Abstract
The present study was conducted with an intent to evaluate the protective effect of butanolic fraction of Delphinium brunonianum on fructose mediated metabolic abnormalities in rats. Rats in all groups except control group were fed on 10% fructose for 6 weeks; however, rats in the treated group also received butanolic fraction for the last 3 weeks, along with the fructose. Moreover, phytoconstituents present in butanolic fraction were analyzed using LC-MS. All doses of butanolic fraction profoundly reduce the fructose-induced blood pressure, sympathetic over-activity, and weight gain. Furthermore, butanolic fraction prominently reduces the glucose intolerance and hyperinsulinemia in fructose-fed rats. On treatment with butanolic fraction, oxidative enzymes and the functionality of the aorta was also restored. Phytochemical analysis revealed the presence of several active constituents including bergenin, scopolin, rutinoside, kaempferol, coumaric acid, apigenin, and gingerol. In conclusion, butanolic fraction of Delphinium brunonianum has the potential to prevent and recover the fructose-induced metabolic perturbations.
Keywords: metabolic syndrome, oxidative stress, sympathetic over-activity, insulin resistance, LC-MS analysis
1. Introduction
Metabolic syndrome is characterized by a cluster of metabolic disorders including hypertension, hyperinsulinemia, dyslipidemia, and obesity. Metabolic syndrome is triggered by several genetic and environmental factors; however, the etiology of this syndrome is still unknown [1]. Currently, metabolic syndrome is a global health issue as each factor predisposes individuals to cardiovascular diseases and diabetes [2]. The use of herbal medicine is as old as the existence of man. Current appraises have propounded that, in many emerging countries, approximately 70–80% of the population reckons laboriously on medicinal plants in their vicinity to encounter their basic health care needs [3]. The literature showed that approximately four billion people around the world are using THM (traditional herbal medicine) for their healthcare needs [4]. In the contemporary world, the use of medicinal plants is of enormous public interest due to the increasing cost of allopathic drugs and associated ADRs. Furthermore, aboriginal plants serve as a source for the discovery of new drugs by providing templates for designing new drugs [5]. Several active constituents have been identified, isolated from medicinal plants, and are being used as a successful treatment option [6]. Delphinium brunonianum is distributed at the altitude range of 3500 to 6000 m in Pamir, Afghanistan, the Himalayan region, and south-east Tibet. Empirically, this species is being used for the treatment of several anomalies, including fever, headache, stomach ache, and for the purpose of blood purification [7,8]. Phytochemical investigation on this species has shown it to contain many valuable phytoconstituents [8,9]. Earlier, we reported the diuretic effect of Delphinium brunonianum, and crude extract has also been assessed for its effect on fructose-mediated hypertension and metabolic alterations [10,11]. Taking this into account, we further extended this study and evaluated the effects of butanolic fraction of Delphinium brunonianum on fructose-mediated perturbations. As we performed these two experiments at the same time in the same laboratory conditions, the results of the fraction-treated groups are compared with the same control group used for the evaluation of crude extract of Delphinium brunonianum [11].
2. Results
2.1. DB-B-Caused Decrease in Blood Pressure in Fructose-Fed Hypertensive Rats
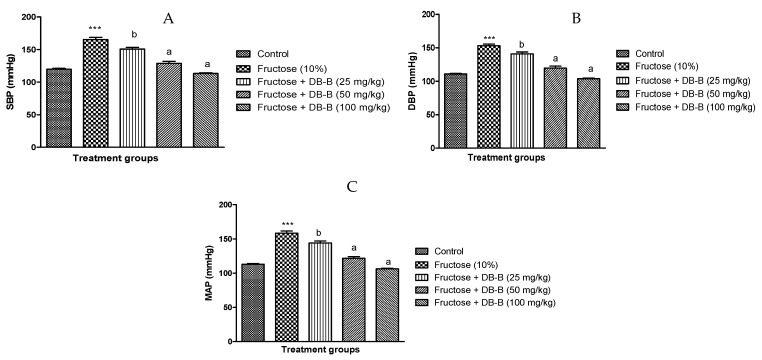
In the disease control group, elevation in blood pressure occurs in rats as compared with animals in control group. After treatment, the rats showed remarkable (p < 0.01–0.001) decreases in systolic, diastolic, and mean arterial pressure compared with the fructose-treated group, as shown in Figure 1.
Figure 1.
DB-B-mediated fall in blood pressure of fructose-fed hypertensive rats: (A) SBP (systolic blood pressure), (B) DBP (diastolic blood pressure), (C) MAP (mean arterial pressure). Results are expressed as mean ± SEM (n = 5). One-way analysis of variance was applied. *** = p < 0.001 when compared with the control group, whereas a = p < 0.001 and b = p < 0.01 in relation to the fructose (10%) group.
2.2. DB-B-Induced Decrease in Sympathetic Over-Activity in Fructose-Fed Hypertensive Rats
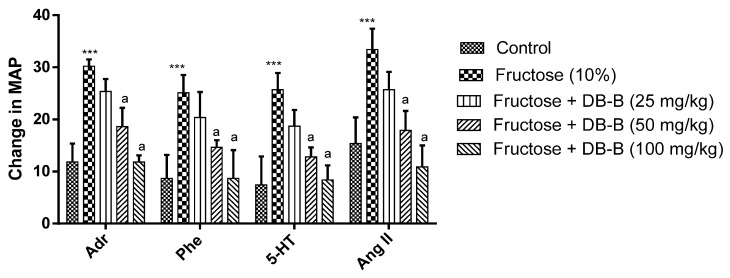
Adrenalin, phenylephrine, serotonin, and angiotensin II significantly increased the mean arterial pressure (MAP) in fructose hypertensive rats in comparison with the control group. Interestingly, butanolic fraction (50, 100 mg/kg) (p < 0.01–0.001) attenuate the increase in MAP by Adr, 5-HT, Phe, and Ang II (Figure 2).
Figure 2.
DB-B-evoked decrease in sympathetic activity in fructose hypertensive rats. All results were analyzed statistically using two-way ANOVA followed by the Bonferrani test using GraphPad Prism. Data is presented as mean ± SEM and *** p < 0.001 in relation to the control group, whereas a = p < 0.001 in comparison with the fructose-treated group.
