Abstract
Anhydrobiotic engineering aims to improve desiccation tolerance in living organisms by adopting the strategies of anhydrobiosis. This was achieved for Escherichia coli and Pseudomonas putida by osmotic induction of intracellular trehalose synthesis and by drying from trehalose solutions, resulting in long-term viability in the dried state.
Organisms able to undergo anhydrobiosis survive the loss of essentially all their water, assuming metabolic dormancy in the dried state and resuming normal functions on rehydration. When dry, such organisms are highly resistant to environmental challenge (2, 7). Although bacteria exhibit variable degrees of desiccation tolerance (9), relatively few genera are recognized as anhydrobiotic, the major exceptions being among the cyanobacteria (10). The ability to confer similar desiccation tolerance on otherwise desiccation-sensitive microorganisms, termed anhydrobiotic engineering (6), has numerous potential biotechnological applications. Studies of anhydrobiosis in baker's yeast suggest that both synthesis and export of the disaccharide trehalose are of crucial importance (2, 5). Since many bacteria accumulate trehalose under certain hyperosmotic culture conditions (11), they are ideally suited for anhydrobiotic engineering. One recent study has shown that osmotically induced trehalose synthesis can increase the rate of survival of desiccation for Escherichia coli (13). In this paper, we demonstrate that when trehalose is present both inside and outside the cell, desiccation tolerance and long-term stability of both E. coli and Pseudomonas putida can be comparable to those of anhydrobiotic organisms.
E. coli MC4100 was grown in M9 medium with 1% glucose and trace elements (0.015 mM FeSO4, 0.015 mM ZnSO4, and 0.015 mM MnSO4), with or without osmotic stress (0.6 M NaCl), and harvested in growth and stationary phases. Intracellular trehalose was measured by gas chromatography, essentially as described previously (12); concentrations were calculated with reference to CFU. Stressed E. coli in growth phase contained the highest level of trehalose (230 μg/109 CFU in a typical experiment), while stressed cells from stationary phase contained somewhat less (150 μg/109 CFU). Unstressed cells from either growth phase or stationary phase did not contain detectable amounts of trehalose (<0.5 μg/109 CFU).
P. putida has been reported to accumulate mannitol as the main compatible solute (8). A study of the compatible solute profile of P. putida KT2440 grown in high-salt medium identical to that used for E. coli, except that 0.4 M NaCl was used, has demonstrated high concentrations of mannitol in early growth phase, which decrease rapidly thereafter. Trehalose is produced in high-salt cultures of P. putida, although not until late growth phase; its concentration then increases during stationary phase (H. Bredholt and A. R. Strøm, unpublished data). Growth-phase, stressed cells contained the highest mannitol concentration (88 μg/109 CFU in a typical experiment; cf. 0.7 μg/109 CFU in stationary phase), whereas stressed cells in the stationary phases contained the highest trehalose level (32 μg/109 CFU; cf. 0.8 μg/109 CFU in growth phase), as measured by gas chromatography. Mannitol was not detectable in unstressed P. putida, and trehalose levels were low (2 to 7 μg/109 CFU).
E. coli and P. putida grown in high-salt or standard medium were vacuum dried from a trehalose solution, using a “quick drying” protocol. Samples from both growth and stationary phases were recovered by gentle centrifugation (4,000 relative centrifugal force) and resuspended in 1 M trehalose (∼109 bacteria/ml), and 100-μl volumes were divided into 7-ml glass serum vials. Vacuum drying for 2 h at 30 to 100 mTorr with a shelf temperature of 30°C was carried out in a modified freeze dryer (Dura-Stop MP; FTS Systems, Stone Ridge, N.Y.); vials were sealed under vacuum. Samples were either rehydrated and plated immediately after drying or were first stored at 30°C for 7 days (Fig. 1). Bacteria were rehydrated with 1 ml of Luria broth at 20°C, serially diluted, and plated on Luria broth agar. Viability is defined as CFU of rehydrated bacteria/CFU of a nondried control, expressed as a percentage.
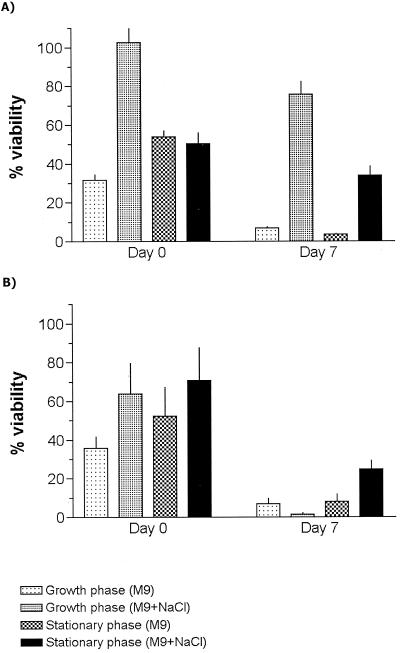
FIG. 1.
