Key Points
Question
Is up-front surgery associated with decreased risk of stroke compared with definitive (chemo)radiotherapy in patients with oropharyngeal squamous cell carcinoma (OPSCC)?
Findings
In this cohort study of 10 436 US veterans diagnosed with OPSCC from 2000 to 2020, cumulative incidence of stroke was 12.5% at 10 years. After propensity score and inverse probability weighting, up-front surgery was associated with a 23% reduced risk of stroke compared with nonsurgical therapy.
Meaning
This study suggests an important additional risk-benefit consideration in the ongoing debate about optimal OPSCC management and should motivate future studies to examine cardiovascular events in this high-risk population.
This cohort study evaluates the association of up-front surgery and stroke risk compared with nonsurgical treatment for oropharyngeal squamous cell carcinoma among US veterans.
Abstract
Importance
Cardiovascular events are an important cause of morbidity in patients with oropharyngeal squamous cell carcinoma (OPSCC). Radiation and chemotherapy have been associated with increased risk of stroke; up-front surgery allows the opportunity for (chemo)radiotherapy de-escalation.
Objective
To evaluate whether up-front surgery was associated with decreased stroke risk compared to nonsurgical treatment for OPSCC.
Design, Setting, and Participants
This cohort study was conducted at the US Veterans Health Administration and examined US veterans diagnosed with nonmetastatic OPSCC from 2000 to 2020. Data cutoff was September 17, 2021, and data analysis was performed from October 2021 to February 2022.
Exposures
Up-front surgical treatment or definitive (chemo)radiotherapy as captured in cancer registry.
Main Outcomes and Measures
Cumulative incidence of stroke, accounting for death as a competing risk; and association between up-front surgery and stroke risk. After generating propensity scores for the probability of receiving surgical treatment and using inverse probability weighting (IPW) to construct balanced pseudo-populations, Cox regression was used to estimate a cause-specific hazard ratio (csHR) of stroke associated with surgical vs nonsurgical treatment.
Results
Of 10 436 patients, median (IQR) age was 61 (56-67) years; 10 329 (99%) were male; 1319 (13%) were Black, and 7823 (75%) were White; 2717 received up-front surgery, and 7719 received nonsurgical therapy with definitive (chemo)radiotherapy. The 10-year cumulative incidence of stroke was 12.5% (95% CI, 11.8%-13.3%) and death was 57.3% (95% CI, 56.2%-58.4%). Surgical patients who also received (chemo)radiotherapy had shorter radiation and chemotherapy courses than nonsurgical patients. After propensity score and IPW, the csHR of stroke for surgical treatment was 0.77 (95% CI, 0.66-0.91). This association was consistent across subgroups defined by age and baseline cardiovascular risk factors.
Conclusions and Relevance
In this cohort study, up-front surgical treatment was associated with a 23% reduced risk of stroke compared with definitive (chemo)radiotherapy. These findings present an important additional risk-benefit consideration to factor into treatment decisions and patient counseling and should motivate future studies to examine cardiovascular events in this high-risk population.
Introduction
Efforts to de-escalate treatment for oropharyngeal squamous cell carcinomas (OPSCC) have largely focused on mitigating toxicities related to functional outcomes such as dysphagia and xerostomia.1,2,3 Several large epidemiologic studies have shown that survivors of head and neck cancer also face substantial competing risks from cardiovascular and cerebrovascular disease, which can equal or exceed their risk of cancer-specific mortality.4,5,6,7 Cancer therapy may potentiate these risks: radiation therapy to the neck has been associated with increased rates of carotid artery stenosis,8 carotid intima medial thickness,9 and stroke in both younger10,11 and older12,13 patient cohorts. In a SEER-Medicare study of head and neck cancer patients over 65 treated with radiotherapy, 10-year incidence of cerebrovascular events was 34%, with a 50% increased risk compared with patients treated with surgery alone.12 Chemotherapy agents including cisplatin can also be toxic to the heart and vasculature.14 Thus, cardiovascular risk reduction is important not only for older patients with traditional shared risk factors for OPSCC such as smoking and alcohol consumption,15 but also for younger patients with HPV-positive disease who have longer life expectancies16,17 during which to experience therapy-induced complications.18
Compared with definitive radiotherapy-based treatment for OPSCC, up-front surgical approaches including trans-oral robotic surgery afford the opportunity to de-escalate or avoid radiotherapy and chemotherapy.19,20 Patients who undergo up-front surgery may still receive radiation or chemoradiation if high-risk features are seen on pathology, but radiation used in the adjuvant setting is generally administered at a lower dose than definitive radiotherapy. Indeed, several retrospective studies have suggested that postoperative radiotherapy, in contrast to definitive radiotherapy, is not associated with an increased risk of stroke in head and neck cancer.10,12 However, there is wide clinical practice variation and continuing debate regarding optimal treatment and de-escalation strategies for OPSCC, and it remains unknown whether up-front surgical therapy may reduce the risk of cerebrovascular events in this population.
