Abstract
Background
Leg ulcers are a frequent complication in patients with the inherited hemoglobin disorders. In thalassemia, the literature is limited, and factors associated with the development of leg ulcers in hemoglobin E (HbE) beta thalassemia, the most common form of severe beta-thalassemia worldwide, have not previously been reported.
Methods
We reviewed all available medical records of patients with HbE beta thalassemia to document the onset of leg ulcers at the 2 largest treatment centers in Sri Lanka. We reviewed the literature to identify studies reporting outcomes of interventions for ulcers in severe thalassemia.
Results
Of a total of 255 actively registered patients with HbE thalassemia in the 2 centers, 196 patient charts were evaluable. A leg ulcer with a documented date of onset was recorded in 45 (22%) of 196 evaluable patients, aged (mean ± SEM) 22.2 ± 1.4 years. Most had been irregularly transfused; steady-state hemoglobin was 6.4 ± 0.2 g/dL. Treatment achieving healing in 17 patients included transfusions, antibiotics, oral zinc, wound toileting, and skin grafting.
Conclusion
Leg ulcers may be more common in HbE beta thalassemia than in other forms of thalassemia. A systematic approach to treatment will be needed to document the prevalence and factors placing such patients at risk for leg ulcers. Controlled trials to evaluate the optimal treatment of this common complication are indicated.
Keywords: Ulcer, Thalassemia, Beta-thalassemia, Hemoglobin E beta thalassemia
Introduction
Thalassemia is one of the two most common monogenic diseases in the world. The genotype of hemoglobin E thalassemia (HbE thalassemia) is present in approximately one-half of patients with severe beta-thalassemia worldwide, with the highest frequencies observed throughout Asia [1], including Sri Lanka [2]. HbE thalassemia is also now the most common form of severe beta-thalassemia identified in US newborn screening programs [3].
HbE thalassemia has a highly variable clinical phenotype, ranging from a mild anemia to a clinical course during which transfusions may be required from infancy. Its clinical variability, and the paucity of long-term studies of its natural history, makes the optimal management of HbE thalassemia uncertain. In higher resource countries, most patients are regularly transfused [4]; by contrast, many patients in lower resource countries are transfused irregularly, some only during episodes of infection or surgery, or during pregnancy [1, 3]. In many such patients, complications of anemia and ineffective erythropoiesis are commonly reported. Complications arising in patients with HbE thalassemia [2] that are related primarily to the basic pathophysiology of the disease include hepatosplenomegaly, facial deformity, extramedullary erythropoiesis, and a history of serious infection. We and others [2, 4, 5, 6, 7] have reported additional complications in HbE thalassemia which may develop following splenectomy (including elevated pulmonary artery pressure, increased platelet counts, and venous thromboembolism); and those arising from poorly controlled iron loading (including delayed growth and sexual maturation, hypothyroidism, and diabetes mellitus).
The development of leg ulcers in patients with the inherited hemoglobinopathies may increase disability, and disrupt quality of life. The prevalence and factors influencing leg ulceration have not previously been extensively examined in thalassemia, and no previous examination of this complication in those with HbE thalassemia has been reported.
We examined all available medical records at the 2 centers in Sri Lanka managing the highest numbers of patients with HbE thalassemia, including a cohort of patients observed since 1996 [2, 8]. Our goal was to identify patients who had sustained the complication of a leg ulcer and to determine factors affecting the development of this complication.
Methods
All the available medical records of patients with HbE thalassemia were reviewed over 2 consecutive months, at 2 centers in Sri Lanka (The National Thalassemia Center, Kurunegala; The Adult Thalassemia Program, Ragama) with the highest numbers of patients with HbE thalassemia in the country.
Ethical permission for the study was obtained from University of Colombo and the Central Oxford Research Ethics Committee (first obtained in 1996 and extended in 2006, 2013, and 2018). Written informed consent was not sought from individual patients because no experimental intervention was implemented.
Of a total of 255 actively registered patients with HbE thalassemia in the 2 centers, 59 charts could not be located or had incomplete data, usually related to the retention of medical records by patients. In the remaining 196 patient charts, data were collected by 2 independent reviewers (P.C. and S.J.). Patients in whom the date of onset of a new leg ulcer was documented were included; patients in whom an ulcer was recorded, but the date of onset was not documented, were excluded. When documentation of the record of a leg ulcer was considered ambiguous, consensus was reached by both reviewers.
We collected available records of: age; gender; history of onset of the ulcer; description (laterality, location); a record of healing, nonhealing, and ulcer recurrence (recurrence was ultimately excluded as a variable because of the frequent ambiguity as to whether descriptions of an ulcer at serial visits were related to the original, or a new, ulcer); blood transfusion regimen; splenectomy status; estimates of iron overload (serum ferritin concentration); hemoglobin and platelet count at ulcer onset; and fetal hemoglobin. No values of reticulocyte counts, LDH, and bilirubin concentrations were available in most charts, even in infrequently transfused patients in whom documentation of trends in these endpoints might have been of interest. Using data from a larger database of HbE thalassemia patients currently under development, we compared selected baseline characteristics of 151 patients with no documentation of a leg ulcer, to those of the 45 patients of this study (Table 1).
Table 1.
