Abstract
When cells of a type II methanotrophic bacterium (Methylocystis strain LR1) were starved of methane, both the Km(app) and the Vmax(app) for methane decreased. The specific affinity (aos) remained nearly constant. Therefore, the decreased Km(app) in starved cells was probably not an adjustment to better utilize low-methane concentrations.
Microbial oxidation of atmospheric methane (CH4) takes place in most aerobic upland soils (5, 10). Because these soils exhibit a lower half-saturation constant [Km(app)] for CH4 than do pure cultures of methanotrophic bacteria, it has been postulated that the active bacteria are unknown species. These have been popularly dubbed “high-affinity” methane oxidizers (5, 10). Recently, a novel group of pmoA-like sequences was detected in several soils which oxidize atmospheric CH4 (11, 12), and incubation of soils under 14CH4 resulted in the labeling of signature phospholipid fatty acids which differed from those of known type II methanotrophs (18). It is therefore likely that as-yet-uncultured species are involved in atmospheric CH4 uptake. However, it remains unknown whether atmospheric CH4 oxidation is limited to particular species and whether these possess a specialized high-affinity CH4 oxidation enzyme.
We previously demonstrated that high-affinity CH4 oxidation is probably not limited to uncultured methanotrophic groups. We enriched a methane-oxidizing bacterium (strain LR1) from an organic soil and identified it based on 16S rDNA, pmoA, and mxaF gene sequences as a type II methanotrophic species of the Methylosinus/Methylocystis cluster (8). Mixed cultures containing strain LR1, when grown under <275 ppm volume CH4, had a low Km(app) for CH4 (56 to 188 nM) similar to the value measured in soil. This increased to >1 μM when cells were grown under >1% CH4. In the present study, we investigated the kinetics of the isolated bacterium (culture is available upon request). Instead of the time-consuming process of growing the organism under low CH4 mixing ratios, we tested the effect of starving cells of CH4.
Kinetics of strain LR1.
Culture was grown in liquid nitrate-mineral salts (NMS) medium (8) under 10% CH4. Purity was controlled microscopically and by plating onto NMS agar, R2A agar, 10% strength Nutrient Agar, and 10% strength AC Broth (Difco). After 1 to 2 months, the culture was diluted to about 109 cells ml−1 with 0.5 mM phosphate buffer (pH 6.0), and 7.5-ml amounts were added to 13-ml serum vials. The vials were capped with sterile butyl rubber stoppers and incubated with gentle shaking (6 rpm) at 25°C without added CH4. After 1 to 2 weeks, some vials were injected with CH4 to a final mixing ratio of 1% and incubated for a further 24 h (“unstarved”). Others remained without CH4 (“starved”). Cell counts were made using a Neubauer chamber and showed that no population growth occurred during the 24-h incubation with 1% CH4 (data not shown).
For determination of kinetic properties, CH4 was injected into these vials to final mixing ratios ranging from 5 to 1,500 ppm volume. The unstarved vials still contained >0.5% CH4 after 24 h and were first flushed well with air. Vials were shaken at 280 rpm. Starting 5 min after CH4 addition and at 30-min intervals thereafter, CH4 was measured by gas chromatography-flame ionization detection (8). Methane oxidation rates and kinetic parameters were estimated as previously described (8).
Unstarved cells had both a higher Km(app) and a higher Vmax(app) for CH4 than did starved cells (Table 1). Addition of KCl may also have decreased the Km(app). The increase of both Km(app) and Vmax(app) in unstarved culture was about 1 order of magnitude, and the specific affinity (aos) (Vmax(app)/Km(app)) therefore remained nearly constant. The specific affinity is the initial slope of the hyperbolic curve, or the pseudo first-order rate constant, and directly indicates how rapidly the culture metabolized limiting substrate (4). At low CH4 concentrations, the rate of CH4 uptake was therefore similar in starved and unstarved culture.
TABLE 1.
