Abstract
Background and Aims
The aim of this study was to identify the utility of <sup>18</sup>F-fluorodeoxyglucose positron emission tomography/computed tomography (<sup>18</sup>F-FDG-PET/CT) as a predictor of early progressive disease (e-PD) in patients with hepatocellular carcinoma (HCC) treated with atezolizumab plus bevacizumab (Atezo/Bev).
Methods
Twenty consecutive patients with measurable intrahepatic target nodules who received Atezo/Bev treatment were reviewed. The oncological aggressiveness of tumors estimated by <sup>18</sup>F-FDG-PET/CT was analyzed using the rate of e-PD within 12 weeks and early progression-free survival (e-PFS) and overall survival (OS). Multivariate analysis was used to identify potential confounders for PD during Atezo/Bev therapy.
Results
Using the Response Evaluation Criteria in Solid Tumors version 1.1, a tumor-to-normal liver ratio (TLR) ≥2, indicating higher oncological aggressiveness in HCCs, was associated with lower objective response rates compared with TLR values <2 (18% vs. 33%, respectively). Moreover, TLR values ≥2 were significantly associated with higher e-PD rates compared with TLR values <2 (64% vs. 11%, respectively) and worse e-PFS (p = 0.021). In multivariate analysis, TLR ≥2 showed marginal significance as a predictor of e-PD (p = 0.053), and utility as a predictor for worse e-PFS (hazard ratio, 7.153; 95% confidence interval, 1.258–40.689; p = 0.027). In contrast, no significant differences in OS with/without e-PD were observed during the treatment course. In this study, 8 patients experienced e-PD and almost 40% of patients experienced acceptable disease control following subsequent lenvatinib treatment.
Conclusion
Pretreatment <sup>18</sup>F-FDG-PET/CT may be a useful new predictor of e-PD and may enable early decision-making based on early treatment changes following Atezo/Bev treatment of HCC.
Keywords: Hepatocellular carcinoma, Atezolizumab plus bevacizumab, Malignant potential, Poorly differentiated, Rapid progressive disease, 18F-fluorodeoxyglucose positron emission tomography/computed tomography
Introduction
Recently, atezolizumab plus bevacizumab (Atezo/Bev) has become available in Japan as a newly recommended first-line combination of an immune checkpoint inhibitor and a molecularly targeted agent for the treatment of unresectable advanced hepatocellular carcinoma (HCC). This regimen is recommended in other countries and is expected to provide better therapeutic efficacy than previously introduced first-line molecularly targeted agents (sorafenib and lenvatinib) to improve the prognosis of this patient population [1, 2, 3, 4]. Approximately one-third of HCC patients achieve an objective response with Atezo/Bev [3]; this response rate is almost twice as high as that observed with anti-programmed death receptor-1 monotherapy [5, 6]. However, hyperprogressive disease (HPD), defined as accelerated tumor growth during the administration of systemic chemotherapy, has been reported in patients receiving immune checkpoint inhibitors. HPD affects survival in patients with certain malignancies, including lung cancer, melanoma, and head and neck cancers [7, 8, 9, 10, 11, 12]. In HCC, HPD has been observed in patients treated with nivolumab or pembrolizumab, both of which failed to meet phase 3 trial primary endpoints [13, 14]. Recently, in patients with HCC receiving Atezo/Bev, a higher neutrophil-to-lymphocyte ratio (NLR) at baseline has been reported to increase the risk of HPD [15]. However, the characteristics and predictors of HPD in patients with HCC treated with Atezo/Bev remain unclear. Similarly, predictors of early progressive disease (e-PD) during Atezo/Bev therapy are not known. With respect to tumor characteristics, the efficacy of lenvatinib to treat HCCs with high malignant potential (e.g., poorly differentiated type and non-simple nodular type) was recently established based on several clinical reports [16, 17].
Regarding 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging features, 18F-FDG-PET/CT positivity has been reported to be associated strongly with poorly differentiated HCC [18]. Therefore, 18F-FDG-PET/CT–positive HCC is usually a negative predictor of response to various treatments (i.e., surgical resection, transarterial chemoembolization [TACE], and sorafenib) [19, 20, 21, 22]. Recently, we reported a high treatment response rate to lenvatinib in patients who had 18F-FDG-PET/CT–positive HCC [23]. However, the utility of 18F-FDG-PET/CT for predicting the prognosis of patients treated with Atezo/Bev has not been sufficiently evaluated. Therefore, the aim of this study was to evaluate the utility of 18F-FDG-PET/CT for predicting e-PD in patients with unresectable HCC following administration of Atezo/Bev.
Patients and Methods
Study Population
From November 2020 to October 2021, 43 patients received systemic anticancer treatment using Atezo/Bev for unresectable HCC at the Department of Hepatology, Toranomon Hospital, Tokyo, Japan. The inclusion criteria for the study were: (1) dynamic CT or magnetic resonance imaging (MRI) performed prior to initiation of Atezo/Bev, (2) tumors showing hyperenhancement in the arterial phase of dynamic CT or MRI, (3) 18F-FDG-PET/CT performed prior to initiation of Atezo/Bev, (4) Child-Pugh class A liver function at the time of lenvatinib initiation, (5) Barcelona Clinic Liver Cancer (BCLC) stage A-C tumor(s), (6) unresectable HCC in patients not wanting to undergo local ablation or chemoembolization therapy for various reasons (i.e., tumor size, number, and location; extrahepatic metastasis; TACE failure/refractoriness [24]; and various complications), (7) no history of treatment with Atezo/Bev, and (8) an observation period of ≥6 weeks. A total of 20 patients met these inclusion criteria. The study was approved by the institutional review board of our hospital (protocol no. 1438-H/B).