Baseline characteristics for 45 patients with a leg ulcer, compared to 151 patients without a documented leg ulcer
| Patients with documented leg ulcer | Patients without documented leg ulcer | Odds ratio | p value | 95% CI | |
|---|---|---|---|---|---|
| N | 45 | 151 | |||
| Age, years | 37.3±1.6 [18–69] | 23.1 ±0.94 [4–70] | 1.11 | <0.001 | 1.07–1.15 |
| Gender | 21 M | 72 M | 0.92 (M) | 0.747 | 0.47–1.80 |
| Proportion of patients receiving prescheduled (monthly) transfusions | 13% | 40% | 0.23 | 0.002 (0.155 after controlling for age) |
0.09–0.57 |
| Initial hemoglobin, g/dL | 5.83±0.23 [2.0–8.7] (n = 44) |
6.00±0.12 [1.9–9.6] (n= 149) |
0.92 | 0.499 | 0.73–1.17 |
| Initial serum ferritin concentration, µg/L | 1,103+139 [57–4,071] | 1,424±164 [19–11,000] (n= 149) |
0.9999 | 1.0 | 0.9997–1.0001 |
| History of splenectomy | 62% | 40% (n = 150) | 2.57 | 1.24–4.90 | 0.010 (0.55 after controlling for age) |
| History of diabetes | 13.3% | 6.8% (n= 147) | 2.11 | 0.187 | 0.72–6.16 |
Data were compiled and analyzed by one reviewer (V.M.). Continuous variables are presented as mean ± standard error of the mean [range]. Discrete variables are presented as number (%) of patients.
To conduct our systematic search of the literature, we searched Ovid MEDLINE, Embase, and CINAHL from their inception to July 16, 2020, using the keywords “thalassemia” and “leg ulcer” and all appropriate synonyms. Studies were included if they evaluated interventions for leg ulcers in patients with severe thalassemia and reported quantitative or qualitative outcomes. Articles were excluded if they were not written in English, were abstracts or conference papers, did not report original research, or if the full-texts were inaccessible. The exception to this was the inclusion of a paper which focused on treatment of leg ulcers in HbE thalassemia but for which the full-text was inaccessible; information was obtained from the abstract of the paper [9].
Results
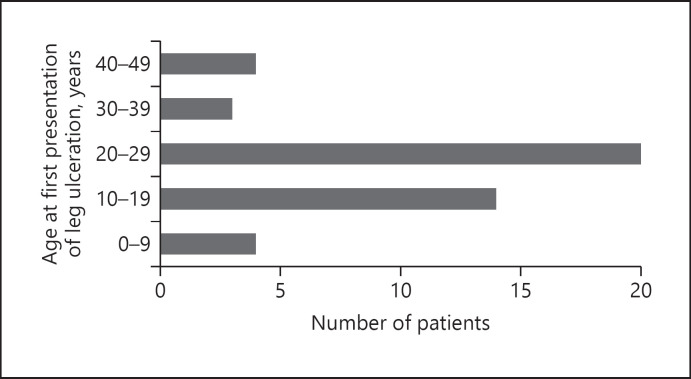
The onset of a new leg ulcer was recorded in 45 (22.2%) of 196 evaluable medical records: 188 patients in Kurunegala and 8 patients in Ragama. Patient characteristics are shown in Table 2. Patients presented with a new leg ulcer at a mean age of 22.2 ± 1.4 years, most between age 20 and 29 years (Fig. 1). The diagnosis of the primary disease of HbE thalassemia at age 12.9 ± 1.9 years is consistent with previous findings [2].
Table 2.
Patient characteristics at the onset of a leg ulcer and during treatment for ulcer
| Age, years Gender | Age at onset of leg ulcer, years | Transfusion (Tx) regimen at ulcer onset | Steady-state Hb at ulcer onset, g/dL (in 31) | Fetal hemoglobin as % of total hemoglobin (in 24) | Patients splenectomized, % | Platelets during ulcer Rx, ×109 (in 26) | Serum ferritin concentration at ulcer diagnosis, ng/mL (in 27) |
|---|---|---|---|---|---|---|---|
| 37.3±1.5 [18–69] 22.2±1.4 [5–49] 21 M 24 F |
On demand: 55.6% ≥8 Tx/year: 15.6% <8 Tx/year: 13.3% |
6.4±0.2 [3.4–8.9] | 31.7+2.1 [5.2–49.2] | Pre-ulcer: 16 (36%) During ulcer treatment: 8 (18%) |
550.6±53.24 [60–1,130] Median: 502.5 |
1,107.7±206.8 [22–8,108] |
Fig. 1.
Age at first presentation of leg ulceration (N = 45). Most patients were diagnosed with a leg ulcer between the ages of 20 and 29 years.
With respect to the factors that may have influenced the development of a leg ulcer, anemia was present in all patients. Steady-state hemoglobin, 6.4 ± 0.2 g/dL, was consistent with the larger cohort of patients including those without ulcers [2]. Fetal hemoglobin concentration was elevated at 31.7 ± 2.1% and mean serum ferritin concentration was 1,108 ± 207 ng/mL, both consistent with findings in the larger cohort including patients without leg ulcers [2]. Of patients who developed a leg ulcer, 55.6% were receiving transfusions “on demand” during periods of infection, intercurrent illness, surgery, and pregnancy only. The remaining patients had been treated with prescheduled transfusions, 15.6% regular transfusions (≥8/year) at ulcer onset, and 13.3% irregular transfusion (<8/year). In 15.6% records, the ongoing regimen of transfusion was undocumented.
Consistent with the age of patients and the practice of splenectomy which was common prior to 2010 in Sri Lanka, over half (53.4%) of patients identified with ulcers had been splenectomized at ulcer diagnosis or underwent splenectomy during ulcer treatment; this proportion was also consistent with the larger cohort including those without ulcers [2].
Consistent with post-splenectomy status, most patients had elevated platelet counts (mean ± SEM 550.6 ± 53.24 × 109) at onset of the ulcer. Hypothyroidism was present in 11.1% patients, and diabetes mellitus in 13.3%, both consistent with those in the larger cohort of patients without ulcers [2].
Overall, 31.1% of patients had a unilateral leg ulcer, 22.2% had bilateral ulcers; in 46.7% of charts, the laterality was unrecorded. The location of ulcers (medial vs. lateral) was also irregularly recorded: 24.4% of patients developed ulcers on the ankle; 24.4% ulcers were reported “on the leg”; in the remainder, location was inconsistently documented. Trauma was specifically reported as an inciting factor to the ulcer development in 15.6% of records.