To address this knowledge gap, we sought to first quantify the risk of stroke in patients with OPSCC without prior cerebrovascular disease in the setting of competing risk of death, and then determine whether treatment with up-front surgery, compared with nonsurgical therapy, was associated with a lower risk of stroke. We investigated these questions in a large contemporary cohort of US veterans, who have a high burden of shared risk factors for head and neck cancer and cardiovascular disease,21,22,23 and a consolidated health record that comprehensively captures oncologic and cardiovascular end points.
Methods
Data Source and Cohort
The electronic health record data of veterans with OPSCC newly diagnosed within the Veterans Health Administration from 2000 to 2020 were obtained and accessed through the Corporate Data Warehouse (CDW). This research protocol was approved by the institutional review boards at the Veterans Affairs (VA) Medical Centers of Philadelphia, Pennsylvania, and Salt Lake City, Utah. These data were collected under waiver of informed consent and Health Insurance Portability and Accountability Act authorization; waiver of informed consent was granted because this study was a retrospective medical record review with deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Complete analytic cases of OPSCC diagnosed during this period were identified from the VA Central Cancer Registry using a combination of site, diagnosis, and procedure codes (eTable in the Supplement). Patients with distant metastatic disease at diagnosis, as well as patients who either did not receive documented cancer therapy or who received only systemic therapy (no surgery or radiation), were excluded. Patients with a history of stroke prior to initiating cancer therapy were also excluded.
Exposures
Treatment modality was determined based on VA Cancer Registry data and defined as up-front surgery or nonsurgical therapy. All nonsurgical patients received definitive chemoradiotherapy or radiation therapy. Patients in the up-front surgery group received surgery either alone or with radiation or chemoradiation. Dates of chemotherapy and radiation therapy administration were obtained from tables within CDW; durations of up to 90 days were considered as initial definitive or adjuvant therapy.
Outcome
Our primary outcome was time from treatment initiation to first occurrence of stroke or transient ischemic attack (TIA), death, or loss to follow-up. Incident stroke or TIA was defined as 2 or more outpatient diagnosis codes, or 1 or more inpatient diagnosis code, with first date of diagnosis used (eTable in the Supplement).24,25 Death was ascertained from vital status and other tables within CDW.
Covariates
Patient-level information including age, sex, race, smoking status, marital status, national deprivation index based on neighborhood mapping,26,27 performance status, Charlson comorbidity index, and baseline comorbidities (hypertension, hyperlipidemia, obesity, atrial fibrillation, diabetes, carotid artery stenosis, coronary artery disease, and kidney disease) were obtained from tables within CDW. Baseline cardiovascular risk factors were defined using International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coding definitions adapted from prior VA-based studies (eTable in the Supplement).28,29,30 Cancer-related variables including diagnosis year, T and N stage were obtained from Cancer Registry data.
Statistical Analysis
To quantify the risk of stroke in the setting of competing risks of mortality, we used a competing risks model to estimate the cumulative incidence of stroke with death as a competing event. We calculated 5- and 10-year cumulative incidence estimates with 95% CIs for both stroke and death.
Next, we assessed the association between treatment modality and risk of incident stroke. To account for observed confounders and baseline imbalance between patients treated with surgical and nonsurgical approaches, we estimated a propensity score for the probability of receiving surgical treatment, using logistic regression incorporating a full list of potential confounders (age, sex, diagnosis year, race, T and N stage, performance status, Charlson comorbidity index, smoking status, marital status, deprivation index, and baseline hypertension, hyperlipidemia, obesity, atrial fibrillation, diabetes, carotid artery stenosis, coronary disease, and kidney disease) as covariates. Because some variables had missing data, multiple imputation was used for all propensity score–based analyses. Complete data sets were imputed using multiple imputation via chained equations.31 Using the generated propensity scores, inverse probability weighting was used to construct balanced pseudo-populations. The positivity assumption was assessed visually based on overlap in kernel density and box plots for propensity scores in the up-front surgery and nonsurgical therapy groups. Standardized differences were estimated in the inverse probability–weighted population to ensure postweighting balance between the treatment groups for all potential confounders.