Diagnosis of HCC
The diagnosis of HCC was based predominantly on analysis of dynamic CT or MRI images that was governed by a protocol reported elsewhere [16, 25]. Briefly, when a liver nodule showed hyperattenuation in the arterial phase and washout in the portal or delayed phases in dynamic studies, the nodule was diagnosed as an HCC.
Imaging Analysis of HCC Using 18F-FDG-PET/CT
Within 1 month before initiation of lenvatinib, 18F-FDG-PET/CT was performed using a dedicated whole-body PET scanner (Biograph mCT Flow40; Siemens Healthcare, Erlangen, Germany). Synapse Vincent software ver. 4 (Fujifilm Medical Systems, Tokyo, Japan) was used for semiquantitative analysis, with the volume of interest drawn along the outline of the tumor, and the maximum SUV and mean SUV in each intra- and extrahepatic target tumor then calculated. Of the lesions measured, the one with the highest 18F-FDG uptake was selected and used to calculate the tumor-to-normal liver ratio (TLR). We next measured normal liver activity by drawing 3 nonoverlapping spherical 1-cm3 volume of interests on axial PET images of the liver (2 in the right lobe and 1 in the left lobe), avoiding the HCC areas seen on dynamic CT. The TLR was calculated using the following equation: TLR = maximum SUV of the tumor/ mean SUV of normal liver tissue. Based on previous reports [19, 20, 26], we selected a TLR ≥2 to indicate a high malignant potential.
Atezo/Bev Treatment and Adverse Event Assessment
Patients received intravenous Atezo (1200 mg) plus Bev (15 mg/kg) every 3 weeks. Treatment was discontinued following observation of any unacceptable or serious adverse event (AE) or clinical tumor progression. For assessment of AEs, the National Cancer Institute Common Terminology Criteria for AEs, ver. 4.0 [27] was used. Manufacturer guidelines for Atezo/Bev treatment were used.
Treatment Response Evaluation
Treatment response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) [28]. After initiation of Atezo/Bev, we assessed the best tumor response during the 6–24-week period. Treatment responses were assessed independently by an expert hepatologist (Y. Kawamura) and an expert hepatobiliary surgeon (J. Shindoh) who were blinded to the clinical data. Discrepancies between these two examiners were resolved by consensus review that included an additional reviewer (K. Ikeda).
Definition of e-PD
In this study, e-PD was defined as a target tumor showing PD by RECIST 1.1 criteria within 12 weeks of initiation of Atezo/Bev.
Definition of Early Progression-Free Survival
In this study, early progression-free survival (e-PFS) was defined as the time from initiation of Atezo/Bev to e-PD or death from any cause.
Definition of HPD
Tumor growth dynamics were assessed as the ratio of posttreatment tumor growth to pretreatment tumor growth using tumor growth kinetics (TGK) in the same target lesions. TGKPRE was defined as the difference of the sum of the largest diameters of the target lesions (according to RECIST 1.1) per unit of time between pre-baseline and baseline imaging. Similarly, TGKPOST was defined as the difference in the sum of the largest diameters of the target lesions per unit of time between baseline and time of PD diagnosis within 12 weeks of initiation of Atezo/Bev treatment.
The TGK ratio (TGKR) was defined as the ratio of TGKPOST to TGKPRE. A TGKR >1 indicated tumor growth acceleration, while 0< TGKR <1 and TGKR <0 indicated tumor deceleration and tumor shrinkage, respectively. HPD was defined as TGKR ≥2 [8].
Assessments of Hepatic Reserve Function
The Child-Pugh classification [29] and albumin-bilirubin (ALBI) grade were used to assess hepatic reserve function. ALBI grade was calculated based on serum albumin and total bilirubin values using the following formula: ALBI score = (0.66 × log10 bilirubin [µmol/L]) + (−0.085 × albumin [g/L]), with the result defined by the following scores: ≤ −2.60 = Grade 1, > −2.60 to ≤ −1.39 = Grade 2, and > −1.39 = Grade 3 [30]. For more detailed evaluations of patients with Grade 2 ALBI function, we used modified ALBI grading consisting of 4 levels, which included subgrading for grade (2a and 2b) based on an ALBI score of −2.27 as the cutoff, which was previously reported as an indocyanine green retention after 15 min value of 30% [31].
Follow-Up Protocol
Physicians examined patients every 1–3 weeks after initiation of Atezo/Bev, and laboratory biochemical and urine tests were also performed. After initiation of Atezo/Bev, patients underwent dynamic CT or MRI every 6–12 weeks to evaluate responses during the treatment period.
Statistical Analysis
Statistical analysis was performed using IBM SPSS software (ver. 27.0 SPSS Inc., Chicago, IL, USA). Data were expressed as the median and range. Differences in background features between each parameter were analyzed using Fisher's exact test. p values <0.05 were considered statistically significant. e-PFS and overall survival (OS) after introduction of Atezo/Bev were estimated by the Kaplan-Meier method, with values compared using the log-rank test.
To identify factors associated with e-PD and e-PFS after initiation of Atezo/Bev, a multivariate analysis was performed using logistic regression with backward elimination. Among potential independent variables, factors with a marginal association (p < 0.1) in univariate analysis were included in the initial model. Then, after stepwise selection, only factors that showed a statistically significant association with e-PD at p < 0.1 were included in the final model.