2.3. DB-B-Prevented Increase in Body Weight in Fructose-Fed Rats
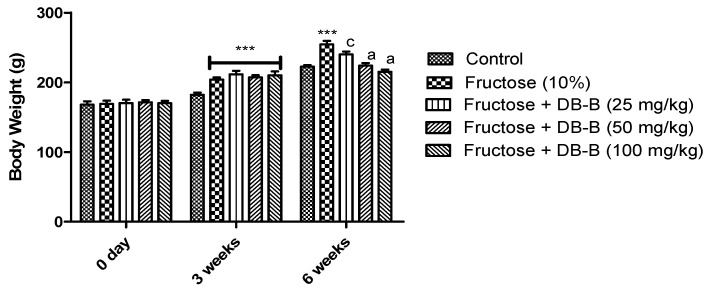
Chronic administration of fructose (10%) increased the body weight of the disease control group. The treatment showed a reduction in body weight as compared with the disease control group (Figure 3).
Figure 3.
Butanolic fraction of D. brunonianum caused a decrease in the body weight of fructose hypertensive anesthetized rats. Data was analyzed statistically using two-way ANOVA followed by the Bonferrani test. Results are presented as mean ± SEM (n = 5) where *** = p < 0.001 compared with the control, while a = p < 0.001 and c = p < 0.05 compared with fructose (10%).
2.4. DB-B-Mediated Alteration in Metabolic Abnormalities Triggered by Fructose
2.4.1. Reversal of Fructose-Elicited Dyslipidemia in Rats
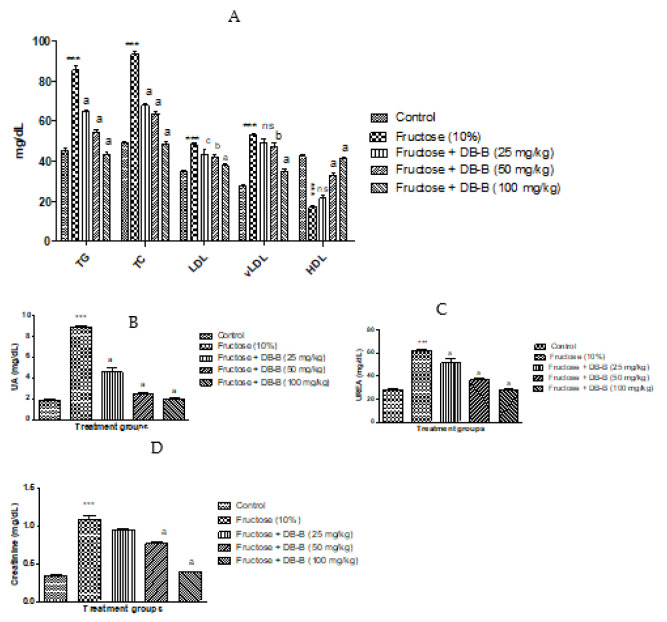
The findings of the present study revealed that there was an increase in the lipid profile of fructose-fed rats compared with the control group, while the level of HDL was significantly decreased in these rats. The increased level of triglyceride was significantly (p < 0.001) lowered by oral treatment with DB-B (25, 50, 100 mg/kg). The magnitude of total cholesterol was significantly (p < 0.01–0.001) attenuated by all doses of DB-B. Similarly, DB-B induced a profound (p < 0.05–0.001) decrease in LDL and vLDL. All doses of DB-B exerted a marked increase in the serum level of HDL. A total of 25 mg/kg of DB-B induced a slight modification in the level of vLDL and HDL, but that was statistically non-significant (Figure 4A).
Figure 4.
Amelioration of fructose-linked metabolic disturbances by oral administration of DB-B: (A) alteration in lipid profile, (B) decrease in level of uric acid, (C) level of urea, (D) decrease in level of creatinine. Each point is presented as mean ± SEM of five values where *** = p < 0.001 as compared with the control, while a = p < 0.001, b = p < 0.01, c = p < 0.05, and ns = non-significant in comparison with the results of the fructose (10%) group. Here, UA: uric acid, DB-B: butanolic fraction of D. brunonianum, TG: triglyceride, TC: total cholesterol, LDL: low-density lipoprotein, vLDL: very low-density lipoprotein, and HDL: high-density lipoprotein.
2.4.2. Decrease in Level of Uric Acid, Urea, and Creatinine in the Blood of Fructose-Fed Hypertensive Rats
Current findings presented the significant rise in the uric acid level in the blood of fructose-fed rats while DB-B (25, 50, 100 mg/kg) caused a remarkable decrease (p < 0.01–0.001) in the level of uric acid as shown in Figure 4B. As shown in Figure 4C, the level of urea in the blood of rats in the control was 27.906 ± 0.815 mg/dL, which was significantly increased after the chronic administration of fructose for a period of 6 weeks that was 61.796 ± 0.670 mg/dL. Treatment of DB-B caused a prominent (p < 0.05–0.001) decrease in the level of urea. A similar dose-dependent fall in the creatinine level was observed in fructose hypertensive rats after the oral administration of DB-B (Figure 4D).
2.4.3. Reversal of Glucose Intolerance and Hyperinsulinemia
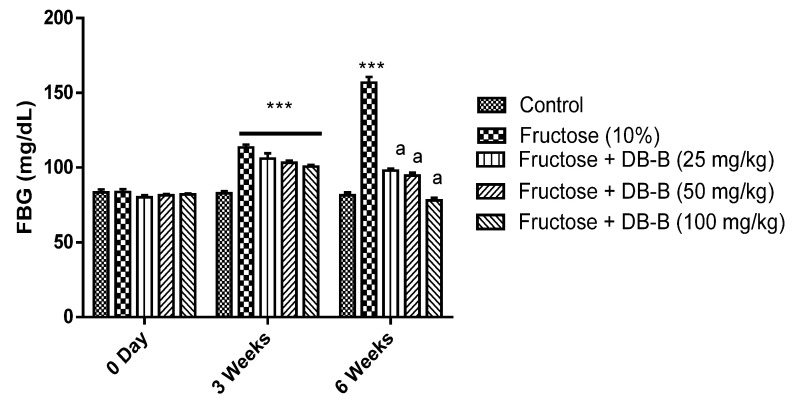
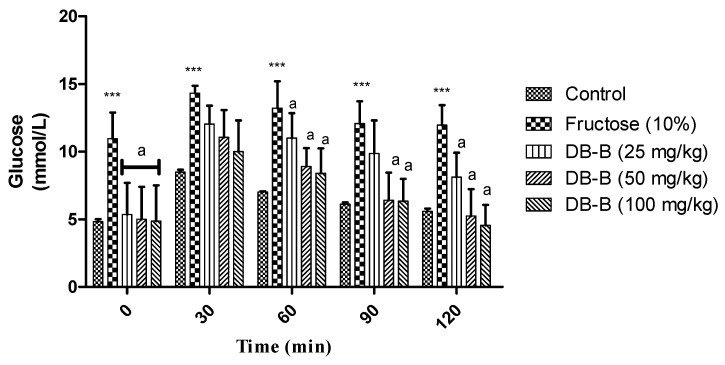
In fructose-fed rats, an increase in fasting blood glucose was recorded (113.4 ± 1.887 and 155 ± 5.0 mg/dl, respectively). However, the treatment with DB-B showed a significant (p < 0.05–0.001) reduction in the level of FBG (Figure 5). DB-B exerted the significant decline in blood-glucose intolerance (Figure 6).