Finally, to test our hypothesis that up-front surgery was associated with a decreased risk of stroke, Cox regression of the inverse probability–weighted population was used to estimate the cause-specific hazard ratio of stroke associated with receiving surgical vs nonsurgical treatment. Because we were interested in the etiologic association between treatment type and stroke, rather than prognostic questions, we explicitly chose Cox regression and cause-specific hazard over Fine and Gray methodology and subdistribution hazard. Proportional hazards assumption was assessed using Schoenfeld residuals. We generated weighted Kaplan-Meier failure curves for stroke for the up-front surgery and nonsurgical pseudopopulations. Analyses using inverse probability weighting were conducted within each of the multiply imputed data sets. Results were combined across imputations using Rubin rules.
As a secondary analysis, we also investigated the association between up-front surgery and stroke risk in several subgroups of interest: younger patients (aged 65 years or younger) vs older patients (aged 65 years or more), and patients with and without a baseline history of hypertension, diabetes, and hyperlipidemia. We first investigated whether covariate balance across treatment groups was preserved within each subgroup. Next, after balance was confirmed, we conducted Cox regression of the weighted population to estimate the cause-specific hazard ratio of stroke associated with surgical vs nonsurgical treatment and plotted Kaplan-Meier curves within each subgroup.
Stata version 17 (StataCorp) was used to conduct all analyses from October 2021 to February 2022. Statistical significance was defined as a 2-sided P < than .05.
Results
Cohort Description and Baseline Characteristics
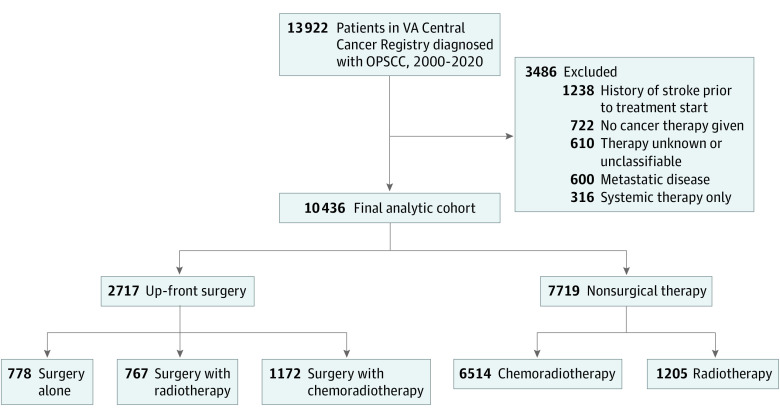
Of 13 922 US veterans diagnosed with squamous cell carcinoma of the oropharynx (OPSCC) from 2000 to 2020 who were identified as complete analytic cases in the cancer registry, 3486 were excluded due to metastatic disease at diagnosis (n = 600), unknown or no cancer therapy administered (n = 1332), systemic treatment alone (n = 316), or history of stroke prior to treatment start (n = 1238), in whom subsequent codes for stroke were thought to be unreliable as indicators of new as opposed to prevalent events. Our analytic cohort thus consisted of 10 436 patients, and the median (IQR) age was 61 (56-67) years; 10 329 (99%) were male, 1319 (13%) were Black, 7823 (75%) were White; and 3963 of 5179 (77%) had a history of smoking. Of these 10 436 patients, 2717 received up-front surgery (with or without radiation/chemotherapy), and 7719 received nonsurgical definitive therapy (radiotherapy or chemoradiotherapy) (Figure 1). The majority of up-front surgery patients either received adjuvant radiotherapy (28% [767 of 2717]) or chemoradiotherapy (43% [1172 of 2717]), in line with prior studies.19,32 As expected, patients treated with up-front surgery had lower stage disease, lower Charlson comorbidity index, and better ECOG performance status (Table 1). Unsurprisingly given these baseline differences, patients treated with up-front surgery had better overall survival than those treated with nonsurgical therapy (median [IQR] overall survival 109.9 [33.3-219.8] months vs 61.3 [15.6-152.1] months) (eFigure 1 in the Supplement). Compared with patients treated with nonsurgical therapy, those treated with up-front surgery who also received (chemo)radiation had a significantly shorter mean (SD) duration of both radiotherapy (45.4 [14.1] days vs 48.2 [13.8] days) and chemotherapy (31.7 [21.9] days vs 33.4 [21.5] days).
