Abstract
Background
Patients admitted to general wards are inherently at risk of deterioration. Thus, tools that can provide early detection of deterioration may be lifesaving. Frequent remote patient monitoring (RPM) has the potential to allow such early detection, leading to a timely intervention by health care providers.
Objective
This study aimed to assess the potential of a novel wearable RPM device to provide timely alerts in patients at high risk for deterioration.
Methods
This prospective observational study was conducted in two general wards of a large tertiary medical center. Patients determined to be at high risk to deteriorate upon admission and assigned to a telemetry bed were included. On top of the standard monitoring equipment, a wearable monitor was attached to each patient, and monitoring was conducted in parallel. The data gathered by the wearable monitors were analyzed retrospectively, with the medical staff being blinded to them in real time. Several early warning scores of the risk for deterioration were used, all calculated from frequent data collected by the wearable RPM device: these included (1) the National Early Warning Score (NEWS), (2) Airway, Breathing, Circulation, Neurology, and Other (ABCNO) score, and (3) deterioration criteria defined by the clinical team as a “wish list” score. In all three systems, the risk scores were calculated every 5 minutes using the data frequently collected by the wearable RPM device. Data generated by the early warning scores were compared with those obtained from the clinical records of actual deterioration among these patients.
Results
In total, 410 patients were recruited and 217 were included in the final analysis. The median age was 71 (IQR 62-78) years and 130 (59.9%) of them were male. Actual clinical deterioration occurred in 24 patients. The NEWS indicated high alert in 16 of these 24 (67%) patients, preceding actual clinical deterioration by 29 hours on average. The ABCNO score indicated high alert in 18 (75%) of these patients, preceding actual clinical deterioration by 38 hours on average. Early warning based on wish list scoring criteria was observed for all 24 patients 40 hours on average before clinical deterioration was detected by the medical staff. Importantly, early warning based on the wish list scoring criteria was also observed among all other patients who did not deteriorate.
Conclusions
Frequent remote patient monitoring has the potential for early detection of a high risk to deteriorate among hospitalized patients, using both grouped signal-based scores and algorithm-based prediction. In this study, we show the ability to formulate scores for early warning by using RPM. Nevertheless, early warning scores compiled on the basis of these data failed to deliver reasonable specificity. Further efforts should be directed at improving the specificity and sensitivity of such tools.
Trial Registration
ClinicalTrials.gov NCT04220359; https://clinicaltrials.gov/ct2/show/NCT04220359
Keywords: remote patient monitoring, noninvasive monitoring, general ward, early warning score system, patient deterioration, clinical prediction, wearable devices, uHealth
Introduction
Validated tools for the early identification of high risk for clinical deterioration in hospitalized patients, or early warning score (EWS) systems, would be of high medical value in clinical practice. A large meta-analysis of previous studies tried to evaluate the effectiveness of rapid response teams for the reduction of in-hospital death in such circumstances [1]. The analysis failed to reach firm conclusions owing to the low quality of design and subsequent outcomes of such studies. One potential cause could be the fact that in different clinical scenarios (eg, general wards vs surgical departments) the clinical circumstances, the classifications used to define the clinical deterioration, and the competencies of the clinical staff are heterogenous [2,3]. Therefore, ideally, early detection technologies and applied prediction algorithms should be tailored for specific patient populations and clinical scenarios.
A common method, used worldwide in general-internal medicine departments, for the early identification of deterioration is placing the patient in a telemetry bed. A retrospective analysis of the effectiveness and potential abuse of this method found that when analyzed retrospectively, only one-quarter of telemetry days during hospitalization were deemed appropriate [4]. Moreover, they described that eliminating unnecessary telemetry days would result in significant cost saving. Interestingly, they did not find any cases of deterioration among patients who were not connected to telemetry devices. This shows that the medical staff was highly professional yet too sensitive, having admitted patients to the telemetry bed frequently.
Possible ways to generate an early risk identification flag is to rely on automatically grouped physiological signals incorporated into different scoring systems, or using artificial intelligence (AI)-based computerized algorithms, rather than counting on follow-up observations by professional staff members. For example, a multicenter retrospective analysis of electronic health records’ data from all patients admitted to 5 US hospitals during the years 2008-2013 showed that prediction of the composite outcome of in-hospital cardiac arrest, the need for intensive care unit (ICU) transfer, and death within 24 hours of observation were higher when conducted using a computerized score [5]. This was also found in the setting of the high-acuity area of an emergency department [6]. Nevertheless, training and competency of professional staff members are key components in every program intended to assimilate computerized predictive tools in hospital departments [7].
