Abstract
Carbohydrate-active enzymes (CAZymes) are an important characteristic of bacteria in marine systems. We herein describe the CAZymes of Paenibacillus algicola HB172198T, a novel type species isolated from brown algae in Qishui Bay, Hainan, China. The genome of strain HB172198T is a 4,475,055 bp circular chromosome with an average GC content of 51.2%. Analysis of the nucleotide sequences of the predicted genes shows that strain HB172198T encodes 191 CAZymes. Abundant putative enzymes involved in the degradation of polysaccharides were identified, such as alginate lyase, agarase, carrageenase, xanthanase, xylanase, amylases, cellulase, chitinase, fucosidase and glucanase. Four of the putative polysaccharide lyases from families 7, 15 and 38 were involved in alginate degradation. The alginate lyases of strain HB172198T exhibited the maximum activity 152 U/mL at 50 °C and pH 8.0, and were relatively stable at pH 7.0 and temperatures lower than 40 °C. The average degree of polymerization (DP) of the sodium alginate oligosaccharide (AOS) degraded by the partially purified alginate lyases remained around 14.2, and the thin layer chromatography (TCL) analysis indicated that it contained DP2-DP8 oligosaccharides. The complete genome sequence of P. algicola HB172198T will enrich our knowledge of the mechanism of polysaccharide lyase production and provide insights into its potential applications in the degradation of polysaccharides such as alginate.
Keywords: Paenibacillus algicola, genome, polysaccharide lyase, alginate lyase, oligosaccharide
1. Introduction
Complex polysaccharides, including alginate, agar, carrageenan, chitin, cellulose and pectin, etc., are the major components of seaweed cell walls and intercellular spaces, and are generally refractory to degradation [1,2]. Most marine polysaccharides (MPs) are structural components of the cell walls of macroalgae, such as alginate in brown algae (Phaeophyceae) and agar and carrageenan in red algae (Rhodophyceae). Alginate is widely distributed, mainly in brown seaweeds such as Laminaria, Sargassum and Macrocystis [3]. It is a water-soluble and acidic polysaccharide, consisting of α-l-guluronic acid (G) and β-d-mannuronic acid (D) in three different arrangements, such as homopolymeric G (PolyG), homopolymeric M (PolyM), alternating GM or random heteropolymeric G/M stretches (polyMG) [4,5]. Alginate, agar and carrageenan are the three typical polysaccharides of marine origin, and are commercially used as thickening, gelling, texturing and stabilizing agents in food, cosmetics and pharmaceuticals [6,7]. On the other hand, algal oligosaccharides, the enzymatic degradation products of MPs, have wide biological activities and used in different fields, such as industry, agriculture and medicine [8,9,10].
The biodegradation of seaweed is crucial to marine ecology and is a key step in material cycles, especially in the carbon cycle [11]. Polysaccharide-degrading bacteria are key players in the global carbon cycle and algal biomass recycling. Many kinds of MP-degrading marine bacteria have been isolated and revealed as the key players in the algal biomass recycling and global carbon cycle, such as Pseudoalteromonas [7], Alteromonas [12], Agarivorans [13], Paenibacillus [14], Vibrio and Zobellia [15,16]. Members of genus Paenibacillus produce many kinds of extracellular enzymes, e.g., chitosanase [17], glucanase [18], cellulase/mannanase/xylanase [19], xanthanase [20] and chitinase [21], which can be used in a wide range of industrial fields. However, only a few species in this genus have been reported to possess alginate lyases, namely, Paenibacillus sp. LJ-23 from brown algae [22] and Paenibacillus sp. strain MY03 [23], Paenibacillus sp. S29 [24] and Paenibacillus sp. str. FPU-7 [25] from soil.
Alginate lyases can cleave alginate at the hexuronic acid residue sites and release the 4,5-unsaturated hexuronic acid residue at the non-reducing terminus; they attract attention for their broad biotechnological applications, especially in the preparation of biologically active alginate oligosaccharides (AOSs) and the production of biofuels directly from macroalgal biomass [26,27]. According to substrate specificity, alginate lyases are divided into polyM-specific lyases (EC 4.2.2.3), polyG-specific lyases (EC 4.2.2.11) and polyMG-specific lyases (EC 4.2.2), which can degrade polyG, polyM and polyMG blocks of alginate, respectively [28]. Based on the carbohydrate-active enzyme (CAZyme) database (www.cazy.org, accessed on 2 March 2022), alginate lyases are grouped into 14 polysaccharide lyase (PL) families: PL5, PL6, PL7, PL14, PL15, PL17, PL18, and the recently identified families PL31, PL32, PL34, PL36, PL38, PL39 and PL41 [13,21]. CAZyme are the key to promoting carbohydrate catabolism in marine heterotrophic bacteria. Based on genome sequencing and functional annotation, the genomes of several alginate-degrading marine bacteria have been assembled and the CAZyme genes annotated; those such as Zobellia russellii and Z. barbeyronii [16], Flammeovirga pacifica WPAGA1 [1], Microbulbifer strain HZ11 [29] and Paenibacillus sp. str. FPU-7 [21] have been reported.
Previously, we described Paenibacillus algicola HB172198T, a novel species isolated from brown algae in Qishui Bay, Hainan, China, with the capability of producing alginate lyase [14]. In the present study, general characteristics of its complete genome sequence are reported, and the genome annotation revealed that diverse CAZymes could degrade various polysaccharides that are constituents of plant and algal cell wall, not just alginate. In addition, we further analyzed the putative polysaccharide lyases (PLs) that are involved in several polysaccharide degradation processes, and investigated the properties of alginate lyase in culture supernatant.
2. Results
2.1. Screening and Identification of Strain HB172198T
Based on the screening results by agar plate method, strain HB172198T from brown seaweed in Qishui Bay, Hainan, China, showed significant alginate lyase activity. Under the action of 1-M CaCl2, a gelation reaction white halo and white ring was observed on the plate, which indicated that the strain secreted alginate lyases (Figure S1). The type of calcium ion-dependent reactions on the agar plate containing alginate shows the substrate specificity of alginate lyase [30]. Colonies are circular, light yellow and approximately 1 mm in diameter when grown on a marine agar 2216 (Difco Laboratories, Detroit, MI, USA) plate at 30 °C for 48 h. Cells are Gram-stain-variable, facultatively anaerobic, motile rods (1.8–4.8 × 0.5–0.8 μm) with a polar and a lateral flagella (Figure S2). Phylogenetic analysis of 16S rRNA gene sequences (1474 bp, GenBank No. MG994973) indicated that strain HB172198T belonged to the genus Paenibacillus, and the closest phylogenetically related species was Paenibacillus lemnae NBRC 109972T (97.6% similarity). Based on the combined phylogenetic relatedness and phenotypic and genotypic features, strain HB172198T was identified as a novel species of the genus Paenibacillus, for which the name Paenibacillus algicola sp. nov. is proposed. The type strain is HB172198T (=CGMCC 1.13583T = JCM 32683T) [14].