Figure 1. Study Inclusion Flow Diagram.
OPSCC indicates oropharyngeal squamous cell carcinoma; VA, Veterans Affairs.
Table 1. Patient Characteristics in Unadjusted and Inverse Probability Weighted Populations.
| Characteristic | Observed | Weighted and imputeda | ||||
|---|---|---|---|---|---|---|
| No. (%)b | SMD | No. (%)b | SMD | |||
| Surgery | Nonsurgical treatment | Surgery | Nonsurgical treatment | |||
| No. | 2717 | 7719 | 2717 | 7719 | ||
| Age, median (IQR) | 60 (55-65) | 62 (56-67) | −0.20 | 62 (56-67) | 61 (56-67) | 0.00 |
| Male | 2679 (98.6) | 7650 (99.1) | −0.05 | 2694 (99.2) | 7644 (99.0) | 0.01 |
| Year of diagnosisc | ||||||
| 2000-2005 | 919 (33.8) | 1986 (25.7) | −0.16 | 835 (30.7) | 2158 (28.0) | −0.02 |
| 2006-2010 | 699 (25.7) | 2112 (27.4) | 674 (24.8) | 2115 (27.4) | ||
| 2011-2015 | 701 (25.8) | 2273 (29.4) | 737 (27.1) | 2150 (27.8) | ||
| 2016-2020 | 398 (14.6) | 1348 (17.5) | 471 (17.3) | 1297 (16.8) | ||
| Race | ||||||
| Black | 291 (12.1) | 1028 (14.9) | −0.08 | 380 (14.0) | 1119 (14.5) | −0.02 |
| White | 2079 (86.5) | 5744 (83.6) | 2304 (84.8) | 6484 (84.0) | ||
| Otherd | 33 (1.4) | 101 (1.5) | 33 (1.2) | 116 (1.5) | ||
| Missing | 314 | 846 | NA | NA | ||
| Clinical tumor stage | ||||||
| Tx or T0 | 496 (19.2) | 265 (3.6) | −0.83 | 205 (7.5) | 563 (7.3) | 0.06 |
| T1 | 815 (31.6) | 1083 (14.8) | 533 (19.6) | 1425 (18.5) | ||
| T2 | 827 (32.1) | 2595 (35.4) | 854 (31.4) | 2752 (35.7) | ||
| T3 | 255 (9.4) | 1545 (21.1) | 429 (15.8) | 1420 (18.4) | ||
| T4 | 211 (7.8) | 1833 (25.0) | 695 (25.6) | 1559 (20.2) | ||
| Missing | 137 | 399 | NA | NA | ||
| Clinical nodal stage | ||||||
| N0 | 1036 (40.1) | 1275 (17.4) | −0.54 | 624 (23.0) | 1751 (22.7) | 0.03 |
| N1 | 434 (16.8) | 1080 (14.7) | 417 (15.4) | 1193 (15.4) | ||
| N2 | 1006 (39.0) | 4522 (61.8) | 1453 (53.5) | 4370 (56.6) | ||
| N3 | 108 (4.2) | 445 (6.1) | 222 (8.2) | 405 (5.3) | ||
| Missing | 133 | 397 | NA | NA | ||
| ECOG performance status | ||||||
| 0 | 483 (56.5) | 1531 (46.5) | −0.21 | 1318 (48.5) | 3768 (48.8) | 0.02 |
| 1 | 324 (37.9) | 1483 (45.0) | 1171 (43.1) | 3356 (43.5) | ||
| 2 or greater | 38 (4.4) | 216 (6.6) | 179 (6.6) | 460 (6.0) | ||
| Missing | 1862 | 4425 | NA | NA | ||
| Charlson comorbidity index | ||||||
| 0 | 1104 (40.6) | 2929 (37.9) | −0.08 | 1057 (38.9) | 2987 (38.7) | 0.00 |
| 1 | 647 (23.8) | 1689 (21.9) | 594 (21.9) | 1715 (22.2) | ||