Unlike the standard spot-check vital sign measurements that are conducted over a short period and could miss changes in parameters, frequent and automated vital sign collection for longer periods using remote patient monitoring (RPM) platforms with data transmission into algorithm-based computerized systems will potentially be better equipped to detect early changes and alerts of various risks [8-11]. It is also accepted that such systems would be of benefit if they are simple and easy to use, frequently measure multiple vital signs, incorporated into the workflow of the health care providers, improve patient outcomes, and would be of help to the medical staff in addition to other measures to improve patient surveillance [11-14]. We expect monitoring systems and early warning scores to be sensitive and specific. The currently used EWS systems, which are collected infrequently by the medical teams, are known to have a relatively high sensitivity and low specificity [15].
This study aimed to assess whether frequent RPM has the potential for early detection of the risk to deteriorate, using grouped signal-based scores, compared to clinical detection of deterioration by the medical staff.
Methods
Study Design and Overview
This prospective observational clinical study with retrospective analysis of the data was conducted in 2 general wards of a large tertiary medical center. Patients determined to be at high risk to deteriorate upon admission and assigned to a telemetry bed were included, after signing an informed consent form. On top of the standard telemetry overhead monitoring devices used in the general ward (Mindray; e PM 10M), a wireless, wearable monitor was attached to the chest of each patient, and monitoring was conducted in parallel. Multimedia Appendix 1 shows a CONSORT (Consolidated Standards of Reporting Trials) flowchart of the study.
Medical treatment was provided on the basis of standard monitoring system only, as the data gathered by the wearable monitor were analyzed retrospectively, with the medical staff being blinded to it in real time. The physiological data from the wearable monitors were collected automatically every 5 minutes during the first 72 hours from admission, with no personally identifiable information besides serial numbers of the devices. Inclusion criteria were adults (aged >18 years) transferred from the emergency department and admitted to the general wards, who were determined to be at an increased risk for clinical and physiological deterioration during the first 72 hours from admission by the attending physicians (eg, patients who were hemodynamically or respiratory unstable in the emergency department, patients suspected of arrhythmia or acute coronary syndrome, and those suspected with infection and signs of sepsis). Exclusion criteria were lack of informed consent, physicians' assessment that patients will not stay in the general ward for the entirety of the first 72 hours, and technical inability to attach the chest monitor to the patients. Furthermore, patients already defined as necessitating lifesaving procedures were not included in the study.
Study Setting
Designated communication routers were deployed and installed in the departments to ensure continuous monitoring, data transmission, and automatic data collection of all measurements. Data were transferred through Bluetooth from the devices to the routers and through Wi-Fi from the routers to a data cloud. Data gathered during the first 72 hours post admission, including physiological parameters measured by the wearable monitors and clinical data and vital sign data collected using the standard-of-care devices and documented in the electronic medical record system, were retrospectively analyzed at the end of the collection phase.
Early Warning of the Risk for Deterioration
Several EWSs of the risk for deterioration were used. These included (1) the National Early Warning Score (NEWS, described in Multimedia Appendix 2) [16-19]; (2) Airway, Breathing, Circulation, Neurology, and Other (ABCNO) score (described in Multimedia Appendix 3) [20]; and (3) deterioration criteria defined by the clinical team as what they expect to have from a device providing continuous monitoring (a “wish list” score, described in Multimedia Appendix 4). In all three scoring systems, the risk scores were calculated every 5 minutes using the frequent data collected by the wearable devices. Data generated by these early warning scores were compared with those obtained from the clinical records of actual deterioration among these patients. Actual clinical deterioration of patients was defined by the medical staff as (1) needing cardiopulmonary resuscitation, (2) needing to be transferred to the ICU, (3) dead or dying, or (4) deteriorating as defined by the ABCNO criteria, relying on measurements from currently used devices in the wards and without using data derived from the wearable monitors.
The Wearable Monitoring Platform
Frequent monitoring was achieved using a wireless, noninvasive, wearable reflective photoplethysmography-based sensor (BB-613WP, Biobeat Technologies Ltd). The data were automatically transmitted immediately upon capture to a cloud-based web platform repository. Patients’ values recorded every 5 minutes included 13 physiological parameters, including heart rate, blood oxygen saturation, respiratory rate, cuffless blood pressure, stroke volume, cardiac output, cardiac index, systemic vascular resistance, heart rate variability, pulse pressure, mean arterial pressure, temperature, and single-channel electrocardiograms [21-25].
Statistical and Data Analysis
We compared various EWS systems using the data collected via the continuous wearable monitoring system, with the exact time as recorded in the electronic medical record, where patients deteriorated, as detected by the medical teams.