Figure 5.
D. brunonianum reversed the fructose-induced rise in the level of blood glucose. Each bar is presented as mean ± SEM of five values where *** = p < 0.001 as compared with the control, while a = p < 0.001, in comparison with the results of the fructose (10%) group.
Figure 6.
DB-B induced a decrease in glucose intolerance in fructose-fed hypertensive rats. Data was analyzed statistically using two-way ANOVA followed by the Bonferrani test. Results are expressed as mean ± SEM (n = 5) where a = p < 0.001 with respect to fructose (10%), whereas *** = p < 0.001 with respect to the control.
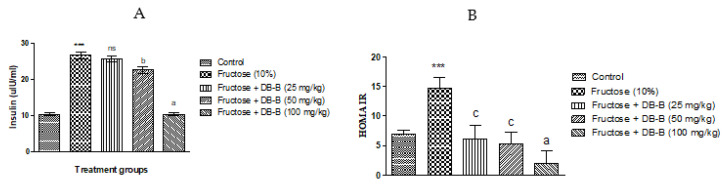
Furthermore, DB-B in doses of 50 and 100 mg/kg inverted the rise in insulin level in fructose-fed rats. (Figure 7A).
Figure 7.
DB-B caused a decrease in serum insulin and HOMA-IR in fructose-fed rats: (A) decrease in serum insulin, and (B) HOMA-IR. Results were analyzed statistically using one-way ANOVA followed by the Bonferrani test. Results are plotted as mean ± SEM (n = 5) where a = p < 0.001 and b = p < 0.01, c = p < 0.05, and ns = non-significant when compared with fructose (10%), whereas *** = p < 0.001 compared with the control.
The homeostatic model of the assessment of insulin resistance offered that IR was more pronounced in the disease control group and it was significantly (p < 0.05–0.001) decreased in DB-B-treated rats (Figure 7B).
2.4.4. D. brunonianum-Evoked Protective Effect on Oxidative Stress Marker in Fructose-Treated Hypertensive Rats
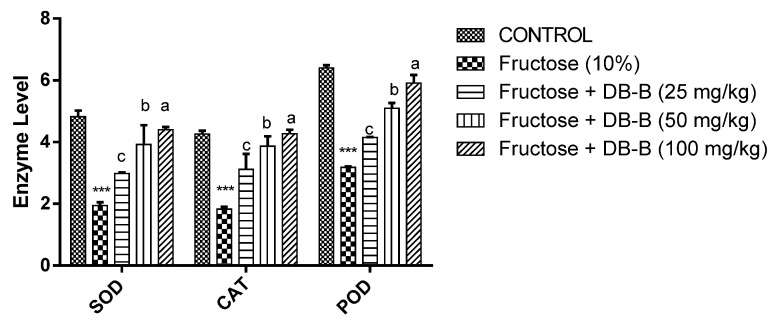
As presented in Figure 8, the findings of this study showed that the level of markers of oxidative stress were significantly (p < 0.001) increased in fructose-treated groups. The treatment significantly reinstated the levels of these enzymes.
Figure 8.
DB-B-elicited restoration of enzymes of the defense system altered by fructose All results were analyzed statistically using two-way ANOVA followed by the Bonferrani test. Data is presented as mean ± SEM of n = 5. Herein, *** represents p < 0.001 in comparison with the control group, whereas a = p < 0.001, b = p < 0.005, and c = p < 0.05 in relation to the fructose (10%) group. DB-B = butanolic fraction of D. brunonianum.
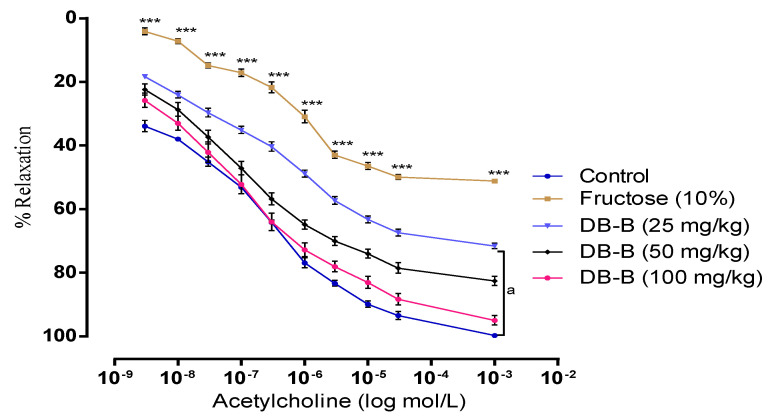
2.4.5. D. brunonianum-Induced Reversal of Acetylcholine-Mediated Vasorelaxation in PE Preconstricted Aortic Tissues
The findings of the current investigation had clearly revealed that oral administration of DB-B (25, 50, 100 mg/kg) and DB-Aq (100, 200, 400 mg/kg) protected endothelium from damage as in these groups acetylcholine relaxed the aortic tissue more significantly compared with the control group who only received fructose (p < 0.05–0.001) (Figure 9).
Figure 9.
DB-B-mediated reversal of acetylcholine-evoked vasorelaxation in PE preconstricted aortic tissues. Data obtained was analyzed statistically using two-way ANOVA followed by the Bonferrani test on GraphPad Prism. Here, each point represents the mean ± SEM of n = 5. *** represents p < 0.001 in comparison with the control group, whereas a = p < 0.001 with respect to the fructose (10%) group. DB-B = butanolic fraction of D. brunonianum.
2.5. Phytochemical Analysis of DB-B Using LC-MS
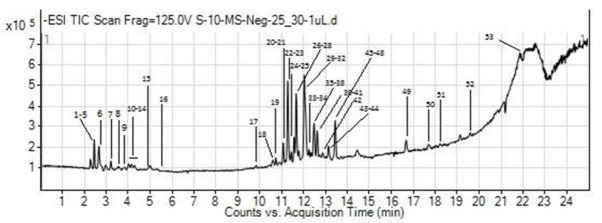
LC-MS analysis of DB-B revealed the presence of 53 active moieties belonging to different chemical classes. The major compounds found in the chemical analysis of DB-B belonged to flavonoid, phenol, alkaloid, and tannins classes (Figure 10 and Table 1).
Figure 10.
LC–MS chromatogram of DB–B.
Table 1.