Results
Overview
Table 1 summarizes the baseline characteristics of the study population. The median age was 72.5 years and 16 (80%) patients were male. The median size of the largest tumor was 27.5 mm (range, 11.0–74.5 mm) and the median tumor number was 6 (range, 1–50). Of the 20 patients, 2 (10%) with BCLC stage A disease received Atezo/Bev due to tumor location, TACE failure/refractoriness, and patient preference; 10 (50%) patients presented with BCLC stage C disease (macrovascular invasion [n = 4]) (Vp2, n = 2; Vp4 = 1; and Vb2, n = 1); while 6 had extrahepatic metastasis. Seven patients (35%) had a history of treatment with lenvatinib and 11 patients (55%) had a TACE failure/refractoriness status. About the treatment line of Atezo/Bev, 13 of 20 (65%) patients received as first-line treatment, and the remaining patients received Atezo/Bev treatment as second to fourth-line treatment. Seven patients had died at the time of database lock (December 14, 2021), with the median duration of Atezo/Bev administration being 3.2 months and a median observation period of 7.4 months.
Table 1.
Clinical profiles and laboratory data of patients with HCC treated with Atezo/Bev
| Patient characteristics and laboratory data | |
| Patients, n | 20 |
| Males:females, n | 16:4 |
| Age, years (range)† | 72.5 (52–88) |
| HCV: HBV: NonB, NonC | 9:1:10 |
| Performance status 0:1, n (%) | 16 (80):4 (20) |
| Platelet count, ×103/µL (range)† | 121 (78–248) |
| Albumin, g/dL (range)† | 3.8 (3.4–4.5) |
| Total bilirubin, mg/dL (range)† | 0.9 (0.5–2.9) |
| Prothrombin activity, % (range)† | 86.4 (71.3–107.9) |
| AST, IU/L (range)† | 33 (18–214) |
| AFP, µg/L (range)† | 46.6 (2.1–30,353.0) |
| DCP, AU/L (range)† | 90.0 (14.0–27,045.0) |
| Child-Pugh score 5:6, n (%) | 12 (60):8 (40) |
| mALBI score (1:2a:2b:3), n (%) | 3 (15):9 (45):8 (40):0 (0) |
| History of lenvatinib treatment, n (%) | 7 (35) |
| Treatment line of Atezo/Bev 1st:2nd:3rd, 4th, n (%) | 13 (65):3 (15):3 (15):1 (5) |
| Tumor characteristics | |
| Largest tumor diameter, mm (range)† | 27.5 (11.0–74.5) |
| Tumors, n, n (range) | 6 (1–50) |
| Macrovascular invasion, n (%) | 4 (20) |
| Extrahepatic metastasis, n (%) | 6 (30) |
| BCLC stage A:B:C, n (%) | 2 (10):8 (40):10 (50) |
| TACE failure/refractoriness, n (%) | 11 (55) |
| 18F-FDG-PET/CT findings | |
| TLR ≥ 2.0, n (%) | 11 (55) |
AFP, alpha-fetoprotein; Atezo/Bev, atezolizumab plus bevacizumab; BCLC, Barcelona Clinic Liver Cancer; AST, aspartate aminotransferase; DCP, des-γ-carboxy prothrombin; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IU, international units; mALBI, modified albumin-bilirubin; NonB, NonC, neither HBV nor HCV infection present; TACE, transarterial chemoembolization; TLR, tumor-to-normal liver standardized uptake value ratio.
Data expressed as median (range).
Best Treatment Response and e-PD Rate after Initiation of Atezo/Bev according to TLR Value on 18F-FDG-PET/CT
At first, a cut-off TLR of 2 was used to define HCCs with high malignant potential. Based on TLR values calculated by RECIST 1.1, the objective response rate for tumors with TLR values or ≥2 and <2 was 18% and 33%, respectively; this difference was not statistically significant (p = 0.617) (Table 2). Based on TLR values calculated by RECIST 1.1, the e-PD rate for TLR values of ≥2 and <2 was 64% and 11%, respectively; this difference was statistically significant (p = 0.028) (Table 3). About, overall best treatment response calculated by RECIST 1.1, objective response rate was 25% (online suppl. Table 1; see www.karger.com/doi/10.1159/000523850 for all online suppl. material).
Table 2.
Evaluation of best treatment response after initiation of Atezo/Bev, grouped according to 18F-FDG-PET/CT findings and analysis of imaging features using RECIST 1.1
| 18F-FDG-PET/CT TLR | Response evaluation using RECIST 1.1, n (%) |
||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | ||
| TLR ≥2.0 (n = 11) | 0 (0) | 2 (18) | 6 (55) | 3 (27) | |
| TLR < 2.0 (n = 9) | 0 (0) | 3 (33) | 6 (67) | 0 (0) | |
| ORR, % | ||||
|---|---|---|---|---|
| TLR ≥2.0 (n = 11) TLR < 2.0 (n = 9) |
18 33 |
18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; Atezo/Bev, atezolizumab plus bevacizumab; CR, complete response; CT, computed tomography; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TLR, tumor-to-normal liver ratio.
Table 3.
Evaluation of e-PD after initiation of Atezo/Bev according to 18F-FDG-PET/CT findings using RECIST 1.1
| 18F-FDG-PET/CT TLR | Rate of e-PD, n (%) |
|
|---|---|---|
| e-PD positive | e-PD negative | |
| TLR ≥2.0 (n = 11) | 7 (64) | 4 (36) |
| TLR < 2.0 (n = 9) | 1 (11) | 8 (89) |
18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; Atezo/Bev, atezolizumab plus bevacizumab; e-PD, early progressive disease; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumors; TLR, tumor-to-normal liver ratio.