Baseline physical parameters were calculated by averaging the first 12 measurements. Continuous data are expressed as mean (SD) values if normally distributed or median (IQR) values if skewed. Categorical variables are presented as frequency (%) values. Between-group comparisons of numerical values were carried out using an independent samples t test, followed by the Levene test for equality of variances. Chi-square and the Fisher exact test were used for between-group comparisons of categorial parameters. Early warning based on NEWS was defined as the initial time point in which the score was above 5. Early warning based on the ABCNO score and the local medical staff deterioration (“wish list”) criteria was defined as being detected when two consecutive measurements were above or below the defined thresholds (see Multimedia Appendices 3 and 4). Patients were included in the final analysis if more than 200 sessions of measurements (each session includes 13 physiological parameters and an EWS score) were carried out per patient, which was considered the minimal volume of data to analyze deterioration during the monitoring period. We did not treat any missing data; once patients had more than 200 measurement sessions, they were considered eligible for inclusion and further analysis.
The actual clinical deterioration events detected by the medical teams on site were collected, and the sensitivity and specificity of early warning by the wearable monitoring platform based on the 3 approaches—NEWS, ABCNO score, and “wish list” criteria of changes in parameters—were assessed post hoc, relying on the combination of the documented events in the electronic medical records of the patients and the physiological data collected by the wearable monitoring system. The investigators who made these post hoc assessments were blinded to the clinical outcomes of participating patients. Another element assessed was the warning time defined as the difference (in hours) from the early detection by any of these approaches to the actual clinical detection of deterioration as documented by the medical staff. All descriptive statistical analyses were performed using SPSS (version 25; IBM Corp).
Within the context of this study, readings regarded as “spurious readings” (basic definitions of either bad signals or signals defined as out of the sensor's measurement range) were automatically removed by the monitoring platform's algorithm and not included in the analysis. Thus, all collected measurements were regarded as valid. The next step was to aggregate the 15-minute data (using all data points) into hourly measurement aggregates using Python's data analysis library [26] and to match the data with the clinical data for each subject, considering also their demographic characteristics and their medical history.
Ethics Approval
This study was approved by the institutional review board of the Sheba Medical Center, Israel (MOH_2020-07-12_009133).
Results
A total of 410 patients, fulfilling the preliminary definition by the attending physicians as being at a high risk to deteriorate during the first 72 hours after admission, were initially recruited. The median patient age was 71 (IQR 62-78) years. Of the recruited 410 patients, 217 had undergone more than 200 measurement sessions using the wearable monitors during their hospitalization (average monitoring time 48 hours, range 25-131 hours) and thus were included in the final analysis. In total, 13 parameters were collected within each measurement session, resulting in approximately 3700 measurements per day per for each of the 217 patients included in the final analysis. When considering at least 2 days of the monitoring period, the number of data points collected during the study crossed 2,000,000 altogether. Of the 217 patients, 130 (59.9%) were male. Demographic details of the participants (upon admission) are provided in Table 1.
Table 1.
Demographic data of study participants with >200 measurements upon admission (N=217).
| Characteristics | No deterioration (n=193) | Deteriorated (n=24) | P value | ||
| Age (years), mean (SD) | 70.3 (15.2) | 71.8 (13.1) | .65 | ||
| Sex (male/female), n/n | 115/79 | 16/8 | .52 | ||
| BMI (kg/m2), mean (SD) | 27.1 (5.4) | 24.8 (6.2) | .05 | ||
| Ethnicity, n | .82 | ||||
|
|
Ashkenazy | 83 | 11 |
|
|
|
|