List of compounds identified by LC-MS analysis of DB-B.
| S.NO | RT (min) |
Base Peak m/z |
Peak Height |
Proposed Compound | Molecular Formula |
Molecular Mass |
Volume |
|---|---|---|---|---|---|---|---|
| 1 | 2.652 | 217.0481 | 46,409 | bis(4-fluorophenyl)-Methanone | C13 H8 F2 O | 218.0534 | 187,240 |
| 2 | 2.668 | 215.0321 | 5966 | 2-C-Methyl-D-erythritol 4-phosphate | C5 H13 O7 P | 216.0395 | 24,028 |
| 3 | 2.672 | 181.0712 | 18,085 | D-Sorbitol | C6 H14 O6 | 182.0785 | 76,215 |
| 4 | 2.697 | 179.0557 | 9855 | L-Galactose | C6 H12 O6 | 180.0631 | 48,970 |
| 5 | 2.753 | 135.0299 | 8636 | D-threonic acid | C4 H8 O5 | 136.0371 | 40,365 |
| 6 | 2.972 | 366.1153 | 12,110 | Met Ser Met | C13 H25 N3 O5 S2 | 367.1229 | 76,009 |
| 7 | 3.214 | 133.0147 | 48,234 | D-(+)-Malic acid | C4 H6 O5 | 134.022 | 233,663 |
| 8 | 3.57 | 290.0883 | 7253 | Sarmentosin epoxide | C11 H17 N O8 | 291.0956 | 75,515 |
| 9 | 3.832 | 253.0929 | 7434 | Galactosylglycerol | C9 H18 O8 | 254.1001 | 46,868 |
| 10 | 4.028 | 128.0356 | 34,200 | N-Acryloylglycine | C5 H7 N O3 | 129.0429 | 208,646 |
| 11 | 4.152 | 243.0619 | 7285 | Uridine | C9 H12 N2 O6 | 244.0691 | 49,247 |
| 12 | 4.292 | 188.0562 | 8154 | Glutarylglycine | C7 H11 N O5 | 189.0635 | 51,869 |
| 13 | 4.292 | 129.0198 | 6037 | Glutaconic acid | C5 H6 O4 | 130.027 | 39,902 |
| 14 | 4.293 | 173.0091 | 14,206 | Dehydroascorbic acid | C6 H6 O6 | 174.0164 | 91,494 |
| 15 | 4.995 | 117.0195 | 20,991 | Erythrono-1,4-lactone | C4 H6 O4 | 118.0268 | 139,568 |
| 16 | 5.681 | 129.0194 | 6310 | Glutaconic acid | C5 H6 O4 | 130.0267 | 50,742 |
| 17 | 9.83 | 131.0355 | 11,447 | 3-Hydroxy-3-methyl-2-oxo-Butyric acid | C5 H8 O4 | 132.0428 | 58,944 |
| 18 | 10.632 | 293.1244 | 10,448 | Ethyl (S)-3-hydroxybutyrate glucoside | C12 H22 O8 | 294.1317 | 43,798 |
| 19 | 10.732 | 327.0723 | 17,123 | Bergenin | C14 H16 O9 | 328.0795 | 84,427 |
| 20 | 11.07 | 353.0885 | 54,518 | Scopolin | C16 H18 O9 | 354.0957 | 369,833 |
| 21 | 11.071 | 467.0816 | 6864 | Castamollissin | C20 H20 O13 | 468.0887 | 34,751 |
| 22 | 11.277 | 609.1477 | 130,491 | Robinetin 3-rutinoside | C27 H30 O16 | 610.1547 | 1,136,964 |
| 23 | 11.283 | 175.0611 | 6144 | 3-propylmalic acid | C7 H12 O5 | 176.0685 | 28,288 |
| 24 | 11.437 | 340.1558 | 11,223 | Carboxyterbinafine derivative2 | C20 H23 N O4 | 341.163 | 68,196 |
| 25 | 11.437 | 403.151 | 10,458 | Desmethylnimodipine | C20 H24 N2 O7 | 404.1586 | 55,189 |
| 26 | 11.565 | 593.1513 | 47,204 | Luteolin 7-rhamnosyl(1- > 6)galactoside | C27 H30 O15 | 594.1586 | 266,559 |
| 27 | 11.662 | 651.157 | 105,640 | Kaempferol 3-(6″-acetylglucoside)-7-glucoside | C29 H32 O17 | 652.1642 | 849,836 |
| 28 | 11.78 | 609.1452 | 15,662 | Robinetin 3-rutinoside | C27 H30 O16 | 610.1524 | 90,280 |
| 29 | 12.016 | 295.0453 | 38,925 | Mono-trans-p-coumaroylmesotartaric acid | C13 H12 O8 | 296.0527 | 394,426 |
| 30 | 12.051 | 635.1622 | 25,444 | Fujikinetin 7-O-laminaribioside | C29 H32 O16 | 636.1699 | 152,900 |
| 31 | 12.052 | 651.1574 | 86,304 | Kaempferol 3-(6″-acetylglucoside)-7-glucoside | C29 H32 O17 | 652.1647 | 852,487 |
| 32 | 12.089 | 186.1143 | 12,154 | KAPA | C9 H17 N O3 | 187.1216 | 70,741 |
| 33 | 12.231 | 593.1528 | 5324 | Luteolin 7-rhamnosyl(1- > 6)galactoside | C27 H30 O15 | 594.16 | 28,075 |
| 34 | 12.254 | 463.0881 | 19,178 | 8-Hydroxyluteolin 8-glucoside | C21 H20 O12 | 464.0951 | 108,984 |
| 35 | 12.336 | 635.1623 | 13,934 | Fujikinetin 7-O-laminaribioside | C29 H32 O16 | 636.1698 | 78,444 |
| 36 | 12.448 | 635.1623 | 9397 | Fujikinetin 7-O-laminaribioside | C29 H32 O16 | 636.1692 | 52,438 |
| 37 | 12.473 | 693.1673 | 49,263 | Isoscutellarein 7-(6‴-acetylallosyl-(1- > 2)-6″-acetylglucoside) | C31 H34 O18 | 694.1745 | 405,976 |
| 38 | 12.478 | 555.1977 | 5318 | punaglandin 1 | C27 H37 Cl O10 | 556.2062 | 33,134 |
| 39 | 12.62 | 693.1672 | 41,258 | Isoscutellarein 7-(6″-acetylallosyl-(1- > 2)-6″-acetylglucoside) | C31 H34 O18 | 694.1743 | 287,244 |
| 40 | 12.717 | 597.2099 | 8960 | 12S-acetoxy-punaglandin 1 | C29 H39 Cl O11 | 598.2179 | 47,677 |
| 41 | 12.766 | 163.0399 | 7568 | m-Coumaric acid | C9 H8 O3 | 164.047 | 33,265 |
| 42 | 12.859 | 677.171 | 11,272 | Apigenin 7-(4″,6″-diacetylalloside)-4′-alloside | C31 H34 O17 | 678.1785 | 66,085 |
| 43 | 13.133 | 677.1739 | 7170 | Apigenin 7-(4″,6″-diacetylalloside)-4′-alloside | C31 H34 O17 | 678.1804 | 40,991 |
| 44 | 13.137 | 447.0937 | 11,848 | 6-Hydroxyluteolin 5-rhamnoside | C21 H20 O11 | 448.1007 | 70,221 |
| 45 | 13.431 | 121.029 | 6423 | 3-Hydroxybenzaldehyde | C7 H6 O2 | 122.0362 | 28,930 |
| 46 | 13.437 | 237.0393 | 6940 | Dipyrocetyl | C11 H10 O6 | 238.0465 | 31,193 |
| 47 | 13.438 | 699.3497 | 96,124 | Septentriodine | C37 H52 N2 O11 | 700.3569 | 634,267 |
| 48 | 13.705 | 269.1032 | 7341 | Idebenone Metabolite (Benzenebutanoic acid,2,5-dihydroxy-3,4-dimethoxy-6-methyl-) | C13 H18 O6 | 270.1104 | 40,569 |
| 49 | 16.669 | 221.1184 | 34,676 | (6S)-dehydrovomifoliol | C13 H18 O3 | 222.1257 | 211,059 |
| 50 | 17.71 | 293.1769 | 12,618 | Gingerol | C17 H26 O4 | 294.1842 | 81,405 |
| 51 | 18.219 | 309.1711 | 10,585 | methyl 8-[2-(2-formyl-vinyl)-3-hydroxy-5-oxo-cyclopentyl]-octanoate | C17 H26 O5 | 310.1785 | 70,372 |