2.2. Genome Specifics
The complete genome of strain HB172198T was determined and one circular chromosome was obtained, with the GenBank/EMBL/DDBJ accession number CP040396. A total of 87,777 reads were analyzed, with an average read length of 21,386 bp, totaling 1.88 Gb, and 418× coverage depth. Strain HB172198T presents a genome of 4,475,055 bp with chromosomal G + C content of 51.2%. A total of 4182 genes were predicted, including 4001 protein-coding genes and 80 tRNA and 27 rRNA sequences. The general features of the HB172198T genome are shown in Table 1 and Figure 1. The gene functions were classified with COG and KEEG databases, and it was shown that a total of 2950 proteins had clear biological functions, 1842 proteins had KEGG homologous genes and 2946 proteins had COG classification. The most common genes in COG annotation are related to the basic functions of bacterial cells. The highest proportion of genes includes carbohydrate transport and metabolism, amino acid transport and metabolism, transcription, cell motility and secretion.
Table 1.
General features of the P. algicola HB172198T genome.
| Category | Number |
|---|---|
| Genome size (bp) | 4,475,055 |
| G+C content (%) | 51.2% |
| Total genes predicted | 4182 |
| Protein-coding genes | 4001 |
| tRNA genes | 80 |
| rRNA genes | 27 |
| 5S rRNA | 9 |
| 16S rRNA | 9 |
| 23S rRNA | 9 |
| ncRNAs | 4 |
| Pseudo genes (total) | 70 |
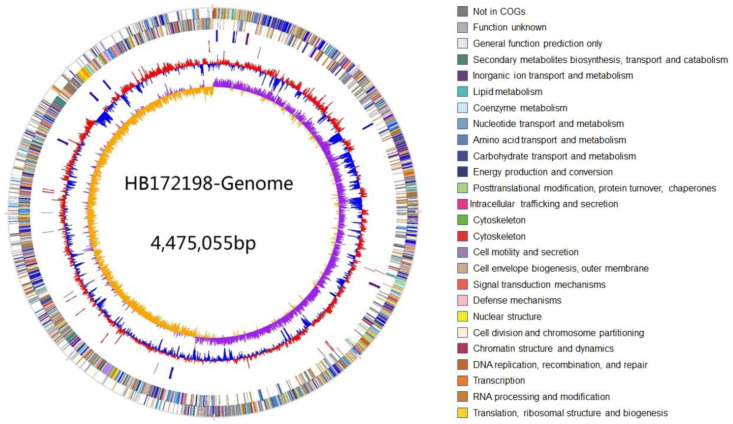
Figure 1.
Graphical map of strain HB172198T genome. From the outside to the center: The outer two circles illustrate predicted coding sequences on the plus and minus strands, respectively, colored by functional categories according to COG classification. The 3rd circle displays tRNA (red) and rRNA (blue). The 4th circle represents mean centered G + C content of the genome (red—above mean; blue—below mean). The 5th circle (innermost) represents GC skew (G − C)/(G + C) calculated using a 2 kb window in steps of 1 kb.
2.3. Genetic Basis of Polysaccharide Degradation
CAZymes are the most important enzymes for polysaccharide degradation. To search for genes related to polysaccharide-degrading enzymes in the genome of P. algicola HB172198T, the carbohydrate-related genes were annotated on the basis of the CAZyme database. Strain HB172198T has 191 CAZymes, including 80 glycoside hydrolases (GHs), 9 polysaccharide lyases (PLs), 53 glycosyl transferases (GTs), 11 carbohydrate esterases (CEs) and 38 carbohydrate-binding modules (CBMs). The PLs are classified into seven families: 7, 8, 12, 15, 31, 38 and 42. The proportion of CAZymes in strain HB172198T is about 4.8%, which is consistent with the statistics of Mann et al. [31]. In general, CAZymes seldom exceed 5% in the genomes of bacteria that specialize in carbohydrate degradation, and typically account for not more than about 2% in most bacterial genomes [32]. The capability of strain HB172198T to degrade various polysaccharides was evident from the annotation of the genome. The genome encodes many kinds of enzymes capable of degrading a diverse range of algal and plant cell wall polysaccharides such as alginate, agar, carrageenan, fucoidan, chitin, xylan, glycosaminoglycan, pullulan, lichenstarch, cellulose, glucan and starch (Table 2). All of these enzymes belong to GHs and PLs.
Table 2.
Diverse genes related to polysaccharide degradation identified in the genome of P. algicola HB172198T.
