) NaCl (saline), shaken thoroughly for 30 min at room temperature, and serially diluted in saline. Duplicate 0.1-ml aliquots were plated for viable counts on XLD agar (Oxoid, Basingstoke, England) and Galle-Chrysoidin-Glycerol (GCG) agar (SIFIN, Berlin, Germany). At later time points, 10-g samples of soil were suspended in 100 ml of buffered peptone water (BPW; Oxoid) or BPW containing ferrioxamine E (50 ng ml−1) and incubated with shaking at 37°C for 24 h before plating on selective agar as described above. In all cases, recovered bacteria were checked by agglutination with omnivalent Salmonella-Serum (SIFIN).
Serovar Typhimurium strain M307 (Table 1) remained culturable on selective media, as measured by direct plating or after incubation in BPW, for 84 days in a nonsterile soil microcosm and for 410 days in sterilized soil (Fig. 1a). This difference, which was observed consistently, is presumably due to competition by indigenous microorganisms in the nonsterile environment, including perhaps the production of antibiotics by soil streptomycetes (14). Prior incubation of soil samples in BPW containing ferrioxamine E, however, resulted in observable growth on selective plates for an additional 371 and 342 days, respectively. By contrast, strain RK102, which lacks the ferrioxamine receptor and so cannot utilize ferrioxamines as sources of iron (Table 1), survived approximately 130 days in a nonsterile soil microcosm, as indicated by growth after incubation in BPW, regardless of the presence of ferrioxamine E (Fig. 1a).
TABLE 1.
Serovar Typhimurium strains used in this study
| Strain | Characteristics | Source or reference |
|---|---|---|
| ATCC 14028 | Type strain | ATCCa |
| M307 | LT1 phage typing reference strain | PHLSb |
| SL1344 | Histidine auxotroph (hisG4) | 5 |
| TA2700 | Deficient in hydroxamate siderophore uptake (fhuC) and enterobactin synthesis (ent) | J. B. Neilands, W. Rabsch (11) |
| RK102 | Lacks ferrioxamine receptor (foxA); nalidixic acid resistant | 6 |
ATCC, American Type Culture Collection, Manassas, Va.
PHLC, Public Health Laboratory Service, London, England.
FIG. 1.
Effect of ferrioxamine E on serovar Typhimurium strains in soil and water microcosms. (a) Microcosms of the strains indicated in sterile or nonsterile humic soil were sampled at intervals; open bars indicate the maximum time during which bacterial growth was observed on Salmonella selection media either directly or following incubation in BPW, and solid bars indicate growth on selective media following incubation in BPW supplemented with ferrioxamine E. (b) Microcosms of the strains indicated in sterile double-distilled water were sampled at intervals; open bars indicate the maximum time during which bacteria were recovered on TSA-pyruvate after incubation in BPW, while solid bars indicate recovery following incubation in BPW supplemented with ferrioxamine E. Sampling intervals varied depending on the observed decline in viable cell counts but were initially approximately monthly, then approximately weekly, and finally, particularly in the case of water microcosms, daily. The data shown in this figure are representative of several experiments with various serovar Typhimurium strains that showed essentially the same results.
Water microcosms were made by inoculating 1.5-liter batches of sterile double-distilled water with fresh TSA cultures at 105 to 106 CFU ml−1 and stored at room temperature in normal laboratory light conditions. Viable cell counts were measured at intervals by plating serially diluted samples (0.1 ml) in triplicate on TSA containing 0.1% (wt/vol) sodium pyruvate (to avoid further stress by selective media). Recovered colonies were checked by subculturing them onto GCG agar and by agglutination using omnivalent Salmonella-Serum. Although considerable interstrain variation was observed (for reasons we cannot explain), progressive reduction in viable counts as measured by direct plating was a consistent feature of water microcosms of all strains tested (Fig. 1b). When colonies were no longer recoverable by direct plating, 60-ml samples of water microcosms were inoculated into 90 ml of 1.67-fold-concentrated BPW and incubated with shaking at 37°C for 24 h before plating them on TSA-pyruvate plates. After incubation in BPW, wild-type strains ATCC14028 and SL1344 (Table 1) were recovered from water microcosms for 119 and 79 days, respectively; incubation in the presence of ferrioxamine, however, allowed recovery of these strains for an additional 35 and 41 days, respectively. On the other hand, incubation in the presence of ferrioxamine E had no observable effect on the recovery of ferrioxamine-uptake-deficient mutant strains TA2700 or RK102 (Fig. 1b).