| 52 | 19.584 | 277.1809 | 7965 | 6-Paradol | C17 H26 O3 | 278.1881 | 50,566 |
| 53 | 21.808 | 265.1489 | 22,964 | Lauryl hydrogen sulfate | C12 H26 O4 S | 266.1563 | 362,120 |
3. Discussion
A steady increase in blood pressure was recorded in this appraisal on the chronic treatment of fructose for a period of six weeks. Furthermore, findings of our study revealed that butanolic fraction of D. brunonianum exerted a prominent decrease in the fructose-induced elevation in blood pressure.
Results of the current experiment showed that response to the intravenous injection of sympathomimetic agents was much reduced in rats treated with DB-B. Hence, it could be proposed that this curative effect might be associated with the sympathetic blockade by DB-B. Data obtained in these experiments has been supported by several similar studies, such as a research conducted on rats subjected to chemical sympathectomy that showed that a functional sympathetic nervous system is crucial for the development of fructose-elicited hypertension [12]. Several lines of evidence support this speculation that hypertension elicited in fructose-fed rats results from an increased degree of insulin resistance [13,14]. The findings of the present study showed that the oral administration of DB-B showed a deterioration in the effect of fructose on the level of insulin in fructose-treated rats. This probably explains that the blood-pressure-lowering effect offered by DB-B may be associated, at least in part, to preventing/reversing fructose-mediated insulin resistance. The results of the present study are similar to previously published studies [15,16,17,18]. In addition, a variety of antihypertensive agents successfully treated the high blood pressure accompanied with hyperinsulinemia [19], supporting the value of the aboriginal plant tested in this study.
Meanwhile, we analyzed the level of lipids in the serum of DB-B-treated fructose hypertensive rats. We found that DB-B reversed and prevented fructose-linked hyperlipidemia. In addition, there are several evidences in the literature which implicate that dietary fructose directly converts to fatty acids and then to triglycerides and cholesterol, supporting the results of our study [20,21]. Coumaric acid was identified in DB-B through LC-MS analysis; in early reports, it has been demonstrated that coumaric acid decreased the level of low-density lipoprotein and, therefore, it could be proposed that the lipid lowering effect of this plant may partially be due to the presence of this compound [22].
Hyperuricemia is considered to be one of the major contributing factors in the development of fructose-induced hypertension [23]. Considering this hypothesis, we wished to evaluate the serum level of uric acid in fructose- and DB-B-treated rats. Interestingly, data obtained showed a marked elevation in the level of serum uric acid on the oral administration of fructose; however, DB-B efficiently abrogated the increase in the magnitude of uric acid. In agreement with our results, previously, it has been reported that the raised level of uric acid due to the over-feeding of fructose leads to an impaired endothelial function which will ultimately causes vasocostriction and high blood pressure [24,25]. Earlier, it was observed by different investigators that the serum level of creatinine and urea was disturbed by the chronic intake of fructose, and different natural and synthetic products successfully abolished this effect of fructose along with other metabolic perturbations [26]. Furthermore, it has been established that rutin contributed to lowering the serum level of glucose, urea, and creatinine, as chemical analysis of DB-B evidenced the presence of derivative of rutin; thus, it could be deduced that rutin may partially contribute to the pharmacological effects of DB-B [27]. We further extend our study to investigate the effect of fructose treatment on the vascular endothelium of hypertensive rats. In this way, our results suggested that the endothelial function was significantly compromised in rats feeding on fructose since the vasorelaxant effect of acetylcholine was abolished in phenylephrine-contracted aortic tissues dissected from fructose hypertensive rats; these findings are in compliance with the previous reports that there is an impairment in endothelium-mediated vasodilatation in models of metabolic syndrome [28,29,30,31]. Meanwhile, the concurrent oral administration of DB-B significantly increased the ach-evoked vasorelaxation in fructose-fed rats; therefore, we can hypothesize that the antihypertensive effect of these plants in fructose-fed rats may likely be, at least, in part associated with its vasoprotective effect. Dehydroascorbic acid has been found in DB-B, and ascorbic acid has been reported for its protective effect on endothelium. Therefore, it could be assumed that this active principle could be responsible, at least in part, for the protective effect of DB-B [32]. In addition, it has also been reported that apigenin regulates glucose, lipid metabolism, and associated endothelial dysfunction [33]. Hence, the restoration of the endothelial function with the treatment of DB-B may partially be due to the presence of apigenin.