Table 1 shows that a lower proportion (13%) of patients with a history of a leg ulcer had been maintained on a regimen of prescheduled transfusions, compared to the 151 patients without documentation of a leg ulcer, of whom 40% had been maintained on a regimen of prescheduled transfusions (p = 0.02). A total of 62% of patients with leg ulcers had undergone splenectomy, while 40% of patients with no documentation of a leg ulcer had undergone splenectomy (p = 0.01). The incidence of diabetes mellitus was higher but not statistically different (at the p = 0.05 level) in those who developed a leg ulcer (13.3%) when compared to that of 6.8%, in 147 (of 151) evaluable patients in whom a leg ulcer was not recorded (p = 0.187; OR: 2.11; CI: 0.72–6.16; Table 1). There was no statistically significant difference in initial serum ferritin concentration, the only estimate of iron loading available in most patients, between the patients with, and without, a documented leg ulcer. It is important to note that the values for the 45-patient cohort are different in Table 1 compared to Table 2 for transfusion status, hemoglobin, and serum ferritin concentration. This is because in Table 2 values at ulcer onset are recorded, whereas in Table 1, baseline values at first visit are recorded. For splenectomy status, Table 2 shows those who were splenectomized before or during ulcer treatment, whereas Table 1 includes lifetime splenectomy status.
Approaches to Treatment
The treatment approach was documented in 22 patients and undocumented in 23 patients. Many treatments were given contemporaneously, often adding another treatment without analysis of the results of the first treatment, resulting in considerable difficulty in assessing the responses to specific treatments. Monthly transfusions had been initiated after the onset of an ulcer in 16 patients. Antibiotics were administered (in 7 patients); in 4 patients, zinc supplements were introduced at the onset of monthly transfusions. In 3 patients, “wound toilet treatment” was introduced. In 3 patients, surgical skin grafts, and in 1 patient, oral corticosteroids, were prescribed.
Treatment Outcomes
Healing was reported as complete in 17 patients. Five of the 17 had received transfusions; one had been administered antibiotics with zinc supplements; one had been given zinc supplements and “wound toilet treatment”; 2 had undergone skin grafting. In 8 of the 17 patients in whom healing was recorded, the treatment that had led to healing was undocumented.
Healing was reported as not complete in 19 patients. Of these, 4 had been treated with regular transfusions; 2 with a combination of transfusions and antibiotics; one each had been started on one of the following regimens: zinc supplements; transfusions and zinc supplements; a skin graft and “wound toilet treatment”; transfusions plus hydrocortisone; antibiotics and zinc supplements. In the other 8 patients in whom the ulcer was nonhealed, treatment(s) were undocumented.
Discussion
Leg ulceration, in patients with the inherited hemoglobinopathies sickle cell disease and thalassemia, is reported to increase disability and disrupt quality of life. The prevalence in sickle cell disease, which is likely influenced by climate [10], is widely variable between 2.5% and 80% [11] and has been associated with an increased risk of death [12]. Despite this high prevalence and risk, optimal management of leg ulcers in sickle cell disease remains uncertain [10, 13], with no non-biased, randomized trial data to guide best treatment practices [11].
Yet an even larger gap exists in thalassemia in which it is difficult even to securely estimate the prevalence of leg ulceration. We identified a total of 23 studies which have reported treatments for leg ulcers in patients with major forms of beta thalassemia [9, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] and severe alpha thalassemia (Hemoglobin H disease) [35].
Only one study has focused treatment on patients with HbE thalassemia [9]. Eighteen of the 23 studies were published over 10 years ago; most were small with a median sample size of 1 patient, with only 3 studies reporting findings in >10 patients. Although the prevalence of leg ulcers may be increased in tropical climates, there are relatively few publications in thalassemia from these areas, with one study reported from each of India and Pakistan and 2 from Thailand. There are no other studies from Sri Lanka which have reported leg ulcers in thalassemia. Overall, outside of Sri Lanka, leg ulcers are reported as more common in thalassemia intermedia (with prevalences varying from 4.7% in a single-center study [12] to 8% in a large review [11]), than in thalassemia major. Reports of ulcers in Hemoglobin H disease are limited to single patients [13].
Our study is one of few to examine the treatment of leg ulcers in HbE thalassemia, the most common genotype of severe beta-thalassemia worldwide. HbE thalassemia arises from compound heterozygosity of the abnormal hemoglobin E and a β-thalassemia allele, and has a complex pathophysiology derived from globin chain imbalance, ineffective erythropoiesis, hemolysis, oxidative damage, and shortened red cell survival [36, 37, 38]. We have previously shown that patients with HbE thalassemia, related to the decreased oxygen affinity of hemoglobin E, have an ability to right-shift their oxygen dissociation curve [39], and related to improved tissue oxygenation, HbE thalassemia patients might be expected to have a lower prevalence of leg ulceration than patients with thalassemia not involving hemoglobin E. However, nearly 1 in 5 evaluable patients in our cohort developed a leg ulcer during their clinical course and because we eliminated records in which no date of onset of the ulcer was recorded, this almost certainly represents an underestimate and is higher than that reported in small studies of other forms of severe beta thalassemia.