Sephardi | 104 | 13 |
|
|
|
|
Arabic | 6 | 0 |
|
|
|
|
Other | 1 | 0 |
|
|
| Blood oxygen saturation (%), mean (SD) | 92.9 (10.9) | 95.5 (2.2) | .30 | ||
| Respiratory rate (breaths/min), mean (SD) | 17.7 (3.5) | 17.2 (2.2) | .50 | ||
| Temperature (°C), mean (SD) | 37.3 (0.6) | 37.2 (0.6) | .31 | ||
| Heart rate (beats/min), mean (SD) | 80.9 (17.7) | 79.9 (17.4) | .80 | ||
| Systolic blood pressure (mm Hg), mean (SD) | 129.3 (24.4) | 131.0 (25.1) | .75 | ||
| Diastolic blood pressure (mm Hg), mean (SD) | 68.7 (14.0) | 72.2 (14.3) | .24 | ||
| Pulse pressure (mm Hg), mean (SD) | 60.6 (20.9) | 58.8 (24.3) | .69 | ||
| Mean arterial pressure (mm Hg), mean (SD) | 88.9 (15.2) | 91.8 (14.7) | .37 | ||
| Stroke volume (mL), mean (SD) | 72.0 (13.6) | 74.7 (17.0) | .37 | ||
| Cardiac output (L/min), mean (SD) | 5.7 (0.9) | 5.8 (0.9) | .39 | ||
| Cardiac index (L/min/m2), mean (SD) | 3.1 (0.6) | 3.1 (0.9) | .49 | ||
| Systemic vascular resistance (dynes•s/cm5), mean (SD) | 1283.0 (256.1) | 1305.3 (319.9) | .70 | ||
| Background diagnosis, n (%) | |||||
|
|
Ischemic heart disease | 61 (31.6) | 11 (46) | .17 | |
|
|
Hypertension | 119 (73) | 17 (71) | .50 | |
|
|
Congestive heart failure | 21 (10.9) | 4 (17) | .49 | |
|
|
Diabetes mellitus | 77 (39.9) | 9 (38) | >.99 | |
|
|
Obesity | 21 (10.9) | 0 (0) | .14 | |
|
|
Valve disease | 15 (7.8) | 2 (8) | >.99 | |
|
|
Chronic obstructive pulmonary disease | 38 (19.7) | 2 (8) | .26 | |
|
|
Asthma | 12 (6.2) | 2 (8) | .66 | |
|
|
Cerebrovascular accident | 26 (13.5) | 3 (13) | >.99 | |
|
|
Chronic kidney disease | 48 (24.9) | 6 (25) | >.99 | |
|
|
Epilepsy | 2 (1.0) | 0 (0) | >.99 | |
|
|
Previous surgery | 107 (55.4) | 12 (50) | .67 | |
|
|
Arrhythmia | 60 (31.1) | 8 (33) | .82 | |
|
|
Anemia | 27 (14.0) | 3 (13) | >.99 | |
|
|
Active malignancy | 40 (20.7) | 6 (25) | .60 | |
|
|
Past malignancy | 11 (5.7) | 3 (13) | .19 | |
|
|
Thyroid | 27 (14.0) | 4 (17) | .76 | |
|
|
Pacemaker | 13 (6.7) | 1 (4) | >.99 | |
|
|
Depression | 8 (4.1) | 0 (0) | .60 | |
|
|
Bronchiectasis or cystic fibrosis | 2 (1.0) | 2 (8) | >.99 | |
|
|
COVID-19 | 2 (1.0) | 1 (4) | .30 | |
| Length of stay (days), mean (SD) | 2.2 (2.3) | 2.4 (0.8) | .61 | ||
Baseline measurements were not significantly different between patients who deteriorated and those who did not. Actual clinical deterioration was detected by the medical staff in 24 of the 217 (11.1%) patients (Table 2).
Table 2.
Comparison of different tools for early detection of patient deterioration (relating to first-time alerts only).
| Tools | Patients in whom an early risk alert was generated by the scoring system, n (%) | Time of detection prior to actual clinical deterioration (hours), n | |
|
|
No deterioration (n=193) | Deteriorated (n=24) |
|
| National Early Warning Score | 150 (77.7) | 16 (67) | 29 |
| Airway, Breathing, Circulation, Neurology, and Other scorea | 162 (83.9) | 18 (75) | 38 |
| The clinical definition of deterioration by local medical staff (“wish list”) | 193 (100) | 24 (100) | 40 |
aA locally implemented version of the Airway, Breathing, Circulation, Disability, Exposure criteria for identification of patients' deterioration, as described in Multimedia Appendix 3.
When analyzing the frequent data collected by the wearable monitors, the NEWS method provided an early warning in 16 of the 24 (67%) patients who deteriorated at 29 hours on average before actual deterioration was detected by the medical staff (an example from one patient is shown in Figure 1). The ABCNO criteria were met in 18 of the 24 (75%) patients at 38 hours on average before actual deterioration was detected by the medical staff. Early warning based on the “wish list” criteria was detected in all 24 patients at 40 hours on average before it was detected by the medical staff.
Figure 1.
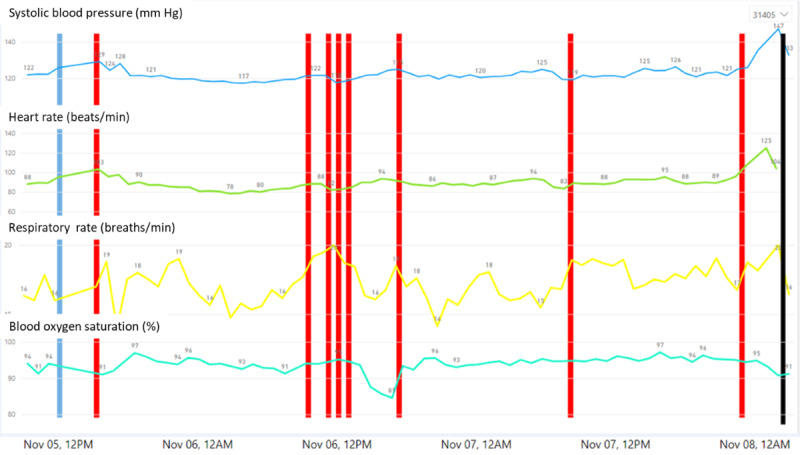
Trends of continuous data gathered by the monitoring platform. Sample of the monitoring data from a single patient, showing systolic blood pressure (mm Hg), heart rate (beats/min), respiratory rate (breaths/min), blood oxygen saturation (%), and markings of warnings and prediction. The black line indicates the time of actual clinical detection of deterioration by the medical staff. Red lines indicate times at which high-risk warnings were provided by the platform using the National Early Warning Score.