In addition to the above-mentioned parameters, it has been clearly presented by previous research that high exposure to fructose is associated with the over-production of reactive oxygen species, suggesting that the development of metabolic disorders in humans is due to the consumption of sugar beverages and fructose-rich diets [34,35]. In the present study, the level of SOD (superoxide dismutase) was recorded as in a normal range in rats receiving both fructose and DB-B; however, the activity of serum SOD was significantly lowered in rats feeding only on fructose. It has been revealed in previous reports that SOD is involved in scavenging the O−2 free radical and, thus, helps in reducing blood pressure [36]. Furthermore, treatment with DB-B significantly blunted the deleterious effect of fructose on POD (peroxidase) and CAT (catalase), the supportive enzymes of the defense system involved in the catalysis of hydrogen per oxide and O2. Chemical analysis of DB-B revealed the presence of a large number flavonoids, and it has been well recognized that flavonoids exert antioxidant effects [37]. Robinetin 3-rutinoside, kaempferol 3-(6″-acetylglucoside)-7-glucoside, coumaric acid, malic acid, scopoline, and dehydroascorbic acid were found in DB-B. These compounds have been reported for their antioxidant potential; therefore, it could be proposed that the preventive effect of DB-B on oxidative stress may likely be due to these bioactive constituents [38,39,40,41]. Bergenin and the derivative of apigenin were identified in DB-B, and earlier studies evidenced the antioxidant potential of apigenin and bergenin; thus, it could be linked with the antioxidant activity of DB-B [42,43]. Therefore, on the basis of previous studies, it could be deduced that the phytochemicals may, at least in part, play a role in the antioxidant activity of DB-B, and it could be presented that this antioxidant activity of DB-B may be responsible for their antihypertensive effect in fructose-fed rats.
4. Material and Methods
4.1. Animals
Sprague dawley male rats weighing 170–250 g were housed in an animal house at the University of Sargodha (UOS), Sargodha, Pakistan, and maintained at a standard diet and conditions. Before the commencement of the experiments, ethical clearance for handling and experimentation on animals was obtained from the Institutional Animal Ethics Committee, University of Sargodha (Approval no. IAEC/UOS/2016/35). Additionally, all protocols complied with the declarations of the National Research Council [11].
4.2. Chemicals and Drugs Used
All the drugs and chemicals used for the experiments were of standard analytical grade.
4.3. Plant Collection and Preparation of Extract
Aerial parts of Delphinium brunonianum were collected from Shinkyote Gilgit Baltistan in the months of June to July, 2016, and authenticated by Dr Sher Wali Khan, Assistant professor in the Department of Botany, Karakoram International University, Gilgit Baltistan. Specimens of the plant catalogued as DB-16-11 were preserved in the herbarium of the College of Pharmacy, University of Sargodha. In order to prepare the crude extract, powdered aerial parts of Delphinium brunonianum were macerated in an aqueous ethanol mixture (30:70) and extracted thrice (72 h each). The obtained extract was filtered, and the solvent was eliminated by a rotary evaporator. The obtained extract was stored in a tightly closed container. Afterwards, the aqueous ethanolic extract (crude extract) was subjected to subsequent fractionation with organic solvents [10]. Butanolic fraction (DB-B; yield 15%), used in this study, was stored in capped containers and maintained at 4 °C, until use for analysis. The dose of DB-B was freshly prepared in distilled water at the time of administration. All doses (according to body weight) were administered via oral route.
4.4. Effect of Butanolic Fraction of Delphinium brunonianum on Fructose-Induced Hypertension and Metabolic Alterations in Rats
Rats in all groups, except the control group, were given 10% fructose in drinking water for the first 3 weeks, whereas the treatment groups received the DB-B (25, 50, 100 mg/kg) for the next 3 weeks (in total 6 weeks of study); however, the rats in the fructose-treated group were fed on only fructose. At the end of 6 weeks, blood pressure was measured using an invasive blood pressure measuring technique. In order to evaluate the effect on sympathetic over-activity, rats from all groups were evaluated for vascular reactivity to various agents such as adrenaline (1 g/kg), phenylephrine (1 g/kg), and angiotensin II (25 ng/kg), and serotonin (1 g/kg) was also computed to scrutinize the function of the adrenergic nervous system [44,45,46].
4.5. Alteration in Fructose-Triggered Metabolic Abnormalities
The fresh serum from rats of all groups was subjected to assess level of lipids, insulin, uric acid, urea, creatinine using standard biochemical kits. Furthermore, body weight and fasting blood glucose was measured at different intervals during the whole study [11].
4.6. Evaluation of DB-B-Mediated Reversal of Glucose Intolerance and Insulin Resistance in Fructose-Fed Rats
At the end of the foregoing experiment, rats were deprived of food for 12 h and the oral glucose tolerance test was conducted using glucometer. After the glucose load (50% solution of glucose (1 mL/100 g B.W)), the level of blood glucose was measured at 0, 30, 60, 90, and 120 min. Later on, HOMA-IR (homeostatic model assessment-insulin resistance) was calculated by the equation given in [47]:
| insulin (u/mL) × glucose (mM/L)/22.5 |
4.7. Analysis of Alteration in the Level of Enzymes of the Oxidative System
For this purpose, serum level of catalase, superoxide dismutase, and peroxidase were estimated in blood samples of all rats from all treatment and control groups by adopting the method described by Younis and coworkers [48].
4.8. Effect of Butanolic Fraction of D. brunonianum on Fructos- Mediated Dysfunction of Vascular Endothelium
To delineate the effect of DB-B on fructose-mediated endothelium damage, the thoracic aorta of all animals was observed for vascular reactivity. Vascular reactivity of aortic rings with intact endothelium was assessed by relaxation induced by acetylcholine against the pre-contraction of phenylephrine (1 µM) [18,49].
4.9. Phytochemical Analysis of DB-B
Phytochemicals present in DB-B were analyzed through RP UHPLC-MS following the method of Saleem et al. [50]. Briefly, UHPLC of an Agilent 1290 Infinity liquid chromatography system accompanied with Agilent 6520 Accurate-Mass Q-TOF mass spectrometer with dual ESI source was used in this study, whereas the column used was Agilent Zorbax Eclipse XDB-C18, narrow-bore 2.1 × 150 mm (3.5 μm P/N: 930990-902), which was kept at 25 °C. The auto-sampler was maintained at 4 °C. A total of 1.0 μL of the sample was injected at a flow rate of 0.5 mL/min, while 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) were used as the mobile phase. The sample was run for 25 min and an extra 5 min were given. Full-scan mass spectrometry analysis was conducted on m/z 100–1000, applying a negative electrospray ion source. The recorded results were processed with Agilent Mass Hunter Qualitative Analysis B.05.00 (Metabolomics-2017-00004.m). The compounds were identified by Search Database: METLIN_AM_PCDL-Ne 170502.cdb, with parameters such as match tolerance: 5 ppm, positive ions: +H, +Na, +NH4, and negative ions: H.
4.10. Statistical Analysis
Data was analyzed using one-way/two-way (ANOVA) followed by an appropriate post-test, either Dunnet or Bonfferoni, using GraphPad Prism. p < 0.05 were considered as statistically significant.