| 2 or greater | 966 (35.6) | 3101 (40.2) | 1066 (39.2) | 3016 (39.1) | ||
| Smoking status | ||||||
| Current | 827 (63.4) | 3077 (63.5) | 0.01 | 1747 (64.3) | 4903 (63.5) | −0.02 |
| Former | 185 (14.2) | 707 (14.6) | 397 (14.6) | 1122 (14.5) | ||
| Never | 293 (22.5) | 1063 (21.9) | 573 (21.1) | 1695 (22.0) | ||
| Missing | 1413 | 3844 | NA | NA | ||
| Married | 1128 (41.5) | 2872 (37.2) | 0.09 | 1024 (37.7) | 2949 (38.2) | −0.01 |
| Deprivation index | 70 (48, 88) | 71 (48, 88) | −0.03 | 69 (49, 87) | 69 (49, 87) | 0.00 |
| Hypertension | 1499 (55.2) | 4548 (58.9) | −0.08 | 1565 (57.6) | 4469 (57.9) | −0.01 |
| Hyperlipidemia | 1344 (49.5) | 3753 (48.6) | 0.02 | 1302 (47.9) | 3762 (48.7) | −0.02 |
| Diabetes | 528 (19.4) | 1619 (21.0) | −0.04 | 539 (19.8) | 1579 (20.5) | −0.02 |
| Obesity | 482 (17.7) | 1354 (17.5) | 0.01 | 489 (18.0) | 1353 (17.5) | 0.01 |
| Atrial fibrillation | 32 (1.2) | 137 (1.8) | −0.05 | 42 (1.5) | 124 (1.6) | −0.01 |
| Carotid artery stenosis | 17 (0.6) | 52 (0.7) | −0.01 | 17 (0.6) | 50 (0.6) | 0.00 |
| Coronary artery disease | 489 (18.0) | 1584 (20.5) | −0.06 | 535 (19.7) | 1529 (19.8) | 0.00 |
| Kidney disease | 88 (3.2) | 329 (4.3) | −0.05 | 110 (4.0) | 308 (4.0) | 0.00 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NA, not applicable; SMD, standardized mean difference.
Based on inverse probability weighted pseudo-populations averaged across 10 imputed data sets.
Median (IQR) for continuous variables; No. (% of nonmissing data) for categorical variables.
Due to delays in cancer registry abstraction and reporting, VA Cancer Registry data are only up to date to 2016.
Includes Alaskan Native or American Indian, Asian, Native Hawaiian or Other Pacific Islander.
Cumulative Incidence of Stroke and Death
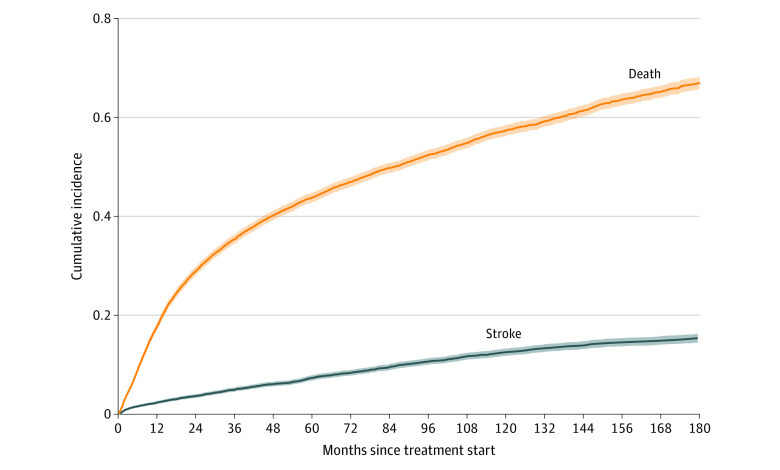
Cumulative incidence functions for stroke (event of interest) and death (competing event) are shown in Figure 2. In this model, across the entire cohort, the cumulative incidence of stroke was 7.37% (95% CI, 6.87%-7.90%) at 5 years and 12.52% (95% CI, 11.81%-13.25%) at 10 years; the cumulative incidence of death was 43.80% (95% CI, 42.82%-44.77%) at 5 years and 57.32% (95% CI, 56.24%-58.38%) at 10 years.
Figure 2. Cumulative Incidence of Stroke and Death in United States Veterans With OPSCC.
Shaded regions indicate 95% CIs; OPSCC, oropharyngeal squamous cell carcinoma.