Kinetic coefficients of starved and unstarved cultures of Methylocystis strain LR1 in several trialsa
| Trial | Measurement time (h) | Km(app) (nM CH4) | Vmax(app) (10−9 nmol cell−1 h−1) |
aos (10−9 ml cell−1 h−1)
|
|
|---|---|---|---|---|---|
| Vmax(app)/Km(app) | Regression | ||||
| Trial 1 | |||||
| Cells starved 12 days | 1 | 298 | 2.61 | 8.75 | 4.65 |
| Starved + KCla | 1 | 138 | 1.22 | 8.84 | 5.27 |
| Unstarved | 1 | 3,170 | 18.7 | 5.90 | 4.33 |
| Trial 2 | |||||
| Cells starved 34 days | 2 | 281 | 0.935 | 3.32 | 2.09 |
| Starved + KClb | 2 | 226 | 0.380 | 1.68 | 0.95 |
| Unstarved | 1.5 | 12,600 | 27.8 | 2.21 | 2.12 |
| Trial 3 | |||||
| Cells starved 10 days | 2 | 336 | 2.97 | 8.88 | 4.73 |
| Freshc | 2 | 2,190 | 13.27 | 6.08 | 4.39 |
| Trial 4 | |||||
| Cells starved 19 days | 2 | 329 | 0.779 | 2.36 | 1.39 |
| Unstarved | 1.5 | 3,400 | 21.7 | 6.38 | 4.81 |
Each calculation was based on data from 12 to 54 individual vials. Michaelis-Menten kinetic coefficients are shown, as well as the specific affinity (aos) either calculated (Vmax(app)/Km(app)) or estimated by linear regression of the CH4 oxidation rate versus the dissolved CH4 concentration for all data points less than Km(app).
KCl was added to a final concentration of 250 mM immediately before test.
Cells were not preshaken and unstarved, but were taken directly from growing stock culture containing >1% CH4.
These results extend our previous observations on strain LR1 by demonstrating that (i) the Km(app) can vary in pure culture, (ii) starvation of CH4 decreases the Km(app), (iii) the specific affinity (aos) changes little with Km(app), and (iv) Km(app) varies in rapid (<2 h) tests without added chloramphenicol (previous tests were run over several days and chloramphenicol was necessary to prevent enzyme production). As previously discussed (8), the variable Km(app) could have resulted because type II methanotrophs possesses different forms of methane monooxygenase (MMO): a particulate (pMMO) and a soluble (sMMO) form. Multiple catalytic forms of pMMO also exist, depending on Cu availability (15, 17). However, while the Km(app) values of these MMOs are different (10, 15, 21), all measured values in pure culture are above 0.8 μM. The values measured in strain LR1 are as low as 56 to 336 nM (present results and reference 8).
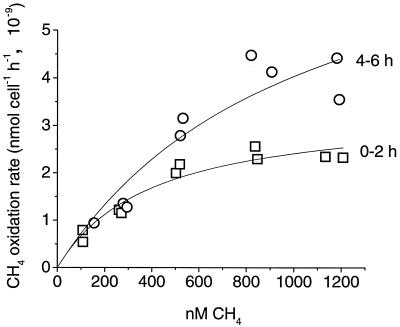
Multiple enzymes may be responsible for the variable Km(app) in LR1, but because the aos per cell remained constant, it cannot be concluded that a high-affinity enzyme was induced to better utilize limiting CH4. This possibility is consistent with the data only if reactivation of inactive cells and expression of a lower-affinity enzyme compensated for each other in the unstarved culture and caused the aos to remain constant. However, if this were so, a biphasic kinetic curve should have been evident, and this was never observed. A better explanation for our results is that measured Km(app) values do not always represent true, constant enzyme properties. Because of diffusion limitation in experimental systems, many reported Km(app) values are gross overestimates (14). In order to control for this in our experiments, the CH4 oxidation rate constants in the linear portion of the hyperbolic curve were also often measured with culture diluted 50%. These rate constants were close to 50% of the rate constants in undiluted culture (in six cases, rate constants were 42, 54, 61, 73, 74, and 81%), indicating that there was only a slight limitation in CH4 movement from the gas phase to the liquid phase. We also used the minimum incubation times which yielded reproducible data (<2 h) to estimate CH4 oxidation rates. To illustrate the effect of longer incubation times, we measured an initial 0 to 2 h rate at five CH4 concentrations in a 10-day-starved culture, and then after 4 h (during which the CH4 declined <40%) reinjected enough CH4 to bring each vial back to its initial CH4 concentration and measured a 4 to 6 h rate (Fig. 1). The CH4 oxidation rate increased with time, most strikingly at the higher CH4 concentrations. This trend leads to an overestimation of the Km(app) with increasing incubation times.