| Catabolic Enzymes | Enzyme Family | No. of Enymes |
|---|---|---|
| Alginate lyase | PL7 | 1 |
| PL15 | 1 | |
| PL38 | 2 | |
| β-Agarase | GH50 | 1 |
| GH86 | 1 | |
| ι-Carrageenase | GH82 | 1 |
| β-1,4-Endo-glucanase | GH9 | 1 |
| β-Glucosidase | GH3 | 4 |
| α-Amylase | GH2 | 1 |
| GH13 | 3 | |
| GH13|CBM34 | 1 | |
| Pullulanase | CBM48|GH13|CBM41 | 1 |
| CBM41|CBM41|CBM48|GH13|CBM41|GH13 | 1 | |
| Lichenase | GH16 | 1 |
| Endo-1,4-β-xylanase | CBM22|GH10|CBM9 | 1 |
| CBM22|GH10|CBM9|CBM9 | 1 | |
| GH11 | 1 | |
| Xylan 1,4-β-xylosidase | GH43 | 3 |
| GH52 | 1 | |
| α-Glucosidase | GH4 | 1 |
| GH13 | 1 | |
| CBM34|GH13 | 1 | |
| Heparinase | PL12 | 1 |
| Chitinase | GH18 | 1 |
| α-L-fucosidase | GH29 | 1 |
| Glucan endo-1,3-β-D-glucosidase | CBM54|GH16|CBM4|CBM4|CBM4|CBM4 | 1 |
| Glycosaminoglycan polysaccharide lyase | PL8 | 1 |
| α-Galactosidase | GH4 | 1 |
| β-Galactosidase | GH36 | 1 |
| GH2 | 4 | |
| GHnc|CBM66 | 1 | |
| α-L-rhamnosidase | GH78 | 3 |
| α-Mannosidase | GH38 | 2 |
| GH125 | 1 | |
| α-Phosphotrehalase | GH13 | 1 |
| Arabinanase | GH117 | 1 |
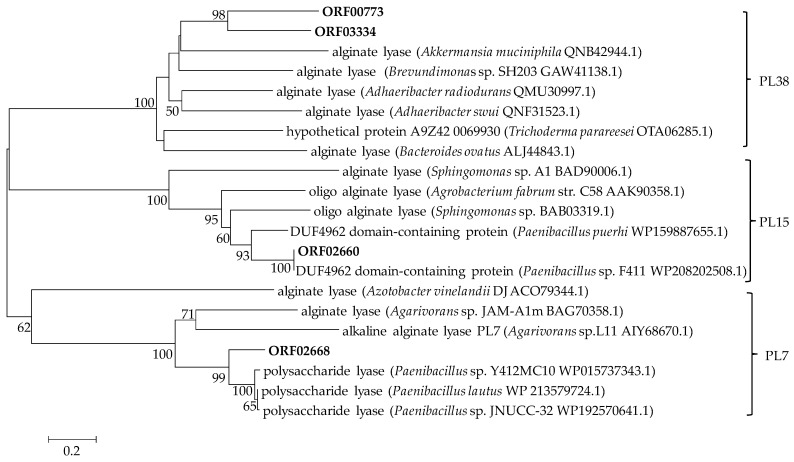
Further analysis revealed that four of the putative PLs, ORF00773 (accession number: QCT01494.1), ORF02660 (PL15, accession number: QCT03372.1), ORF02668 (PL7, accession number: QCT03380.1) and ORF3334 (PL38, accession number: QCT04045.1), were involved in alginate degradation. The open reading frames (ORFs) of the orf02660, orf02668, orf00773 and orf03334 genes consist of 2319, 936, 3042 and 4833 bp nucleotides; encode 772, 312, 1013 and 1611 amino acids; and contain alginate lyase domains of the PL15, 7, 38 and 38 families, respectively (Table S1). Aly38A was the first alginate lyase belonging to the PL38 family obtained from Agarivorans sp. B2Z047 that has been found to degrade alginate [13]. In the CAZy database, a total of 1383 proteins are classified as PL38 family members. Only two of them, CUL-I and TpPL38A, have been characterized as endo-β-1,4-glucuronan lyase, and CUL-I also exhibits alginate lyase activity as well [32,33]. Detailed analysis of the CAZyme information of Paenibacillus species was rarely reported. Paenibacillus sp. strain MY03 from root soil of cypress had the capability of metabolizing polysaccharides of marine algae and animals. Various polysaccharidase genes related to seaweed degradation were found in its genome, including a glucoamylase, a mannanase, an alginate lyase, 3 putative agarases, 4 glucanases and 10 xylanases [23]. However, further analysis based on the dbCAN server (http://cys.bios.niu.edu/dbCAN2, accessed on 8 May 2022) revealed that five of the putative PLs were involved in alginate degradation, namely two PL6, one PL14 and two PL15, which was inconsistent with Liu et al. [23]. No genomic data for other Paenibacillus species with the ability to degrade alginate were found on the NCBI webpage. To further confirm the attribution of the alginate lyases, a phylogenetic tree was constructed according to the amino acid sequences of the four enzymes and other reported alginate lyases. As shown in Figure 2, ORF02660 and ORF02668 were clearly located in the clade with the PL15 and PL7 families, respectively; moreover, ORF00773 and ORF03334 were located in the clade with the PL38 family and formed a distinct branch, which was consistent with the results predicted by the CAZy database.
Figure 2.
Neighbor-joining molecular phylogenetic tree of alginate lyases belonging to PL7, PL15 and PL38 families based on predicted amino acid sequences. Bootstrap values (1000 replicates) are shown as percentages at each node for values. The scale bar represents 0.2 nucleotide substitutions per position. Putative alginate lyases of strain HB172198T are highlighted in bold.
Additionally, two agar-degrading genes belonging to the GH50 and GH86 families were predicted, whose members are known in the CAZy database for β-agarase and porphyrinase activities [34]. Agarose is generally hydrolyzed by GH86 family β-agarase to generate neoagarotetraose and neoagarohexaose, while neoagarooligosaccharide is generally hydrolyzed by GH50 family β-agarase to generate neoagarobiaose [35]. Both of the β-agarases in strain HB172198T would degrade agar to neoagarobiaose. The ι-carrageenase identified in strain HB172198T belongs to the GH82 family. It is reported that all members of the GH82 family demonstrate carrageenase activity against ι-carrageenan.
Furthermore, two cellulase genes were identified in strain HB172198T. One β-1,4-endo-glucanase (GH9 family) and four β-glucosidases (GH3 family) were found in the genome of strain HB172198T. Generally, cellulose hydrolysis is achieved by the synergistic action of endo-glucanase, exo-glucanase and β-glucosidase.
Some bacteria were reported as degraders of xylan and called xylanases. P. algicola HB172198T can also degrade xylan, which is a group of hemicelluloses found in plant cell walls and some algae [36]. Seven putative xylanases exist in strain HB172198T, including three putative endo-1,4-β-xylanases (GH10 and GH11 families), and four putative xylan 1,4-β-xylosidases (GH43 and GH52 families). Two endo-1,4-β-xylanases are grouped with the GH10 family, which are modular enzymes. CBM9 and CBM22 modules were observed and the modular structure of xylanases facilitates the binding of enzymes to substrates.
A total of five α-amylase and two pullulanase genes were identified. One α-amylase is classified in the GH2 family and three in GH13 families, whereas the fifth one is a modular enzyme belonging to the GH13 family that is appended with carbohydrate-binding modules CBM34. The two pullulanases are both modular enzymes belonging to the GH13 family with additional carbohydrate-binding modules CBM48 and CBM41.
Besides the enzymes responsible for degrading these above-mentioned polysaccharides, numerous other saccharide-degrading enzymes were predicted from the P. algicola HB172198T genome, including α-l-fucosidase, lichenase, glucan endo-1,3-β-d-glucosidase, α-glucosidase, α-galactosidase, β-galactosidase, α-l-rhamnosidase, α-mannosidase, arabinanase and α-phosphotrehalase. These masses of CAZymes comprise a complex system for carbohydrate catabolism in strain HB172198T.