Association Between Treatment Modality and Stroke Risk
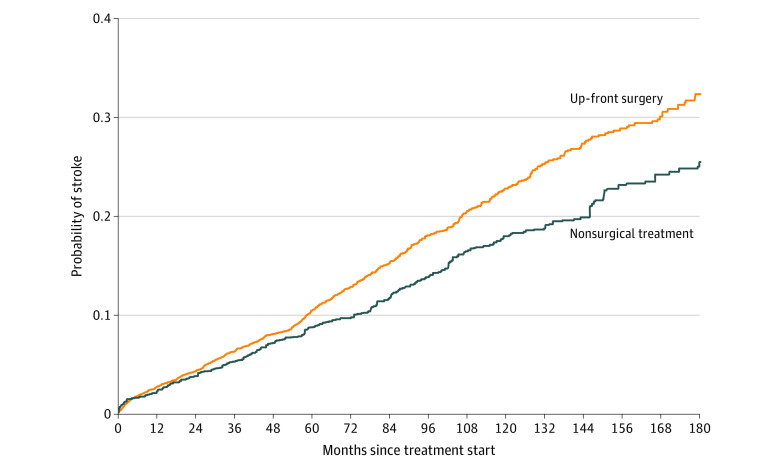
Estimated propensity scores for the surgical and nonsurgical treated populations displayed good overlap. After inverse probability weighting, balance between groups was achieved for all covariates, with standardized mean differences less than 0.1 (Table 1; eFigure 2 in the Supplement). The inverse probability weighted cause-specific hazard ratio of stroke associated with surgical treatment was 0.77 (95% CI, 0.66-0.91) (Table 2). Weighted Kaplan-Meier curves for the up-front surgery and nonsurgical treatment inverse probability weighted groups are presented in Figure 3.
Table 2. Cause-Specific Hazard Ratios Associated With Up-front Surgery, Nonsurgical Treatment, Overall Population, and Key Subgroups.
| Characteristic | No. | Cause-specific HR (95% CI) |
|---|---|---|
| Entire cohort | 10 436 | 0.77 (0.66-0.91) |
| Age, y | ||
| >65 | 3063 | 0.70 (0.49-0.99) |
| ≤65 | 7373 | 0.80 (0.67-0.96) |
| History of hypertension | ||
| Yes | 6047 | 0.83 (0.68-1.01) |
| No | 4389 | 0.68 (0.51-0.90) |
| History of hyperlipidemia | ||
| Yes | 5097 | 0.76 (0.61-0.94) |
| No | 5339 | 0.79 (0.62-1.01) |
| History of diabetes | ||
| Yes | 2147 | 0.70 (0.52-0.96) |
| No | 8289 | 0.80 (0.66-0.96) |
Abbreviation: HR, hazard ratio.
Figure 3. Incidence of Stroke in Patients Undergoing Up-front Surgery vs Nonsurgical Treatment.
Weighted Kaplan-Meier failure curves of stroke incidence, inverse probability weighted pseudopopulations. Numbers at risk are not reported for inverse probability weighted and multiply imputed datasets.
Subgroup Analyses
We next assessed this association between up-front surgery and stroke risk in subgroups defined by age and prevalent cardiovascular risk factors. Covariate balance in inverse probability weighted pseudopopulations was maintained across strata of all subgroups of interest. Cause-specific hazard ratios associated with up-front surgery within subgroups are shown in Table 2; weighted Kaplan-Meier curves by treatment for each subgroup are shown in eFigure 3 in the Supplement. All subgroups except for patients with a history of hypertension and without a history of hyperlipidemia had a significantly decreased risk of stroke with up-front surgery. Proportional hazards assumption was met for all subgroups by Schoenfeld residual testing.
Discussion
Cardiovascular and cerebrovascular disease represent important causes of morbidity and mortality in patients with head and neck cancer.5,6,12 Our study of more than 10 000 veterans with OPSCC without a prior history of stroke showed a 12.5% 10-year cumulative incidence of stroke in the setting of competing mortality risk. This finding is consistent with other studies that have also reported high rates of cardiovascular risk factors,15 carotid artery stenosis,8,33 and stroke11 in patients with head and neck squamous cell carcinoma.
Given prior studies linking neck radiation to stroke risk, as well as increasing use of surgery for OPSCC, we sought to evaluate whether a management strategy of up-front surgery was associated with a lower risk of stroke. We found that up-front surgery was associated with a 23% reduction in hazard of stroke compared with nonsurgical therapy, an effect that was seen across subgroups, including younger patients and those without prior cardiovascular risk factors. More than a quarter of up-front surgery patients avoided chemotherapy and radiotherapy altogether, and the remainder underwent shorter courses of radiotherapy and chemotherapy than nonsurgical patients treated with definitive (chemo)radiotherapy. The observed stroke risk reduction associated with up-front surgery may reflect a combination of treatment avoidance as well as some observed de-escalation of chemotherapy and radiotherapy. We attempted to account for imbalances between groups (ie, healthier patients more likely to be offered surgery) with propensity score and inverse probability weighting methods that incorporated cardiovascular risk factors and comorbidities, but unmeasured confounding remains a possible contributor to the observed difference.