e-PFS after Initiation of Atezo/Bev according to TLR Value on 18F-FDG-PET/CT
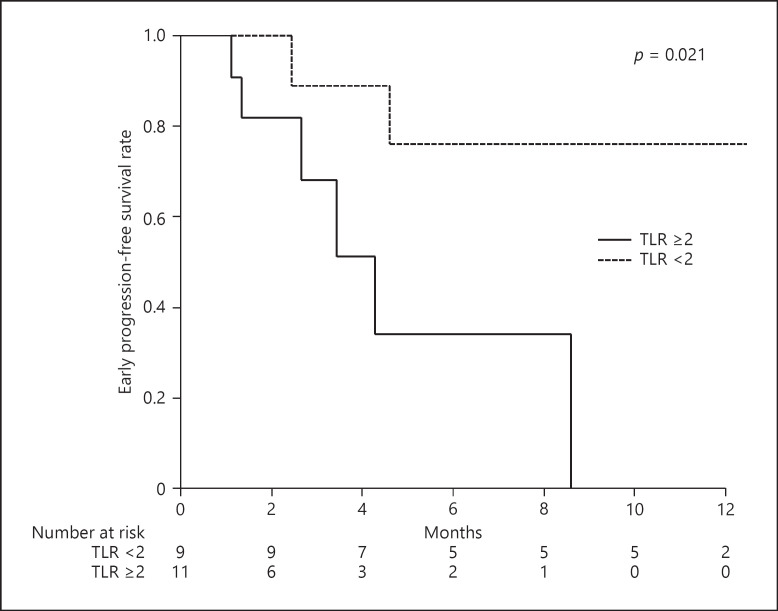
Figure 1 shows e-PFS according to TLR value; a significant difference was observed between groups (p = 0.021). On the other hand, overall e-PFS was 57% for 6 months and 47% for 12 months (online suppl. Fig. 1).
Fig. 1.
e-PFS according to TLR value on 18F-FDG-PET/CT prior to introduction of Atezo/Bev.
OS after Initiation of Atezo/Bev according to Presence of e-PD
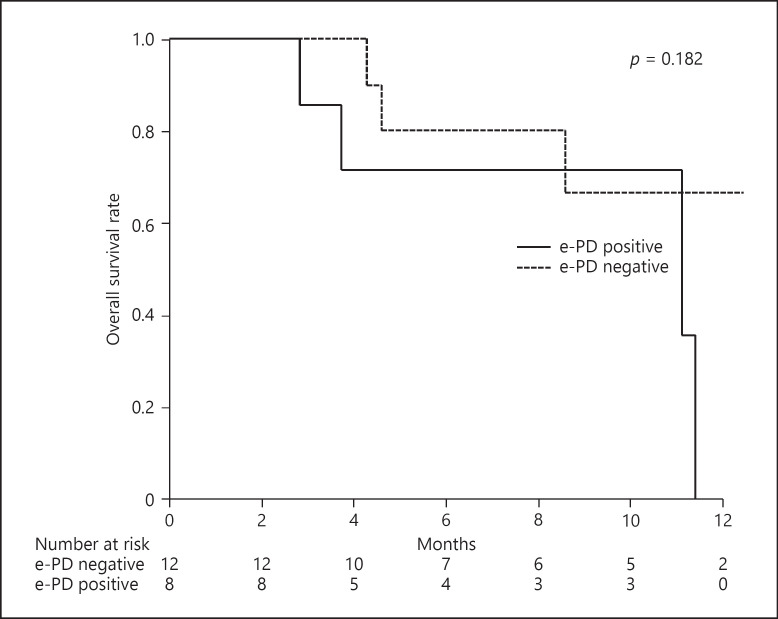
Figure 2 shows OS according to the presence of e-PD. The difference between groups was not statistically significant (p = 0.182). On the other hand, overall OS was 76% for 6 months and 38% for 12 months (online suppl. Fig. 2).
Fig. 2.
PFS according to TLR value on 18F-FDG-PET/CT prior to introduction of Atezo/Bev.
Treatment Course after Diagnosis of e-PD
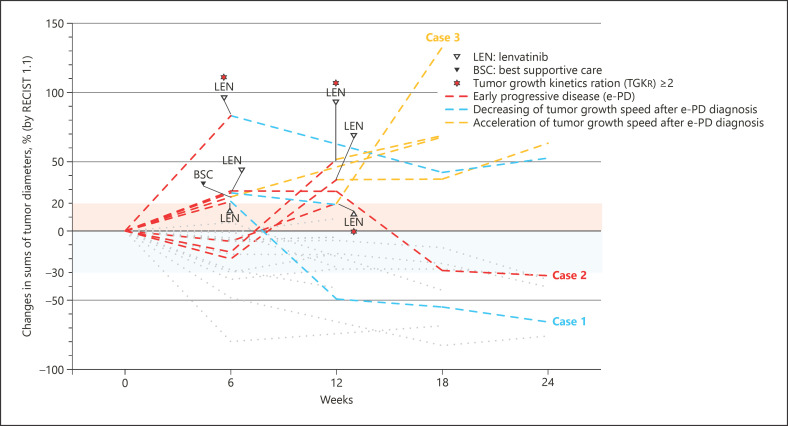
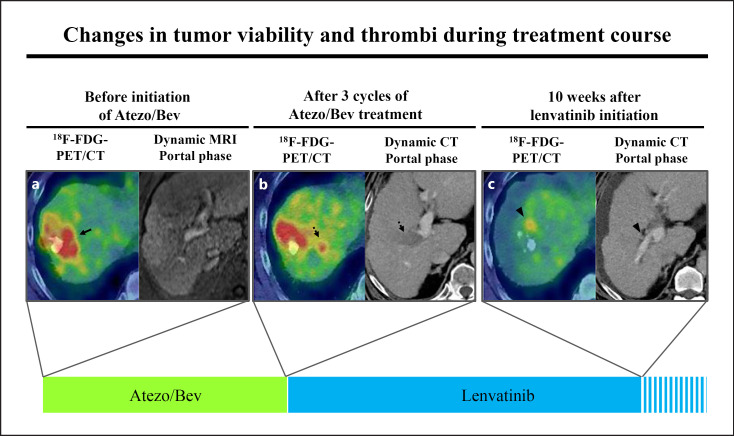
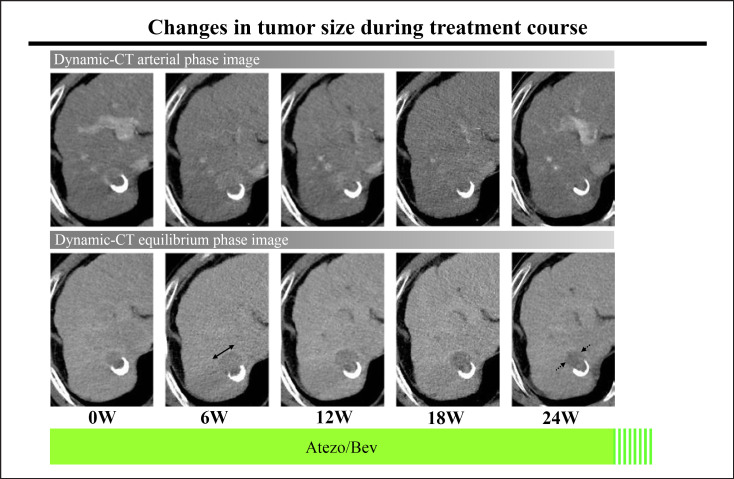
Figure 3 shows changes in tumor diameter during the treatment period using RECIST 1.1. Among patients who experienced e-PD, 6 patients received subsequent lenvatinib therapy; 3 of these patients experienced tumor shrinkage and 3 experienced further disease progression from the time of e-PD diagnosis. In addition, 1 patient showed marked tumor shrinkage, decreasing 18F-FDG accumulation, and tumor thrombus disappearance (Fig. 3, Case 1; Fig. 4).
Fig. 3.
Changes in sums of tumor diameters were evaluated by RECIST 1.1 during Atezo/Bev treatment.
Fig. 4.
Changes in tumor viability and thrombi during the Atezo/Bev treatment course. a Before initiation of Atezo/Bev, the tumor was 18F-FDG-PET/CT–positive (arrow). b After three cycles of Atezo/Bev, the tumor showed rapid progression and massive portal vein invasion (dotted arrow). c Ten weeks after lenvatinib initiation, the tumor showed marked shrinkage and decreasing FDG accumulation (arrowhead).
One patient experienced tumor shrinkage while receiving Atezo/Bev treatment. Therefore, this patient potentially had pseudoprogression during immunotherapy (Fig. 3, Case 2; Fig. 5). Among patients who experienced e-PD, only 1 patient showed 18F-FDG-PET/CT–negative result (TLR <2) at the time of Atezo/Bev initiation (Fig. 3, Case 3).
Fig. 5.