2.4. Test of Carbohydrate Utilization
The OD600 of the fermentation broth was measured after 48 h incubation at 180 rpm and 30 °C on an orbital shaker. Cell growth represents that the tested carbohydrates can be utilized by the strain as a carbon source for its growth. Excitingly, all the tested carbon sources could be utilized for the growth of strain HB172198T as the sole carbon source, such as alginate, carrageenan, agar, chitin, starch, cellulose, hemicellulose, xylan, xanthan, sucrose, lactose, maltose, glucose, rhamnose, fructose, xylose and arabinose (Table S2). For the complex polysaccharides, strain HB172198T grew best on alginate (OD600 = 1.152), followed by chitin, carrageenan, xanthan, xylan, starch, agar, hemicellulose and cellulose. Therefore, the results indicate that strain HB172198T harbors powerful enzymes for utilizing various simple and complex carbohydrates, which would enable it to adapt to various environments.
2.5. Enzymatic Properties of Alginate Lyase
Fermentation was carried out using the optimal fermentation medium and condition; the supernatant was obtained by centrifugation, with the alginate lyase activity of 152 U/mL determined by the ultraviolet absorption method. The culture supernatant was precipitated by saturation of ammonium sulfate (80%) to prepare extracellular alginate lyase. The specific activity was increased to 1839 U mg/L from the initial 152 U mg/L, with a yield of 12.1.
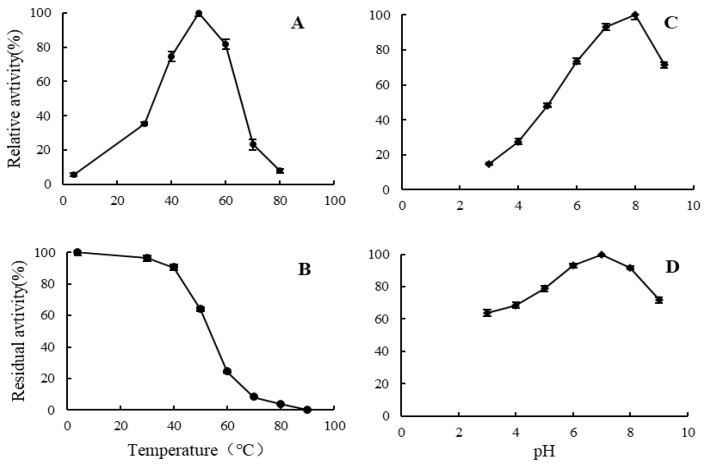
The effects of pH and temperature on the activity of the partially purified alginate lyase were examined. As shown in Figure 3A, the alginate lyase from strain HB172198T exhibited maximum enzymatic activity at 50 °C. Nearly 80% of the highest activity was manifested at the temperature range of 40–60 °C, while almost no detectable activity was observed at 4 °C and 80 °C. The thermostability of alginate lyases was determined at a temperature ranging from 4 to 90 °C (Figure 3B). The alginate lyases were relatively stable at temperatures lower than 40 °C; approximately 100% of the activity was maintained after incubation at less than 40 °C for 1 h. As the temperature rose above 40 °C, the activity declined dramatically; and the vast majority of the activity was lost above 70 °C. As shown in Figure 3C, the activity was the highest at pH 8.0, and above 70% of the maximum activity when the pH value was between 7.0 and 9.0. The activity was the most stable at pH 7.0, above 90% of the activity was retained at pH 6–8 and about 60% of the activity at pH 3.0 and pH 9.0 (Figure 3D). These results indicate that the alginate lyases have good pH stability.
Figure 3.
The biochemical characteristics of alginate lyases. (A) Effect of different temperatures on the activity (4–80 °C). (B) Effect of different temperatures on the stability (4–90 °C). (C) Effect of different pH levels on the activity (pH 3–9). (D) Effect of different pH levels on the stability (pH 3–9). The highest activity was taken as 100%. Data are given as the means ± standard deviation, n = 3.
Results of various metal ions and compounds on alginate lyase showed that the ions (1-mM) of Ca2+, Mg2+, NH4+ and Fe2+ displayed a slightly promoted effect on the enzyme activity, but Zn2+, Mn2+ and urea had weak inhibitory effect. Ba2+ showed some inhibitory effect with 80.3% of the relative activity, and EDTA directly reduced the enzymatic activity to 27.1% of the control group.
The substrate specificity was detected by measuring the increased absorbance at 235 nm of the unsaturated uronic acids that were generated from the oligomers via a β-elimination reaction. According to the results of the substrate specificity assay, the alginate lyases from strain HB172198T exhibited higher activity with polyM than with alginate and polyG (Figure S3). The ability to degrade polyG was only 56.0% of that of alginate, while the ability to degrade polyM was 2.52 times that of alginate. Obviously, the enzymes could act more significantly on polyM than polyG and sodium alginate. This suggested that the enzymes were suitable for the production of mannuronate oligosaccharides from polyM blocks, and the production of oligosaccharides from sodium alginate.
2.6. Enzymatic Degradation of Sodium Alginate
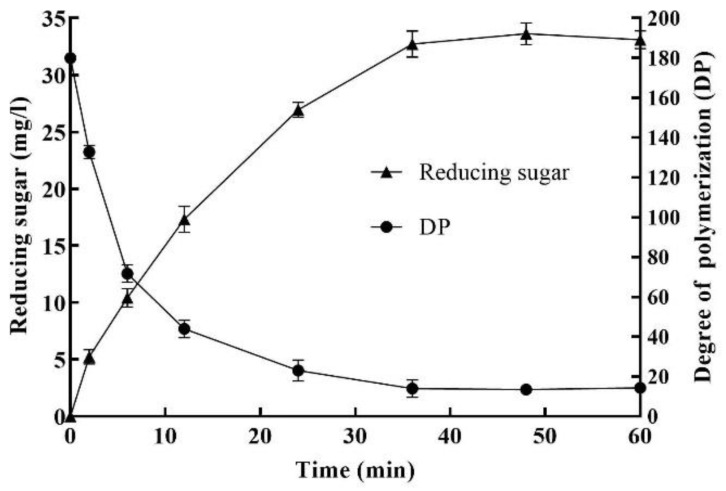
The extent of polysaccharide degradation was determined using the partially purified alginate lyase, the contents of reducing and total sugars were monitored and the average DP of the alginate fragments was calculated. Under the optimized conditions of 1.2% sodium alginate, 18.60 U/mL enzyme, pH 7.0 and 45 °C for 36 h, the yield of reducing sugar and the average DP are shown in Figure 4. The action of the enzyme solution on sodium alginate resulted in the release of reducing sugars, with a 50% increase in reducing sugars during 12 h incubation. With the increase of enzymatic hydrolysis time, the content of reducing sugar increased rapidly, and the average DP of AOS decreased rapidly. After 36 h, both of them were gradually stabilized, the reducing sugar content reached a maximum value of 34.0 mg/L, and the average DP of oligosaccharides reached a minimum value of 14.2. After hydrolysis for 36 h, there was no obvious increase in the yield of reducing sugars, partially because the enzyme lost its activity after being incubated at 45 °C for a long time.