In total, 193 patients did not experience clinical deterioration during the index hospitalization. However, NEWS provided early warning alerts in 150 of the 193 (77.7%) patients, ABCNO criteria were met in 162 of the 193 (83.9%) patients, and when following the “wish list” criteria, all 193 patients who did not deteriorate had early warning alerts.
When measuring the sensitivity and specificity of the methods applied, NEWS revealed a sensitivity of 67% and specificity of 22%; ABCNO score, 75% and 16%; and the “wish list” criteria, 100% and 0%, respectively (Table 3).
Table 3.
Comparison of the sensitivity and specificity of different tools of early detection of deterioration in patients with >200 measurements (N=217).
|
|
Sensitivity, % | Specificity, % |
| National Early Warning Score | 67 | 22 |
| Airway, Breathing, Circulation, Neurology, and Other scorea | 75 | 16 |
| The clinical definition of deterioration by local medical staff (“wish list”) | 100 | 0 |
aA locally implemented version of the Airway, Breathing, Circulation, Disability, Exposure criteria for identification of patients' deterioration, as described in Multimedia Appendix 3.
Discussion
Principal Findings
In this study, we have assessed an automated frequent RPM platform with several integrated EWS systems. Alerts were provided many hours before patients have clinically deteriorated; however, similar alerts were also provided for patients who did not deteriorate, questioning the suitability of these EWS systems when frequent monitoring is available. EWS systems are used by health care providers to identify the early signs of clinical deterioration and initiate prompt intervention and management, including nursing staff attention, notifying the clinicians, or activating a rapid response team [27]. A numeric value is assigned to several physiologic parameters, and a composite score is derived and used to identify a patient at risk of deterioration. Most are based on an aggregate weighted system in which the elements are assigned different points for the degree of physiological abnormalities, such as those presented in Multimedia Appendices 2-4. Previous observational studies have suggested that patients often show signs of clinical deterioration up to 24 hours before a serious medical event necessitating an intervention [28]. Delays in care or insufficient treatment of patients on general hospital wards may result in increased admissions to the ICU, cardiac arrest, increased length of hospital stay, or death [28]. The purpose of the EWS scores is to ensure timely and appropriate management of deteriorating patients in general hospital wards. Moreover, for the complex patient population admitted in the general ward and the medical staff treating them, an early warning could be the difference between prevention and late response to decompensation. We also show that when using current early warning systems in a frequent measurement mode, the sensitivity is high, yet the specificity is low, potentially leading to provider fatigue in real-world settings. This is further emphasized when using the “wish list” definitions provided by the medical staff, which has led to an early warning in all 217 patients included in the final analysis, including the 193 patients who had no actual clinical deterioration. This clearly shows that the “wish list” criteria cannot be used for early warning. Previous studies have also shown the relatively high sensitivity of EWS systems; yet, among all patients, the specificity was low [29]. Moreover, in many cases, they provide too many alerts leading to alert fatigue [30].
These results might lead to claims suggesting that clinical judgment is more effective than any EWS system, highlighting the importance of holistic patient care and good clinical judgment. However, it seems that by further improving these EWS systems, sensitivity could be kept high, while specificity would be higher. This was not achieved yet, but preliminary data from various studies implementing big data analysis of multiple physiologic parameters collected automatically and frequently already show promise in early detection of clinically significant changes, and this could eventually result in the desired combination for future EWSs [24,31].
Limitations
Though a limitation of this study is that the health care providers were not using the RPM system in real time and did not react to the warnings it provided, it is expected that in a real-world scenario, once an early warning is provided, the medical staff will intervene and provide the relevant medical support, thus changing the clinical course of the patients from that moment. Importantly, we do not know whether the alerts provided by the different early warning tools resolved spontaneously or whether patients received medical treatment coincidentally at the same time, leading to patient improvement and the resolution of the alert.
At a more practical level, we show that using an RPM system with frequent measurements is feasible in the acute care setting within the general ward. Though 410 patients were recruited, continuous monitoring was achieved properly in only 217 patients (more than 200 measurement sessions) owing to mis-attachment of the wearable monitoring devices. We assume that the reason for that was the blinding of the medical staff from access to the real-time data. Though upon attaching the monitoring devices, the research team ensured that the sensors were well attached and transmitted the data properly, from that moment on, there was no real-time and continuous indication on the quality of the signal. We did see substantial improvement with time, showing that a positive learning curve was rapidly reached and that the devices are simple to use. Moreover, in a real-time scenario, where the medical staff will rely on such a wearable monitoring system, they will immediately receive a notification of an improper signal and will reattach the sensor.