Pseudoprogression of HCC during Atezo/Bev treatment. The tumor showed rapid progression at 6 weeks after Atezo/Bev initiation. However, the tumor size gradually decreased until 24 weeks after Atezo/Bev initiation.
Predictors of e-PD and Unfavorable e-PFS after Introduction of Atezo/Bev
Table 4 summarizes the results of multivariate analysis for predictive factors for e-PD. Of the 14 variables tested, 18F-FDG-PET/CT positivity (TLR ≥2) (odds ratio, 14.485; 95% confidence interval [CI], 0.961–218.314; p = 0.053) and lenvatinib treatment history (odds ratio, 8.673; 95% CI, 0.686–109.734; p = 0.095) showed marginal significance as predictors of e-PD during Atezo/Bev treatment. Moreover, in multivariate analysis for predictive factors for unfavorable e-PFS, 18F-FDG-PET/CT positivity (TLR ≥2) (hazard ratio, 7.153; 95% CI, 1.258–40.689; p = 0.027), and des-γ-carboxy prothrombin level (hazard ratio, 1.012; 95% CI, 1.002–1.0022; p = 0.021) were significantly associated with poor e-PFS (Table 5).
Table 4.
Predictive factors for e-PD
| p value* | Coefficients† | SE | Wald χ2 | OR | 95% CI | |
|---|---|---|---|---|---|---|
| 18F-FDG-PET/CT-positive (TLR ≥ 2) | 0.053 | 2.673 | 1.384 | 3.730 | 14.485 | 0.961–218.314 |
| History of lenvatinib treatment | 0.095 | 2.160 | 1.295 | 2.784 | 8.673 | 0.686–109.734 |
18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; Atezo/Bev, atezolizumab plus bevacizumab; CI, confidence interval; SE, standard error; TLR, tumor-to-normal liver ratio; OR, odds ratio. Multivariate logistic regression was applied using stepwise backward selection. Of the potential predictors, factors showing a marginal association (p < 0.1) with e-PD after introduction of Atezo/Bev in univariate analysis were included in the initial model. The factors that showed no or limited statistically significant association (p > 0.1) adjusted for the remaining factors in the model were then deleted from the model in a stepwise fashion. The following 14 variables were tested (p values in univariate analysis): age (0.063), sex (0.501), etiology (HCV vs. other) (0.153), mALBI grade (0.255), prothrombin activity (0.212), serum alpha-fetoprotein (0.562), plasma des-γ-carboxy prothrombin (0.554), tumor diameter (0.979), tumor number (0.949), macrovascular invasion (0.650), extrahepatic metastasis (0.552), 18F-FDG-PET/CT positivity (TLR ≥2) (0.032), TACE failure/refractoriness (0.714), and lenvatinib treatment history (0.046).