In all inherited hemoglobinopathies, one of the primary risk factors influencing ulcer development is likely severe untreated, or undertreated, chronic anemia. Reflecting the transfusion practices of the past 20 years in Sri Lanka, anemia is present in all our patients with HbE thalassemia. Over half of our patients had been treated with “on demand” and/or irregularly scheduled, transfusions; one in 6 had received monthly transfusions. The severity of anemia in patients who developed ulcers (mean steady-state hemoglobin 6.4 ± 0.2 g/dL) was not different from the group as a whole [2]. In parallel, however, the elevated concentrations of fetal hemoglobin were similar to those which may aggravate tissue hypoxia, and possibly increase the formation of leg ulcers, as observed in patients with sickle cell disease [18, 20]. While some studies had suggested that elevated concentrations of fetal hemoglobin may protect against the development of leg ulcers in patients with sickle cell disease [40], as well as with nontransfusion-dependent thalassemia [41], other studies in sickle cell disease have suggested a lack of a protective effect of higher fetal hemoglobin or of hydroxyurea, which augments fetal hemoglobin, against the development of leg ulcers [12]. In our patients, mean fetal hemoglobin of 31.7% was slightly less than that (53.8 ± 7.6%) in the 13 studies of thalassemia patients who developed leg ulcers which have reported this variable [14, 17, 18, 20, 21, 22, 25, 26, 28, 30, 31, 32, 34].
Other risk factors for the development of leg ulcers may include ongoing red cell hemolysis resulting in consumption of nitric oxide and impaired endothelial function [42]. In patients with HbE thalassemia, significant reduction in plasma nitric oxide metabolites and in prostaglandin E2 levels [43], and evidence of markers for endothelial cell activation and injury [44], have been reported. In this study reticulocyte counts, LDH and bilirubin concentrations were not available in most patients with leg ulcers. This is by contrast to reports in those with sickle cell disease and leg ulcers [45]. Further studies in HbE thalassemia, including prospective analyses in our patients, may provide more information about the relationship of these markers of accelerated hemolysis to the risk of the development of leg ulcers.
In tropical climates, including Sri Lanka, exposure may lead to higher risk of infection aggravating leg ulcers [46]. On a related note, trauma has been reported as an inciting incident for the onset of leg ulcers in patients with sickle cell disease [11]. One in 6 of our patients reported trauma as an inciting incident. Other risk factors may also be extrapolated from studies of sickle cell disease in which increased venous pressure, resulting in slow healing, has been cited as a risk factor for ulcers [47]. Although no studies in thalassemia have correlated elevated venous pressures to leg ulcers, significant elevations in pulmonary artery pressures have been reported in HbE thalassemia patients, particularly those who have been splenectomized [48, 49, 50, 51].
A chronic low-grade hypercoagulable state in sickle cell disease arising from elevated platelet counts and increased endothelial adhesion has been reported in sickle cell disease patients who develop leg ulcers [13, 52]. Data suggest that splenectomized patients with HbE thalassemia may also experience a chronic, low-grade hypercoagulable state [53], probably influenced by post-splenectomy elevated platelet counts, reduced levels of protein C and protein S, a reactive red cell surface and abnormal endothelium [44, 48, 54, 55], which may be ameliorated by blood transfusions [49]. This may increase a propensity for vascular perturbation, consistent with the observed increased risk of venous thromboembolism in splenectomized patients with HbE thalassemia [56], and of leg ulcers in splenectomized patients with nontransfusion-dependent thalassemia [57]. In our patients, more than half of whom had been splenectomized, platelet counts recorded at ulcer onset were elevated at 550.6 ± 53.24 × 109, similar to that (587.2 ± 111.2 × 109/L) in the 7 thalassemia ulcer studies in which this information was provided [14, 15, 26, 28, 31, 32, 34].
Generally, leg ulcers are a disease of young adults; our patients at diagnosis of the ulcer were 22.2 ± 1.4 years, younger than the mean age (30.9 ± 3.6 years) of reported onset of ulcers in patients with other forms of thalassemia [15, 16, 17, 18, 19, 21, 22, 24, 25, 26, 27, 28, 29, 30, 34].
One study suggested that iron overload plays a role in the pathophysiology of ulcers through generation of oxidative stress, as well as a factor perpetuating chronicity of the ulcer [14]. In our patients, serum ferritin concentration at diagnosis of ulcer was elevated at 1,108 ± 207 µg/L, similar to the serum ferritin concentration (1,045 ± 86 ng/mL) of the large cohort with HbE thalassemia [2] and that (1,247.0 ± 405.1 ng/mL) in the 5 studies of ulcers in thalassemia which have reported this variable [14, 28, 29, 31, 34].
In sickle cell disease and thalassemia intermedia, one risk factor influencing ulcer development is likely undertreated chronic anemia. Data from a larger database of HbE thalassemia patients currently under development were used to compare selected baseline characteristics of 151 patients with no documentation of a leg ulcer to those of the 45 patients of this study. Some differences were identified which may guide future prospective work. A lower proportion (13%) of patients with a history of a leg ulcer had been maintained on a regimen of prescheduled transfusions, compared to 40% of patients without documentation of a leg ulcer. Although the initial mean hemoglobin concentration in the patients with a documented leg ulcer was not significantly different from that in the patients without a leg ulcer, we did not have access to the steady-state hemoglobin over time in most patients. It is possible that, because those with leg ulcers were less regularly transfused, their steady-state hemoglobin concentrations would have been lower and their concentrations of fetal hemoglobin higher (both representing a possible risk factor for the development of a leg ulcer) than in those without a history of a leg ulcer. Patients with a documented leg ulcer had also undergone splenectomy at a higher rate. As discussed, splenectomy may aggravate a chronic, low-grade hypercoagulable state [49], probably influenced by post-splenectomy elevated platelet counts, which were unavailable in most of the larger cohort for comparison.
Finally, it is well recognized that ulcers are common in diabetic patients [58]. While the pathophysiology of HbE thalassemia likely represented the primary reason for the development of leg ulcers in these patients; another factor representing a risk for the development of leg ulcers in the nonthalassemia population is diabetes mellitus. The incidence of diabetes mellitus was higher, but not statistically different (at the p = 0.05 level), in those who developed a leg ulcer when compared to the 147 (of 151) evaluable patients in whom a leg ulcer was not recorded. In further prospective studies, it would be interesting to evaluate educational and income levels (on which we have no current information) in patients who did or did not develop a leg ulcer; these are reported to influence the risk for development of leg ulcers in patients with diabetes in Sri Lanka [59].