FIG. 1.
CH4 oxidation rates at five CH4 concentrations in a 10-day-starved culture of Methylocystis strain LR1, measured 0 to 2 h (□) and 4 to 6 h (○) after adding CH4.
Although we minimized these methodological problems, there are further ways in which an apparent Km in a complex system can vary. The Km(app) of MMO for CH4 is affected by the CH4 association and dissociation constants, the rate of CH4 diffusion across the cell envelope, and the concentrations of cosubstrates (O2 and reductant). One explanation for the lower Km(app) in starved cells is low reductant availability. The mechanism of pMMO is not well elucidated, but sMMO follows a catalytic sequence in which O2, CH4, and NADH sequentially bind (9, 20). If in starved cells CH4 binds normally but the overall catalytic cycle is slowed by NADH limitation, the rate-limiting step may cease to be the association and dissociation of CH4 to the enzyme (i.e., the affinity constant) and instead become the reaction rate (i.e., the kinetic constant). In such a case, both the Vmax(app) and the Km(app) for CH4 decrease (19). To illustrate this, the following reaction diagram has been simplified to consider only CH4 as a reactant. The binding of O2 and NADH and the formation of methanol are considered by the rate constant kp:
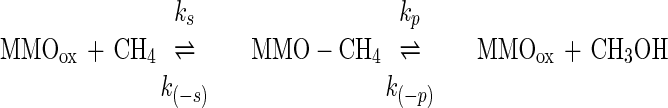 |
Here, Km(app) = [k(−s) + kp]/[ks + k(−p)]. Limitation of reductant will decrease the reaction rate constant kp and in turn decrease Km(app) (i.e., cause a higher apparent affinity). The above model is a very simple explanation of the observed variability. The truth may of course be more complex.
Bender and Conrad (2) observed that incubation of various soils under 20% CH4 increased methanotrophic Km(app) and Vmax(app) values by 1 to 3 orders of magnitude, but increased methanotrophic cell counts only 3- to 10-fold. When specific affinities were calculated (Table 2), two of these soils (a meadow cambisol and a cultivated cambisol) had a similar pattern as LR1—that aos varied little despite large changes (30- to 100-fold) of Km(app). A third soil (forest luvisol) had a much lower aos after enrichment than before, suggesting that a different population had become active in CH4-enriched soil. Comparisons must be cautiously made, but these data suggest that the pattern noted in LR1 is applicable to some, but not all, soils.
TABLE 2.
Michaelis-Menten apparent half-saturation constants for CH4 oxidation in three soils, and calculated specific affinities from data given in reference 2a
| Sample | Km(app) (nM CH4) | aos (10−9 ml cell−1 h−1) |
|---|---|---|
| Cultivated cambisol | ||
| Fresh | 50.6 | 0.0039 |
| +20% CH4 | 91 | 0.017 |
| 1,740 | 0.016 | |
| Meadow cambisol | ||
| Fresh | 49.9 | 0.050 |
| +20% CH4 | 12.6 | 0.051 |
| 4,560 | 0.027 | |
| Forest luvisol | ||
| Fresh | 29.7 | 0.505 |
| +20% CH4 | 470 | 0.136 |
| 27,900 | 0.025 |
Data are given for fresh soil and soils preincubated 3 weeks under 20% CH4. In preincubated soils, a dual kinetic was evident, with both a high-affinity (second row for each soil) and a low-affinity (third row for each soil) activity.
It is clear from this and other work (3, 8) that the Km(app) for CH4 changes with culture conditions. Nevertheless, our lowest measured Km(app) is still higher than the lowest measured values in soil of about 10 nM (8), so we hesitate to conclude that no true high-affinity MMO exists. Calculations based on maintenance energy requirements suggest that methanotrophic bacteria cannot survive on atmospheric CH4 without a more efficient CH4-oxidizing system (6). However, soil methanotrophs may not consume only atmospheric methane but also alternate substrates, such as methanol (3, 13), or CH4 produced in anaerobic soil microsites (1, 7, 16, 22). Our present results show that the observed high-affinity activity in soil cannot in itself be taken as proof that the responsible bacteria are novel oligotrophic species with a specialized form of MMO.