Based on the likelihood test adjusted for other factors in the final model.
Estimated coefficient for the variable and associated SE.
Table 5.
Predictive factors for e-PFS
| p value* | Coefficients† | SE | Wald χ2 | HR | 95% CI | |
|---|---|---|---|---|---|---|
| 18F-FDG-PET/CT positivity (TLR ≥ 2) DCP + 100 AU/L | 0.027 0.021 | 1.968 0.012 | 0.887 0.005 | 4.921 5.322 | 7.153 1.012 | 1.258–40.689 1.002–1.022 |
18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; Atezo/Bev, atezolizumab plus bevacizumab; CI, confidence interval; DCP, des-γ-carboxy prothrombin; HR, hazard ratio; SE, standard error; TLR, tumor-to-normal liver ratio; mALBI, modified albumin-bilirubin. Multivariate Cox regression was applied using stepwise backward selection. Of the potential predictors, factors showing a marginal association (p < 0.1) with e-PFS after introduction of Atezo/Bev in univariate analysis were included in the initial model. The factors that showed no or limited statistically significant association (p > 0.1) adjusted for the remaining factors in the model were then deleted from the model in a stepwise fashion. The following 14 variables were tested (p values in univariate analysis): age (0.580), sex (0.768), etiology (HCV vs. other) (0.102), mALBI grade (0.546), prothrombin activity (0.142), serum alpha-fetoprotein (0.030), plasma des-γ-carboxy prothrombin (0.026), tumor diameter (0.203), tumor number (0.618), macrovascular invasion (0.414), extrahepatic metastasis (0.508), 18F-FDG-PET/CT positivity (TLR ≥2) (0.037), TACE failure/refractoriness (0.827), and lenvatinib treatment history (0.052).
Based on the likelihood test adjusted for other factors in the final model.
Estimated coefficient for the variable and associated SE.
Frequency of Grade ≥3 AEs following Initiation of Atezo/Bev
With respect to Grade 3 AEs, 3 of 20 patients (15%) experienced proteinuria, 1 of 20 (5%) experienced hypertension, 1 of 20 (5%) experienced fatigue, 1 of 20 (5%) experienced dizziness, 1 of 20 (5%) experienced bone infection, and 1 of 20 (5%) experienced ascites, respectively. With respect to Grade 4 or greater AEs, 1 of 20 (5%) experienced myocardial infarction, 1 of 20 (5%) experienced interstitial pneumonia, and 1 of 20 (5%) experienced sepsis. Finally, 2 patients died during the treatment course of AE (interstitial pneumonia and sepsis).
Discussion
18F-FDG-PET/CT is considered useful for predicting the degree of histological differentiation. 18F-FDG-PET/CT positivity has been reported to be strongly associated with poorly differentiated HCCs [18] and is therefore often a negative predictor of response to various treatments [19, 20, 21, 22]. Moreover, the utility of 18F-FDG-PET/CT for predicting patient outcomes, including HPD during immunotherapy, has been reported in other cancers [32, 33, 34, 35, 36, 37, 38]. However, as we reported previously [23, 39], 18F-FDG-PET/CT positivity (TLR ≥2) was correlated with a better response to lenvatinib, and PFS was similar in patients with 18F-FDG-PET/CT–negative disease (TLR <2). In contrast, in the present study, 18F-FDG-PET/CT positivity (TLR ≥2) was significantly associated with a high e-PD rate and poor e-PFS compared with a 18F-FDG-PET/CT–negative result (TLR <2) to Atezo/Bev treatment. In multivariate analysis, 18F-FDG-PET/CT positivity (TLR ≥2) and previous history of lenvatinib treatment showed marginal significance as factors predictive of e-PD. This study cohort had a high rate of previous lenvatinib treatment (35%). Overall, 8 of 20 (40%) patients experienced e-PD during the treatment period, and 5 of these 8 (63%) patients received lenvatinib immediately before Atezo/Bev treatment initiation. As we reported previously, in some patients with FDG accumulation changes, 18F-FDG-PET/CT–negative disease becomes positive during lenvatinib treatment [40]. Therefore, clinical history may have affected the present results. In addition, in this study, 3 of 20 (15%) patients showed HPD (TGKR ≥2) (Fig. 3); all 3 of these patients had previously received lenvatinib, and in 2 of 3 (67%) patients, readministration of lenvatinib controlled the rate of disease progression. As in the previous reports [41, 42], readministration of lenvatinib after diagnosed disease progression during immunotherapies has the potential of disease control effect in some patients. Recently, other researchers reported the utility of NLRs for predicting patient outcomes following Atezo/Bev treatment of HCC [15, 43, 44, 45]. Furthermore, Maesaka et al. [15] reported an NLR cut-off value of ≥3 as a useful predictor for HPD. However, in the present cohort, no significant differences in NLR were observed between the e-PD and non-e-PD populations (median NLR, 3.29 vs. 2.91, p = 0.624). This difference may have been due to the small number of cases in the current study. In addition, it should be noted that previously reported NLR cut-off values for predicting patient outcomes differ between studies [15, 43, 44, 45], and NLRs change due to various patient conditions (e.g., infections and certain medications, particularly steroid-based immunosuppressive regimens). Therefore, more robust NLR cut-off values are desirable for use in daily clinical practice. In contrast, TLR values acquired on 18F-FDG-PET/CT are useful predictive factors of patient outcomes, with a well-established cut-off value of ≥2 [18, 19, 20, 26]. In general, this value is not affected by patient conditions that do not alter blood glucose levels. Hence, TLR value on 18F-FDG-PET/CT appears to be a useful predictor of e-PD and e-PFS during Atezo/Bev treatment of HCC, and it has the potential to be widely used globally. A large-scale, multicenter study is required to evaluate the significance of TLRs prior to Atezo/Bev treatment.