Figure 4.
Effect of enzymolysis time on the enzymatic hydrolysis of sodium alginate under the optimized conditions of 1.2% sodium alginate, 18.60 U/mL enzyme, pH 7.0 and 45 °C.
TLC was used to detect AOS with low DPs. As the hydrolysis process proceeded, AOS with different DPs appeared (Figure S4). Lanes 1-8 mean the digested samples with enzymolysis times of 0, 2, 6, 12, 24, 36, 48 and 60 h, respectively. When incubated for 2 and 6 h, small amounts of low DP oligosaccharides began to appear. When incubated for 24–48 h, AOS with small DP showed higher content, which was consistent with the detection results of reducing sugars. In addition, the oligosaccharide content decreased to some extent after 60 h of incubation. Results showed that the AOS with various low degrees of polymerization (DPs 2–8) were continuous. In general, only oligosaccharides below DP8 can be developed under the TLC conditions employed. There was no monosaccharide in the TLC results, indicating that the four alginate lyases produced by strain HB172198T were all endolytic lyases.
3. Materials and Methods
3.1. Materials and Strains
The polysaccharides of sodium alginate, carrageenan, agar, cellulose, chitin, starch, glucan, hemicellulose, xylan and xanthan were purchased from Sangon (Shanghai, China). PolyM and polyG (purity > 97%) were purchased from Qingdao Haizhida Biotech Co., Ltd. (Qingdao, China). Other chemicals and reagents used in this study were of analytical grade.
3.2. Screening and Identification of Strain HB172198T
Brown seaweed samples were collected from Qishui Bay, Hainan, China (19°38′6″ N, 111°0′21″ E). For the isolation of spore-forming bacteria, the mashed sample was incubated at 80 °C for 15 min to kill any vegetative cells. Then, suspension liquid was serially diluted with sterile saline water and spread on modified 2216E agar (MA; Difco) supplemented with 0.5% sodium alginate. The alginate lyase activity was preliminarily screened with the agar plate method using 1-M calcium chloride as the enzyme-producing indicator. The substrate specificity of alginate lyase can be determined by discriminating between the types of gelation (i.e., halo or ring formation) caused by the interaction between calcium ions and depolymerized alginates [30]. The bacterial isolate HB172198T was picked and identified using a polyphasic approach [14].
3.3. Genome Sequencing and Annotation
Cells of strain HB172198T were cultured overnight in 2216E medium (MB; Difco). DNA was extracted using TIANamp Bacteria DNA Kit (Qiagen, DP302) following the manufacturer’s protocol. The quality and size of genomic DNA were determined by 0.8% agarose gel electrophoresis, NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA) and Qubit version 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA). A high-quality genome sequence of strain HB172198T was obtained using the PacBio RSII system (Pacific Biosciences, Menlo Park, CA, USA) and Illumina X10 (San Diego, CA, USA) at the Chinese National Human Genome Center (Shanghai, China). The 10 kb library of inserts was constructed by using DNA Template Prep Kit 4.0 and sequenced on the Pacbio RSII system (Pacific Biosciences, Menlo Park, CA, USA). The pair-end library of the 300 bp insert was constructed by using the TruSeqTM DNA Sample Prep Kit-Set A and sequenced on Illumina X10 (Illumina, San Diego, CA, USA). The clean data from Illumina sequencing were corrected for the assembly of PacBio by HGAP v. 23 to generate one contig without gaps. Protein-coding sequences were predicted with Glimmer version 3.02 software [37]; transfer RNA (tRNA) and ribosomal RNA (rRNA) were predicted with tRNAScan [38] and RNAmmer [39]. Functional annotation of the predicted protein-coding genes was performed against the non-redundant protein (NR) database and the GO [40], COG [41], KEGG [42] databases, respectively. The CAZymes and carbohydrate-binding modules were predicted using the BLASTP and CAZy database (http://www.cazy.org/, accessed on 25 February 2022) [34]. The signal peptide was predicted using the SingalP server (https://services.healthtech.dtu.dk/service.php?SignalP-5.0, accessed on 26 February 2022) [43]. The theoretical isoelectronic point (pI) and molecular weight (Mw) were predicted online (http://web.expasy.org/protparam/, accessed on 26 February 2022). The protein domain prediction was performed with the Simple Modular Architecture Research Tool (SMART) (http://web.expasy.org/protparam/, accessed on 26 February 2022). The neighbor-joining phylogenetic tree was generated based on the reported alginate lyases using MEGA version 7.0 [44].
3.4. Utilizing Abilities of Carbohydrate
The alginate-degrading strain HB172198T, which was classified into the genus Paenibacillus, was further investigated regarding its ability to utilize a range of different carbohydrates as sole carbon source in a marine minimal medium (MMM; w/v: 0.5% (NH4)2SO4, 0.2% K2HPO4, 2% NaCl, 0.1% MgSO4·7H2O, 0.001% FeSO4·7H2O, pH 7.5), containing 0.5% of each mono-, oligo- or polysaccharide substrate. The following carbohydrates were used as sole carbon source: polysaccharides such as sodium alginate, carrageenan, agar, colloidal chitin, starch, cellulose, hemicellulose, xylan and xanthan; disaccharides such as sucrose, lactose and maltose; monosaccharides such as glucose, rhamnose, fructose, xylose and arabinose. After incubating for 48 h on an orbital shaker at 180 rpm and 30 °C, the OD600 was detected to determine whether the strain could utilize the tested carbohydrate as the carbon source for its growth.