Treatment Outcomes
Treatment approaches to leg ulcers have been variable in major forms of thalassemia. Skin grafting has achieved partial healing in some [15, 20, 29], but not all studies [18]. Using this approach, 2 of our patients achieved complete healing while one did not. Multiple topical therapies have been used over several decades. Partial or complete healing has been reported using a combination of antibiotics, aluminum acetate, pinch grafts, petroleum strips, ace bandages, and an Unna boot [15], ascorbic acid [16], gelatin sponge powder [18], eusol and paraffin dressings [13], xanthinol nicotinate [19], perilesional injections of granulocyte-macrophage colony-stimulating factor [60], and a combination of compression bandages, hydrocolloids, and silver-coated dressings [29]. Failures of healing have been reported with local application of oxygen and silver nitrate [17] and topical antibiotics [14].
The most common systemic therapies have included blood transfusions and platelet-derived growth factor. Transfusions have been suggested to be nonprotective for the development of leg ulcers in sickle cell disease [12] but were reported as protective for leg ulcers in patients with NTDT [61]. All studies in thalassemia have been small, with variable results from rapid healing with a 100% recurrence rate [14], to slower but complete healing [28, 30], including when combined with antibiotics [34]. One-third of our patients were started on a regular (monthly) transfusion regimen after the diagnosis of an ulcer; 5 were recorded as having their ulcer healed while in the others nonhealing was documented, or healing was not documented. Platelet-rich plasma treatment in one small [25] and one relatively large study [32] was reported as associated with successful healing in many patients without recurrence.
Granulocyte-macrophage colony-stimulating factor therapy has been associated with both partial [14] and complete [27] healing of leg ulcers. Other systemic therapies have included, pentoxifylline combined with deferoxamine in a relatively large study in which reduced healing time was associated with recurrence [23]; and pentoxifylline alone, associated with complete, albeit short-term, healing [22]. In the only (other) study that had focused on ulcers in patients with HbE thalassemia, dilazep was associated with complete or partial healing in most [9]. Other treatments associated with complete or partial healing of ulcers in patients with thalassemia include hyperbaric oxygen therapy [21], arginine butyrate [24], hydroxyurea [26], and luspatercept [33].
Conclusion
This study, the largest to examine the complication of leg ulcers in any patients with any form of severe beta thalassemia, is also among the few to evaluate patients with HbE thalassemia; we could identify only one other study in the literature focused on this most common form of severe beta thalassemia. We observed, in 2 large patient centers in Sri Lanka that 1 in 5 evaluable patients with HbE thalassemia developed a leg ulcer over their clinical course. This is a higher incidence than reported in other forms of beta thalassemia, according to our literature review which was striking for the paucity of studies of leg ulcers in thalassemia, including few from low- and middle-income countries, where thalassemia is endemic.
Currently, there is no clear benefit to any of the treatment approaches, of those attempted both in our study and other studies, for leg ulcers in thalassemia. Given both the broad and nonsystematic nature of treatments explored in this retrospective study, as well as the relative paucity of other studies of leg ulcers in HbE thalassemia, and in other forms of thalassemia, there is considerable difficulty in providing evidence-based recommendations for the treatment of leg ulcers in HbE thalassemia. This gap in knowledge with respect to the optimal treatment of a complication, which from this study appears in HbE thalassemia to be relatively common, might be addressed in simple prospective comparative studies. Controlled trials including of promising avenues of treatment planned or underway in studies of leg ulcers in sickle cell disease [62], might be undertaken to guide the best practices of care in this important, and common, complication. From the data available in these patients and in the literature, we believe that a clinical trial comparing 3 promising therapies − simple transfusions, platelet-rich plasma, and topical therapies − might be considered. While successful in some studies, treatment with G-CSF may be an impractical avenue of treatment in low-resource settings outside of a research study.
In settings in which research studies may be impractical, we would recommend that the same 3 avenues of intervention: a short-term (6 months) regimen of monthly transfusions; or infusions of platelet-rich plasma; or systematic and careful toileting with topical therapies, should be considered. Especially because one substantial limitation in the present analysis was that many treatment outcomes were undocumented, any treatment (transfusions, plasma, or intensive topical therapies) should be evaluated before another treatment is added. In this and the evaluation of other treatments for other complications, resources to improve systematic collection of data and recording of treatment outcomes such as those under evaluation in other chronic disorders in lower resource settings [63], may be an important first step in centers of thalassemia throughout Asia.
Statement of Ethics
The study was approved by the Oxford Tropical Research Ethics Committee Study number (Corec 95-010; OXTREC reference 515-13), the University Health Network Research Ethics Board (18-6246; 18-6242), and by the Institutional Review Board of the University of Colombo. Signed informed consents were judged by the research Ethics Committees as not required for this study, a retrospective chart review.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
No funding was received for this study.
Author Contributions
David Weatherall and Nancy Olivieri conceived and planned the study. Nancy Olivieri acquired funding for the study. Anuja Premawardhena and Sachith Mettananda provided resources. Sanasi M. Jayawardena and Priya Chandrakumaran collected the original data. Vikita Mehta, Abirami Kirubarajan, Amir Sabouhanian, Sanasi M. Jayawardena, Priya Chandrakumaran, Nila Thangavelu, Shawn Khan, Angela Allen, and Nancy Olivieri curated and analysed the data. David Weatherall, Anuja Premawardhena, Sachith Mettananda, and Nancy Olivieri were responsible for follow-up of patients, clinical assessments, and entry of data. Amir Sabouhanian and Nancy Olivieri were responsible for methodology and software. Anuja Premawardhena, Angela Allen, Sachith Mettananda, Refai Cader, Dayananda Bandara, David Weatherall, and Nancy Olivieri helped administer the project. Anuja Premawardhena, Sachith Mettananda, Dayananda Bandara, Angela Allen, David Weatherall, and Nancy Olivieri supervised the work. Amir Sabouhanian, Abirami Kirubarajan, Vikita Mehta, Nila Thangavelu, Shawn Khan, Sanasi M. Jayawardena, Priya Chandrakumaran, and Nancy Olivieri validated the data. Vikita Mehta, Abirami Kirubarajan, Amir Sabouhanian, and Nancy Olivieri drafted the manuscript. Vikita Mehta, Abirami Kirubarajan, Amir Sabouhanian, and Nancy Olivieri verified the underlying data. All the authors reviewed and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
We wish to acknowledge the support from the medical and nursing officers, laboratory personnel, clinic volunteers in Kurunegala and Ragama as well as the interest, support, and contributions of the patients and families attending these clinics.