In terms of efficiency, once connected, the data were seamlessly and automatically transferred into the data collection repository and, in parallel, could have been presented on the monitoring screens of the department. This part was not available to the medical staff as they were blinded to real-time monitoring data. EWS compliance is often found to be poor for several reasons, including misinterpretation or incorrect calculation of the scores and poor communications [32]. However, this becomes irrelevant when using an automatically generated and transmitted EWS score.
Further development and future studies are needed to provide an advanced EWS tool that would have higher sensitivity and specificity, making it a better-suited tool in real-world settings, focusing on presymptomatic warnings of potential patient deterioration, to be used as a preventive measure and as a medical decision support tool in both the outpatient and in-hospital settings. The combination of frequently collected multiple physiological parameters, an advanced algorithm, and timely alerts could potentially provide medical staff peace of mind, knowing that they are called only when there is an imminent threat of clinical importance. Moreover, improved prediction of deterioration would have vast positive outcomes when considering the low availability of telemetry beds in hospitals.
Another limitation of the study is that we did not have continuous measurements for all patients during the whole monitoring period. Nonetheless, the data set is much larger than what usually is collected within the general wards, and it still provides important insights. This should be further optimized in future studies on this subject.
Conclusions
To conclude, frequent RPM allows for early detection of physiological changes with potential clinical significance. The integration of an EWS system may provide another layer of clinical awareness, serving as an important decision support tool for early medical intervention. Current scoring systems have high sensitivity but low specificity and warrant further development when combined with frequent multiparameter monitoring. The frequency of measurements alone, though providing a better understanding of trajectories of various vital signs, is not enough to provide an improved EWS score, and in practice, this might be translated into a high rate of alarms, complicating its hospital implementation.
Future systems, which would rely on frequent collection and calculation of the EWS score, could provide better sensitivity and specificity and should be better adjusted to provide tailored scores for the prevention of different medical conditions.
Acknowledgments
This study was funded by the Israel Innovation Authority (grant 64695). The funding source had no active role in this study.
Abbreviations
- ABCNO
Airway, Breathing, Circulation, Neurology, and Other
- AI
artificial intelligence
- EWS
early warning score
- ICU
intensive care unit
- NEWS
National Early Warning Score
- RPM
remote patient monitoring
CONSORT Patient and Analysis Flow.
National Early Warning Score (NEWS).
Medical Emergency Team activation criteria.
Deterioration criteria are defined by the clinical teams of the general wards assuming continuous monitoring available.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Rocha HAL, Alcântara ACC, Rocha SGMO, Toscano CM. Effectiveness of rapid response teams in reducing intrahospital cardiac arrests and deaths: a systematic review and meta-analysis. Rev Bras Ter Intensiva. 2018;30(3):366–375. doi: 10.5935/0103-507X.20180049. https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-507X2018000300366&lng=en&nrm=iso&tlng=en .S0103-507X2018000300366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunch JL, Groves PS, Perkhounkova Y. Realistic Evaluation of a Rapid Response System: Context, Mechanisms, and Outcomes. West J Nurs Res. 2019 Apr;41(4):519–536. doi: 10.1177/0193945918776310. [DOI] [PubMed] [Google Scholar]
- 3.Le Lagadec MD, Dwyer T. Scoping review: The use of early warning systems for the identification of in-hospital patients at risk of deterioration. Aust Crit Care. 2017 Jul;30(4):211–218. doi: 10.1016/j.aucc.2016.10.003.S1036-7314(16)30133-3 [DOI] [PubMed] [Google Scholar]
- 4.Chong-Yik R, Bennett AL, Milani RV, Morin DP. Cost-Saving Opportunities with Appropriate Utilization of Cardiac Telemetry. Am J Cardiol. 2018 Nov 01;122(9):1570–1573. doi: 10.1016/j.amjcard.2018.07.016.S0002-9149(18)31488-7 [DOI] [PubMed] [Google Scholar]