3.5. Detection of Alginate Lyase Activity and Enzymatic Properties
Strain HB172198T was propagated at 30 °C and 180 rpm using the optimized liquid medium, which contained: sodium alginate 7.50 g/L, tryptone 13.57 g/L, NaCl 29.75 g/L, MgSO4·7H2O 0.08 g/L, pH 7.0. After 36 h of incubation, cells were removed by centrifugation at 10,000 rpm, 4 °C for 15 min. The supernatant was taken as the crude enzyme solution to detect alginate lyase activity with the ultraviolet absorption method [45]. One unit of enzyme activity was defined as an increase of 0.01 in absorbance per min at 235 nm.
In the following, the operating temperature of alginate lyase was maintained at 4 °C unless otherwise stated. The cell-free supernatant was precipitated by 80% saturation of ammonium sulfate and kept overnight. The precipitated protein was collected by centrifugation (10,000 rpm, 30 min) and dissolved in 0.05 M phosphate-citrate buffer at pH 7.0. This enzyme solution was dialyzed in a dialysis bag (MWCO: 12,000–14,000 Da) against the same buffer four times, changing the dialysis buffer every six hours. The dialyzed supernatant was used as a partially purified alginate lyase for the enzymatic property.
To determine the optimal temperature, the enzyme activity was measured at various temperatures (4 °C, 30–80 °C at 10 °C increments) and pH 7.0. To test thermal stability, the enzyme was preincubated at various temperatures and pH 7.0 for 1 h. To determine the optimal pH, the enzyme activity was measured at various pHs using citrate-phosphate (pH 3.0–7.0) and Tris-HCl (pH 8.0–9.0) buffers at 40 °C. To test pH stability, the enzyme was preincubated at various pHs (pH 3.0–9.0, with increments of 1) at 4 °C for 24 h. The highest activity was taken as 100%. To determine the metal ions and compounds, the enzyme activity was measured at 1-mM KCl, CaCl2, NH4Cl, FeSO4, MgCl2, ZnSO4, BaCl2, MnSO4, urea and ethylenediamine tetraacetic acid (EDTA), respectively. The enzyme activity without the treatment or addition of extra substances was defined as 100%. All reactions were performed in triplicate. After each treatment, the enzyme activity was estimated by measuring the absorbance at 235 nm.
The enzyme activity assays of sodium alginate, polyM and polyG were defined for investigating the substrate specificity. The amount of yielded unsaturated uronic acid was monitored by recording the absorbance of the reaction mixture at 235 nm, using sodium alginate as the reference (100%) [46].
3.6. Preparation and Detection of the Enzymatic Degradation of Alginate
To elucidate the effect of the enzymes concerning polysaccharide, alginate degradation was performed with 12 g/L sodium alginate in 50 mM phosphate buffer (pH 7.0) as a substrate. The experiments were carried out under the optimized conditions of 1.2% sodium alginate, 18.60 U/mL enzyme, pH 7.0 and 45 °C. At intervals (0, 2, 6, 12, 24, 36, 48 and 60 h), aliquot samples (10 mL) were taken, boiled for 10 min to denature the enzymes and the polysaccharides were precipitated overnight with three times the volume of ethanol. After centrifugation at 10,000 rpm for 15 min, the supernatant was taken, lyophilized and then redissolved in distilled water to reduce sugar, total sugar and TLC testing. The content of reducing sugar was determined by using 3,5-dinitrosalicylic acid (DNS) colorimetry [47]. The content of total carbohydrate was determined with the phenol-sulfuric acid method described by Doubois et al. [48]. The average DP of the alginate fragments was calculated by dividing the total sugar content by the reducing sugar content. To determine the enzymatic depolymerization pattern of sodium alginate, AOSs were also measured with the TLC method on a silica gel high-performance TLC plate (Merck, Germany) with the solvent system (1-butanol/formic acid/water 4:5:1) and visualized by heating at 110 °C for 5 min after spraying with 10% (v/v) sulfuric acid in ethanol [49]. The guluronic acid sodium salt monomers, trimers and pentamers (1 mg/mL) (Qingdao Bozhi Huili Biotech, Qingdao, China) were used as standards.
4. Conclusions
In this work, the genome of a novel alginate lyase-producing marine bacterium, designated Paenibacillus algicola HB172198T, was sequenced. The assembled fine genome contains 4,475,055 bp with G + C content of 51.2%. Among 4182 genes, 4001 protein-coding genes and 80 tRNA and 27 rRNA sequences were predicted. Analysis of nucleotide sequence of predicted gene using the CAZymes Analysis Toolkit indicated that strain HB172198T encodes 191 CAZymes, including 80 glycoside hydrolases, 11 carbohydrate esterases, 9 polysaccharide lyases and 38 carbohydrate-binding modules. In addition, abundant putative enzymes involved in degrading polysaccharide were found, including alginate lyases, agarase, carrageenase, cellulase, xylanases, amylase, pullulanase, chitinase, xanthanase, fucosidase, lichenase, glucanase, etc. The crude extracellular alginate lyase activity of strain HB172198T reached 152 U/mL using the optimized liquid medium at 30 °C and 180 rpm for 36 h. The average DP of oligosaccharide-degrading sodium alginate was maintained at about 14.2, and the oligosaccharide components of DP2-DP8 were present as well. Our results show that Paenibacillus algicola HB172198T is therefore a source of potential MP-degrading biocatalysts for biorefinery applications and oligosaccharide preparation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20060388/s1: Figure S1: Gelation reactions of strain HB172198T observed on the plate covered by the CaCl2 solution; Figure S2: Transmission electron micrograph of cells of strain HB172198T from a 2-day-old culture on MA. Bar, 2 µm; Figure S3: Substrate specificity of the alginate lyases from strain HB172198T; Figure S4: TLC detection results of AOS; Table S1: Characteristics of the alginate lyases identified in the genome of strain HB172198T; Table S2: The ability of carbohydrates-utilization by Strain HB172198T.