References
- 1.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–6. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Premawardhena A, Fisher CA, Olivieri NF, de Silva S, Arambepola M, Perera W, et al. Haemoglobin E beta thalassaemia in Sri Lanka. Lancet. 2005;366:1467–70. doi: 10.1016/S0140-6736(05)67396-5. [DOI] [PubMed] [Google Scholar]
- 3.Olivieri NF, Pakbaz Z, Vichinsky E. HbE/β-thalassemia: basis of marked clinical diversity. Hematol Oncol Clin North Am. 2010;24:1055–70. doi: 10.1016/j.hoc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Rees DC, Styles L, Vichinsky EP, Clegg JB, Weatherall DJ. The hemoglobin E syndromes. Ann N Y Acad Sci. 1998;850:334–43. doi: 10.1111/j.1749-6632.1998.tb10490.x. [DOI] [PubMed] [Google Scholar]
- 5.Viprakasit V, Jansutjawan S. Survival and causes of death in patients with alpha- and beta-thalassaemia in a developing country: the first report from Thailand. EHA Library; 2015. p. p. P383. Abstract. Available from: https://library.ehaweb.org/eha/2015/20th/100623/vip.viprakasit.survival.and.causes.of.death.in.patients.with.lpha-.and.html. [Google Scholar]
- 6.Natesirinilkul R, Charoenkwan P, Nawarawong W, Boonsri S, Tantivate P, Wongjaikum S, et al. Hypercoagulable state as demonstrated by thromboelastometry in hemoglobin E/beta-thalassemia patients: association with clinical severity and splenectomy status. Thromb Res. 2016;140:125–31. doi: 10.1016/j.thromres.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Ekwattanakit S, Siritanaratkul N, Viprakasit V. A prospective analysis for prevalence of complications in Thai nontransfusion-dependent Hb E/β-thalassemia and α-thalassemia (Hb H disease) Am J Hematol. 2018;93((5)):623–9. doi: 10.1002/ajh.25046. [DOI] [PubMed] [Google Scholar]
- 8.de Silva S, Fisher CA, Premawardhena A, Lamabadusuriya SP, Peto TE, Perera G, et al. Thalassaemia in Sri Lanka: implications for the future health burden of Asian populations. Lancet. 2000;355:786–91. doi: 10.1016/s0140-6736(99)08246-x. [DOI] [PubMed] [Google Scholar]
- 9.Opartkiattikul N, Sukpanichnant S, Wanachiwanawin W, Fucharoen S, Funahara Y, Sumiyoshi A, et al. A double-blind placebo control trial of dilazep in beta-thalassemia/hemoglobin E patients. Southeast Asian J Trop Med Public Health. 1997;28((Suppl 3)):167–71. [PubMed] [Google Scholar]
- 10.Monfort JB, Senet P. Leg ulcers in sickle-cell disease: treatment update. Adv Wound Care. 2020;9:348–56. doi: 10.1089/wound.2018.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martí-Carvajal AJ, Knight-Madden JM, Martinez-Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database Syst Rev. 2014;2014:Cd008394. doi: 10.1002/14651858.CD008394.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol. 2010;85:831–3. doi: 10.1002/ajh.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serjeant GR, Serjeant BE, Mohan JS, Clare A. Leg ulceration in sickle cell disease: medieval medicine in a modern world. Hematol Oncol Clin North Am. 2005;19:943–56, viii–ix. doi: 10.1016/j.hoc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Matta BN, Abbas O, Maakaron JE, Koussa S, Daderian RH, Taher AT. Leg ulcers in patients with β-thalassaemia intermedia: a single centre's experience. J Eur Acad Dermatol Venereol. 2014;28:1245–50. doi: 10.1111/jdv.12211. [DOI] [PubMed] [Google Scholar]
- 15.Zelickson AS. Leg ulcers associated with mediterranean anemia (thalassemia major and minor) AMA Arch Derm. 1957;76((3)):351–6. doi: 10.1001/archderm.1957.01550210077009. [DOI] [PubMed] [Google Scholar]
- 16.Afifi AM, Ellis L, Huntsman RG, Said MI. High dose ascorbic acid in the management of thalassaemia leg ulcers: a pilot study. Br J Dermatol. 1975;92:339–41. doi: 10.1111/j.1365-2133.1975.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 17.Ganor S, Cohen T. Leg ulcers in a family with both beta thalassaemia and glucose-6-phosphate dehydrogenase deficiency. Br J Dermatol. 1976;95:203–6. doi: 10.1111/j.1365-2133.1976.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 18.Stevens DM, Shupack JL, Javid J, Silber R. Ulcers of the leg in thalassemia. Arch Dermatol. 1977;113:1558–60. [PubMed] [Google Scholar]
- 19.Afifi AM, Adnan M, Taha M, Amasha ME. Xanthinol nicotinate in the management of leg ulcers associated with haemoglobinopathies. Curr Med Res Opin. 1979;6:309–13. doi: 10.1185/03007997909109443. [DOI] [PubMed] [Google Scholar]
- 20.Gimmon Z, Wexler MR, Rachmilewitz EA. Juvenile leg ulceration in beta-thalassemia major and intermedia. Plast Reconstr Surg. 1982;69:320–5. doi: 10.1097/00006534-198202000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Richmond MN, Galbraith KA, Rogerson JT. Hyperbaric oxygen for a thalassaemic leg ulcer. J R Army Med Corps. 1987;133:23–5. doi: 10.1136/jramc-133-01-04. [DOI] [PubMed] [Google Scholar]