Viability of bacteria after vacuum drying from a 1 M trehalose solution. E. coli was cultured to growth phase or stationary phase in M9 with or without 0.6 M NaCl (A), and P. putida was cultured to growth phase or stationary phase in M9 with or without 0.4 M NaCl (B), as shown by the key. A quick drying protocol was used. Samples were assayed immediately after drying (Day 0) or after 7 days of storage at 30°C (Day 7). Values are the mean (+ the standard deviation) of four determinations.
Strikingly, with one exception, E. coli and P. putida gave remarkably similar results after immediate rehydration (Fig. 1), regardless of whether bacteria had been osmotically stressed. The exception was the E. coli culture in high-salt medium sampled in growth phase, where a very high proportion of cells remained viable (Fig. 1A). However, after storage for one week at 30°C, differences became more apparent. For E. coli, osmotically stressed bacteria maintained significantly higher viability than those from an equivalent growth phase in standard medium (P < 0.05; two-tailed Student's t test). Growth-phase cells from high-salt cultures also performed better (P < 0.05) than equivalent stationary-phase cells, in which the trehalose concentration is lower (Fig. 1A). For P. putida, higher (P < 0.05) viability after 1 week of storage was observed for stationary-phase, stressed cells compared to results for other samples, again correlating with the presence of intracellular trehalose. Mannitol in osmotically stressed growth-phase P. putida cultures did not appear to improve viability at day 7 (Fig. 1B).
The residual water content of dried bacteria after quick drying was ∼10% (wt/wt) according to coulometric Karl- Fischer titration (Aquapal III; CSC Scientific Co., Inc., Fairfax, Va.). Since the water content of dried anhydrobiotic organisms is usually lower than 10% (2, 7), it was postulated that more extensive drying could increase the long-term stability of the dried bacteria, particularly for P. putida. An experiment was therefore carried out whereby bacteria were sampled at four stages of an extended vacuum-drying procedure: 2 h at 30°C (quick drying, as above); 15 h at 30°C (“primary drying”); primary drying followed by an increase in shelf temperature of 2.5°C/min, holding for 15 min every 2°C, up to a final temperature of 60°C (“secondary drying”); and secondary drying to 80°C. E. coli and P. putida with maximal intracellular trehalose concentrations were dried from 1 M trehalose and sampled for residual moisture content and viability at each stage of the drying process; further samples were also stored at 30°C for up to 28 days. As expected, more extensive drying decreased the water content to the following amounts: for quick drying, 9 to 11%, for primary drying, 6 to 7.5%, for secondary drying to 60°C, 4.5 to 6%, and for secondary drying to 80°C, 1.5 to 2.5% (wt/wt) residual water.
For E. coli, viability remained approximately constant over time, regardless of sample water content (within the measured range): essentially no loss of viability was seen from day 1 to day 28 of storage at 30°C (Fig. 2A). In the experiment shown, a relatively constant viability of ∼50% was observed; over a series of experiments, some variability in the survival rate was seen, with viability ranging from 40 to 70%. It is worth noting that the first sampling point for this experiment was at day 1, even for quick-dried samples, which were effectively stored (at 30°C) for ∼20 h prior to rehydration. The viability of quick-dried E. coli organisms after this storage period was consistently below the range observed when they were rehydrated and assayed immediately (70 to 100%; e.g., see Fig. 1A). We attribute this to further drying in the vial during the initial storage period, due to redistribution of some residual cellular water to extracellular trehalose or to the vapor phase.
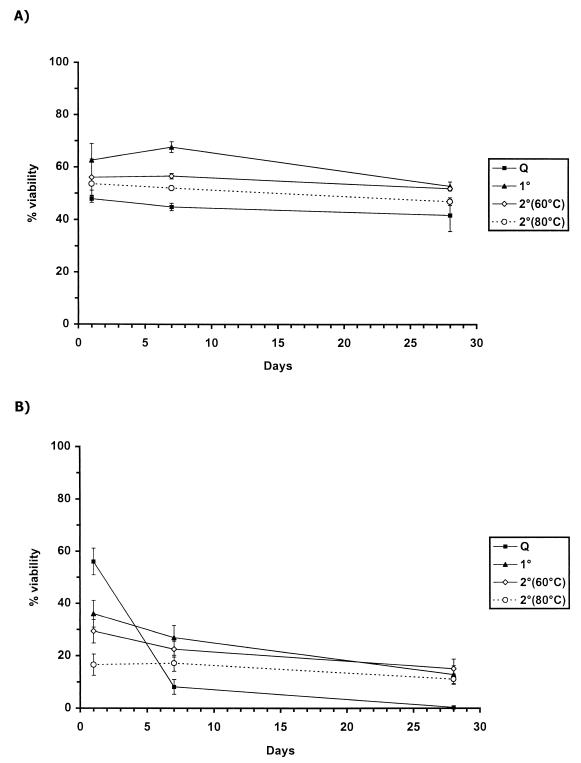
FIG. 2.