This study had some limitations. First, it was a retrospective, single-center study that enrolled a relatively small series of patients. Second, the diagnosis of HCC was based exclusively on imaging analysis. Third, the follow-up period was relatively short compared with that of the global Phase III IMbrave150 study [4] (median follow-up period, 7.4 vs. 15.6 months, respectively). It was therefore not possible to conduct a high-quality prognostic analysis. Fourth, although 18F-FDG-PET/CT analysis is an optional imaging tool, it cannot be performed as easily compared as other types of imaging studies, such as CT or MRI, for various reasons, including the relatively high cost and the small number and uneven distribution of instruments required for the scans. Therefore, a large-scale, multicenter study is required to evaluate the utility of 18F-FDG-PET/CT for predicting overall prognosis and e-PD in patients with HCC receiving Atezo/Bev.
In conclusion, pretreatment 18F-FDG-PET/CT positivity may be a useful new predictor of e-PD and e-PFS and may enable early decision-making based on early treatment changes following Atezo/Bev treatment of HCC. For patients with a high TLR (≥2) prior to initiation of Atezo/Bev treatment, careful imaging follow-up should be conducted to identify e-PD.
Statement of Ethics
This retrospective, noninterventional study was approved by the Institutional Review Board, Toranomon Hospital (protocol No. 1438-H/B). The study was performed in accordance with the Declaration of Helsinki. Because the data were anonymized and the opt out option was disclosed on our institution's homepage (https://toranomon.kkr.or.jp/crc/files/uploads/2020/06/rinken_1438HB-3.pdf), the requirement for additional informed consent to participate in this study was deemed unnecessary according to the Japanese national regulations “Ethical Guidelines for Medical and Health Research Involving Human Subjects” (https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf).
Conflict of Interest Statement
Yusuke Kawamura, MD, PhD reports honoraria from Eisai Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Masahiro Kobayashi, MD reports honoraria from Eisai Co., Ltd. Junichi Shindoh, MD, PhD reports honoraria from Eisai Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Hiromitsu Kumada, MD, PhD reports honoraria from Eisai Co., Ltd. The other authors declare no conflicts of interest.
Funding Sources
This study was supported by Okinaka Memorial Institute for Medical Research and the Japanese Ministry of Health, Labour and Welfare.
Author Contributions
Yusuke Kawamura, MD, PhD: study concept and design, acquisition of data, statistical analysis, and drafting of the manuscript. Masahiro Kobayashi, MD: acquisition of data and statistical analysis. Junichi Shindoh, MD, PhD: acquisition of data and critical revision. Masaru Matsumura, MD, PhD: acquisition of data. Satoshi Okubo, MD, PhD: acquisition of data. Nozomu Muraishi, MD: acquisition of data. Shunichiro Fujiyama, MD: acquisition of data. Tetsuya Hosaka, MD: acquisition of data. Satoshi Saitoh, MD: acquisition of data. Hitomi Sezaki, MD: acquisition of data. Norio Akuta, MD, PhD: acquisition of data. Fumitaka Suzuki, MD, PhD: acquisition of data. Yoshiyuki Suzuki, MD, PhD: acquisition of data. Kenji Ikeda, MD, PhD: acquisition of data, statistical analysis, and study supervision. Yasuji Arase, MD, PhD: acquisition of data. Masaji Hashimoto, MD, PhD: acquisition of data. Hiromitsu Kumada, MD, PhD: acquisition of data.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was supported, in part, by grants from the Ministry of Health, Labour and Welfare in Japan and the Japan Agency for Medical Research and Development.
References
- 1.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39((3_Suppl l)):267–7. [Google Scholar]
- 5.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–5. [Google Scholar]
- 6.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase iii trial. J Clin Oncol. 2020 Jan 20;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 7.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017 Apr 15;23((8)):1920–8. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 8.Saada-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017 Jul 1;28((7)):1605–11. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018 Nov 1;4((11)):1543–52. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanjanapan Y, Day D, Wang L, Al-Sawaihey H, Abbas E, Namini A, et al. Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer. 2019 Apr 15;125((8)):1341–9. doi: 10.1002/cncr.31999. [DOI] [PubMed] [Google Scholar]
- 11.Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019 Jul 1;30((7)):1104–13. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang F, Zhong M, Yarden Y, Fu L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer. 2020 May 2;19((1)):81. doi: 10.1186/s12943-020-01200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019 May;49((10)):1323–33. doi: 10.1111/apt.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ, Kang B, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2021 Feb;74((2)):350–9. doi: 10.1016/j.jhep.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Maesaka K, Sakamori R, Yamada R, Tahata Y, Imai Y, Ohkawa K, et al. Hyperprogressive disease in patients with unresectable hepatocellular carcinoma receiving atezolizumab plus bevacizumab therapy. Hepatol Res. 2021 Dec 16;52((3)):298–307. doi: 10.1111/hepr.13741. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. Pretreatment heterogeneous enhancement pattern of hepatocellular carcinoma may be a useful new predictor of early response to lenvatinib and overall prognosis. Liver Cancer. 2020;9:1–18. doi: 10.1159/000505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9:1–16. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007 Jan 15;13((2 Pt 1)):427–33. doi: 10.1158/1078-0432.CCR-06-1357. [DOI] [PubMed] [Google Scholar]