- 22.Tercedor J, Herranz MT, Ródenas JM, García-Lora E, Skiljo M, García-Mellado V. Pentoxifylline treatment for beta-thalassemia intermedia leg ulcer. Int J Dermatol. 1992;31:747–8. doi: 10.1111/j.1365-4362.1992.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 23.Angelides NS, Angastiniotis C, Pavlides N. Effect of pentoxifylline on treatment of lower limb ulcers in patients with thalassemia major. Vasc Surg. 1993;27((7)):519–24. doi: 10.1177/000331979204300702. [DOI] [PubMed] [Google Scholar]
- 24.Sher GD, Olivieri NF. Rapid healing of chronic leg ulcers during arginine butyrate therapy in patients with sickle cell disease and thalassemia. Blood. 1994;84:2378–80. [PubMed] [Google Scholar]
- 25.Josifova D, Gatt G, Aquilina A, Serafimov V, Vella A, Felice A. Treatment of leg ulcers with platelet-derived wound healing factor (PDWHFS) in a patient with beta thalassaemia intermedia. Br J Haematol. 2001;112:527–9. doi: 10.1046/j.1365-2141.2001.02540-2.x. [DOI] [PubMed] [Google Scholar]
- 26.Gamberini MR, Fortini M, De Sanctis V. Healing of leg ulcers with hydroxyurea in thalassaemia intermedia patients with associated endocrine complications. Pediatr Endocrinol Rev. 2004;2((Suppl 2)):319–22. [PubMed] [Google Scholar]
- 27.Stavrianeas N, Xifteri A, Kavatha D, Vrettou H. Topical effectiveness of filgrastim in beta-thalassaemia leg ulcer. Dermatology. 2005;211:173. doi: 10.1159/000086456. [DOI] [PubMed] [Google Scholar]
- 28.Aessopos A, Kati M, Tsironi M, Polonifi E, Farmakis D. Exchange blood transfusions for the treatment of leg ulcerations in thalassemia intermedia. Haematologica. 2006;91:ECR11. [PubMed] [Google Scholar]
- 29.Fracchia E, Elkababri M, Cantello C, Gori A, Partsch H, Forni GL. Venous-like leg ulcers without venous insufficiency in congenital anemia: successful treatment using compression bandages. Dermatol Surg. 2010;36:1336–40. doi: 10.1111/j.1524-4725.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- 30.Levin C, Koren A. Healing of refractory leg ulcer in a patient with thalassemia intermedia and hypercoagulability after 14 years of unresponsive therapy. Isr Med Assoc J. 2011;13:316–8. [PubMed] [Google Scholar]
- 31.Dixit S, Agrawal PR, Sharma DK, Singh RP. Closure of chronic non healing ankle ulcer with low level laser therapy in a patient presenting with thalassemia intermedia: Case report. Indian J Plast Surg. 2014;47:432–5. doi: 10.4103/0970-0358.146642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afradi H, Saghaei Y, Kachoei ZA, Babaei V, Teimourian S. Treatment of 100 chronic thalassemic leg wounds by plasma-rich platelets. Int J Dermatol. 2017;56:171–5. doi: 10.1111/ijd.13443. [DOI] [PubMed] [Google Scholar]
- 33.Piga A, Perrotta S, Gamberini MR, Voskaridou E, Melpignano A, Filosa A, et al. Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with β-thalassemia. Blood. 2019;133:1279–89. doi: 10.1182/blood-2018-10-879247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zafar S, Saleem K, Rashid A. An unusual presentation of a patient with leg ulcers: a Case Report. Cureus. 2019;11:e6293. doi: 10.7759/cureus.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daneshmend TK, Peachey RD. Leg ulcers in alpha-thalassaemia (haemoglobin H disease) Br J Dermatol. 1978;98:233–5. doi: 10.1111/j.1365-2133.1978.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 36.Orkin SH, Kazazian HH, Jr, Antonarakis SE, Ostrer H, Goff SC, Sexton JP. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982;300:768–9. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- 37.Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, et al. A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood. 2000;96:2606–12. [PubMed] [Google Scholar]
- 38.Datta P, Basu S, Chakravarty SB, Chakravarty A, Banerjee D, Chandra S, et al. Enhanced oxidative cross-linking of hemoglobin E with spectrin and loss of erythrocyte membrane asymmetry in hemoglobin Ebeta-thalassemia. Blood Cells Mol Dis. 2006;37:77–81. doi: 10.1016/j.bcmd.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Allen A, Fisher C, Premawardhena A, Peto T, Allen S, Arambepola M, et al. Adaptation of anemia in hemoglobin E-B thalassemia. Blood. 2010;116:5368–70. doi: 10.1182/blood-2010-06-289488. [DOI] [PubMed] [Google Scholar]
- 40.Koshy M, Entsuah R, Koranda A, Kraus AP, Johnson R, Bellvue R, et al. Leg ulcers in patients with sickle cell disease. Blood. 1989;74:1403–8. [PubMed] [Google Scholar]
- 41.Musallam KM, Sankaran VG, Cappellini MD, Duca L, Nathan DG, Taher AT. Fetal hemoglobin levels and morbidity in untransfused patients with β-thalassemia intermedia. Blood. 2012;119:364–7. doi: 10.1182/blood-2011-09-382408. [DOI] [PubMed] [Google Scholar]
- 42.Mack AK, Kato GJ. Sickle cell disease and nitric oxide: a paradigm shift? Int J Biochem Cell Biol. 2006;38:1237–43. doi: 10.1016/j.biocel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satitthummanid S, Uaprasert N, Songmuang SB, Rojnuckarin P, Tosukhowong P, Sutcharitchan P, et al. Depleted nitric oxide and prostaglandin E(2) levels are correlated with endothelial dysfunction in β-thalassemia/HbE patients. Int J Hematol. 2017;106:366–74. doi: 10.1007/s12185-017-2247-8. [DOI] [PubMed] [Google Scholar]