Author Contributions
Conceptualization, Y.H. and S.B.; Data curation, Z.Z., X.Z. and R.G.; Formal analysis, H.H., Z.Z. and Z.W.; Funding acquisition, H.H. and Y.H.; Investigation, R.G. and S.B.; Methodology, Z.Z., Z.W. and J.Z.; Project administration, H.H., Y.H. and S.B.; Software, H.H.; Supervision, Y.H. and S.B.; Writing—original draft, H.H. and Z.Z.; Writing—review & editing, H.H. and X.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by grants from the Key Research and Development Project of Hainan Province (ZDYF2020182), the Financial Fund of the Ministry of Agriculture and Rural Affairs of China (NFZX2021, NHYYSWZZZYKZX2020), and Central Public-interest Scientific Institution Basal Research Fund from Chinese Government (1630052019014).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gao B.L., Jin M., Li L., Qu W., Zeng R.Y. Genome sequencing reveals the complex polysaccharide-degrading ability of novel deep-sea bacterium Flammeovirga pacifica WPAGA1. Front. Microbiol. 2017;8:600–614. doi: 10.3389/fmicb.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martone P.T., Estevez J.M., Lu F., Ruel K., Denny M.W., Somerville C., Ralph J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009;19:169–175. doi: 10.1016/j.cub.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Wang M.P., Chen L., Zhang Z.J., Wang X.J., Qin S., Yan P.S. Screening of alginate lyase-excreting microorganisms from the surface of brown algae. AMB Express. 2017;7:74–82. doi: 10.1186/s13568-017-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong T., Preston L., Schiller N. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000;54:289–340. doi: 10.1146/annurev.micro.54.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhein-Knudsen N., Meyer A.S. Chemistry, gelation, and enzymatic modification of seaweed food hydrocolloids. Trends Food Sci. Technol. 2021;109:608–621. doi: 10.1016/j.tifs.2021.01.052. [DOI] [Google Scholar]
- 7.Chen X.L., Dong S., Xu F., Dong F., Li P.Y., Zhang X.Y., Zhou B.C., Zhang Y.Z., Xie B.B. Characterization of a new cold-adapted and salt-activated polysaccharide lyase family 7 alginate lyase from Pseudoalteromonas sp. SM0524. Front. Microbiol. 2016;7:1120–1128. doi: 10.3389/fmicb.2016.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y.H., Yin H.Y., Zhao X.M., Wang W.X., Du Y.G., He A.L., Sun K.G. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014;113:446–454. doi: 10.1016/j.carbpol.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 9.Yang J.H., Bang M.A., Jang C.H., Jo G.H., Jung S.K., Ki S.H. Alginate oligosaccharide enhances LDL uptake via regulation of LDLR and PCSK9 expression. J. Nutr. Biochem. 2015;26:1393–1400. doi: 10.1016/j.jnutbio.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Ghadam P., Akhlaghi F., Ali A.A. One-step purification and characterization of alginate lyase from a clinical Pseudomonas aeruginosa with destructive activity on bacterial biofilm. Iran J. Basic Med. Sci. 2017;20:467–473. doi: 10.22038/IJBMS.2017.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal-Melgosa S., Lagatora M., Sichert A., Priest T., Pätzold J., Hehemann J.H. Not digested: Algal glycans move carbon dioxide into the deep-sea. bioRxiv. 2022 doi: 10.1101/2022.03.04.483023. [DOI] [Google Scholar]
- 12.Neumann A.M., Balmonte J.P., Berger M., Giebel H.A., Arnosti C., Voget S., Simon M., Brinkhoff T., Wietz M. Different utilization of alginate and other algal polysaccharides by marine Alteromonas macleodii ecotypes. Environ. Microbiol. 2015;17:3857–3868. doi: 10.1111/1462-2920.12862. [DOI] [PubMed] [Google Scholar]
- 13.Sun X.K., Gong Y., Shang D.D., Liu B.T., Du Z.J., Chen G.J. Degradation of alginate by a newly isolated marine bacterium Agarivorans sp. B2Z047. Mar. Drug. 2022;20:254. doi: 10.3390/md20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J., Mo K.L., Zheng Z.G., Wang Z.X., Hu Y.H., Zou X.X., Gu H.J., Wang H.Y., Bao S.X., Huang H.Q. Paenibacillus algicola sp. nov., a novel marine bacterium producing alginate lyase. Int. J. Syst. Evol. Microbiol. 2020;70:5087–5092. doi: 10.1099/ijsem.0.004385. [DOI] [PubMed] [Google Scholar]
- 15.Martin M., Barbeyron T., Martin R., Portetelle D., Michel G., Vandenbol M. The cultivable surface microbiota of the brown alga Ascophyllum nodosum is enriched in macroalgal-polysaccharide-degrading bacteria. Front. Microbiol. 2015;24:1487–1500. doi: 10.3389/fmicb.2015.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chernysheva N., Bystritskaya E., Likhatskaya G., Nedashkovskaya O., Isaeva M. Genome-wide analysis of PL7 alginate lyases in the genus Zobellia. Molecules. 2021;26:2387. doi: 10.3390/molecules26082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S.N., Wagenknecht M., Nareddy P.K., Bhuvanachandra B., Niddana R., Balamurugan R., Swamy M.J., Moerschbacher B.M., Podile A.R. Amino groups of chitosan are crucial for binding to a family 32 carbohydrate binding module of a chitosanase from Paenibacillus elgii. J. Biol. Chem. 2016;36:18977–18990. doi: 10.1074/jbc.M116.721332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong M.J., Yang Y.J., Tang X.H., Shen J.D., Xu B., Li J.J., Wu Q., Zhou J.P., Ding J.M., Han N.Y., et al. NaCl-, protease-tolerant and cold-active endoglucanase from Paenibacillus sp. YD236 isolated from the feces of Bos frontalis. Springerplus. 2016;5:746–757. doi: 10.1186/s40064-016-2360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phakeenuya V., Ratanakhanokchai K., Kosugi A., Tachaapaikoon C. A novel multifunctional GH9 enzyme from Paenibacillus curdlanolyticus B-6 exhibiting endo/exo functions of cellulase, mannanase and xylanase activities. Appl. Microbiol. Biotechnol. 2020;104:2079–2096. doi: 10.1007/s00253-020-10388-3. [DOI] [PubMed] [Google Scholar]
- 20.Ashraf S., Soudi M.R., Ghadam P. Production of xanthanases by Paenibacillus spp.: Complete xanthan segradation and possible applications. Iran J. Biotechnol. 2017;15:120–127. doi: 10.15171/ijb.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh T. Structures and functions of carbohydrate-active enzymes of chitinolytic bacteria Paenibacillus sp. str. FPU-7. Biosci. Biotechnol. Biochem. 2021;85:1314–1323. doi: 10.1093/bbb/zbab058. [DOI] [PubMed] [Google Scholar]