- 19.Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006 Sep;30((9)):1736–41. doi: 10.1007/s00268-005-0791-5. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol. 2012 Jan;19((1)):156–62. doi: 10.1245/s10434-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 21.Song MJ, Bae SH, Lee SW, Song DS, Kim HY, Yoo IR, et al. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2013 Jun;40((6)):865–73. doi: 10.1007/s00259-013-2366-2. [DOI] [PubMed] [Google Scholar]
- 22.Sung PS, Park HL, Yang K, Hwang S, Song MJ, Jang JW, et al. 18F-fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur J Nucl Med Mol Imaging. 2018 Mar;45((3)):384–91. doi: 10.1007/s00259-017-3871-5. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. 18F-fluorodeoxyglucose uptake in hepatocellular carcinoma as a useful predictor of an extremely rapid response to lenvatinib. Liver Cancer. 2019:1–9. doi: 10.1159/000503577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87((Suppl 1)):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y, Ikeda K, Shindoh J, Kobayashi Y, Kasuya K, Fujiyama S, et al. No-touch ablation in hepatocellular carcinoma has the potential to prevent intrasubsegmental recurrence to the same degree as surgical resection. Hepatol Res. 2019;49((2)):164–176. doi: 10.1111/hepr.13254. [DOI] [PubMed] [Google Scholar]
- 26.Hyun SH, Eo JS, Lee JW, Choi JY, Lee KH, Na SJ, et al. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona clinic liver cancer stages 0 and a hepatocellular carcinomas: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging. 2016 Aug;43((9)):1638–45. doi: 10.1007/s00259-016-3348-y. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute . Division of cancer treatment and diagnosis. cancer therapy evaluation program. Adverse events/CTCAE. Available from: https://ctep.cancer.gov/protocol Development/electronic_applications/ctc.htm#ctc_40 (accessed January 21, 2021) [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45((2)):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60((8)):646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015 Feb 20;33((6)):550–8. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka A, Kumada T, Tsuji K, Takaguchi K, Itobayashi E, Kariyama K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019 Mar;8((2)):121–9. doi: 10.1159/000488778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito K, Schoder H, Teng R, Humm JL, Ni A, Wolchok JD, et al. Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. 2019 Apr;46((4)):930–9. doi: 10.1007/s00259-018-4211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castello A, Rossi S, Mazziotti E, Toschi L, Lopci E. Hyperprogressive disease in patients with non-small cell lung cancer treated with checkpoint inhibitors: the role of (18)F-FDG PET/CT. J Nucl Med. 2020 Jun;61((6)):821–6. doi: 10.2967/jnumed.119.237768. [DOI] [PubMed] [Google Scholar]
- 34.Chardin D, Paquet M, Schiappa R, Darcourt J, Bailleux C, Poudenx M, et al. Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: a prospective study. J Immunother Cancer. 2020 Jul;8((2)):e000645. doi: 10.1136/jitc-2020-000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamoto R, Zaba LC, Rosenberg J, Reddy SA, Nobashi TW, Davidzon G, et al. Prognostic value of volumetric PET parameters at early response evaluation in melanoma patients treated with immunotherapy. Eur J Nucl Med Mol Imaging. 2020 Nov;47((12)):2787–95. doi: 10.1007/s00259-020-04792-0. [DOI] [PubMed] [Google Scholar]
- 36.Seban RD, Moya-Plana A, Antonios L, Yeh R, Marabelle A, Deutsch E, et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur J Nucl Med Mol Imaging. 2020 Sep;47((10)):2301–12. doi: 10.1007/s00259-020-04757-3. [DOI] [PubMed] [Google Scholar]
- 37.Wong A, Callahan J, Keyaerts M, Neyns B, Mangana J, Aberle S, et al. 18F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging. 2020 May 14;20((1)):36. doi: 10.1186/s40644-020-00313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamoto R, C Zaba L, Rosenberg J, Arani Reddy S, W Nobashi T, Ferri V, et al. Imaging characteristics and diagnostic performance of 2-deoxy-2-[(18)F]fluoro-D-glucose PET/CT for melanoma patients who demonstrate hyperprogressive disease when treated with immunotherapy. Mol Imaging Biol. 2021 Feb;23((1)):139–47. doi: 10.1007/s11307-020-01526-4. [DOI] [PubMed] [Google Scholar]
- 39.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Muraishi N, et al. Pretreatment positron emission tomography with 18F-fluorodeoxyglucose may be a useful new predictor of overall prognosis following lenvatinib treatment. Oncology. 2021;99:1–11. doi: 10.1159/000516565. [DOI] [PubMed] [Google Scholar]
- 40.Yamashige D, Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, et al. Potential and clinical significance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for evaluating liver cancer response to lenvatinib treatment. Oncology. 2020 Nov;18:1–8. doi: 10.1159/000510754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoki T, Kudo M, Ueshima K, Morita M, Chishina H, Takita M, et al. Exploratory analysis of lenvatinib therapy in patients with unresectable hepatocellular carcinoma who have failed prior PD-1/PD-L1 checkpoint blockade. Cancers. 2020 Oct 20;12((10)):3048. doi: 10.3390/cancers12103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kudo M. Sequential therapy for hepatocellular carcinoma after failure of atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2021 Apr;10((2)):85–93. doi: 10.1159/000514312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheon J, Yoo C, Hong JY, Kim HS, Lee DW, Lee MA, et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42((3)):674–81. doi: 10.1111/liv.15102. [DOI] [PubMed] [Google Scholar]
- 44.Chuma M, Uojima H, Hattori N, Arase Y, Fukushima T, Hirose S, et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: a multicenter analysis. Hepatol Res. 2022;52((3)):269–80. doi: 10.1111/hepr.13732. [DOI] [PubMed] [Google Scholar]
- 45.Eso Y, Takeda H, Taura K, Takai A, Takahashi K, Seno H. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr Oncol. 2021 Oct 14;28((5)):4157–66. doi: 10.3390/curroncol28050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.