- 44.Butthep P, Rummavas S, Wisedpanichkij R, Jindadamrongwech S, Fucharoen S, Bunyaratvej A. Increased circulating activated endothelial cells, vascular endothelial growth factor, and tumor necrosis factor in thalassemia. Am J Hematol. 2002;70:100–6. doi: 10.1002/ajh.10101. [DOI] [PubMed] [Google Scholar]
- 45.Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, et al. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133((5)):570–8. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacFarlane DE, Baum KF, Serjeant GR. Bacteriology of sickle cell leg ulcers. Trans R Soc Trop Med Hyg. 1986;80:553–6. doi: 10.1016/0035-9203(86)90137-9. [DOI] [PubMed] [Google Scholar]
- 47.Mohan JS, Vigilance JE, Marshall JM, Hambleton IR, Reid HL, Serjeant GR. Abnormal venous function in patients with homozygous sickle cell (SS) disease and chronic leg ulcers. Clin Sci. 2000;98:667–72. [PubMed] [Google Scholar]
- 48.Sonakul D, Pacharee P, Laohapand T, Fucharoen S, Wasi P. Pulmonary artery obstruction in thalassaemia. Southeast Asian J Trop Med Public Health. 1980;11:516–23. [PubMed] [Google Scholar]
- 49.Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78:139–45. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 50.Phrommintikul A, Sukonthasarn A, Kanjanavanit R, Nawarawong W. Splenectomy: a strong risk factor for pulmonary hypertension in patients with thalassaemia. Heart. 2006;92:1467. doi: 10.1136/hrt.2005.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singer ST, Kuypers FA, Styles L, Vichinsky EP, Foote D, Rosenfeld H. Pulmonary hypertension in thalassemia: association with platelet activation and hypercoagulable state. Am J Hematol. 2006;81:670–5. doi: 10.1002/ajh.20640. [DOI] [PubMed] [Google Scholar]
- 52.Cacciola E, Musso R, Giustolisi R, Cacciola E, Alessi M. Blood hypercoagulability as a risk factor for leg ulcers in sickle cell disease. Blood. 1990;75:2467–8. [PubMed] [Google Scholar]
- 53.Atichartakarn V, Angchaisuksiri P, Aryurachai K, Onpun S, Chuncharunee S, Thakkinstian A, et al. Relationship between hypercoagulable state and erythrocyte phosphatidylserine exposure in splenectomized haemoglobin E/beta-thalassaemic patients. Br J Haematol. 2002;118:893–8. doi: 10.1046/j.1365-2141.2002.03711.x. [DOI] [PubMed] [Google Scholar]
- 54.Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N, et al. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom. 2004;57:23–31. doi: 10.1002/cyto.b.10064. [DOI] [PubMed] [Google Scholar]
- 55.Angchaisuksiri P, Atichartakarn V, Aryurachai K, Archararit N, Chuncharunee S, Tiraganjana A, et al. Hemostatic and thrombotic markers in patients with hemoglobin E/β-thalassemia disease. Am J Hematol. 2007;82:1001–4. doi: 10.1002/ajh.20945. [DOI] [PubMed] [Google Scholar]
- 56.Keawvichit R, Khowawisetsut L, Chaichompoo P, Polsrila K, Sukklad S, Sukapirom K, et al. Platelet activation and platelet-leukocyte interaction in β-thalassemia/hemoglobin E patients with marked nucleated erythrocytosis. Ann Hematol. 2012;91:1685–94. doi: 10.1007/s00277-012-1522-2. [DOI] [PubMed] [Google Scholar]
- 57.Taher A, Musallam K, Karimi M, El-Beshlawy A, Belhoul K, Daar S, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115:1886–92. doi: 10.1182/blood-2009-09-243154. [DOI] [PubMed] [Google Scholar]
- 58.Nelzén O, Bergqvist D, Lindhagen A. High prevalence of diabetes in chronic leg ulcer patients: a cross-sectional population study. Diabet Med. 1993;10:345–50. doi: 10.1111/j.1464-5491.1993.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 59.Kumarasinghe AS, Sudharshani W, Priyadharshika H, Shamini P. Predictors of diabetic foot and leg ulcers in a developing country with a rapid increase in the prevalence of diabetes mellitus. PLoS One. 2013;8((11)):e80856. doi: 10.1371/journal.pone.0080856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voskaridou E, Kyrtsonis MC, Loutradi-Anagnostou A. Healing of chronic leg ulcers in the hemoglobinopathies with perilesional injections of granulocyte-macrophage colony-stimulating factor. Blood. 1999;93:3568–9. [PubMed] [Google Scholar]
- 61.Taher AT, Musallam KM, El-Beshlawy A, Karimi M, Daar S, Belhoul K, et al. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br J Haematol. 2010;150:486–9. doi: 10.1111/j.1365-2141.2010.08220.x. [DOI] [PubMed] [Google Scholar]
- 62.Mandal P, Ghosh M, Bhattacharyya M. Does profile of Hemoglobin Eβ-thalassemia patients change after splenectomy? Experience of a tertiary thalassemia care centre in Eastern India. Indian J Hematol Blood Transfus. 2015;31:446–52. doi: 10.1007/s12288-014-0498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olivieri NF, Mehta V, Theivendrampillai S, Bl G. DEPICT health thalassemia to improve management of thalassemia in Sri Lanka. Pediatr Blood Cancer. 2020;67:S37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.