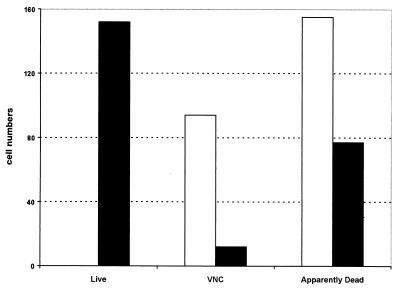
Bacteria in water microcosms were sampled on dark Nucleopore polycarbonate membrane filters (Corning Glass Works, Corning, N.Y.) and stained with the LIVE/DEAD BacLight Bacterial Viability kit L-7012 (Molecular Probes Europe, Leiden, The Netherlands). Cells were observed with an Optiphot-2 (Nikon Tokyo, Japan) fluorescence microscope, and eight fields of each sample were enumerated by the image system LUCIA (Nikon). The two-color fluorescence dye of the LIVE/DEAD system distinguishes living and apparently dead bacteria as green and red cells, respectively (Fig. 2). We also observed brownish orange bacteria that we assumed were cells in the VNC state, but it is not clear whether the color is evidence of an intermediate level of stress injury or of dormancy. In addition, all samples were assayed microscopically with the redox dye 5-cyano-2,3-ditolyl tetrazolium chloride and counterstained with DAPI (4′,6′-diamidino-2-phenylindole) (13) in order to confirm the numbers of living cells (red fluorescent) relative to the total cell count. Samples of a water microcosm of strain SL1344 taken after the time that colonies were no longer observable (by direct plating or following incubation in BPW, i.e., 89 days) contained no detectable living cells, and approximately equal numbers of VNC and apparently dead cells (Fig. 2). Analysis following incubation of the same samples in the presence of ferrioxamine E, however, showed the presence of many living cells and significantly fewer VNC and apparently dead types, despite the fact that no growth had occurred during the incubation period.
FIG. 2.
Resuscitation of serovar Typhimurium strain SL1344 from water microcosms by ferrioxamine E. Microcosm samples taken at 89 days after inoculation were analyzed by using the LIVE/DEAD BacLight Bacterial Viability kit L-7012 for the presence of live, VNC, and apparently dead cells (green, brownish orange, red, respectively), either directly (open bars) or following incubation in BPW containing ferrioxamine E (solid bars). Three replicate experiments gave essentially the same results, with deviations of means for each cell type of no greater than 5%.
The loss of culturability we observed in soil and water microcosms is similar to that reported by others (1, 7, 10). The novel observation here, however, is that incubation of microcosm samples in the presence of ferrioxamine E is apparently able to resuscitate stressed serovar Typhimurium cells, although it should be noted that our experiments do not conclusively demonstrate a direct link. We suggest that the uptake of ferrioxamine is required, since the effect was not seen with two mutants lacking components of the uptake mechanism for ferrioxamines. Moreover, microcosms of Escherichia coli strains, which do not utilize ferioxamines as sources of iron (6), also do not show extended culturability upon incubation with ferrioxamine E (data not shown). It is unlikely that these effects are due to ferrioxamine E degradation as a source of utilizable carbon, as was recently reported for desferrioxamine B with a Spirillum spp.-like bacterium (15), since Salmonella strains did not grow in minimal media in which the only organic component was ferrioxamine (unpublished data). Rather, we propose that the phenomenon depends upon the fact that ferrioxamine E (an iron-saturated complex with 1:1 stoichiometry) not only supplies iron to bacteria that may have been severely iron starved for prolonged periods but also, by balancing release (e.g., by reduction) and use (in respiration, growth, etc.) of ferric ions, may prevent the generation of damaging free radicals at a time when stressed cells are in a particularly vulnerable state (4). Studies are in progress to determine the mechanism of this effect, but meanwhile we recommend the use of a preincubation medium supplemented with ferrioxamine E for improved detection of Salmonella in environmental, food, and clinical samples.