Long-term viability of trehalose-containing E. coli (A) or P. putida (B) vacuum dried from 1 M trehalose solutions using quick (Q), primary (1°), or secondary (2°) drying protocols. Secondary drying was taken to either 60 or 80°C, as indicated in the key. Values are the mean (± the standard deviation) of four determinations.
For P. putida, a different pattern was observed (Fig. 2B). There was increased loss of viability at day 1 as the residual water content was reduced, but bacteria became increasingly resistant to further loss during storage. The difference in the degrees of dryness required to achieve long-term stability for E. coli and P. putida could be due to the intracellular trehalose content: assuming a bacterial cell volume of ∼2 fl, the trehalose concentration in E. coli is of the order of 300 mM prior to drying; in P. putida this is closer to 40 mM. A higher intracellular trehalose concentration might inhibit Maillard reactivity more efficiently or promote a higher intracellular glass transition temperature, thereby allowing greater latitude in the degree of desiccation required for long-term stability.
A recent report suggests that for Lactococcus lactis, extracellular mannitol acts as a desiccation protectant (4), but there is no information on the efficacy of intracellular mannitol. Our data suggest that for P. putida it is less effective than trehalose, perhaps because mannitol has a tendency to crystallize rather than forming the glassy matrix by which trehalose confers at least part of its protective effect (3).
Anhydrobiotic engineering of gram-negative bacteria can be achieved, therefore, with high intracellular and extracellular concentrations of trehalose prior to drying. Where intracellular trehalose levels are limiting, best results correlate with minimal residual water content. Intracellular trehalose can be accumulated by induction of endogenous trehalose synthase genes or by a genetic engineering approach, analogous to a recent attempt using a sucrose synthase gene (1).
Acknowledgments
This work was supported by EC grant BIO4-CT98-0283. A.T. is the Anglian Water Senior Research Fellow of Pembroke College, University of Cambridge.
REFERENCES
- 1.Billi D, Wright D J, Helm R F, Prickett T, Potts M, Crowe J H. Engineering desiccation tolerance in Escherichia coli. Appl Environ Microbiol. 2000;66:1680–1684. doi: 10.1128/aem.66.4.1680-1684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowe J H, Hoekstra F A, Crowe L M. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 3.Crowe J H, Carpenter J F, Crowe L M. The role of vitrification in anhydrobiosis. Annu Rev Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Efiuvwevwere B J O, Gorris L G M, Smid E J, Kets E P W. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl Microbiol Biotechnol. 1999;51:100–104. [Google Scholar]
- 5.Eleutherio E C A, De Araujo P S, Panek A D. Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim Biophys Acta. 1993;1156:263–266. doi: 10.1016/0304-4165(93)90040-f. [DOI] [PubMed] [Google Scholar]
- 6.García de Castro A, Lapinski J, Tunnacliffe A. Anhydrobiotic engineering. Nat Biotech. 2000;18:473. doi: 10.1038/75237. [DOI] [PubMed] [Google Scholar]
- 7.Keilin D. The problem of anabiosis or latent life: history and current concept. Proc R Soc Ser B. 1959;150:149–191. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- 8.Kets E P W, Galinski E A, de Wit M, de Bont J A M, Heipieper H J. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J Bacteriol. 1996;178:6665–6670. doi: 10.1128/jb.178.23.6665-6670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potts M. Desiccation tolerance in prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potts M. The anhydrobiotic cyanobacterial cell. Physiol Plant. 1996;97:788–794. [Google Scholar]
- 11.Strøm A R. Osmoregulation in the model organism Escherichia coli: genes governing the synthesis of glycine betaine and trehalose and their use in metabolic engineering of stress tolerance. J Biosci. 1998;23:437–445. [Google Scholar]
- 12.Welsh D T, Reed R H, Herbert R A. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J Gen Microbiol. 1991;137:745–750. doi: 10.1099/00221287-137-4-745. [DOI] [PubMed] [Google Scholar]
- 13.Welsh D T, Herbert R A. Osmotically induced intracellular trehalose, but not glycine betaine accumulation promotes desiccation tolerance in Escherichia coli. FEMS Microbiol Lett. 1999;174:57–63. doi: 10.1111/j.1574-6968.1999.tb13549.x. [DOI] [PubMed] [Google Scholar]