- 22.Wang M.P., Chen L., Lou Z.Y., Yuan X.T., Pan G.P., Ren X.Y., Wang P.Y. Cloning and characterization of a novel alginate lyase from Paenibacillus sp. LJ-23. Mar. Drugs. 2022;20:66. doi: 10.3390/md20010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H.H., Cheng Y.Y., Gu J.Y., Wang Y.H., Li J.G., Li F.C., Han W.J. Draft genome sequence of Paenibacillus sp. strain MY03, a terrestrial bacterium capable of degrading multiple marine-derived polysaccharides. Genome Announc. 2017;5:e00678-17. doi: 10.1128/genomeA.00678-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurakake M., Kitagawa Y.H., Okazaki A., Shimizu K. Enzymatic properties of alginate lyase from Paenibacillus sp. S29. Appl. Biochem. Biotechnol. 2010;183:1455–1464. doi: 10.1007/s12010-017-2513-5. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T., Nakagawa E., Yoda M., Nakaichi A., Hibi T., Kimoto H. Structural and biochemical characterisation of a novel alginate lyase from Paenibacillus sp. str. FPU-7. Sci. Rep. 2019;9:14870. doi: 10.1038/s41598-019-51006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang Y.Z., Zhao X., Liu L.L., Yu G.L. Applications of mass spectrometry to structural analysis of marine oligosaccharides. Mar. Drugs. 2014;12:4005. doi: 10.3390/md12074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng D., Jiang C., Xu J., Liu Z., Mao X. Characteristics and applications of alginate lyases. Int. J. Biol. Macromol. 2020;164:1304–1320. doi: 10.1016/j.ijbiomac.2020.07.199. [DOI] [PubMed] [Google Scholar]
- 28.Kam N., Park Y.J., Lee E.Y., Kim H.S. Molecular identification of a polyM-specific alginate lyase from Pseudomonas sp. strain KS-408 for degradation of glycosidic linkages between two mannuronates or mannuronate and guluronate in alginate. Can. J. Microbiol. 2011;57:1032–1041. doi: 10.1139/w11-106. [DOI] [PubMed] [Google Scholar]
- 29.Sun C., Chen Y.J., Zhang X.Q., Pan J., Cheng H., Wu M. Draft genome sequence of Microbulbifer elongatus strain HZ11, a brown seaweed-degrading bacterium with potential ability to produce bioethanol from alginate. Mar. Genomics. 2014;18:83–85. doi: 10.1016/j.margen.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Hisano T., Nishimura M., Yamashita T., Imanaka T., Muramatsu T., Kimura A., Murata K. A simple method for determination of substrate specificity of alginate lyases. J. Fermen. Bioeng. 1994;78:182–184. doi: 10.1016/0922-338X(94)90261-5. [DOI] [Google Scholar]
- 31.Mann A.J., Hahnke R.L., Huang S.X., Werner J., Xing P., Barbeyron T., Huettel B., Stuber K., Reinhardt R., Harder J., et al. The genome of the alga-associated marine flavobacterium Formosa agariphila KMM 3901T reveals a broad potential for degradation of algal polysaccharides. Appl. Environ. Microbiol. 2013;79:6813–6822. doi: 10.1128/AEM.01937-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi M., Konno N., Suzuki T., Fujii Y., Kodama Y., Isogai A., Habu N. A bacterial endo-β-1,4-glucuronan lyase, CUL-I from Brevundimonas sp. SH203, belonging to a novel polysaccharide lyase family. Protein Expr. Purif. 2020;166:105502–105506. doi: 10.1016/j.pep.2019.105502. [DOI] [PubMed] [Google Scholar]
- 33.Pilgaard B., Vuillemin M., Munk L., Holck J., Meier S., Wilkens C., Meyer A.S. Discovery of a novel glucuronan lyase system in Trichoderma parareesei. Appl. Environ. Microbiol. 2022;88:e01819-21. doi: 10.1128/AEM.01819-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drula E., Garron M.L., Dogan S., Lombard V., Henrissat B., Terrapon N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022;50:D571–D577. doi: 10.1093/nar/gkab1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi W.J., Chang Y.K., Hong S.K. Agar degradation by microorganisms and agar-degrading exzymes. Appl. Microbiol. Biotechnol. 2012;94:917–930. doi: 10.1007/s00253-012-4023-2. [DOI] [PubMed] [Google Scholar]
- 36.Ebringerová A., Heinze T. Xylan and xylan derivatives-biopolymers with valuable properties, 1. Naturally occurring xylans structures, isolation procedures and properties. Macromol. Rapid Commun. 2000;21:542–556. doi: 10.1002/1521-3927(20000601)21:9<542::AID-MARC542>3.0.CO;2-7. [DOI] [Google Scholar]
- 37.Delcher A.L., Bratke K.A., Powers E.C., Salzberg S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowe T.M., Eddy S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagesen K., Hallin P., Rødland E.A., Staerfeldt H.H., Rognes T., Ussery D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatusov R.L., Natale D.A., Garkavtsev I.V., Tatusova T.A., Shankavaram U.T., Rao B.S., Kiryutin B., Galperin M.Y., Fedorova N.D., Koonin E.V. The COG database: New developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29:22–28. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanehisa M., Goto S., Hattori M., Aoki-Kinoshita K.F., Itoh M., Kawashima S., Katayama T., Araki M., Hirakawa M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006;34:354–357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen H. Predicting secretory proteins with SignalP. Methods Mol. Biol. 2017;1611:59–73. doi: 10.1007/978-1-4939-7015-5_6. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budi S.W., van Tuinen D., Arnould C., Dumas-Gaudot E., Gianinazzi-Pearson V., Gianinazzi S. Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil-borne pathogenic fungi. Appl. Soil Ecol. 2000;15:191–199. doi: 10.1016/S0929-1393(00)00095-0. [DOI] [Google Scholar]
- 46.Huang H.Q., Li S., Bao S.X., Mo K.L., Sun D.M., Hu Y.H. Expression and characterization of a cold-adapted alginate lyase with exo/endo-type activity from a novel marine bacterium Alteromonas portus HB161718T. Mar. Drugs. 2021;19:155. doi: 10.3390/md19030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller G.L. Use of dinitrosalicylic acid for determina tion of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 48.Albalasmeh A.A., Berhe A.A., Ghezzehei T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013;97:253–261. doi: 10.1016/j.carbpol.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 49.Kim D.E., Lee E.Y., Kim H.S. Cloning and characterization of alginate lyase from a marine bacterium Streptomyces sp. ALG-5. Marine Biotechnol. 2009;11:10–16. doi: 10.1007/s10126-008-9114-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.