5. Conclusions
In conclusion, the findings of the current study proposed that butanolic fraction of Delphinium brunoninaum was found to restore the fructose-mediated hypertension and metabolic abnormalities. DB-B significantly prevented sympathetic over-activity and insulin resistance. As described in the current study, DB-B could efficiently restore the oxidative enzymes and endothelium integrity. Hence, butanolic fraction of Delphinium brunonianum has the potential to prevent the fructose-mediated metabolic abnormalities, and it could serve as a promising source for the development of new therapeutically useful compounds. Nonetheless, further investigation and the isolation of active principles identified in LC-MS analysis is required to validate the mechanism of action
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education, in Saudi Arabia, and the central laboratory at Jouf University for supporting this study.
Author Contributions
Conceptualization, Writing—Review and Editing, Funding Acquisition, S.N.A.B.; Methodology, Investigation, Writing—Original Draft Preparation, Writing—Review and Editing, H.A.; Supervision, Conceptualization, Writing—Review and Editing, A.; Writing—Review and Editing, M.H.A.; H.M.I.; H.E., M.A.E. and K.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Before the commencement of the experiments, ethical clearance for handling and experimentation on animals was obtained from the Institutional Animal Ethics Committee, University of Sargodha (Approval no. IAEC/UOS/2016/35).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors’ work was supported through grant number “375213500” from the Deputyship for Research and Innovation, Ministry of Education, in Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feldeisen S.E., Tucker K.L. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007;32:46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimi-Mameghani M., Asghari-Jafarabadi M., Rezazadeh K. TCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2018;16:329–334. doi: 10.1016/j.joim.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Abdala S., Martín-Herrera D., Benjumea D., Gutiérrez S.D. Diuretic activity of some Smilax canariensis fractions. J. Ethnopharmacol. 2012;140:277–281. doi: 10.1016/j.jep.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Mahady G.B. Global harmonization of herbal health claims. J. Nutr. 2001;131:1120S–1123S. doi: 10.1093/jn/131.3.1120S. [DOI] [PubMed] [Google Scholar]
- 5.Kamboj V.P. Herbal medicine. Curr. Sci. 2000;78:35–39. [Google Scholar]
- 6.Samuelsson G., Bohlin L. Drugs of Natural Origin: A Treatise of Pharmacognosy. CRC Press Inc.; Boca Raton, FL, USA: 2017. [Google Scholar]
- 7.Ghimire S. Medicinal and aromatic plants in the Nepal Himalaya: Status, use, sale and Conservation. Wildlife. 1999;1:42–52. [Google Scholar]
- 8.Tripathee H.P., Sharma R.P., Timilsina Y.P., Pathak R., Devkota K.P. An assessment of ethnomedicinal use, chemical constituents analysis and bioactivity evaluation on high altitude medicinal plant Delphinium brunonianum of Manang district. Nepal J. Sci. Technol. 2012;12:111–118. doi: 10.3126/njst.v12i0.6488. [DOI] [Google Scholar]
- 9.Khan A., Hassan M., Ali S. Secondary Metabolite Studies of Some Selected Plants of District Gilgit, Gilgit-Baltistan. Int. J. Pharmacogn. Phytochem. Res. 2014;6:4. [Google Scholar]
- 10.Asif H., Ahmad M.I., Alotaibi N.H., Alharbi K.S., Bukhari S.N.A., Saleem H., Locatelli M. Phytochemical analysis and reappraisal of diuretic activity of Delphinium brunonianum Royle and its mode of action in experimental rats. Pak. J. Pharm. Sci. 2020;33:1833–1838. [PubMed] [Google Scholar]
- 11.Asif H., Alamgeer A., Bukhari I.A., Vohra F., Afzal S., Khan S.W., Niazi Z.R. Phytochemical analysis of crude extract of Delphinium brunonianum and its effect on hypertension and metabolic perturbations in fructose fed rats. Nat. Prod. Res. 2019;5:2982–2986. doi: 10.1080/14786419.2019.1679134. [DOI] [PubMed] [Google Scholar]
- 12.Verma S., Bhanot S., Mc Neill J.H. Sympathectomy prevents fructose-induced hyperinsulinemia and hypertension. Eur. J. Pharmacol. 1999;373:R1–R4. doi: 10.1016/S0014-2999(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 13.Tran L.T., Yuen V.G., McNeill J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 14.Elkayam A., Mirelman D., Peleg E., Wilchek M., Miron T., Rabinkov A., Oron-Herman M., Rosenthal T. The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am. J. Hypertens. 2003;16:1053–1056. doi: 10.1016/j.amjhyper.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Ahangarpour A., Mohammadian M., Dianat M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran. J. Med. Sci. 2012;37:181. [PMC free article] [PubMed] [Google Scholar]
- 16.Vikrant V., Grover J., Tandon N., Rathi S., Gupta N. Treatment with extracts of Momordica charantia and Eugenia jambolana prevents hyperglycemia and hyperinsulinemia in fructose fed rats. J. Ethnopharmacol. 2001;76:139–143. doi: 10.1016/S0378-8741(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 17.Dimo T., Azay J., Tan P.V., Pellecuer J., Cros G., Bopelet M., Serrano J.J. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 2001;76:215–221. doi: 10.1016/S0378-8741(01)00229-X. [DOI] [PubMed] [Google Scholar]
- 18.Dimo T., Rakotonirina S.V., Tan P.V., Azay J., Dongo E., Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 2002;83:183–191. doi: 10.1016/S0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 19.Rösen P., Ohly P., Gleichmann H. Experimental benefit of moxonidine on glucose metabolism and insulin secretion in the fructose-fed rat. J. Hypertens. 1997;15:S31–S38. doi: 10.1097/00004872-199715011-00004. [DOI] [PubMed] [Google Scholar]
- 20.Bantle J.P. Dietary fructose and metabolic syndrome and diabetes. J. Nutr. 2009;139:1263S–1268S. doi: 10.3945/jn.108.098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zang L.-Y., Cosma G., Gardner H., Shi X., Castranova V., Vallyathan V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell Physiol. 2000;279:C954–C960. doi: 10.1152/ajpcell.2000.279.4.C954. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T., Tuttle K.R., Short R.A., Johnson R.J. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat. Rev. Nephrol. 2005;1:80. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 24.Feig D.I. Hyperuricemia and hypertension. Adv. Chronic Kidney Dis. 2012;19:377–385. doi: 10.1053/j.ackd.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Khosla U.M., Zharikov S., Finch J.L., Nakagawa T., Roncal C., Mu W., Krotova K., Block E.R., Prabhakar S., Johnson R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 26.Olatunji L.A., Okwusidi J.I., Soladoye A.O. Antidiabetic effect of Anacardium occidentale. Stem-bark in fructose-diabetic rats. Pharm. Biol. 2005;43:589–593. doi: 10.1080/13880200500301712. [DOI] [Google Scholar]