Acknowledgments
This work was supported by the British-German Academic Research (ARC) Programme of the British Council and the Deutscher Akademischer Austauschdienst (project 354 awarded to R.R. and P.H.W.) and by Medical Research Council Collaborative Studentship CS93 15 awarded to R.A.K. in association with Medeva Group Research.
We are grateful to H.-P. Schnebli, Novartis, Ltd., Basel, Switzerland, for providing ferrioxamine E and to J. B. Neilands (University of California at Berkeley) for supplying strain TA2700.
REFERENCES
- 1.Chmielewski R A N, Frank J F. Formation of viable but nonculturable Salmonella during starvation in chemically defined solutions. Lett Appl Microbiol. 1995;20:380–384. doi: 10.1111/j.1472-765x.1995.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 2.Colwell R R, Brayton P, Herrington D, Tall B, Huq A, Levine M M. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 3.Dixon B. Viable but nonculturable. ASM News. 1998;64:372–373. [Google Scholar]
- 4.Dodd C E R, Sharman R L, Bloomfield S F, Booth I R, Stewart G S A B. Inimical processes: bacterial self-destruction and sub-lethal injury. Trends Food Sci Technol. 1997;8:238–241. [Google Scholar]
- 5.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 6.Kingsley R A, Reissbrodt R, Rabsch W, Ketley J M, Tsolis R M, Everest P, Dougan G, Bäumler A J, Roberts M, Williams P H. Ferrioxamine-mediated iron(III) utilization by Salmonella enterica. Appl Environ Microbiol. 1999;65:1610–1618. doi: 10.1128/aem.65.4.1610-1618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh P, Morris N Z, Wellington E M H. Quantitative molecular detection of Salmonella typhimurium in soil and demonstration of persistence of an active but non-culturable population. FEMS Microbial Ecol. 1998;27:351–363. [Google Scholar]
- 8.Oliver J D. The viable but non-culturable state in the human pathogen Vibrio vulnificans. FEMS Microbiol Lett. 1995;133:203–208. doi: 10.1111/j.1574-6968.1995.tb07885.x. [DOI] [PubMed] [Google Scholar]
- 9.Pless P, Reissbrodt R. Improvement of Salmonella detection on motility enrichment media by ferrioxamine E-supplementation of pre-enrichment culture. Int J Food Microbiol. 1995;27:147–159. doi: 10.1016/0168-1605(94)00160-8. [DOI] [PubMed] [Google Scholar]
- 10.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabsch W. Characterization of the catecholate indicator strain S. typhimurium TA2700 as an ent fhuC double mutant. FEMS Microbiol Lett. 1998;163:79–84. doi: 10.1111/j.1574-6968.1998.tb13029.x. [DOI] [PubMed] [Google Scholar]
- 12.Reissbrodt R, Vielitz E, Kormann E, Rabsch W, Kühn H. Ferrioxamine E-supplemented pre-enrichment and enrichment media improve various isolation methods for Salmonella. Int J Food Microbiol. 1996;29:81–91. doi: 10.1016/0168-1605(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez G G, Phipps D, Ishiguro K, Ridgway H F. Use of a fluorescence redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turpin P E, Dhir V K, Maycroft K A, Rowlands C, Wellington E M H. The effect of Salmonella species on the survival of Streptomyces in soil. FEMS Microbial Ecol. 1992;101:271–280. [Google Scholar]
- 15.Winkelmann G, Schmidtkunz K, Rainey F A. Characterization of a novel Spirillum-like bacterium that degrades ferrioxamine-type siderophores. Biometals. 1996;9:78–83. doi: 10.1007/BF00188094. [DOI] [PubMed] [Google Scholar]