Acknowledgments
P.F.D. was supported by a stipendium from the Max Planck Society and a grant from the EC RTD Programme Biotechnology.
REFERENCES
- 1.Andersen B L, Bidoglio G, Leip A, Rembges D. A new method to study simultaneous methane oxidation and methane production in soils. Global Biogeochem Cycles. 1998;12:587–594. [Google Scholar]
- 2.Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270. [Google Scholar]
- 3.Benstead J, King G M, Williams H G. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1998;64:1091–1098. doi: 10.1128/aem.64.3.1091-1098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Button D K. Nutrient uptake by microorganisms according to kinetic parameters from theory as related to cytoarchitecture. Microbiol Mol Biol Rev. 1998;62:636–645. doi: 10.1128/mmbr.62.3.636-645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad R. Soil microorganisms oxidizing atmospheric trace gases (CH4, CO, H2, NO) Ind J Microbiol. 2000;39:193–203. [Google Scholar]
- 7.Dunfield P F, Topp E, Archambault C, Knowles R. Effect of nitrogen fertilizers and moisture content on CH4 and N2O fluxes in a humisol: measurements in the field and intact soil cores. Biogeochemistry. 1995;29:199–222. [Google Scholar]
- 8.Dunfield P F, Liesack W, Henckel T, Knowles R, Conrad R. High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl Environ Microbiol. 1999;65:1009–1014. doi: 10.1128/aem.65.3.1009-1014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassner G T, Lippard S J. Component interactions in the soluble methane monooxygenase system from Methylococcus capsulatus (Bath) Biochemistry. 1999;38:12768–12785. doi: 10.1021/bi990841m. [DOI] [PubMed] [Google Scholar]
- 10.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henckel T, Jäckel U, Schnell S, Conrad R. Molecular analyses of novel methanotrophic communities in forest soil that oxidize atmospheric methane. Appl Environ Microbiol. 2000;66:1801–1808. doi: 10.1128/aem.66.5.1801-1808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes A J, Roslev P, McDonald I R, Iversen N, Henriksen K, Murrell J C. Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol. 1999;65:3312–3318. doi: 10.1128/aem.65.8.3312-3318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen S, Priemé A, Bakken L. Methanol improves methane uptake in starved methanotrophic microorganisms. Appl Environ Microbiol. 1998;64:1143–1146. doi: 10.1128/aem.64.3.1143-1146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joergensen L, Degn H. Mass spectrometric measurements of methane and oxygen utilization by methanotrophic bacteria. FEMS Microbiol Lett. 1983;20:331–335. [Google Scholar]
- 15.Lontoh S, Semrau J D. Methane and trichloroethylene degradation by Methylosinus trichosporium OB3b expressing particulate methane monooxygenase. Appl Environ Microbiol. 1998;64:1106–1114. doi: 10.1128/aem.64.3.1106-1114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald J A, Skiba U, Sheppard L J, Hargreaves K J, Smith K A, Fowler D. Soil environmental variables affecting the flux of methane from a range of forest, moorland and agricultural soils. Biogeochemistry. 1996;34:113–132. [Google Scholar]
- 17.Nguyen H T, Elliott S J, Yip J H, Chan S I. The particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a novel copper-containing three-subunit enzyme. J Biol Chem. 1998;273:7957–7966. doi: 10.1074/jbc.273.14.7957. [DOI] [PubMed] [Google Scholar]
- 18.Roslev P, Iversen N. Radioactive fingerprinting of microorganisms that oxidize atmospheric methane in different soils. Appl Environ Microbiol. 1999;65:4064–4070. doi: 10.1128/aem.65.9.4064-4070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segel I H. Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 20.Siegbahn P E M, Crabtree R H, Nordlund P. Mechanism of methane monooxygenase—a structural and quantum chemical perspective. J Biol Inorg Chem. 1998;3:314–317. [Google Scholar]
- 21.Sipkema E M, de Koning W, Ganzeveld K J, Janssen D B, Beenackers A A C M. Experimental pulse technique for the study of microbial kinetics in continuous culture. J Biotechnol. 1998;64:159–176. [Google Scholar]
- 22.Yavitt J B, Fahey T J, Simmons J A. Methane and carbon dioxide dynamics in a northern hardwood ecosystem. Soil Sci Soc Am J. 1995;59:796–804. [Google Scholar]