- 27.Kamalakkannan N., Prince P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006;98:97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Lozada L.G., Tapia E., Jiménez A., Bautista P., Cristóbal M., Nepomuceno T., Soto V., Ávila-Casado C., Nakagawa T., Johnson R.J. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Ren. Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 29.Panchal S.K., Wong W.-Y., Kauter K., Ward L.C., Brown L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition. 2012;28:1055–1062. doi: 10.1016/j.nut.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Verma S., Bhanot S., Yao L., McNeill J.H. Defective endothelium-dependent relaxation in fructose-hypertensive rats. Am. J. Hypertens. 1996;9:370–376. doi: 10.1016/0895-7061(95)00392-4. [DOI] [PubMed] [Google Scholar]
- 31.Deedwania P.C. Mechanisms of endothelial dysfunction in the metabolic syndrome. Curr. Diabetes Rep. 2003;3:289–292. doi: 10.1007/s11892-003-0019-8. [DOI] [PubMed] [Google Scholar]
- 32.May J.M. How does ascorbic acid prevent endothelial dysfunction? Free Radic. Biol. Med. 2000;28:1421–1429. doi: 10.1016/S0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 33.Ren B., Qin W., Wu F., Wang S., Pan C., Wang L., Zeng B., Ma S., Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Vanhoutte P. Endothelial dysfunction in cardiovascular disease; Proceedings of the Southeast Asian-Western Pacific Regional Meeting of Pharmacologists; Busan, Korea. 19–23 August 2003. [Google Scholar]
- 35.Hecker P.A., Galvao T.F., O’Shea K.M., Brown B.H., Henderson Jr R., Riggle H., Gupte S.A., Stanley W.C. High-sugar intake does not exacerbate metabolic abnormalities or cardiac dysfunction in genetic cardiomyopathy. Nutrition. 2012;28:520–526. doi: 10.1016/j.nut.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng P.-W., Lee H.-C., Lu P.-J., Chen H.-H., Lai C.-C., Sun G.-C., Yeh T.-C., Hsiao M., Lin Y.-T., Liu C.-P. Resveratrol inhibition of Rac1-derived reactive oxygen species by AMPK decreases blood pressure in a fructose-induced rat model of hypertension. Sci. Rep. 2016;6:25342. doi: 10.1038/srep25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Tu Y.-C., Lian T.-W., Hung J.-T., Yen J.-H., Wu M.-J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006;54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Wahab M., El-Mahdy M.A., Abd-Ellah M.F., Helal G., Khalifa F., Hamada F. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res. 2003;48:461–465. doi: 10.1016/S1043-6618(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 39.De Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R.M.B. Hypoglycemic Effect and Antioxidant Potential of Kaempferol-3, 7-O-(α)-dirhamnoside from Bauhinia f orficata Leaves. J. Nat. Prod. 2004;67:829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 40.Yen G.-C., Duh P.-D., Tsai H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002;79:307–313. doi: 10.1016/S0308-8146(02)00145-0. [DOI] [Google Scholar]
- 41.Hung T.M., Na M., Thuong P.T., Su N.D., Sok D., Song K.S., Seong Y.H., Bae K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006;108:188–192. doi: 10.1016/j.jep.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Khan H., Amin H., Ullah A., Saba S., Rafique J., Khan K., Ahmad N., Badshah S.L. Antioxidant and antiplasmodial activities of bergenin and 11-O-galloylbergenin isolated from Mallotus philippensis. Oxidative Med. Cell. Longev. 2016;2016:1051925. doi: 10.1155/2016/1051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nafisi S., Hashemi M., Rajabi M., Tajmir-Riahi H.A. DNA adducts with antioxidant flavonoids: Morin, apigenin, and naringin. DNA Cell Biol. 2008;27:433–442. doi: 10.1089/dna.2008.0735. [DOI] [PubMed] [Google Scholar]
- 44.Bellamkonda R., Karuna R., Rao B.S.B., Haritha K., Manjunatha B., Silpa S., Saralakumari D. Beneficiary effect of Commiphora mukul ethanolic extract against high fructose diet induced abnormalities in carbohydrate and lipid metabolism in wistar rats. J. Tradit. Complementary Med. 2018;8:203–211. doi: 10.1016/j.jtcme.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De AF Da R.D.C., de Souza P., Crestani S., Júnior A.G., Boligon A.A., Athayde M.L., da Silva-Santos J.E. Hypotensive and diuretic effect of the butanolic soluble fraction of the hydroethanolic extract of bark of Scutia buxifolia Reissek in rats. J. Ethnopharmacol. 2015;172:395–401. doi: 10.1016/j.jep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Mohan M., Jaiswal B.S., Kasture S. Effect of Solanum torvum on blood pressure and metabolic alterations in fructose hypertensive rats. J. Ethnopharmacol. 2009;126:86–89. doi: 10.1016/j.jep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Moraes É.A., da Silva Marineli R., Lenquiste S.A., Queiroz V.A.V., Camargo R.L., Borck P.C., Carneiro E.M., Júnior M.R.M. Whole sorghum flour improves glucose tolerance, insulin resistance and preserved pancreatic islets function in obesity diet-induced rats. J. Funct. Foods. 2018;45:530–540. doi: 10.1016/j.jff.2017.03.047. [DOI] [Google Scholar]
- 48.Younis W., Alamgeer, Schini-Kerth. V.B., Junior A.G., Muhammad M. Cardioprotective effect of Asphodelus tenuifolius Cav. on blood pressure and metabolic alterations in glucose-induced metabolic syndrome rats–An ethnopharmacological approach. J. Ethnopharmacol. 2018;214:168–178. doi: 10.1016/j.jep.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Maneesai P., Bunbupha S., Kukongviriyapan U., Prachaney P., Tangsucharit P., Kukongviriyapan V., Pakdeechote P. Asiatic acid attenuates renin-angiotensin system activation and improves vascular function in high-carbohydrate, high-fat diet fed rats. BMC Complementary Altern. Med. 2016;16:123. doi: 10.1186/s12906-016-1100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleem H., Htar T.T., Naidu R., Nawawi N.S., Ahmad I., Ashraf M., Ahemad N. Biological, chemical and toxicological perspectives on aerial and roots of Filago germanica (L.) huds: Functional approaches for novel phyto-pharmaceuticals. Food Chem. Toxicol. 2019;123:363–373. doi: 10.1016/j.fct.2018.11.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in article.