Limitations
This cohort study has limitations to consider. First, the US veterans population is not completely generalizable to the OPSCC population overall, particularly given known higher rates of tobacco and alcohol consumption in veterans with HNSCC.23 Owing to the demographic makeup of veterans with HNSCC, men represented more than 99% of the cohort. We were also not able to reliably ascertain HPV status in the VA data structure. However, recent analyses suggest that the proportion of HPV+ OPSCC in the veteran population is comparable to that of the general population.34 Thus, our contemporary cohort (patients treated from 2000 to2020) likely represents a predominantly HPV+ OPSCC population. We also did not have data on chemotherapy or radiation doses, and we had to use duration of therapy as a proxy for treatment intensity. There was a substantial amount of missing data in covariates including smoking status and performance status; because these data were not missing completely at random, we used multiple imputation by chained equations, which produces unbiased estimates under missing at random missingness.31 Finally, as with all retrospective administrative data, there is a risk of misclassification in variables obtained from ICD-9, ICD-10, and Cancer Registry coding.
Given the aforementioned limitations, these hypothesis-generating results merit validation in other large databases. As important next steps, these findings highlight the importance of cardiovascular risk mitigation efforts in survivors of head and neck cancer and provide support for future de-escalation trials that may explicitly investigate cardiovascular and cerebrovascular outcomes in treatment groups.
Conclusions
To our knowledge, this cohort study is the first to investigate whether up-front surgery may mitigate cardiovascular risk, an increasingly recognized long-term complication of chemoradiotherapy for head and neck cancer. Our analysis of US veterans with OPSCC found a 12.5% cumulative incidence of stroke overall at 10 years, and a significant protective association between up-front surgery and incident risk of stroke. Up-front surgery and definitive (chemo)radiotherapy represent 2 standard treatment approaches for OPSCC that provide similar oncologic outcomes but carry distinct acute and long-term toxicity profiles.35 For patients who are eligible for either up-front surgery or nonsurgical therapy, this study suggests an additional risk-benefit consideration when counseling patients about toxicity tradeoffs and contributes a large body of observational data to the ongoing debate about optimal management for OPSCC.
eTable 1. Coding Definitions
eFigure 1. Kaplan-Meier Curves for Overall Survival, Up-Front Surgery vs Nonsurgical Treatment Groups
eFigure 2. Propensity Score Overlap Between Unweighted and Weighted Treatment Groups
eFigure 3. Stroke Incidence by Surgical vs Non-Surgical Treatment in Key Subgroups, Weighted Kaplan-Meier Curves
References
- 1.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002). J Clin Oncol. 2021;39(9):956-965. doi: 10.1200/JCO.20.03128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swisher-McClure S, Lukens JN, Aggarwal C, et al. A phase 2 trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus-related squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2020;106(4):725-732. doi: 10.1016/j.ijrobp.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 3.Mehanna H, Robinson M, Hartley A, et al. ; De-ESCALaTE HPV Trial Group . Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51-60. doi: 10.1016/S0140-6736(18)32752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120(10):1507-1513. doi: 10.1002/cncr.28588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller CD, Wang SJ, Thomas CR Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973-1998. Cancer. 2007;109(7):1331-1343. doi: 10.1002/cncr.22563 [DOI] [PubMed] [Google Scholar]
- 6.Rose BS, Jeong JH, Nath SK, Lu SM, Mell LK. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29(26):3503-3509. doi: 10.1200/JCO.2011.35.7301 [DOI] [PubMed] [Google Scholar]
- 7.Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10(6):1956-1962. doi: 10.1158/1078-0432.CCR-03-1077 [DOI] [PubMed] [Google Scholar]
- 8.Dorth JA, Patel PR, Broadwater G, Brizel DM. Incidence and risk factors of significant carotid artery stenosis in asymptomatic survivors of head and neck cancer after radiotherapy. Head Neck. 2014;36(2):215-219. doi: 10.1002/hed.23280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gujral DM, Chahal N, Senior R, Harrington KJ, Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014;110(1):31-38. doi: 10.1016/j.radonc.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Chu CN, Chen SW, Bai LY, Mou CH, Hsu CY, Sung FC. Increase in stroke risk in patients with head and neck cancer: a retrospective cohort study. Br J Cancer. 2011;105(9):1419-1423. doi: 10.1038/bjc.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282-288. doi: 10.1200/JCO.2002.20.1.282 [DOI] [PubMed] [Google Scholar]