- 5.Green M, Lander H, Snyder A, Hudson P, Churpek M, Edelson D. Comparison of the Between the Flags calling criteria to the MEWS, NEWS and the electronic Cardiac Arrest Risk Triage (eCART) score for the identification of deteriorating ward patients. Resuscitation. 2018 Feb;123:86–91. doi: 10.1016/j.resuscitation.2017.10.028. http://europepmc.org/abstract/MED/29169912 .S0300-9572(17)30682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pullinger R, Wilson S, Way R, Santos M, Wong D, Clifton D, Birks J, Tarassenko L. Implementing an electronic observation and early warning score chart in the emergency department: a feasibility study. Eur J Emerg Med. 2017 Dec;24(6):e11–e16. doi: 10.1097/MEJ.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 7.Prgomet M, Cardona-Morrell M, Nicholson M, Lake R, Long J, Westbrook J, Braithwaite J, Hillman K. Vital signs monitoring on general wards: clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int J Qual Health Care. 2016 Sep;28(4):515–521. doi: 10.1093/intqhc/mzw062.mzw062 [DOI] [PubMed] [Google Scholar]
- 8.Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic Yield of Extended Cardiac Patch Monitoring in Patients with Stroke or TIA. Front Neurol. 2014;5:266. doi: 10.3389/fneur.2014.00266. doi: 10.3389/fneur.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin B, Wong AM, Tseng KC. Community-Based ECG Monitoring System for Patients with Cardiovascular Diseases. J Med Syst. 2016 Apr;40(4):80. doi: 10.1007/s10916-016-0442-4.10.1007/s10916-016-0442-4 [DOI] [PubMed] [Google Scholar]
- 10.Posthuma LM, Downey C, Visscher MJ, Ghazali DA, Joshi M, Ashrafian H, Khan S, Darzi A, Goldstone J, Preckel B. Remote wireless vital signs monitoring on the ward for early detection of deteriorating patients: A case series. Int J Nurs Stud. 2020 Apr;104:103515. doi: 10.1016/j.ijnurstu.2019.103515.S0020-7489(19)30322-0 [DOI] [PubMed] [Google Scholar]
- 11.Muralitharan S, Nelson W, Di S, McGillion M, Devereaux PJ, Barr NG, Petch J. Machine Learning-Based Early Warning Systems for Clinical Deterioration: Systematic Scoping Review. J Med Internet Res. 2021 Feb 04;23(2):e25187. doi: 10.2196/25187. https://www.jmir.org/2021/2/e25187/ v23i2e25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkinson PJ, Barber VS, Price JD, Hann A, Tarassenko L, Young JD. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia. 2006 Nov;61(11):1031–1039. doi: 10.1111/j.1365-2044.2006.04818.x. doi: 10.1111/j.1365-2044.2006.04818.x.ANA4818 [DOI] [PubMed] [Google Scholar]
- 13.Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation. 2016 May;102:1–5. doi: 10.1016/j.resuscitation.2016.02.005. http://europepmc.org/abstract/MED/26898412 .S0300-9572(16)00077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taenzer AH, Perreard IM, MacKenzie T, McGrath SP. Characteristics of Desaturation and Respiratory Rate in Postoperative Patients Breathing Room Air Versus Supplemental Oxygen: Are They Different? Anesth Analg. 2018 Mar;126(3):826–832. doi: 10.1213/ANE.0000000000002765. [DOI] [PubMed] [Google Scholar]
- 15.Brunetti E, Isaia G, Rinaldi G, Brambati T, De Vito D, Ronco G, Bo M. Comparison of Diagnostic Accuracies of qSOFA, NEWS, and MEWS to Identify Sepsis in Older Inpatients With Suspected Infection. J Am Med Dir Assoc. 2022 May;23(5):865–871.e2. doi: 10.1016/j.jamda.2021.09.005.S1525-8610(21)00820-3 [DOI] [PubMed] [Google Scholar]
- 16.Credland N, Dyson J, Johnson MJ. Do early warning track and trigger tools improve patient outcomes? A systematic synthesis without meta-analysis. J Adv Nurs. 2021 Mar;77(2):622–634. doi: 10.1111/jan.14619. [DOI] [PubMed] [Google Scholar]
- 17.Bilben B, Grandal L, Søvik S. National Early Warning Score (NEWS) as an emergency department predictor of disease severity and 90-day survival in the acutely dyspneic patient - a prospective observational study. Scand J Trauma Resusc Emerg Med. 2016 Jun 02;24:80. doi: 10.1186/s13049-016-0273-9. https://sjtrem.biomedcentral.com/articles/10.1186/s13049-016-0273-9 .10.1186/s13049-016-0273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alam N, Vegting IL, Houben E, van Berkel B, Vaughan L, Kramer MHH, Nanayakkara PWB. Exploring the performance of the National Early Warning Score (NEWS) in a European emergency department. Resuscitation. 2015 May;90:111–115. doi: 10.1016/j.resuscitation.2015.02.011.S0300-9572(15)00078-7 [DOI] [PubMed] [Google Scholar]
- 19.Ehara J, Hiraoka E, Hsu H, Yamada T, Homma Y, Fujitani S. The effectiveness of a national early warning score as a triage tool for activating a rapid response system in an outpatient setting: A retrospective cohort study. Medicine (Baltimore) 2019 Dec;98(52):e18475. doi: 10.1097/MD.0000000000018475. doi: 10.1097/MD.0000000000018475.00005792-201912270-00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith D, Bowden T. Using the ABCDE approach to assess the deteriorating patient. Nurs Stand. 2017 Nov 29;32(14):51–63. doi: 10.7748/ns.2017.e11030.36 [DOI] [PubMed] [Google Scholar]