- 12.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119-5125. doi: 10.1200/JCO.2008.16.6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong JC, Kruser TJ, Gondi V, et al. Risk of cerebrovascular events in elderly patients after radiation therapy versus surgery for early-stage glottic cancer. Int J Radiat Oncol Biol Phys. 2013;87(2):290-6. doi: 10.1016/j.ijrobp.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 14.Patanè S. Cardiotoxicity: cisplatin and long-term cancer survivors. Int J Cardiol. 2014;175(1):201-202. doi: 10.1016/j.ijcard.2014.04.238 [DOI] [PubMed] [Google Scholar]
- 15.Okoye C, Bucher J, Tatsuoka C, et al. Cardiovascular risk and prevention in patients with head and neck cancer treated with radiotherapy. Head Neck. 2017;39(3):527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261-269. doi: 10.1093/jnci/djn011 [DOI] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfister DG, Fury MG. New chapter in our understanding of human papillomavirus-related head and neck cancer. J Clin Oncol. 2014;32(30):3349-3352. doi: 10.1200/JCO.2014.56.5754 [DOI] [PubMed] [Google Scholar]
- 19.Ferris RL, Flamand Y, Weinstein GS, et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol. 2022;40(2):138-149. doi: 10.1200/JCO.21.01752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma DJ, Price KA, Moore EJ, et al. Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus-associated oropharynx squamous cell carcinoma. J Clin Oncol. 2019;37(22):1909-1918. doi: 10.1200/JCO.19.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryar CD, Herrick K, Afful J, Ogden CL. Cardiovascular disease risk factors among male veterans, U.S., 2009-2012. Am J Prev Med. 2016;50(1):101-105. doi: 10.1016/j.amepre.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709. [PMC free article] [PubMed] [Google Scholar]
- 23.Mashberg A, Boffetta P, Winkelman R, Garfinkel L. Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72(4):1369-1375. doi: [DOI] [PubMed] [Google Scholar]
- 24.Navar-Boggan AM, Rymer JA, Piccini JP, et al. Accuracy and validation of an automated electronic algorithm to identify patients with atrial fibrillation at risk for stroke. Am Heart J. 2015;169(1):39-44.e2. doi: 10.1016/j.ahj.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Niesner K, Murff HJ, Griffin MR, et al. Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology. 2013;24(2):334-335. doi: 10.1097/EDE.0b013e3182821e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the Neighborhood Atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.University of Wisconsin School of Medicine and Public Health . 2015. Area Deprivation Index v2.0. Accessed October 25, 2019. https://www.neighborhoodatlas.medicine.wisc.edu/
- 28.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. ; Veterans Aging Cohort Study . HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227-1234. doi: 10.1097/QAD.0b013e32832bd7af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paisible A-L, Chang C-CH, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209-216. doi: 10.1097/QAI.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Parikh RB, Hubbard RA, et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. 2021;4(2):e210070-e210070. doi: 10.1001/jamanetworkopen.2021.0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 32.Zhan KY, Puram SV, Li MM, et al. National treatment trends in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Cancer. 2020;126(6):1295-1305. doi: 10.1002/cncr.32654 [DOI] [PubMed] [Google Scholar]
- 33.Steele SR, Martin MJ, Mullenix PS, Crawford JV, Cuadrado DS, Andersen CA. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187(5):594-598. doi: 10.1016/j.amjsurg.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 34.Shay SG, Chang E, Lewis MS, Wang MB. Characteristics of human papillomavirus-associated head and neck cancers in a veteran population. JAMA Otolaryngol Head Neck Surg. 2015;141(9):790-796. doi: 10.1001/jamaoto.2015.1447 [DOI] [PubMed] [Google Scholar]
- 35.Nichols AC, Theurer J, Prisman E, et al. Randomized trial of radiotherapy versus transoral robotic surgery for oropharyngeal squamous cell carcinoma: long-term results of the ORATOR trial. J Clin Oncol. 2022;40(8):866-875. doi: 10.1200/JCO.21.01961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Coding Definitions
eFigure 1. Kaplan-Meier Curves for Overall Survival, Up-Front Surgery vs Nonsurgical Treatment Groups
eFigure 2. Propensity Score Overlap Between Unweighted and Weighted Treatment Groups
eFigure 3. Stroke Incidence by Surgical vs Non-Surgical Treatment in Key Subgroups, Weighted Kaplan-Meier Curves