- 21.Nachman D, Gepner Y, Goldstein N, Kabakov E, Ishay AB, Littman R, Azmon Y, Jaffe E, Eisenkraft A. Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Sci Rep. 2020 Sep 30;10(1):16116. doi: 10.1038/s41598-020-73172-3. doi: 10.1038/s41598-020-73172-3.10.1038/s41598-020-73172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachman D, Constantini K, Poris G, Wagnert-Avraham L, Gertz SD, Littman R, Kabakov E, Eisenkraft A, Gepner Y. Wireless, non-invasive, wearable device for continuous remote monitoring of hemodynamic parameters in a swine model of controlled hemorrhagic shock. Sci Rep. 2020 Oct 19;10(1):17684. doi: 10.1038/s41598-020-74686-6. doi: 10.1038/s41598-020-74686-6.10.1038/s41598-020-74686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atzmon Y, Ben Ishay E, Hallak M, Littman R, Eisenkraft A, Gabbay-Benziv R. Continuous Maternal Hemodynamics Monitoring at Delivery Using a Novel, Noninvasive, Wireless,PPG-Based Sensor. J Clin Med. 2020 Dec 22;10(1) doi: 10.3390/jcm10010008. https://www.mdpi.com/resolver?pii=jcm10010008 .jcm10010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenkraft A, Maor Y, Constantini K, Goldstein N, Nachman D, Levy R, Halberthal M, Horowitz NA, Golan R, Rosenberg E, Lavon E, Cohen O, Shapira G, Shomron N, Ishay AB, Sand E, Merin R, Fons M, Littman R, Gepner Y. Continuous Remote Patient Monitoring Shows Early Cardiovascular Changes in COVID-19 Patients. J Clin Med. 2021 Sep 17;10(18) doi: 10.3390/jcm10184218. https://www.mdpi.com/resolver?pii=jcm10184218 .jcm10184218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar-On E, Segal G, Regev-Yochay G, Barkai G, Biber A, Irony A, Luttinger A, Englard H, Grinberg A, Katorza E, Rahav G, Afek A, Kreiss Y. Establishing a COVID-19 treatment centre in Israel at the initial stage of the outbreak: challenges, responses and lessons learned. Emerg Med J. 2021 May;38(5):373–378. doi: 10.1136/emermed-2020-209639. http://europepmc.org/abstract/MED/33771818 .emermed-2020-209639 [DOI] [PubMed] [Google Scholar]
- 26.McKinney W. Data Structures for Statistical Computing in Python. 9th Python in Science Conference (SciPy 2010); June 28 - July 3, 2010; Austin, TX. 2010. https://pandas.pydata.org/ [DOI] [Google Scholar]
- 27.Whittington J, White R, Haig KM, Slock M. Using an automated risk assessment report to identify patients at risk for clinical deterioration. Jt Comm J Qual Patient Saf. 2007 Sep;33(9):569–574. doi: 10.1016/s1553-7250(07)33061-4.S1553-7250(07)33061-4 [DOI] [PubMed] [Google Scholar]
- 28.McGaughey J, Alderdice F, Fowler R, Kapila A, Mayhew A, Moutray M. Outreach and Early Warning Systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD005529. doi: 10.1002/14651858.CD005529.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Forster S, Housley G, McKeever TM, Shaw DE. Investigating the discriminative value of Early Warning Scores in patients with respiratory disease using a retrospective cohort analysis of admissions to Nottingham University Hospitals Trust over a 2-year period. BMJ Open. 2018 Jul 30;8(7):e020269. doi: 10.1136/bmjopen-2017-020269. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=30061434 .bmjopen-2017-020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennell TI, Cimino JJ. A Potential Answer to the Alert Override Riddle: Using Patient Attributes to Predict False Positive Alerts. AMIA Annu Symp Proc. 2019;2019:532–541. http://europepmc.org/abstract/MED/32308847 . [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein N, Eisenkraft A, Arguello CJ, Yang GJ, Sand E, Ishay AB, Merin R, Fons M, Littman R, Nachman D, Gepner Y. Exploring Early Pre-Symptomatic Detection of Influenza Using Continuous Monitoring of Advanced Physiological Parameters during a Randomized Controlled Trial. J Clin Med. 2021 Nov 08;10(21) doi: 10.3390/jcm10215202. https://www.mdpi.com/resolver?pii=jcm10215202 .jcm10215202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Lagadec MD, Dwyer T, Browne M. The efficacy of twelve early warning systems for potential use in regional medical facilities in Queensland, Australia. Aust Crit Care. 2020 Jan;33(1):47–53. doi: 10.1016/j.aucc.2019.03.001.S1036-7314(18)30359-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Patient and Analysis Flow.
National Early Warning Score (NEWS).
Medical Emergency Team activation criteria.
Deterioration criteria are defined by the clinical teams of the general wards assuming continuous monitoring available.


